Abstract
Historically, brain neurochemicals have been broadly classified as energetic or informational. However, increasing evidence implicates metabolic substrates and byproducts as signalling agents, which blurs the boundary between energy and information, and suggests the introduction of a new category for ‘translational’ substances that convey changes in energy state to information. One intriguing example is hydrogen peroxide (H2O2), which is a small, readily diffusible molecule. Produced during mitochondrial respiration, this reactive oxygen species, can mediate dynamic regulation of neuronal activity and transmitter release by activating inhibitory ATP-sensitive K+ (KATP) channels, as well as a class of excitatory non-selective cation channels, TRPM2. Studies using ex vivo guinea pig brain slices have revealed that activity-generated H2O2 can act via KATP channels to inhibit dopamine release in dorsal striatum and dopamine neuron activity in the substantia nigra pars compacta. In sharp contrast, endogenously generated H2O2 enhances the excitability of GABAergic projection neurons in the dorsal striatum and substantia nigra pars reticulata by activating TRPM2 channels. These studies suggest that the balance of excitation vs. inhibition produced in a given cell by metabolically generated H2O2 will be dictated by the relative abundance of H2O2-sensitive ion channel targets that receive this translational signal.
Margaret E. Rice is a Professor in the Department of Neurosurgery and the Department of Neuroscience and Physiology at New York University School of Medicine. She received her PhD in chemistry from the University of Kansas, with a focus on neurochemistry. Her NIH-funded laboratory studies factors that regulate the release of dopamine, a key transmitter in motor and reward pathways in the brain. On-going projects include studies of axonal dopamine release regulation in dorsal and ventral striatum by hydrogen peroxide and by diet and insulin. Her group is also pursuing mechanistic studies of somatodendritic dopamine release, focused on substantia nigra dopamine neurons that degenerate in Parkinson's disease. Dr Rice is a member of the NYU Parkinson and Movement Disorders Center.

Introduction
F. O. Schmitt and Fred Samson introduced the notion of ‘informational’ substances to describe a neurochemical class that is distinct from ‘energetic’ substances (Schmitt, 1984, 1985). In this classification scheme, informational substances include neurotransmitters, exemplified by glutamate and GABA, whereas energetic substances include metabolic substrates, like glucose and oxygen. Increasingly, however, the line between these broad categories has blurred. Glutamate is not only the primary excitatory transmitter in the CNS, but also an important metabolic substrate (e.g. Dienel, 2013), and glucose not only provides energy for cells, but also conveys information that modulates the activity of specific glucose-sensing neurons (Routh, 2010; Thorens, 2012). Adding further complexity to the delineation between information and energy is that oxygen consumption during mitochondrial respiration, an energetic process, generates reactive oxygen species (ROS), including superoxide (•O2−) and hydrogen peroxide (H2O2) (Boveris & Chance, 1973; Dugan et al. 1995; Liu et al. 2002; Bao et al. 2009; Rigoulet et al. 2011; Mailloux et al. 2013) that can act as signalling molecules (Sundaresan et al. 1995; Kamsler & Segal, 2004; Rhee, 2006; Stone & Yang, 2006; Avshalumov et al. 2007; Kishida & Klann, 2007; Miller et al. 2007b; Gerich & Funke, 2009; Groeger et al. 2009; Rigoulet et al. 2011; Rice, 2011; Jeong et al. 2012; Sies, 2014).
Molecules like ROS are at the interface between energy and information, and merit a class of their own. Indeed, they could be viewed as ‘translational’ substances that provide an interpretation of cellular activity within a cell or to neighbouring cells. In the case of ROS in the CNS, increased neuronal activity drives energy demand, which drives metabolism, and thereby generates signalling molecules that can act both within individual neurons (as intracellular signals) and between neurons (as diffusible messengers) to give immediate modulatory feedback about local activity. Other molecules in this class might include ATP, adenosine and glucose.
A particularly strong candidate as a ‘translational’ substance is H2O2. During mitochondrial electron transport, oxygen is first reduced to the free radical •O2−, up to 5% of which leaves the respiratory process (Arnaiz et al. 1999), and can be converted to H2O2 by the enzyme superoxide dismutase or by spontaneous dismutation (see Peuchen et al. 1997). Oxygen consumption is proportional to local activity (Kennedy et al. 1992), with the greatest demand to support ATP-dependent signalling, e.g. informational processes (Engl & Attwell, 2015). The H2O2 produced in this process is well positioned to serve as a dynamic reporter of neuronal activity. Other slower metabolic processes also generate H2O2, including NADPH oxidases (NOXs) that produce •O2−, and thus H2O2 (Babior, 1984; Lambeth, 2004; Infanger et al. 2006; Rhee, 2006; Bedard & Krause, 2007), and monoamine oxidases (MAOs), which produce one molecule of H2O2 for each biogenic amine molecule metabolized (Maker et al. 1981; Azzaro et al. 1985; Cohen, 1994). These additional sources allow a range of timescales for H2O2 signalling that extend from a rapid, subsecond level for mitochondrial H2O2 generation to slower regulation by growth factor activation of NOX, for example, which can proceed over hours, days, or even longer (Miller et al. 2007b).
