Abstract
The spatial relationships between brain and braincase represent a major topic in surgery and evolutionary neuroanatomy. In paleoneurology, neurocranial landmarks are often used as references for brain areas. In this study, we analyze the variation and covariation of midsagittal brain and skull coordinates in a sample of adult modern humans in order to demonstrate spatial associations between hard and soft tissues. The correlation between parietal lobe size and parietal bone size is very low, and there is a marked individual variation. The distances between lobes and bones are partially influenced by the dimensions of the parietal lobes. The main pattern of morphological variability among individuals, associated with the size of the precuneus, apparently does not influence the position of the neurocranial sutures. Therefore, variations in precuneal size modify the distance between the paracentral lobule and bregma, and between the parietal lobe and lambda. Hence, the relative position of the cranial and cerebral landmarks can change as a function of the parietal dimensions. The slight correlation and covariation among these elements suggests a limited degree of spatial integration between soft and hard tissues. Therefore, although the brain influences the cranial size and shape during morphogenesis, the specific position of the cerebral components is sensitive to multiple effects and local factors, without a strict correspondence with the bone landmarks. This absence of correspondent change between brain and skull boundaries suggests caution when making inferences about the brain areas from the position of the cranial sutures. The fact that spatial relationships between cranial and brain areas may vary according to brain proportions must be considered in paleoneurology, when brain anatomy is inferred from cranial evidence.
Keywords: geometric morphometrics, neuroanatomy, paleoneurology, parietal lobes, precuneus, vault
Introduction
The brain and the braincase are partially integrated through their functional and structural relationships (Richtsmeier et al. 2006). Brain growth generates pressures during morphogenesis, inducing changes on the elements of the braincase (Enlow, 1990). At the same time, such forces may be redirected by biomechanical tensors such as the meningeal layers (Moss & Young, 1960) or, on a smaller scale, by the neurons themselves (Van Essen, 1997; Hilgetag & Barbas, 2005), shaping the braincase and the cortex, respectively. The facial block and the cranial base exert further constraints on the neurocranial and cerebral system, adding further factors of correlation (Bookstein et al. 2003; Bastir & Rosas, 2005). In ontogenetic terms, the neural elements, maturing earlier, influence the basal and facial areas, which mature later (Bastir et al. 2006). Nonetheless, later changes of the facial block can induce minor changes in the brain morphology (Neubauer et al. 2009). In evolutionary terms, it is expected that the bone components influence the brain morphology at the endocranial base, whereas in the vault, the reverse situation is more likely, with the cortical tissue shaping the bony elements (Bruner, 2015). Integration plays a major role in phylogenetic and ontogenetic changes, but it seems somehow less decisive in shaping adult intraspecific variation. In adult variability, local factors still have a major role in influencing the endocranial (Bruner & Ripani, 2008) and cerebral (Bruner et al. 2010; Gómez-Robles et al. 2014) shape. In both cases, spatial proximity is the main source of integration suggesting that, at least in morphology, structural factors may be largely a matter of short range physical interactions. Such local influences and anatomical dissociation are therefore major forces in cranial evolution (Bookstein et al. 2003; Mitteroecker & Bookstein, 2008).
The spatial organization of brain and braincase is a relevant issue in medical and evolutionary fields. In microsurgery, the spatial relationships between cranial and cerebral points can supply relevant information during craniotomies and for intraoperative identification of the sulcal patterns (Ribas et al. 2006). The reciprocal influence between soft and hard neurocranial elements is also essential when dealing with pathological conditions altering the timing of growth and development, as in craniosynostoses (Aldridge et al. 2002). In paleoneurology, this information is necessary to provide reliable inferences on brain morphology from neurocranial osteometric landmarks (Holloway et al. 2004; Bruner et al. 2011; Ogihara et al. 2015). Previous analyses have been published which investigate the brain midsagittal shape variation in adult humans using digital anatomy and geometric morphometrics, this plane being relevant in terms of biological organization and human evolution (Bruner et al. 2010, 2014a). However, we will ignore how these brain morphological variations can influence the boundaries of the cranial elements, and to what extent the cranial boundaries can be used to get indirect information on the extension of the underlying brain areas.
The morphogenetic association between vault bones and lobes is due to brain pressure and redistribution of endocranial forces (Moss & Young, 1960; Enlow, 1990) and embryological processes shared by soft and hard tissues (Jiang et al. 2002; Morriss-Kay & Wilkie, 2005). This leads to a correspondence between the general morphology and surface geometry of brain and bones. Nonetheless, beyond the general vault curvature, at present we will ignore the extent to which the expansion of the bone, as delimited by its sutures, is influenced by brain size.
The pattern of suture displacement depends upon local factors and the precise distribution of such morphogenetic forces (Fig. 1). A correspondent growth of lobes and bones will involve proportional changes between these areas. In this case, for example, larger parietal lobes will involve larger parietal bones, and a proportional displacement of the respective sutures. Conversely, a non-linear growth, or a growth based on multiple independent factors, will involve a small or null spatial correlation between cranial and cerebral elements.
Fig. 1.
