Abstract
The aim of this study was to quantitatively evaluate the function of the cranial diploic and spinal epidural veins as cerebrospinal fluid (CSF) drainage pathways by measuring lipocalin-type prostaglandin D synthase (PGDS) and cystatin C (CysC) dissolved in the blood of these veins. This was a prospective study involving 51 consecutive patients, 31 males and 20 females, who underwent 41 cranial and 10 spinal surgeries. Intraoperatively, peripheral venous blood and diploic venous blood, or peripheral venous blood and spinal epidural venous blood samples were simultaneously collected and immediately centrifuged. For all samples, dissolved albumin (for reference), PGDS and CysC were measured using an enzyme-linked immunosorbent assay. The diploic vein/peripheral vein ratios in five cranial locations and epidural vein/peripheral vein ratios were calculated and statistically evaluated for the three biomarkers. For PGDS, the diploic vein/peripheral vein ratio was significantly increased in the frontal (P = 0.011), temporal (P = 0.028), parietal (P = 0.046) and skull base (P = 0.039), while it did not reach statistical significance for CysC. For patients older than 45 years, the diploic vein/peripheral vein ratio for PGDS was significantly decreased in the frontal region (P = 0.028), and the epidural vein/peripheral vein ratio for CysC was significantly decreased (P = 0.014). These results show that the diploic veins constitute CSF drainage pathways with heterogeneous functional intensity at different cranial locations. Compared with the diploic veins, spinal epidural veins seem to drain less CSF. The cranial diploic and spinal epidural veins may jointly function as an alternative, age-related trans-dural CSF drainage system.
Keywords: biomarkers, CSF drainage, diploic veins, spinal epidural venous plexus
Introduction
It has been suggested that arachnoid granulations located in the walls of the cranial dural sinuses, olfactory mucosa, lymphatics and cranial nerve sheaths function as outlets for intracranial cerebrospinal fluid (CSF; Koh et al. 2005; Glimcher et al. 2008; Kapoor et al. 2008; Pollay, 2010). These arachnoid granulations have been explored by neuroimaging (Roche & Warner, 1996; Farb, 2007; Leach et al. 2008; Chen et al. 2011), post mortem dissection (Haybaeck et al. 2008; Chen et al. 2011; Yew et al. 2011), and by using casting material (Johnston et al. 2007) and ex vivo models (Glimcher et al. 2008). However, there have been few reports showing the process of intracranial CSF absorption in vivo (Tsutsumi et al. 2014). A fraction of intracranial arachnoidal structures protruding into the skull and contiguous with the diploic veins is present throughout the cranium; however, their distribution and functional implications have rarely been documented (Tsutsumi et al. 2014). On post mortem specimens, various fine arachnoidal structures are found to protrude into the skull through defects in the dura mater. Tsutsumi et al. (2013, 2014) proposed the hypothesis that these arachnoidal structures and diploic veins may function as alternative CSF drainage routes.
Previous studies have also indicated that the spinal CSF may be absorbed into the bloodstream through spinal arachnoid villi and granulations. Contrast agents with low-molecular weights, such as metrizamide (Golman, 1975; Eldevik, 1983), indium-labeled diethylene triamine pentaacetic acid (Partain et al. 1978) and iopamidol (Seyfert et al. 2003) were detected in the peripheral blood after they were infused into the lumbar subarachnoid space. In contrast, there have been few reports documenting the morphology of spinal arachnoid granulations with a substantially smaller diameter than those in the cranial cavity (Kido et al. 1976; Tubbs et al. 2007). However, in such studies that have been conducted thus far, a close relationship between the arachnoid granulations and intradural (Tubbs et al. 2007) or interdural (Kido et al. 1976) venous structures was suggested.
Lipocalin-type prostaglandin D synthase (PGDS) is an endogenous β chaperone with a molecular weight of 19 kDa that is present in the brain and secreted into the CSF (Yamashima et al. 1997; Urade & Hayashi, 2000). PGDS is thought as a CSF-specific biomarker, and is sensitive to inflammatory demyelinating diseases (Huang et al. 2009), Alzheimer's disease and normal pressure hydrocephalus (Kanekiyo et al. 2007). Cystatin C (CysC), a cysteine proteinase inhibitor with a molecular weight of 13 kDa, is known to be more highly expressed in the CSF compared with plasma (Carrette et al. 2005).