Among the best-studied intracellular targets for slow regulation by H2O2 are redox-sensitive phosphatases that are inactivated by H2O2 and kinases that are activated by this ROS (Klann & Thiels, 1999; Rhee et al. 2005; Woolley et al. 2013). Modulation of phosphatase and kinase activities regulates signal propagation downstream of receptors, so that H2O2 helps fine-tune transmission to further downstream targets, including transcription factors like p53, NF-κB and AP-1, which are also H2O2 sensitive (Groeger et al. 2009; Woolley et al. 2013). Through these processes, H2O2 plays key roles in cell growth and survival. The primary source of H2O2 for these regulatory processes is NOX, which is activated by growth factors and other receptor activators (Rhee et al. 2005; Miller et al. 2007b, 2010; Groeger et al. 2009; Woolley et al. 2013).
In contrast to slow signalling mediated by NOX- and MAO-generated H2O2 that can regulate intracellular signalling cascades, rapid, subsecond signalling by H2O2 originates in mitochondria (Bao et al. 2009) and acts on cellular ion channels (Seutin et al. 1995; Krippeit-Drews et al. 1999; Avshalumov et al. 2003, 2003; Avshalumov & Rice, 2003; Bao et al. 2005; Patel et al. 2011; Lee et al. 2011, 2013). Early physiological studies showed that application of exogenous H2O2 can cause membrane hyperpolarization by activating a K+ conductance in various cell types, including CA1 hippocampal neurons (Seutin et al. 1995) and pancreatic β-cells (Krippeit-Drews et al. 1999). Our laboratory subsequently identified ATP-sensitive K+ (KATP) channels as key inhibitory targets of endogenous, as well as exogenous H2O2 (Avshalumov et al. 2003, 2005; Avshalumov & Rice, 2003; Patel et al. 2011). More recently, we identified a subclass of transient receptor potential (TRP) channels in basal ganglia neurons that are dynamically regulated by H2O2 (Bao et al. 2005; Lee et al. 2011, 2013). Work in our laboratory has focused on rapid signalling by H2O2 in regions of the basal ganglia, particularly the striatum and the substantia nigra pars compacta (SNc) and pars reticulata (SNr). Our cellular focus has been on dopaminergic (DAergic) neurons of the SNc that project to the dorsal striatum, forming the nigrostriatal pathway that plays critical roles in movement and motor learning (Carlsson, 2002; Redgrave et al. 2011), and on GABAergic neurons of the SNr, which are basal ganglia output neurons (Zhou & Lee, 2011).
This review describes how H2O2 rapidly translates a dynamic change in cellular metabolism, specifically mitochondrial oxygen consumption, to a salient signal. The targets of this signal are H2O2-sensitive ion channels that can influence the excitability of the neuron in which H2O2 is generated, as well as neighbouring cellular elements. Most data presented were obtained using ex vivo brain slices prepared from adult male guinea pigs or mice after inducing deep anaesthesia (50 mg kg−1 sodium pentobarbital, i.p.). Methods include fast-scan cyclic voltammetry (FCV) to detect DA release, whole-cell recording to monitor neuronal activity, and fluorescence imaging to indicate H2O2 generation (e.g. Avshalumov et al. 2003, 2005, 2008; Bao et al. 2009; Lee et al. 2011, 2013; Patel & Rice, 2013).
Cellular regulation of H2O2
The previous section discussed sources of H2O2, but it is important to recognize that sinks, including metabolism and diffusion, are critical in shaping patterns of H2O2 signalling, as well. Thus, cellular H2O2 regulation must not only prevent oxidative damage from H2O2 elevation, but also allow intracellular and extracellular concentrations sufficient for a signalling effect to be achieved (Avshalumov et al. 2004; Murphy et al. 2011; Rice, 2011). Absolute basal and dynamic intracellular concentrations of H2O2 remain a matter of debate; the technical and conceptual issues involved have been reviewed elsewhere (Adimora et al. 2010; Murphy et al. 2011; Rice, 2011).
Peroxidase enzymes that metabolize H2O2 include glutathione (GSH) peroxidase, which is found in the cytosol and in mitochondria (Stults et al. 1977), and catalase, which is localized to intracellular peroxisomes (Cohen, 1994; Peuchen et al. 1997; Dringen et al. 2005). Dynamic H2O2 regulation is also provided by peroxiredoxins and thioredoxins, which act in a complicated dance that not only buffers H2O2 levels to prevent potentially toxic consequences of oxidative stress, but also facilitates the elevation of intracellular H2O2 to levels sufficient for signalling (Rhee et al. 2005; Adimora et al. 2010; Rhee & Woo, 2011; Jeong et al. 2012).
Complementing H2O2 regulation by cellular peroxidase enzymes are roles played by the low molecular weight antioxidants GSH and ascorbate. These antioxidants protect against possible pathological consequences of H2O2 elevation through their actions as scavengers of the aggressive hydroxyl radical (•OH) produced from the interaction of H2O2 or •O2− with trace metal ions (Cohen, 1994). Additionally, GSH is an essential cofactor for GSH peroxidase activity, and with other cellular thiols provides critical regulation of peroxiredoxins and thioredoxins through reduction of disulfide bonds involved in activation and inactivation (Stults et al. 1977; Rhee & Woo, 2011; Mailloux et al. 2013). Ascorbate, by contrast, has a higher redox potential than GSH and so cannot reduce oxidized thiols or break disulfide bonds, and does not interact with H2O2 directly (Rice, 2000; Avshalumov et al. 2004; Rhee & Woo, 2011). Notably, ascorbate is the primary low molecular weight antioxidant in neurons (with an intracellular concentration of 10 mm) (Rice & Russo-Menna, 1998), so that it is in a prime position to permit neuronal H2O2 signalling, yet prevent pathological consequences that could occur from unregulated H2O2 generation and •OH production (Rice, 2012).