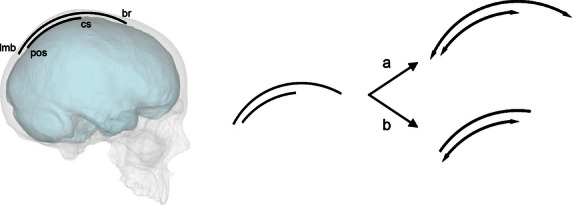
Parietal bones and parietal lobes share morphogenetic processes, displaying a correspondence in terms of curvature and size. However, we ignore whether larger lobes involve proportional larger bone (a) or whether their respective boundaries are not sensitive to reciprocal variations (b). Position of lambda (lmb), bregma (br), central sulcus (cs) and parieto-occipital sulcus (pos) are here approximated for graphic purposes.
To investigate these two alternatives, we analyzed the spatial variations of midsagittal cranial and cerebral landmarks in a sample of adult individuals in order to establish patterns and constraints associated with the relationships between hard and soft tissues according to the normal endocranial variability of our species. A null hypothesis is represented by an absence of association between bones and lobes, in terms of dimensions (as measured by diameters) and spatial position (as measured by landmark coordinates). In this case, larger lobes are not associated with larger bones, and the brain variations do not influence the dimensions of the bones and the position of their sutures. Conversely, with a direct and linear relationship, changes in one of these references (cranial bones or brain lobes) should be associated with corresponding changes in the others. In this case, the spatial relationships between cranial and brain landmarks should remain stable. If brain morphology has a direct influence on the growth of the adjacent bone elements, for example, larger parietal bones should be associated with larger parietal lobes, and the spatial relationships between lobes and bones should remain constant.
Materials and methods
One hundred adult individuals were sampled from the OASIS magnetic resonance (MRI) database (Marcus et al. 2007). The sample comprised 50 males and 50 females, with an age range of 20–40 years. This range was selected to include brains with full maturation and stable cortical morphology (according to Gogtay et al. 2004) but avoiding the following decades in which brain shrinkage can influence the spatial relations between brain and skull topology. MRI signal is based onto the concentration of water or fat, and it is therefore more suited to reveal the morphology of the soft tissues. Although hardly useful to reveal the cranial elements, it can show the position of the cranial sutures because of their connective content (Cotton et al. 2005). Using MRI to reveal sutures and bone boundaries can limit the resolution of the analysis, but nonetheless it represents a useful operational compromise for dealing with soft and hard tissues at once. Integration of tomographic and resonance data would be more suited for the scope of this study but, at present, it is not feasible for large samples in terms of costs, logistics, and X-ray exposure. Because of the noise associated with this operational limit, further research using different techniques will be necessary to supply more detailed data on this topic.
We analyzed the midsagittal section because it has many homologous landmarks for both brain and skull, being largely investigated in evolutionary neuroanatomy (Bruner et al. 2004, 2010). Twenty-three landmarks were sampled in two dimensions from brain and cranial references (Fig. 2). In particular, the boundaries between frontal, parietal, and occipital bones and lobes are of interest for this study to evaluate whether the position of the former can be used to estimate the position of the latter. Landmarks on the vault profile were all sampled along the endocranial surface, independently of the presence of meninges and the cerebrospinal fluid. Scans are below such a degree of resolution, and this minor approximation does not influence the macroanatomical variations we are interested in this study. Although this study concerns the midsagittal elements, landmarks have been localized using the information available throughout the complete MRI stacks. This approach is useful when dealing with individual variations, allowing the recognition on a larger scale of sulci and gyri beyond confounding factors such as the presence of connective and vascular components. Bregma (endobregma) and lambda (endolambda) were localized by following, throughout the MRI stacks, the course of the coronal and lambdoidal sutures. The sutures can be recognized moving along the stacks through transversal or sagittal sections, and bregma and lambda can be recognized as the midsagittal meeting point between the left and right sutures. The position of lambda compared with the location of the parieto-occipital sulcus, and the position of bregma compared with the central sulcus were specifically considered, being used generally to delimit the frontal, parietal and occipital territories. Landmarks were sampled by one researcher (H.A.). Intra-observer error based on five replicas digitized in five independent sessions shows a range of 0.4–1.8 mm, averaging 0.8 mm. Distances between bregma, the central sulcus, the marginal branch of the cingulate sulcus, lambda, and the perpendicular sulcus were quantified by studying the distributions of their distances. The distance between the marginal branch of the cingulate sulcus and the parieto-occipital sulcus represents the length of the precuneus. This diameter is particularly important considering previous results concerning its variation (Bruner et al. 2014a,b; Bruner et al. 2015). The distance between bregma and lambda represents the length of the parietal bone. The distance between the central sulcus and parieto-occipital sulcus represents the length of the parietal lobe. The distance between the central sulcus and bregma represents the overlapping area between parietal bone and frontal lobes. The distance between lambda and the perpendicular sulcus represents the overlapping area between parietal bone and occipital lobes. This last value can be negative, as in a few specimens the perpendicular sulcus can be positioned before lambda, which is under the occipital bone. A preliminary analysis showed a strong correlation between precuneus chord and arc (R = 0.997; P = 0.0001) and parietal bone chord and arc (R = 0.962; P = 0.0001). However, both arcs and chords will be used here as proxies of midsagittal size for bones and lobes in order to take into account the effect of bulging. First, an analysis based on linear correlation among bone and lobe lengths was aimed at investigating the overall proportions between hard and soft elements. Secondly, a shape analysis was performed to evaluate the role of the boundaries of bones and lobes within the main morphological correlation patterns. Shape variation, as represented by a geometrical model based on landmark coordinates, was analyzed according to the principles of geometric morphometrics (Zelditch et al. 2004). Two-dimensional coordinates from 23 cerebral and cranial landmarks (Fig. 2) were normalized by Procrustes superimposition, by translating to a common centroid, scaling to unitary centroid size, and rotated as to minimize the distance between corresponding landmarks (Bookstein, 1991). This registration minimizes the spatial differences within the sample, and the residuals after normalization are available to be analyzed through multivariate statistics. Shape coordinates were analyzed by principal component analysis, to describe and quantify the patterns of covariation among the transformed coordinates. The patterns of variation along the multivariate vectors can be visualized by coordinate displacement and geometrical models, or by using deformation grids based on thin-plate spline function, which interpolates the minimum deformation associated with the differences between configurations or along multivariate axes. As such, shape changes are strictly referred to relative proportions and spatial relationships, and not to the overall dimensions.