There is substantial anatomical and functional continuity between the veins, venous sinuses, and venous plexus of the brain and spine (Tobinick & Vega, 2006). On the other hand, the cerebrospinal dura mater delimits the subarachnoid spaces throughout the central nervous system, constituting broad interfaces between the arachnoid membranes and the extradural venous system. The objective of the present study was to quantitatively evaluate the cranial diploic veins and spinal epidural veins functioning as CSF drainage pathways by measuring PGDS and CysC levels dissolved in the blood of these venous systems.
Materials and methods
This prospective study was performed in accordance with the guidelines for human research of the Juntendo University Urayasu Hospital. Written informed consent was obtained from all patients.
The study included 51 adult patients with intact skull, dural sinuses and bony spinal canal who underwent craniotomy, endonasal transsphenoidal surgery or spinal surgery between December 2012 and November 2014. Patients with pituitary adenoma accompanying bony erosion in the sella turcica were included. Patients who underwent craniospinal surgeries, presented with CSF leakage, or with symptoms of increased intracranial pressure or intracranial hypotension were excluded from the study. The cranial part of the current study did not include patients from the earlier investigation (Tsutsumi et al. 2014). For these 51 patients, peripheral and diploic venous blood, or peripheral and epidural venous blood was simultaneously collected. The cranial locations for collecting the diploic venous blood were classified into frontal, temporal, parietal, occipital and skull base. The topological classification in the cranial convexity followed that of the cerebral lobes underneath. Suboccipital craniotomy and endonasal transsphenoidal surgery in which diploic venous blood was collected from the bony defects in the posterior cranial fossa and sella floor were allotted to the ‘skull base’ category. The diploic venous blood was manually aspirated in 5-mL syringes when drained from the bony edges at the cranial convexity and skull base. The epidural venous blood was manually aspirated in 5-mL syringes under direct vision, by puncturing the ventral or dorsal part of the internal vertebral venous plexus following bony resection at the anterior or posterior approach to the spinal canal. All samples were poured into test tubes without any mounting reagent, and were immediately centrifuged to remove cells and debris, aliquoted and finally stored in polypropylene tubes at −80 °C until ready for biochemical analysis. Albumin, PGDS and CysC were measured in all samples by enzyme-linked immunosorbent assay. Albumin was used as a reference marker, with a larger molecular weight (66 kDa) than PGDS and CysC. The diploic vein/peripheral vein ratios in each cranial site and the spinal epidural vein/peripheral vein ratio were calculated for PGDS and CysC. Furthermore, correlations between these ratios and the patients' age and body mass index were evaluated. The results were analyzed using the Wilcoxon signed-rank test (SPSS 18.0 software package; IBM; New York, NY, USA). A P-value less than 0.05 was considered statistically significant.
Results
General patient characteristics
Of the 51 patients, there were 31 men and 20 women with a mean age of 55 years (range, 25–87 years). The mean body mass index was 22.6 kg m−2, ranging from 16.2 to 31.9 kg m−2. Thirty-six patients underwent craniotomy, five had endonasal transsphenoidal surgery and 10 had spinal surgeries. The number of patients and pathology for each of the cranial and spinal surgeries performed are shown in Tables 1 and 2, respectively. Chiari type 1 malformation in Tables 1 and 2 were different patients.
Table 1.
Pathology and number of patients receiving cranial surgeries
| Pathology | n |
|---|---|
| Meta | 15 |
| Glioma | 7 |
| Meningioma | 5 |
| PT | 5 |
| TN | 2 |
| Neurinoma | 1 |
| Lymphoma | 1 |
| AVM | 1 |
| SAH | 1 |
| ICH | 1 |
| Hemifacial sp. | 1 |
| Chiari | 1 |
| Total | 41 |
AVM, arteriovenous malformation; ICH, intracerebral hemorrhage; PT, Pituitary tumor; SAH, subarachnoid hemorrhage; TN, Trigeminal neuralgia.
Table 2.
Pathology and number of patients receiving spinal surgeries
| Pathology | n |
|---|---|
| CS | 4 |
| LCS | 3 |
| IMCCT | 1 |
| Chiari | 1 |
| SIDEMT | 1 |
| Total | 10 |
CS, cervical spondylosis; IMCCT, intramedullary cervical cord tumor; LCS, lumbar canal stenosis; SIDEMT, sacral intradural extramedullary tumor.