One final point about H2O2 regulation is the extent to which H2O2 can diffuse from a site of generation to act at targets in the same or neighbouring cells. In contrast to other ROS, H2O2 is not a free radical and not an ion. These properties not only limit H2O2 reactivity and extend its lifetime (Cohen, 1994), but also increase its membrane permeability (Ramasarma, 1982; Bienert et al. 2007; Adimora et al. 2010). However, increasing evidence indicates that net cellular H2O2 efflux and entry is governed by cell-specific membrane permeability factors coupled with competing effects of the antioxidant network (Makino et al. 2004; Bienert et al. 2007; Adimora et al. 2010; Miller et al. 2010; Mishina et al. 2011; Bienert & Chaumont, 2014). In particular, the cellular expression of specific aquaporin isoforms, including aquaporins 3 and 8 (Miller et al. 2010; Bienert & Chaumont, 2014), has been shown to facilitate the passive diffusion of H2O2 across membranes, and thereby influence its efficacy as an intracellular signalling agent, as well as a diffusible messenger.
Endogenous H2O2 inhibits axonal and somatodendritic DA release
Regulation of dopamine release from DAergic axons or cell bodies and dendrites (somatodendritic release) can be studied readily in ex vivo brain slices using carbon-fibre microelectrodes with FCV (Rice et al. 2011; Patel & Rice, 2013). For such studies, local electrical stimulation is used to elicit an increase in extracellular DA concentration ([DA]o), which reflects the net influence of DA release and uptake. The use of pulse-train stimulation allows the influence of concurrently released transmitters and local activity changes on [DA]o as the train progresses. For the studies summarized here, stimulation parameters were commonly 30 pulses at 10 Hz, although trains of as few as 7 pulses at 10 Hz are sufficient to reveal modulation by endogenously generated H2O2 in guinea pig dorsal striatum (K. A. Moran & M. E. Rice, unpublished observations).
Using these methods, we found that exogenous H2O2 causes a reversible, 30% suppression of evoked [DA]o in dorsal striatum that is not accompanied by a change in tissue DA content or signs of oxidative damage (Chen et al. 2001). We then examined a role for endogenous H2O2 in DA release regulation by inhibiting GSH peroxidase to amplify levels of dynamically generated H2O2 levels using mercaptosuccinate (MCS). Exposure to MCS causes similar reversible decreases in evoked [DA]o in dorsal and ventral striatum to those seen with exogenous H2O2, again with no change in tissue DA content (Chen et al. 2002; Avshalumov et al. 2008). Significantly, DA release suppression in the dorsal striatum can be reversed by the H2O2 metabolizing enzyme catalase in the continued presence of MCS, confirming H2O2 involvement (Avshalumov et al. 2003). Suppression of evoked [DA]o also persists when DA uptake is inhibited, indicating an effect on DA release, not uptake (Avshalumov et al. 2003).
Although exogenous H2O2 causes suppression of somatodendritic DA release in the SNc and in the adjacent ventral tegmental area (VTA), GSH peroxidase inhibition decreases evoked [DA]o in SNc, but not VTA (Chen et al. 2002). This difference between SNc and VTA is potentially important, because DAergic neurons of the SNc degenerate in Parkinson's disease and in animal models of Parkinson's, whereas those in the VTA are relatively spared (Yamada et al. 1990; Fearnley & Lees, 1991; Betarbet et al. 2000).
Endogenous H2O2 in dorsal striatum is generated downstream of glutamatergic AMPA receptor activation
Local electrical stimulation evokes release of glutamate, GABA and other transmitters, as well as DA. In striatum, GSH peroxidase inhibition by MCS has no effect on [DA]o evoked by a single stimulus pulse (Avshalumov et al. 2003), indicating that modulatory H2O2 must be generated dynamically during initial and subsequent pulses of a stimulus train to inhibit on-going DA release. The generation of this modulatory H2O2 proved to require activation of AMPA receptors (AMPARs), as AMPAR blockade causes a marked increase in pulse-train-evoked [DA]o in guinea pig dorsal striatum (Avshalumov et al. 2003), indicating that physiological glutamate release inhibits axonal DA release in dorsal striatum via AMPARs. An essential role for inhibitory H2O2 in mediating this effect, as well as in mediating the opposing effect on DA release by GABA acting at GABAA receptors, was subsequently shown by the loss of regulation in the presence of exogenous catalase or GSH peroxidase (Avshalumov et al. 2003).
As reviewed elsewhere, the absence of AMPARs and GABAARs on DAergic axons in dorsal striatum led us to postulate that the cellular sources of glutamate-dependent H2O2 generation were not DAergic axons, but rather other striatal neurons, including the predominant striatal neurons, GABAergic medium spiny neurons (MSNs) (Avshalumov et al. 2007, 2008; Rice et al. 2011; Rice, 2011; Patel & Rice, 2012). We tested this hypothesis using single-cell fluorescence imaging of H2O2, in which dihydro-dichlorofluorescein diacetate (H2DCF-diacetate) is loaded into cells via a patch pipette used for whole-cell recording (Avshalumov et al. 2005, 2008). This dye is cleaved by intracellular esterases to form H2DCF, which becomes fluorescent DCF when oxidized. Local stimulation in guinea pig striatal slices activates a single action potential with each stimulus pulse in recorded MSNs (Fig.1A, lower panel). Concurrent imaging of DCF fluorescence confirmed a significant increase in DCF fluorescence intensity (FI) evoked using the same pulse-train stimulation parameters as in DA release studies (Fig.1A, upper panel). Activity-dependent increases in DCF FI are enhanced when GSH peroxidase is inhibited by MCS, and abolished by the addition of exogenous catalase, confirming H2O2 detection (Avshalumov et al. 2008). Consistent with a requirement for glutamate-dependent AMPAR activation in the generation of modulatory H2O2 in dorsal striatum, antagonism of AMPARs prevents stimulus-induced action potentials and H2O2 generation in MSNs (Fig.1B) (Avshalumov et al. 2008). These and other data support a role for dynamically generated H2O2 as a diffusible messenger that is generated in striatal MSNs (and possibly other local neurons), and diffuses to adjacent DAergic axons to inhibit DA release (Avshalumov et al. 2008; Rice, 2011).