Fig. 2.
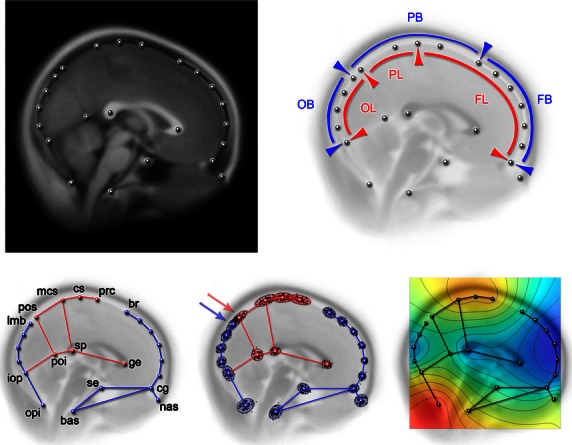
In the upper row, configuration of landmarks used in this analysis plotted on the mean superimposed images from the whole sample, displaying the average brain midsagittal morphology (left) and showing the spatial relationships between frontal, parietal and occipital lobes (FL, PL, OL) and bones (FB, PB, OB). In the lower row, landmarks with labels (red: brain landmarks; blue: cranial landmarks; unlabelled landmarks: semilandmarks) (left), scatterplot after Procrustes superimposition (center), and map of residual variation after registration (red – high; blue – small) (right). bas, basion; br, endobregma; cg, crista galli; cs, central sulcus; ge, genu; iop, interna occipital protuberance; lmb, endolambda; mcs, marginal ramus of the cingulate sulcus; nas, nasion; opi, opisthion; poi, parieto-occipital sulcus (internal); pos, parieto-occipital sulcus (external); prc, precentral sulcus; se, sella; sp, splenium. The arrows show the overlapping of the parieto-occipital sulcus (perpendicular scissure) and endolambda. Cranial and brain landmarks were sampled on the endocranial surface, independently of minor differences due to meningeal thickness, which is nonetheless negligible and not properly visible at the current resolution.
Correlation between different groups of landmarks (blocks) was also further tested by partial least-square correlation based on separate superimpositions (Rohlf & Corti, 2000). Statistics were computed with MorphoJ 1.06f (Klingenberg, 2011) and past 2.17 (Hammer et al. 2001).
Results
Table 1 shows the distribution of the distances between the main anatomical references. According to these values, the precuneus length is definitely more variable (coefficient of variation 20.6) than the parietal bone length (coefficient of variation 5.1). The correlation between parietal lobe and parietal bone lengths is low (chords: R = 0.27; P = 0.01; arcs: R = 0.32; P = 0.001) and the correlation between precuneus length and parietal bone length is even more modest (chords: R = 0.20; P = 0.05; arcs: R = 0.24; P = 0.02). Hence, although a larger precuneus is associated with larger parietal bone, this relationship explains only 4–6% of the latter values, suggesting a considerable individual variation based on different factors. When considering all the parietal lobe, its correlation with the parietal bone explains slightly more (7–10%). There is a moderate negative correlation between the precuneus length and the separation of the boundaries between the parietal bone and lobe (R = −0.37 and P = 0.0002 for both metrics, namely the distance between bregma and central sulcus, and the distance between lambda and the parieto-occipital sulcus). Therefore, the larger the precuneus, the more the boundaries of the lobe approach the boundaries of the bone. This suggests that the extension of the bone is not very sensitive to or associated with the extension of the lobe.
Table 1.
Distribution of the inter-landmark distances (mm)
| Mean | St. dev. | 25° | Median | 75° | |
|---|---|---|---|---|---|
| Bregma-central sulcus | 57.9 | 6.8 | 54.0 | 57.3 | 61.5 |
| Lambda-perpendicular sulcus | 10.1 | 6.9 | 5.7 | 10.3 | 14.5 |
| Parietal bone length | 114.2 | 5.8 | 109.8 | 114.2 | 118.8 |
| Parietal lobe length | 56.6 | 7.3 | 51.5 | 56.8 | 61.3 |
| Precuneus length | 37.0 | 7.6 | 31.3 | 37.3 | 41.4 |
The scatterplot of the Procrustes coordinates (Fig. 2) shows that, although lambda generally lies behind the parieto-occipital sulcus, there is some overlapping in the variations of these two landmarks. In fact, in 10 specimens (10% of the sample), lambda, which is generally located behind the parieto-occipital sulcus, was found to be positioned beyond it, and hence the boundary between the parietal and occipital bone trespasses on the boundary between the parietal and occipital lobes. In contrast, bregma is always very distant from the central and precentral sulci, well above the prefrontal cortex.