Cranial surgery
The cranial locations for collecting the diploic venous blood are shown in Fig. 1. The diploic venous blood was collected with ease in all 41 patients who underwent cranial surgeries. The mean ± standard deviation PGDS levels in the peripheral and diploic venous blood were 162.2 ± 137.5 and 286.9 ± 299.9 ng mL−1 in the frontal area, 130.1 ± 106.8 and 386.8 ± 553.7 ng mL−1 in the temporal area, 118.3 ± 55.2 and 283.5 ± 253.8 ng mL−1 in the parietal area, 194.0 ± 106.6 and 398.0 ± 175.6 ng mL−1 in the occipital area, and 145.9 ± 141.8 and 177.1 ± 113.6 ng mL−1 in the skull base, respectively. The diploic vein/peripheral vein ratio in the frontal, temporal, parietal, occipital and skull base was 2.1 ± 1.5 in frontal, 2.9 ± 2.0, 2.6 ± 1.5, 3.0 ± 2.9 and 1.6 ± 0.8, respectively. The diploic vein/peripheral vein ratio was significantly increased in the frontal (P = 0.011), temporal (P = 0.028), parietal (P = 0.046) and skull base (P = 0.039). For patients older than 45 years, the diploic vein/peripheral vein ratio was significantly decreased in the frontal area (P = 0.028; Fig. 2).
Fig. 1.
Pie chart showing the cranial locations for collecting the diploic venous blood among 41 patients receiving cranial surgeries.
Fig. 2.
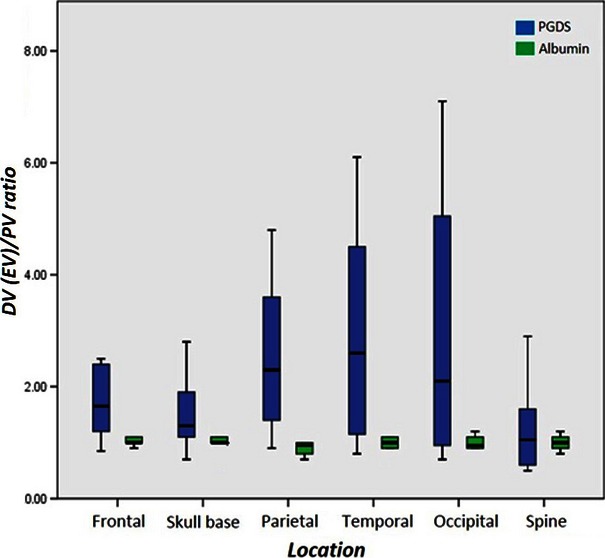
Box plot of diploic (DV) plus spinal epidural (EV) to peripheral vein (PV) ratios of prostaglandin D synthase (PGDS) and albumin concentration in different cranial locations and spine showing a significant increase in the frontal, temporal, parietal and skull base.
The CysC levels in the peripheral and diploic venous blood were 391.3 ± 223.8 and 385.7 ± 196.4 μg L−1 in the frontal area, 390.5 ± 271.1 and 532.8 ± 583.9 μg L−1 in the temporal area, 417.3 ± 183.1 and 377.0 ± 123.8 μg L−1 in the parietal area, 539.8 ± 304.0 μg L−1 in the occipital area, and 383.2 ± 188.5 and 359.3 ± 98.4 μg L−1 in the skull base, respectively. The diploic vein/peripheral vein ratio in the frontal, temporal, occipital and skull base was 1.2 ± 0.5, 1.2 ± 0.4, 1.0 ± 0.3, 1.0 ± 0.2 and 1.0 ± 0.3, respectively, and did not show a significant increase in any area (Fig. 3).
Fig. 3.