Figure 1. Modulatory H2O2 generation in guinea pig striatal medium spiny neurons (MSNs) requires AMPAR activation.
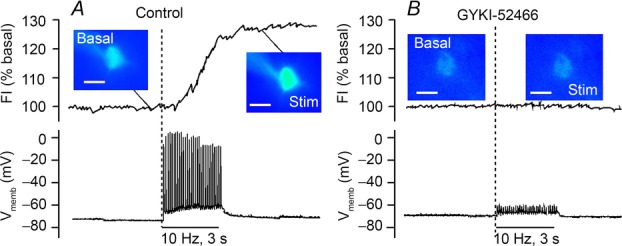
A, local electrical stimulation (30 pulses at 10 Hz; 100 μs, 0.5 mA) causes tetrodotoxin-sensitive action potentials and increased DCF fluorescence intensity (FI) in MSNs in ex vivo slices of guinea pig dorsal striatum. B, AMPAR blockade with GYKI-52466 (50 μm) prevents stimulus-induced spikes and H2O2 generation (modified from Avshalumov et al. 2008).
H2O2 acts via KATP channels in DAergic axons
The targets at which H2O2 acts to regulate nigrostriatal DA release are KATP channels (Avshalumov et al. 2003; Avshalumov & Rice, 2003). Blocking KATP channels with a sulfonylurea receptor antagonist not only enhances pulse-train-evoked [DA]o in striatum, but also prevents the usual H2O2-dependent inhibition of DA release by MCS, as well as the patterns of DA release regulation by AMPAR and GABAAR activation (Avshalumov et al. 2003; Avshalumov & Rice, 2003; Rice, 2011). These data show that KATP channels are required for DA release modulation by H2O2, glutamate and GABA (Avshalumov et al. 2003; Avshalumov & Rice, 2003). Consequently, striatal DA release is decreased by KATP channel openers, whether evoked with single-pulse or pulse-train stimulation (Avshalumov & Rice, 2003; Patel et al. 2011). Although release suppression is seen with an opener selective for either SUR1- or SUR2-subunit-containing KATP channels, only a SUR1-selective opener occludes the effects of MCS, as well as the effects of AMPAR and GABAAR antagonism, on evoked [DA]o showing that DA release regulation by glutamate-dependent H2O2 requires SUR1-based KATP channels (Avshalumov & Rice, 2003).
A key aspect of our hypothesis that H2O2 is a diffusible messenger in the striatum is that KATP channels are located directly on DAergic axons. Our observation that single-pulse-evoked [DA]o is suppressed by KATP channel openers supports direct localization, which was confirmed using immunohistochemical methods showing striatal colocalization of a KATP channel subunit with tyrosine hydroxylase, an enzyme required for DA synthesis (Patel et al. 2011; Patel & Rice, 2012). Together with our previous work, these data indicate that H2O2 is a diffusible messenger, which is generated in striatal MSNs, but acts at KATP channels on DA axons.
It should be emphasized at this point that H2O2-dependent signalling via ion channel activation is fast and transient, with a subsecond to second time scale (Patel et al. 2011; Patel & Rice, 2012). This was assessed using paired-pulse stimulation to evoke [DA]o with pharmacological blockade of KATP channels or amplification of H2O2 levels using GSH peroxidase inhibition. Maximal H2O2-/KATP channel-dependent suppression of DA release is seen 500 ms after an initiating stimulus, with a significant influence persisting until ∼1000 ms (Patel et al. 2011).
Mitochondria are the subcellular source of H2O2 for dynamic striatal signalling
Studies in isolated mitochondria indicate that succinate, a substrate of mitochondrial complex II, drives H2O2 production by back-flow of electrons to complex I, which is prevented by partial complex I inhibition by rotenone (Votyakova & Reynolds, 2001; Liu et al. 2002; Gyulkhandanyan & Pennefather, 2004). We demonstrated that this regulation also occurs in whole cells in brain slices using a reversible H2O2-sensitive dye Redoxfluor-1 (RF1) (Miller et al. 2007a). Succinate caused a rapid increase in RF1 FI in striatal MSNs (Fig.2A and B), which was reversed by co-application of rotenone at a concentration that leads to partial complex I inhibition (50 nm) in the continued presence of succinate (Fig.2B) (Bao et al. 2009). We then examined pulse-train-evoked DA release in this rotenone + succinate cocktail to assess whether the subcellular source of modulatory H2O2 was mitochondrial respiration. Consistent with the expected effect of enhanced H2O2 generation, succinate alone suppressed evoked [DA]o; suppression of DA release was prevented by catalase, confirming H2O2 involvement, and was reversed by rotenone (Bao et al. 2009). Most importantly, rotenone + succinate prevents the usual increase in evoked [DA]o with AMPAR blockade (Fig.2C) and the decrease that usually accompanies GSH peroxidase inhibition by MCS (Fig.2D). By contrast, inhibition of either NADPH oxidase or MAO had no effect on pulse-train-evoked [DA]o or on the usual suppression of DA release seen when GSH peroxidase is inhibited by MCS (Bao et al. 2005). These data show that mitochondrial respiration is the source of H2O2 that can then translate the significance of a glutamate-activated increase in cellular activity and mitochondrial metabolism in MSNs to a signal at DAergic axons to dynamically regulate DAergic transmission.