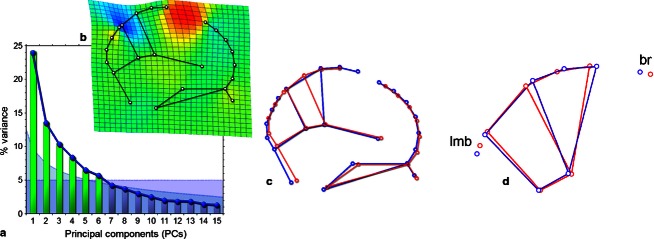
A principal component analysis of the Procrustes coordinates shows a dominant first component and a set of secondary components, showing only minor differences in their relative weight (Fig. 3). According to a broken stick threshold based on random distribution, and to a threshold of 5% of variance explained, at least the first six components are significant. Such minor differences between these secondary components can be interpreted as the result of the scarce morphological integration described in the skull (Bruner & Ripani, 2008) and brain (Bruner et al. 2010; Gómez-Robles et al. 2014) as a whole, and will not be discussed further here. Because of the dominant role of the first component, only this vector will be evaluated in detail, as a reliable biological pattern of covariation in this study. This first principal component explains 25% of the variance, and it is associated with dilation/contraction of the precuneus, displacing the central area anteriorly/posteriorly (Fig. 3). The proportions of the paracentral lobule do not change. This change only influences brain landmarks and not skull landmarks. Accordingly, along this vector, the relative position of the brain and cranial references does change. The dilation (lengthening) of the precuneus mainly occurs anteriorly, displacing the paracentral lobule towards a more forward position. Therefore, it involves a reduction in the distance between the central cortical area and bregma and, to a lesser extent, increases the distance between the parietal lobes and lambda. Hence, the position of the cranial references (bone boundaries) relative to the cerebral references (lobe boundaries) will depend upon the size of the parietal lobes. The rest of the spatial organization is not particularly influenced by this main pattern. The result is the same if we analyze males and females separately.
Fig. 3.
(a) scree plot of the Principal Component Analysis, showing in green the components beyond the broken stick threshold (blue curve) and the 5% variance (violet area); (b) deformation grid show the changes associated with the first component, with expansion factors (red: dilation; blue: compression); (c) the same pattern showed by wireframes; (d) wireframes for the principal component when considering only precuneus, paracentral lobule, bregma (br), and lambda (lmb).
If we analyze only the outer profile (from the internal occipital protuberance to crista galli) or only the cortical block (precuneus and paracentral lobule; Fig. 3d) the result is the same: a first principal component associated with precuneal lengthening/shortening, in which this change is particularly expressed forward, reducing/increasing the distance between the central cortical area and bregma.
A partial least-square correlation between the central cortical block (precuneus and paracentral lobule) and the rest of the configuration is not significant (P = 0.16), further demonstrating a lack of patent reciprocal association between these blocks.
In summary, we observed a modest correlation between parietal bone and lobe length, a large individual variation, and a variable spatial organization between cranial and cerebral elements. Variations of the brain proportions exert a minor influence on the extension of the vault bones. The reciprocal position of cranial and cerebral elements is not constant, and their morphology is sensitive to independent factors. The main pattern of variability is associated with increase of precuneus size, separating the boundaries between the parietal and occipital bone and lobe, and approaching the boundaries between the frontal and parietal bone and lobe.
Discussion
Spatial relationships between brain and sutures
The spatial relationships between brain and braincase represent a basic issue both in evolutionary and medical studies. Previous pioneering analyses in neurosurgery have been performed by dissection of cadavers (Ribas et al. 2006). However, analyses performed with physical dissections often have limits in terms of sample size because of the difficulties in performing these kinds of studies, and limits in terms of anatomical reliability because tissues are not observed in their functional conditions. In this study, we analyzed the geometric relationships between major cranial and cerebral landmarks in a sample of 100 adult humans, using MRI imaging, bivariate analysis, and geometric morphometric multivariate approaches. We computed a bivariate analysis to quantify the degree of correlation between the dimensions and position of the cortical and bone elements of the upper braincase. Then, we computed a shape analysis to investigate the role of these relationships within the major morphological schemes underlying the phenotypic structure.
The bivariate analysis showed that the correlation between parietal bones and lobes is very weak. Larger lobes are associated with larger bones but the correlation is low and there is considerable individual variability. In this sense, the null hypothesis is falsified because of the existence of a correlation, and we can state that larger parietal lobes are associated with larger parietal bones. However, this correlation is slight, suggesting the existence of further independent factors that makes this association feeble. The parietal lobes contribute to the extension of the parietal bones, but only to a limited extent, at least when considering intraspecific adult variability. In individuals with larger parietal lobes, the distance between the boundaries of lobes (sulci) and bones (sutures) is smaller. Therefore, we conclude that there is an allometric pattern in which the enlargement of the parietal bone does not keep pace with the enlargement of the parietal lobe and, by consequence, the boundaries of these two areas become closer. However, the pattern is weak and is influenced by other factors and by individual variation. The fact that the two areas do not show a correspondent variation, and that the correlation is slight, suggests that the spatial position of the cranial and cerebral elements is influenced by independent variables, with a limited integration between hard and soft tissues in the final phenotype.