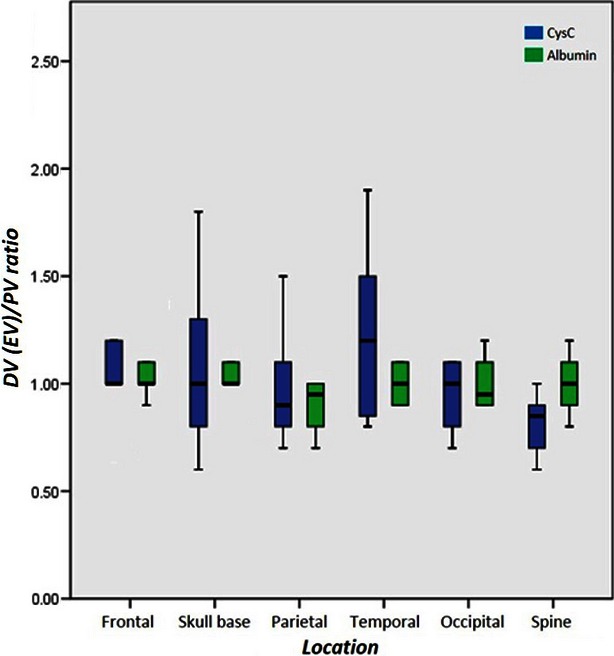
Box plot of diploic (DV) plus spinal epidural (EV) to peripheral vein (PV) ratios of cystatin C (CysC) and albumin concentration in different cranial locations and spine.
For both the PGDS and CysC, the body mass index was not a significant factor for the diploic vein/peripheral vein ratio.
Spinal surgery
Epidural venous blood was collected from the cervical, lumbar and sacral spine levels in six, three and one patient, respectively, that underwent spinal surgery. In four patients, the anterior approach was adopted, while the posterior approach was selected for the remaining six patients (Table 2). The epidural venous blood was collected with ease in all 10 of these patients. The PGDS levels in the peripheral and epidural venous blood were 182.2 ± 94.7 and 230.2 ± 182.9 ng mL−1, respectively. The epidural vein/peripheral vein ratio was 1.3 ± 0.8, which did not show a significant increase (Fig. 2).
The CysC levels in the peripheral and epidural venous blood were 402.8 ± 112.6 and 328.7 ± 102.2 μg L−1, respectively. The epidural vein/peripheral vein ratio was 0.8 ± 0.1, which did not reach a significant increase (Fig. 3). For patients older than 45 years, the epidural vein/peripheral vein ratio was significantly decreased (P = 0.014).
The epidural vein/peripheral vein ratio did not differ significantly between the four patients operated by an anterior approach in the supine position and the six patients who were treated by a posterior approach in the prone position. For both PGDS and CysC, body mass index was not a significant factor for the epidural vein/peripheral vein ratio.
Albumin showed a stable and constant value in the diploic, spinal epidural and peripheral venous blood (Figs 2 and 3).
Discussion
The present study showed that the intracranial CSF may drain into the diploic and spinal epidural veins. The diploic veins showed heterogeneous functional intensity at different cranial locations, and the spinal epidural veins drained relatively less CSF. These results suggest that in addition to the classic CSF drainage pathways through intracranial arachnoid granulations that directly protrude into the dural sinuses, there are alternative systems functioning as trans-dural routes throughout the craniospinal axis. Furthermore, the amounts of CSF draining through the cranial frontal region and spinal axis were shown to decrease in patients more than 45 years old, which was consistent with the two biomarkers assessed, PGDS for the former and CysC for the latter. This result may indicate that the trans-dural CSF absorption in the frontal region and spinal axis may regress with increased age. The difference in significant biomarkers between these two locations may partly be explained by the much smaller size of the spinal arachnoidal structures (Kido et al. 1976) compared with those in the intracranial cavity, which function as CSF outlets with a limited capability to permeate only a small amount of CSF and proteins with lower molecular weights. These results were probably influenced by the architecture, nature of the end group, and molecular weight of PGDS and CysC used for investigation, in addition to the property, distribution and diameter of each arachnoidal structure and connecting diploic vein. Given that all the blood samples were obtained under general anesthesia, drugs and anesthetics used intraoperatively might have influenced the results. Furthermore, interindividual variations in intracranial and intraspinal pressure and intravenous pressure may be an influencing factor.
In the present study, body mass index did not have a significant influence on CSF drainage, which is in contrast to a previous report that documented inverse correlation of iodine contrast transfer from lumbosacral CSF to blood (Seyfert et al. 2003). Furthermore, the epidural vein/peripheral vein ratio did not show a significant difference between the four patients that were operated on in the supine position and the six patients who were operated on in the prone position, in which the pressure in the epidural veins is likely to be increased. Given that all of the blood samples were collected during stable conditions under mechanical ventilation, these results might not appropriately reflect the true physiological condition of the patients. Further investigations with larger samples, using different CSF-specific biomarkers are needed to increase the accuracy of the results obtained in the present study.