Figure 2. Role of mitochondrial H2O2 in dynamic modulation of striatal DA release.
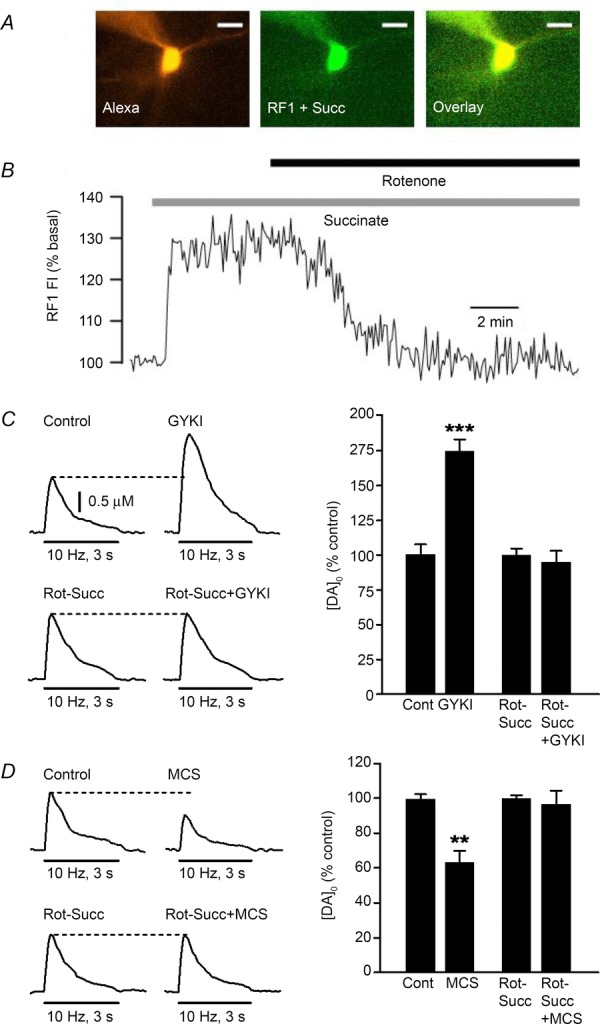
A, superfusion of succinate (5 mm) causes an increase in fluorescence intensity (FI) of the reversible H2O2-sensitive dye RF1 in a medium spiny neuron (MSN) in a guinea pig striatal slice; Alexa Red was included in the pipette solution to allow morphological identification of the imaged MSN (scale bar = 20 μm). B, time course of the increase in RF1 FI in the MSN in A during succinate exposure; the increase in FI was reversed by rotenone, at a concentration (50 nm) that leads to partial inhibition of mitochondrial complex I, in the continued presence of succinate (n = 7). C, representative pulse-train-evoked [DA]o (30 pulses, 10 Hz) in artificial cerebrospinal fluid (aCSF) alone (Control) or in the presence of rotenone + succinate (Rot + Succ), before and after the addition of an AMPAR antagonist, GYKI-52466 (GYKI, 50 μm). Bar graphs show average evoked [DA]o normalized to the starting condition for each experiment; GYKI caused a significant increase in evoked [DA]o (***P < 0.001 vs. same-site control; n = 5), which was prevented by the rotenone–succinate cocktail (P > 0.05 vs. same-site Rot-Succ; n = 6). D, representative pulse-train-evoked [DA]o in aCSF alone or in the presence of Rot-Succ before and after the addition of a GSH peroxidase inhibitor, MCS (1 mm). Bar graphs show average evoked [DA]o normalized to the starting condition for each experiment; MCS caused a significant decrease in evoked [DA]o (**P < 0.01 vs. same-site control; n = 5), which was prevented by the rotenone-succinate cocktail (P > 0.05 vs. same-site Rot-Suc; n = 5).
H2O2 regulates SNc DA neuron activity via KATP channels
DCF imaging in SNc DAergic neurons in guinea pig midbrain slices revealed tonic and activity-dependent H2O2 generation in these spontaneously active cells (Avshalumov et al. 2005). Notably, tonically generated H2O2 has a significant effect on DA cell excitability: depletion of intracellular H2O2 by including catalase in the pipette solution or blockade of KATP channels causes a significant increase in spontaneous firing rate in all DAergic neurons tested. Moreover, catalase in the pipette has no effect when KATP channels are blocked, demonstrating that tonically generated H2O2 regulates DAergic cell activity via KATP channels (Avshalumov et al. 2005). Notably, the pipette backfill solution typically contains 3 mm ATP, which should close KATP channels (Häusser et al. 1991), suggesting that H2O2 may act by decreasing channel sensitivity to ATP; the mechanism of regulation has yet to be elucidated, however (for discussion, see Patel & Rice 2012).