Shape analysis was aimed at considering whether these spatial relationships influence the patterns of correlation which generate the phenotypic variation. In morphometrics, the covariance structure as revealed through multivariate statistics is able to quantify and characterize the strength of the correlation schemes constraining the phenotypic variability, namely the degree and patterns of morphological integration among the anatomical components (Wagner, 1984). Following these principles, studies in two (Bruner et al. 2010) and three (Gómez-Robles et al. 2014) dimensions suggest that the adult brain morphology shows a modest degree of integration, mostly based on local effects and physical proximity. Our data, integrating the cranial component with the brain shape geometry, are in agreement with these previous results, demonstrating only one dominant pattern of covariance, followed by many minor secondary vectors. This main pattern is associated specifically with the relative proportions of the precuneus, displacing back and forth the paracentral lobule formed by the precentral and postcentral areas. This same pattern was described previously using a different sample (Bruner et al. 2014a) and the results from that study are confirmed and reproduced here. This change of the precuneal area is not only a variation in parietal proportions compared with the rest of the brain, but it is also associated with an actual enlargement/reduction of the precuneal cortical surface (Bruner et al. 2015). The present analysis shows that this major morphological component, based on precuneus dimensions, involves brain geometry but without influencing in a corresponding way the bone extension. Therefore, precuneus enlargement/reduction changes secondarily the reciprocal positions of bones and lobes. The changes at the posterior boundary are less conspicuous, most of the spatial adjustment being associated with the displacement of the anterior areas. Once more, these results suggest independence between the cranial and cerebral elements: when the brain proportions change, the cranial boundaries do not change accordingly. Interestingly, no endocranial morphological changes were described in one case study in which a bregmatic bone was so large as to constitute an actual fifth component of the vault morphogenesis (Barberini et al. 2008). That case suggests a remarkable stability of the endocranial morphological system, independent of patent changes of the suture positions and patterns.
Therefore, we conclude that during morphogenesis, the bulging of the parietal lobe can influence the curvature of the parietal bone, but the spatial reciprocal organization of the cranial and cerebral elements varies according to other independent factors. Hence, we probably should distinguish a general morphological integration (form integration) from a more specific spatial integration (relative position of the anatomical elements). It is worth noting that the parietal enlargement associated with the braincase globularity of our species occurs in a very early post-natal stage (Neubauer et al. 2009; Neubauer, 2014) and, beside gross morphological changes, the parietal cortex also matures very early (Gogtay et al. 2004). In later stages, other brain and cranial districts undergo growth and development, changing the spatial relationships previously established (Bastir et al. 2006). With this information in mind, at least two different hypotheses can explain the partial independence between cranial and cerebral elements that we have described in this study among adult individuals: the changes of the spatial relationships between parietal bones and lobes can be achieved either during the parietal morphogenesis or else after this stage (Fig. 4). In the first case, the parietal bulging associated with the globularization stage specific for our species would change the spatial relationships between bones and lobes. The growing parietal volume displaces the frontal cortex, and the central sulcus approaches the frontal bone. In the second case, the parietal bulging would be associated with a corresponding (isometric) growth of the parietal bone. In this stage, there is a tighter integration of parietal bone and lobe. Such correspondence is then lost in successive stages, when the anterior areas (the frontal lobes and the facial block) grow and develop during later morphogenetic steps. Ontogenetic series will be necessary to evaluate these two alternatives.
Fig. 4.
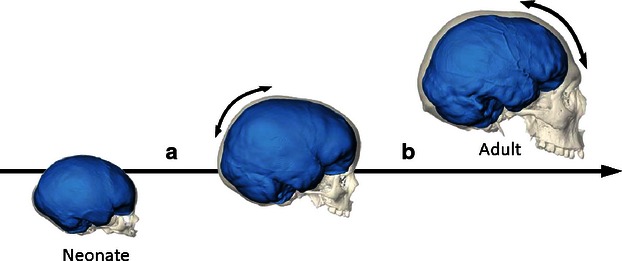
Parietal bulging in modern humans occurs in the early postnatal stage (a), while frontal and facial morphology undergo changes in successive ontogenetic steps (b). The spatial dissociation between cerebral and cranial elements can be the result of the early parietal growth. Alternatively, parietal bones and lobes can be more integrated during this stage, but the spatial association can be lost in the successive steps, after modification of the frontal and facial blocks (digital reconstructions after Neubauer, 2014).