The vertebral venous system is divided into three intercommunicating divisions. The first is the internal vertebral venous plexus, which consists of veins surrounding the spinal dura mater. This part of the plexus is partially supplied by radicular veins arising intradurally and veins distributing in the posterior part of the bony canal. The second division is the basivertebral veins, which comprise the vessels in the vertebral bodies, and the third is the external vertebral venous plexus that surrounds the vertebral column (Groen et al. 1997). The human spinal arachnoid villi and granulations are divided into those located entirely internal to the dura, those that are extended into the dura, and those that penetrate the dura completely. Furthermore, most arachnoid proliferations found along the dural sleeves were located adjacent to the dural sinuses (Groen et al. 1997; Griessenauer et al. 2015). These anatomical findings strongly suggest that the CSF in the spinal subarachnoid space is connected to the epidural veins. Therefore, it was considered that trans-dural CSF drainage in the spinal axis could be monitored by measuring the CSF-specific biomarkers dissolved in the spinal epidural venous blood. Previous studies did not use endogenous biomarkers (Golman, 1975; Partain et al. 1978; Eldevik, 1983; Seyfert et al. 2003).
Despite the shortcomings in the methodology and the relatively low number of subjects included in the present analysis, the authors believe that these results convincingly support the notion that at least a fraction of the intracranial and intraspinal CSF is drained into the cranial diploic and spinal epidural veins throughout the craniospinal axis.
Concluding remarks
The diploic veins constitute CSF drainage pathways with heterogeneous functional intensity at different cranial locations. Compared with the cranial diploic veins, spinal epidural veins appear to drain less CSF. These results suggest that the cranial diploic and spinal epidural veins may jointly function as an alternative, age-related trans-dural CSF drainage system.
Acknowledgments
This work did not receive any grant.
Competing interests
The authors declare no competing interests concerning the materials or methods used in this study or the findings specified in this paper.
References
- Carrette O, Burkhard PR, Hughes S, et al. Truncated cystatin C in cerebrospinal fluid: technical [corrected] artefact or biological process? Proteomics. 2005;5:3060–3065. doi: 10.1002/pmic.200402039. [DOI] [PubMed] [Google Scholar]
- Chen F, Deng XF, Liu B, et al. Arachnoid granulations of middle cranial fossa: a population study between cadaveric dissection and in vivo computed tomography examination. Surg Radiol Anat. 2011;33:215–221. doi: 10.1007/s00276-010-0733-2. [DOI] [PubMed] [Google Scholar]
- Eldevik OP. Elimination of metrizamide from the spinal subarachnoid space: a study of patients with abolished intracranial circulation. Am J Neuroradiol. 1983;4:585–587. [PMC free article] [PubMed] [Google Scholar]
- Farb RI. The dural venous sinuses: normal intraluminal architecture defined on contrast-enhanced MR venography. Neuroradiology. 2007;49:727–732. doi: 10.1007/s00234-007-0250-0. [DOI] [PubMed] [Google Scholar]
- Glimcher SA, Holman DW, Lubow M, et al. Ex vivo model of cerebrospinal fluid outflow across human arachnoid granulations. Invest Ophthalmol Vis Sci. 2008;49:4721–4728. doi: 10.1167/iovs.08-2238. [DOI] [PubMed] [Google Scholar]
- Golman K. Absorption of metrizamide from cerebrospinal fluid to blood: pharmacokinetics in humans. J Pharm Sci. 1975;64:405–407. doi: 10.1002/jps.2600640310. [DOI] [PubMed] [Google Scholar]
- Griessenauer CJ, Raborn J, Foreman P, et al. Venous drainage of the spine and spinal cord: a comprehensive review of its history, embryology, anatomy, physiology, and pathology. Clin Anat. 2015;28:75–87. doi: 10.1002/ca.22354. [DOI] [PubMed] [Google Scholar]
- Groen RJ, Groenewegen HJ, van Alphen HA, et al. Morphology of the human internal vertebral venous plexus: a cadaveric study after intravenous Araldite CY 221 injection. Anat Rec. 1997;249:285–294. doi: 10.1002/(SICI)1097-0185(199710)249:2<285::AID-AR16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Haybaeck J, Silye R, Soffer D. Dural arachnoid granulations and “giant” arachnoid granulations. Surg Radiol Anat. 2008;30:417–421. doi: 10.1007/s00276-008-0345-2. [DOI] [PubMed] [Google Scholar]