Conversely, elevation of H2O2 by exogenous application or GSH peroxidase inhibition causes neuronal hyperpolarization in ∼50% of SNc DAergic neurons examined in guinea pig midbrain. Neurons in this sensitive population, which we call ‘responders’, are also hyperpolarized by an SUR1-subunit-selective KATP channel opener whereas those that are insensitive to H2O2 (‘non-responders’) are hyperpolarized by an SUR2-subunit-selective opener (Fig.3) (Avshalumov et al. 2005). These data indicate that SUR1 expression in SNc DA neurons conveys sensitivity to elevated H2O2, as seen for striatal DA axons (Avshalumov & Rice, 2003; Patel et al. 2011). Overall, these data show that H2O2 plays an auto-regulatory role in SNc DA neurons via the activation of inhibitory KATP channels.
Figure 3. SUR1-containing KATP channels convey enhanced sensitivity to H2O2 elevation in guinea pig SNc DAergic neurons.
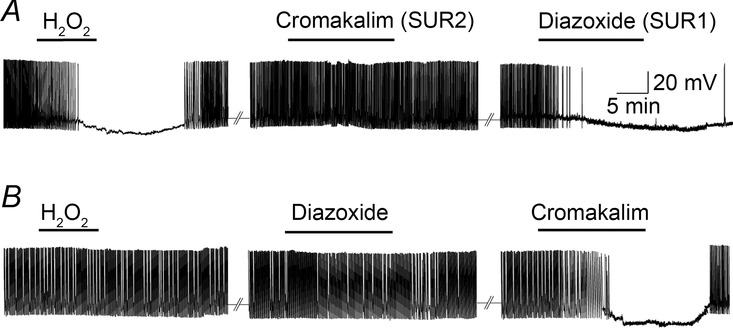
A, exogenous H2O2 (1.5 mm) (or MCS, 1 mm) causes hyperpolarization in ‘responders’; this is mimicked by SUR1-selective KATP-channel opener, diazoxide (60 μm), whereas a SUR2-selective opener, cromakalim (60 μm), has no effect (n = 6). B, a second population of SNc DAergic neurons does not respond to H2O2, MCS, or diazoxide, but hyperpolarizes with cromakalim (n = 6) (modified from Avshalumov et al. 2005).
Activation of TRPM2 channels by H2O2 in GABAergic neurons in the basal ganglia
Increasing evidence points to an additional ion channel target for H2O2, which is a subclass of TRP channels that provides regulation complementary to that provided by KATP channels. Although a variety of TRP channels are expressed in the brain, one subclass, TRPM2 (TRP melastatin 2), is uniquely sensitive to activation by H2O2 (Fleig & Penner, 2004). Activation of these channels by H2O2 leads to an increase in neuronal excitability (Bao et al. 2005; Lee et al. 2011, 2013), rather than the decrease that accompanies activation of KATP channels. How H2O2 activates TRPM2 channels is somewhat better understood than its mechanism at KATP channels, but not without debate. Although there is evidence for direct activation of TRPM2 channels by H2O2 (Wehage et al. 2002), other data argue against this (Tóth & Csanády, 2010). Instead, activation may be mediated by H2O2-dependent elevation of ADP ribose or a synergistic action of H2O2 and ADP ribose (Perraud et al. 2005; Lange et al. 2008).
The first evidence for H2O2-dependent regulation of neuronal excitability by TRP channels emerged in the course of studies to investigate the role of mitochondria as a source of modulatory H2O2 (Bao et al. 2005, 2009). In these studies we found that partial mitochondrial complex I inhibition by nanomolar concentrations of rotenone leads to unregulated generation of H2O2, indicated by single-cell DCF imaging in MSNs in guinea pig dorsal striatum (Fig.4A) (Bao et al. 2005; Avshalumov et al. 2007). Unsurprisingly, this increase in H2O2 leads to suppression of evoked DA release, which is prevented by catalase or by KATP channel blockade (Fig.4B), demonstrating H2O2 and KATP channel involvement. What was more surprising at the time, however, was that simultaneous current-clamp recording in MSNs showed a depolarization and an increase in excitability of those neurons, with a time course that paralleled the increase in DCF FI (Fig.4C) (Bao et al. 2005). Changes in MSN membrane properties were prevented by catalase, confirming H2O2 dependence. Moreover, the increase in excitability was also prevented by flufenamic acid (FFA), a non-selective TRP channel antagonist, implicating H2O2-dependent TRP channel activation in these GABAergic projection neurons (Bao et al. 2005). Although FFA can inhibit several TRP subtypes, as well as other ion channels (Guinamard et al. 2013), recognized targets include H2O2-sensitive TRPM2 channels (Hill et al. 2004). As discussed further below, TRPM2 channels have been identified as the target for H2O2-dependent modulation of SNr GABAergic projection neurons (Lee et al. 2013). Given that TRPM2 channels are expressed in striatal MSNs, it is likely that they also contribute to H2O2 modulation of MSNs (Hill et al. 2006). Blocking KATP channels led to enhanced MSN depolarization during rotenone exposure, indicating that concurrently activated KATP channels in these cells serves to counterbalance the predominant TRP-dependent effects of H2O2 elevation.
Figure 4. Unregulated H2O2 generation during partial mitochondrial complex I inhibition by rotenone inhibits striatal DA release via KATP and excites MSNs via TRP channels.