Parietal enlargement and paleoneurology
Bregma and lambda are the boundaries between the frontal, parietal and occipital bones. The central sulcus and the perpendicular sulcus are the boundaries between the frontal, parietal and occipital lobes. Whereas bones are real biological units associated with specific morphogenetic elements, lobes are conventional units that do not represent actual neuroanatomical entities. Nonetheless, the correspondence between bones and lobes is often used as a possible reference in medicine and paleoneurology. In surgery, cranial landmarks are used as spatial references to plan and perform operations. In paleoneurology, cranial landmarks are used to estimate brain areas. In neurosurgery, current biomedical imaging provides direct support in developing and verifying a proper map of the relationships between skull and brain anatomy. In contrast, in paleoneurology, the soft tissues are lost, and brain shape can only be inferred using endocranial shape. The current analysis suggests that in modern humans, cranial sutures should not be used as fixed references to make inferences as to brain areas, at least according to their specific position. In this sense, ‘average’ distances between brain and skull landmarks may not be informative and may be even misleading, because they do not consider the reciprocal variations of these elements. The overall form of the brain surface (that is, its size and shape) can actually be extrapolated from the endocranial form, because brain growth molds the neurocranial bones directly, most of all at the vault (Moss & Young, 1960; Enlow, 1990). Furthermore, the correspondence between brain morphology and endocranial surface, although not complete, is also sufficiently reliable to localize major anatomical cortical traits on endocasts (Kobayashi et al. 2014a). In contrast, the specific extension of the cortical areas should not be inferred or extrapolated directly from the position of the cranial boundaries alone.
Quantitative scaling rules derived from the observed variation of relative spatial positions, as in the present analysis, could be used to extrapolate brain landmarks from cranial landmarks. Nonetheless, the current study demonstrates that, beyond such a lack of fixed proportions between bones and lobes boundaries, there is also an important individual variation, suggesting that multiple factors are involved in the final phenotype. In adult modern humans, the length of the parietal bone is influenced by the size of the parietal lobes to a very minor extent (7–10%) and this value is even lower when accounting only for the length of the precuneus (4–6%). Arcs displayed larger correlations than chords, revealing a role of the bulging effect; however, the increase of variance explained is scanty (2–3%). This means that, even if the parietal lobe moulds the shape and surface of the parietal bone (Moss & Young, 1960), it influences its longitudinal extensions to a much smaller degree. Correlations at interspecific level are often more pronounced than at intraspecific level, and therefore we may expect that this value, when comparing different hominids, may be larger. It in fact needs to be shown that this result refers to intraspecific adult variation. Intra- and interspecific correlation patterns can be based on very different mechanisms, the former being the result of normal variation and the latter of specific adaptations (Martin & Barbour, 1989). Integration, pleiotropy and polygeny create important connections between intra- and interspecific variability (e.g. Cheverud, 1982, 1996). However, results in one of these two domains should not be strictly regarded as results in the other. In this sense, studies in comparative primatology will be necessary to evaluate whether the patterns observed in the current analysis can be extended beyond the species-specific limits. For example, in macaques there are some stable relationships between cranial and cerebral references, at least when considering the coronal suture and cortical references associated with parasagittal elements of the frontal lobes (Kobayashi et al. 2014b). A similar consideration must be put forward when examining static variation (that is, adult variation, as as in this study) vs. ontogenetic variation. Nonetheless, without this information, we must take into account that vault bones and brain lobes may share shape (curvature) and surface morphology (sulcal traits), but the reciprocal position of their anatomical boundaries and extension is more variable and less reliable.
The shape variations described here are particularly important when considering that the precuneal changes responsible for modern human brain variability are very similar to the pattern of cranial variation associated with the evolution of the human skull (Bruner et al. 2014b). These two patterns are so comparable as to suggest a relation between intra- and interspecific variations. When compared with other hominids, Homo sapiens displays a distinct and specific increase of parietal bone diameters (Bruner et al. 2011). Hence, the pattern described in this study, responsible for the changes in the distances between bones and lobes, may be involved in generating the same cranial differences between modern and non-modern humans.
Non-modern human species lacked the parietal bulging described in modern humans, possibly even presenting a negative allometric trend: the larger the brain, the relatively shorter the parietal areas (Bruner, 2004). If the mismatch between brain and skull landmarks described in this study is also effective at the evolutionary level, we must infer that Neanderthals, having the largest cranial capacity of non-modern human species, probably displayed extreme values along this morphological vector. That is, Neanderthals may have had a bregma that was further away from the central sulcus compared with modern humans.
The parietal bone and the occipital bone are strongly integrated, and bulging of one of these areas is associated with flattening of the other (Gunz & Harvati, 2007). Interestingly, in Neanderthals, the relative shortening of the parietal areas, possibly related to encephalization and parietal constraints in the human genus, is associated with lambdatic supernumerary ossicles, suggesting a degree of morphogenetic imbalance in those areas (Manzi et al. 1996; Bruner, 2014). Taking into account the pattern described in this article, we may wonder whether such a lack of reciprocal adjustment between brain and skull references, associated with structural limits of a large brain size and the integration between the parietal and occipital bones, may be involved in those constraints and consequent morphological instability.
Conclusions
Paleoneurology aims to reconstruct brain form in fossil species by analyzing their endocranial anatomy (Holloway et al. 2004). Unfortunately, cortical imprints on the endocranial surface may be very faint, and much experience is needed to reveal cortical patterns on the endocranial mould. Generally, multiple sources of information are necessary to make such inferences, integrating metric and non-metric inputs from the neighboring cranial and endocranial characters. Despite such uncertainty, endocranial morphology is the only direct evidence of brain change in evolutionary neuroanatomy and we should try to optimize this resource. This study provides evidence that, at least when considering the adult intraspecific variation, the spatial correlation between cranial and cerebral elements is weak. The main source of morphological variation, i.e. the size of the precuneus, alters the reciprocal position of neural and cranial elements. Although the proportions of the parietal lobes are probably crucial in shaping the brain phenotype in both ontogenetic and phylogenetic terms (Bruner et al. 2014a,b, 2015), the extension of the lobes shows only a weak correlation with the extension of the parietal bone. Local influences and multiple factors associated with a non-linear morphogenetic process based on different and independent stages are probably the reason for such a lack of strict correspondence in the position of hard and soft tissues. The lack of strong integration patterns makes any relationship between cerebral and neurocranial boundaries feeble. Scaling rules can tentatively be investigated to evaluate how those boundaries can vary according to the variation of specific brain areas such as, in this case, the parietal cortex. Although brain and braincase show a reciprocal relation in terms of size (volume) and shape (curvature), the position of their anatomical elements is sensitive to independent factors. This independence must be considered when evaluating brain reconstruction in fossil species.