- Huang YC, Lyu RK, Tseng MY, et al. Decreased intrathecal synthesis of prostaglandin D2 synthase in the cerebrospinal fluid of patients with acute inflammatory demyelinating polyneuropathy. J Neuroimmunol. 2009;206:100–105. doi: 10.1016/j.jneuroim.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Johnston K, Walji AH, Fox RJ, et al. Access to cerebrospinal fluid absorption sites by infusion into vascular channels of the skull diplö. J Neurosurg. 2007;107:841–843. doi: 10.3171/JNS-07/10/0841. [DOI] [PubMed] [Google Scholar]
- Kanekiyo T, Ban T, Aritake K, et al. Lipocalin-type prostaglandin D synthase/beta-trace is a major amyloid beta-chaperone in human cerebrospinal fluid. Proc Natl Acad Sci USA. 2007;104:6412–6417. doi: 10.1073/pnas.0701585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor KG, Katz SE, Grzybowski DM, et al. Cerebrospinal fluid outflow: an evolving perspective. Brain Res Bull. 2008;77:327–334. doi: 10.1016/j.brainresbull.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Kido DK, Gomez DG, Pavese AM, Jr, et al. Human spinal arachnoid villi and granulations. Neuroradiology. 1976;11:221–228. doi: 10.1007/BF00328377. [DOI] [PubMed] [Google Scholar]
- Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. 2005;2:6. doi: 10.1186/1743-8454-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach JL, Meyer K, Jones BV, et al. Large arachnoid granulations involving the dorsal superior sagittal sinus: findings on MR imaging and MR venography. Am J Neuroradiol. 2008;29:1335–1339. doi: 10.3174/ajnr.A1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partain CL, Wu HP, Staab EV, et al. A multiregional kinetics model of cerebrospinal fluid. Radiology. 1978;127:705–711. doi: 10.1148/127.3.705. [DOI] [PubMed] [Google Scholar]
- Pollay M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res. 2010;7:9. doi: 10.1186/1743-8454-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche J, Warner D. Arachnoid granulations in the transverse and sigmoid sinuses: CT, MR, and MR angiographic appearance of a normal anatomic variation. Am J Neuroradiol. 1996;17:677–683. [PMC free article] [PubMed] [Google Scholar]
- Seyfert S, Koch HC, Kunzmann V. Conditions of iodine contrast transfer from lumbosacral CSF to blood. J Neurol Sci. 2003;206:85–90. doi: 10.1016/s0022-510x(02)00340-4. [DOI] [PubMed] [Google Scholar]
- Tobinick E, Vega CP. The cerebrospinal venous system: anatomy, physiology, and clinical implications. MedGenMed. 2006;8:53. [PubMed] [Google Scholar]
- Tsutsumi S, Nakamura M, Tabuchi T, et al. Calvarial diploic venous channels: an anatomic study using high-resolution magnetic resonance imaging. Surg Radiol Anat. 2013;35:935–941. doi: 10.1007/s00276-013-1123-3. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S, Ogino I, Miyajima M, et al. Cranial arachnoid protrusions and contiguous diploic veins in CSF drainage. Am J Neuroradiol. 2014;35:1735–1739. doi: 10.3174/ajnr.A4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubbs RS, Hansasuta A, Stetler W, et al. Human spinal arachnoid villi revisited: immunohistological study and review of the literature. J Neurosurg Spine. 2007;7:328–331. doi: 10.3171/SPI-07/09/328. [DOI] [PubMed] [Google Scholar]
- Urade Y, Hayashi O. Prostaglandin D synthase: structure and function. Vitam Horm. 2000;58:89–120. doi: 10.1016/s0083-6729(00)58022-4. [DOI] [PubMed] [Google Scholar]
- Yamashima T, Sakuda K, Tohma Y, et al. Prostaglandin D synthase (beta-trace) in human arachnoid and meningioma cells: role as a cell marker or in cerebrospinal fluid absorption, tumorigenesis, and calcification. J Neurosci. 1997;17:2376–2382. doi: 10.1523/JNEUROSCI.17-07-02376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew M, Dubbs B, Tong O, et al. Arachnoid granulations of the temporal bone: a histologic study of dural and osseous penetration. Otol Neurotol. 2011;32:602–609. doi: 10.1097/MAO.0b013e3182129026. [DOI] [PubMed] [Google Scholar]