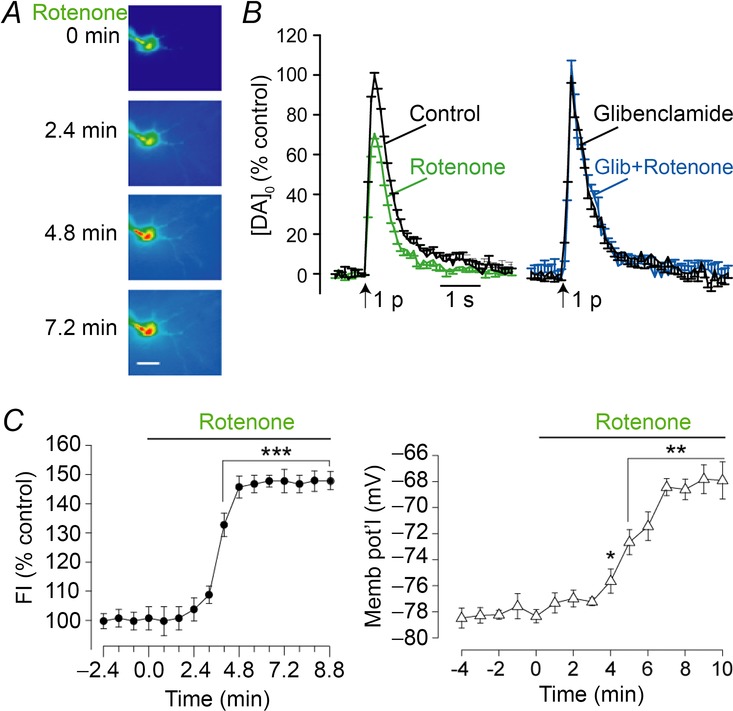
A, DCF fluorescence intensity (FI) in a MSN in a guinea pig striatal slice under control conditions (t = 0) and during exposure to rotenone (50 nm); scale bar is 20 μm. B, average DA release records after single-pulse stimulation elicited at 5 min intervals under control conditions and after 30 min exposure to rotenone (50 nm; n = 7) compared with release in the presence of glibenclamide (Glib; 3 μm) and glibenclamide plus rotenone (n = 5). Data are normalized, with maximum [DA]o under control conditions for each slice taken as 100%. C, average time course of H2O2 generation (DCF FI) recorded in single MSNs during rotenone exposure (n = 13; ***P < 0.001 rotenone vs. basal FI; ANOVA) compared to the time course of rotenone-induced changes in membrane potential (Memb pot'l) in these same MSNs (n = 10; *P < 0.05; **P < 0.01 rotenone vs. control; ANOVA) (modified from Bao et al. 2005).
We then turned our attention to spontaneously active GABAergic projection neurons in the SNr (Lee et al. 2011, 2013). Consistent with opposing regulation of cellular activity by H2O2 acting at TRP vs. KATP channels, exogenous catalase causes a decrease in firing rate of SNr GABAergic neurons in guinea pig midbrain slices, indicating maintenance of excitability by basal levels of H2O2 (Lee et al. 2011). In sharp contrast to the inhibitory effect of H2O2 elevation on SNc DAergic neurons (Fig.3) (Avshalumov et al. 2005), elevation of H2O2 levels causes an increase in the firing rate of SNr GABAergic neurons, whether through amplification of endogenous levels by GSH peroxidase inhibition (Fig.5A, B and E) or exposure to exogenous H2O2 (Lee et al. 2011). Implicating H2O2-sensitive TRPM2 channels in this process, these increases in SNr neuron firing rates are blocked by FFA (Fig.5C and E), as well as when an antibody to the C-terminus of TRPM2 channels is included in the pipette solution (Fig.5D and F). In addition to these studies of functional TRPM2 expression, immunohistochemistry (Fig.5F) and in situ hybridization studies provide anatomical confirmation of TRPM2 channels in guinea pig SNr GABAergic neurons (Lee et al. 2013). Companion immunohistochemical studies of guinea pig midbrain also demonstrated expression of TRPM2 channels in SNc DAergic neurons (Fig.6) (Lee et al. 2013), consistent with other studies showing a functional role for these ion channels in SNc DAergic neurons in rats (Chung et al. 2011), as well as evidence for the presence of TRPM2 in DAergic neurons in mice (Mrejeru et al. 2011).
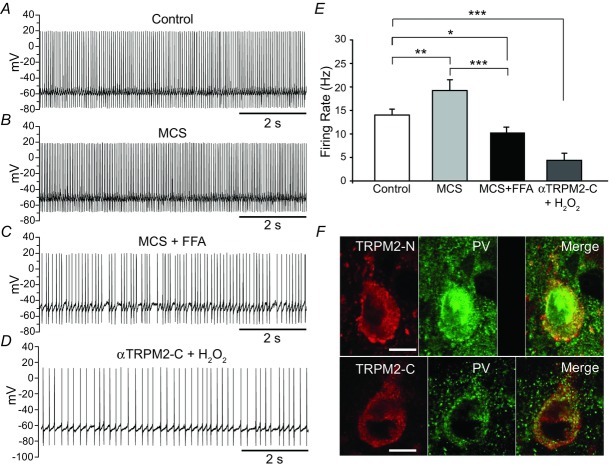
Figure 5. TRPM2 channel blockade reverses H2O2-dependent increases in firing rate in guinea pig SNr GABAergic neurons.