Acknowledgments
This study is funded by a Grant-in-Aid for Scientific Research on Innovative Areas ‘Replacement of Neanderthals by Modern Humans: Testing Evolutionary Models of Learning’ from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (#22101006). Emiliano Bruner and José Manuel de la Cuétara are funded by the Ministerio de Economía y Competitividad, Spain (CGL2012-38434-C03-02) and by the Italian Institute of Anthropology (Isita). Two anonymous referees provided very useful notes improving the structure of the manuscript and the interpretation of the results. We thank Gizéh Rangel and Sofia Pedro for their comments, and Simon Neubauer for the images in Fig. 4. The OASIS project is founded by NIH grants P50AG05681, P01AG03991, P20MH071616, RR14075, RR16594, BIRN002, the Alzheimer's Association, the James S. McDonnell Foundation, the Mental Illness and Neuroscience Discovery Institute, and the Howard Hughes Medical Institute. The authors declare no conflict of interest.
References
- Aldridge K, Marsh JL, Govier D, et al. Central nervous system phenotypes in craniosynostosis. J Anat. 2002;201:31–39. doi: 10.1046/j.1469-7580.2002.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberini F, Bruner E, Cartolari R, et al. An unusually-wide human bregmatic Wormian bone: anatomy, tomographic description, and possible significance. Surg Radiol Anat. 2008;30:683–687. doi: 10.1007/s00276-008-0371-0. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A. Hierarchical nature of morphological integration and modularity in the human posterior face. Am J Phys Anthropol. 2005;128:26–34. doi: 10.1002/ajpa.20191. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A, O'Higgins P. Craniofacial levels and the morphological maturation of the human skull. J Anat. 2006;209:637–654. doi: 10.1111/j.1469-7580.2006.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein FL. Morphometric Tools for Landmark Data: Geometry and Biology. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Bookstein FL, Gunz P, Mitteroecker P, et al. Cranial integration in Homo: singular warps analysis of the midsagittal plane in ontogeny and evolution. J Hum Evol. 2003;44:167–187. doi: 10.1016/s0047-2484(02)00201-4. [DOI] [PubMed] [Google Scholar]
- Bruner E. Geometric morphometrics and paleoneurology: brain shape evolution in the genus Homo. J Hum Evol. 2004;47:279–303. doi: 10.1016/j.jhevol.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Bruner E. Functional craniology, human evolution, and anatomical constraints in the Neanderthal braincase. In: Akazawa T, Ogihara N, Tanabe HC, Terashima H, editors. Dynamics of Learning in Neanderthals and Modern Humans. Vol. 2. Tokyo, Japan: Springer; 2014. pp. 121–129. [Google Scholar]
- Bruner E. Functional craniology and brain evolution. In: Bruner E, editor. Human Paleoneurology. Cham, Switzerland: Springer; 2015. pp. 57–94. [Google Scholar]
- Bruner E, Ripani M. A quantitative and descriptive approach to morphological variation of the endocranial base in modern humans. Am J Phys Anthropol. 2008;137:30–40. doi: 10.1002/ajpa.20837. [DOI] [PubMed] [Google Scholar]
- Bruner E, Saracino B, Ricci F, et al. Midsagittal cranial shape variation in the genus Homo by geometric morphometrics. Coll Antropol. 2004;28:99–112. [PubMed] [Google Scholar]
- Bruner E, Martin-Loeches M, Colom R. Human midsagittal brain shape variation: patterns, allometry and integration. J Anat. 2010;216:589–599. doi: 10.1111/j.1469-7580.2010.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner E, de la Cuétara JM, Holloway R. A bivariate approach to the variation of the parietal curvature in the genus Homo. Anat Rec. 2011;294:1548–1556. doi: 10.1002/ar.21450. [DOI] [PubMed] [Google Scholar]
- Bruner E, Rangel de Lázaro G, de la Cuétara JM, et al. Midsagittal brain variation and shape analysis of the precuneus in adult humans. J Anat. 2014a;224:367–376. doi: 10.1111/joa.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner E, de la Cuétara JM, Masters M, et al. Functional craniology and brain evolution: from paleontology to biomedicine. Front Neuroanat. 2014b;8:19. doi: 10.3389/fnana.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner E, Román FJ, de la Cuétara JM, et al. Cortical surface area and cortical thickness in the precuneus of adult humans. Neuroscience. 2015;286:345–352. doi: 10.1016/j.neuroscience.2014.11.063. [DOI] [PubMed] [Google Scholar]
- Cheverud JM. Relationships among ontogenetic, static, and evolutionary allometry. Am J Phys Anthropol. 1982;59:139–149. doi: 10.1002/ajpa.1330590204. [DOI] [PubMed] [Google Scholar]
- Cheverud JM. Developmental integration and the evolution of pleiotropy. Am Zool. 1996;36:44–50. [Google Scholar]
- Cotton F, Rozzi FR, Vallee B, et al. Cranial sutures and craniometric points detected on MRI. Surg Radiol Anat. 2005;27:64–70. doi: 10.1007/s00276-004-0283-6. [DOI] [PubMed] [Google Scholar]
- Enlow DH. Facial Growth. Philadelphia: Saunders; 1990. [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Robles A, Hopkins WD, Sherwood CC. Modular structure facilitates mosaic evolution of the brain in chimpanzees and humans. Nat Commun. 2014;5:4469. doi: 10.1038/ncomms5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunz P, Harvati K. The Neanderthal ‘chignon’: variation, integration, and homology. J Hum Evol. 2007;52:262–274. doi: 10.1016/j.jhevol.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Paleontol Electronica. 2001;4:1–9. [Google Scholar]
- Hilgetag CC, Barbas H. Developmental mechanics of the primate cerebral cortex. Anat Embryol. 2005;210:411–417. doi: 10.1007/s00429-005-0041-5. [DOI] [PubMed] [Google Scholar]
- Holloway RL, Broadfield DC, Yuan MS. The Human Fossil Record, Vol. 3: Brain Endocast. Hoboken: Wiley; 2004. [Google Scholar]
- Jiang X, Iseki S, Maxson RE, et al. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour. 2011;11:353–357. doi: 10.1111/j.1755-0998.2010.02924.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Matsui T, Haizuka Y, et al. Cerebral sulci and gyri observerd on macaque endocasts. In: Akazawa T, Ogihara N, Tanabe HC, Terashima H, editors. Dynamics of Learning in Neanderthals and Modern Humans. Vol. 2. Tokyo, Japan: Springer; 2014a. pp. 131–137. [Google Scholar]
- Kobayashi Y, Matsui T, Haizuka Y, et al. The coronal suture as an indicator of the caudal border of the macaque monkey prefrontal cortex. In: Akazawa T, Ogihara N, Tanabe HC, Terashima H, editors. Dynamics of Learning in Neanderthals and Modern Humans. Vol. 2. Tokyo, Japan: Springer; 2014b. pp. 139–143. [Google Scholar]
- Manzi G, Vienna A, Hauser G. Developmental stress and cranial hypostosis by epigenetic trait occurrence and distribution: an exploratory study on the Italian Neandertals. J Hum Evol. 1996;30:511–527. [Google Scholar]
- Marcus DS, Wang TH, Parker J, et al. Open Access Series of Imaging Studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci. 2007;19:1498–1507. doi: 10.1162/jocn.2007.19.9.1498. [DOI] [PubMed] [Google Scholar]
- Martin RD, Barbour AD. Aspects of line-fitting in bivariate allometric analyses. Folia Primatol. 1989;53:65–81. doi: 10.1159/000156409. [DOI] [PubMed] [Google Scholar]
- Mitteroecker P, Bookstein F. The evolutionary role of modularity and integration in the hominoid cranium. Evolution. 2008;62:943–958. doi: 10.1111/j.1558-5646.2008.00321.x. [DOI] [PubMed] [Google Scholar]
- Morriss-Kay GM, Wilkie AO. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J Anat. 2005;207:637–653. doi: 10.1111/j.1469-7580.2005.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss ML, Young RW. A functional approach to craniology. Am J Phys Anthropol. 1960;18:281–292. doi: 10.1002/ajpa.1330180406. [DOI] [PubMed] [Google Scholar]
- Neubauer S. Endocasts: possibilities and limitations for the interpretation of human brain evolution. Brain Behav Evol. 2014;84:117–134. doi: 10.1159/000365276. [DOI] [PubMed] [Google Scholar]
- Neubauer S, Gunz P, Hublin JJ. The pattern of endocranial ontogenetic shape changes in humans. J Anat. 2009;215:240–255. doi: 10.1111/j.1469-7580.2009.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogihara N, Amano H, Kikuchi T, et al. Towards digital reconstruction of fossil crania and brain morphology. Anthropol Sci. 2015;123:57–68. [Google Scholar]
- Ribas GC, Yasuda A, Ribas EC, et al. Surgical anatomy of microneurosurgical sulcal key-points. Neurosurgery. 2006;59:S177–S208. doi: 10.1227/01.NEU.0000240682.28616.b2. [DOI] [PubMed] [Google Scholar]
- Richtsmeier JT, Aldridge K, de Leon VB, et al. Phenotypic integration of neurocranium and brain. J Exp Zool B Mol Dev Evol. 2006;306B:360–378. doi: 10.1002/jez.b.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlf FJ, Corti M. The use of two-block partial least squares to study covariation in shape. Syst Biol. 2000;49:740–753. doi: 10.1080/106351500750049806. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Wagner GP. On the eigenvalue distribution of genetic and phenotypic dispersion matrices: evidence for a nonrandom organization of quantitative character variation. J Math Biol. 1984;21:77–95. [Google Scholar]
- Zelditch M, Swiderski D, Sheets D, et al. Geometric Shape Analysis for Biologists: A Primer. San Diego: Elsevier; 2004. [Google Scholar]