A, spontaneous activity of a SNr GABAergic neuron under control conditions. B, amplification of endogenous H2O2 levels by GSH peroxidase inhibition with MCS increases spontaneous firing rate. C, in the presence of a non-specific TRP channel blocker, flufenamic acid (FFA), H2O2 causes a decrease, rather than increase in SNr GABAergic neuron firing rate. D, the effect of elevated H2O2 is also converted to a suppression of spontaneous activity when TRPM2 channels are blocked by including an antibody to the C-terminus of TRMP2 channels (α-TRPM2-C) in the pipette solution. E, summary of the influence of elevated H2O2 on spontaneous SNr neuron firing rate. Increases in firing rate by H2O2 are reversed and suppressed below control levels by FFA or by α-TRPM2-C (*P < 0.05; **P < 0.01; ***P < 0.001) (modified from Lee et al. 2011, 2013). F, immunohistochemical labelling of guinea pig SNr neurons with parvalbumin (PV), a marker for GABAergic neurons, and an antibody to the N- or C-terminus of TRPM2, confirming TRPM2 expression in these cells. Staining is eliminated following pre-adsorption of the primary antibody with its immunogenic peptide (top row, third panel from left); scale bar, 10 μm. (modified from Lee et al. 2013).
Figure 6. TRPM2 channel expression in guinea pig SNc DAergic neurons.
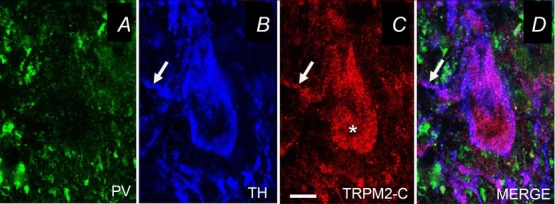
A, Immunohistochemical labelling of guinea pig SNc shows little somatic staining with an antibody to parvalbumin (PV), a marker for GABAergic neurons, but B, abundant staining of most SNc neurons with an antibody to tyrosine hydroxylase (TH), confirming their identity as DAergic neurons. C, TH-immunopositive neurons are also immunopositive for TRPM2, indicated using an antibody to the C-terminus of TRPM2 (TRPM2-C). D, Merged image of PV, TH, and TRPM2-C. Arrows point to TRPM2 in DAergic dendrites; scale bar, 20 μm (modified from Lee et al. 2013).
In addition to regulating the spontaneous firing rate of SN neurons, TRPM2 channels are required for NMDA-induced burst firing in SNr GABAergic neurons (Lee et al. 2013). Notably, we found that H2O2 modulates NMDA-induced burst firing in these cells, leading to an increase in burst duration and a decrease in burst frequency (Fig.7). These findings reveal another modulatory role for H2O2 that could be especially relevant in Parkinson's disease, in which increased burst firing in SNr neurons and increases in ROS both occur (see Lee et al. 2013).
Figure 7. H2O2 modulates NMDA-induced burst firing in SNr GABAergic neurons.
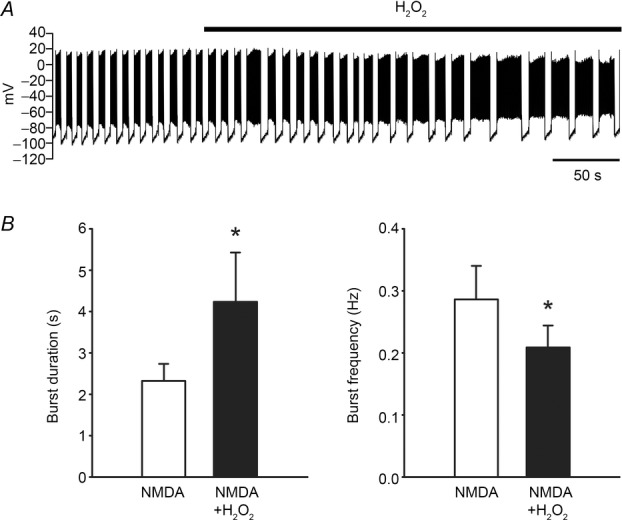
A, activity of a SNr GABAergic neuron exhibiting NMDA-induced (30 μm) burst firing before and after addition of exogenous H2O2 (1.5 mm). B, both exogenous H2O2 and amplified endogenous H2O2 induced by inhibiting GSH peroxidase with MCS (1 mm) caused an increase in burst duration and a decrease in burst frequency (*P < 0.05) (modified from Lee et al. 2013).
Species similarities and differences
Most studies of H2O2 as a dynamic neuromodulator have been conducted using ex vivo brain slices from guinea pigs. Initial experiments suggested species independence of the basic inhibitory effect of H2O2 elevation on pulse-train-evoked DA release in dorsal striatum, with a similar reversible suppression of pulse-train-evoked [DA]o in ex vivo striatal slices from rat, guinea pig and marmoset when GSH peroxidase was inhibited by MCS (Rice et al. 2002; Avshalumov et al. 2003). The Sombers group has further demonstrated H2O2-dependent suppression of DA release in rat striatum in vivo (Spanos et al. 2013). Recent studies have confirmed DA release suppression when endogenous H2O2 is elevated in mouse striatal slices, as well (B. O'Neill, R. Asri, J. C. Patel & M. E. Rice, unpublished observations). However, additional evidence suggests that dynamic regulation of DA release by glutamate, GABA and H2O2 may differ in mouse dorsal striatum with possible H2O2-independent regulation of DA release by these transmitters.
As noted above, studies in ex vivo slices from young adult guinea pig brain show greater sensitivity of SUR1- vs. SUR2-based KATP channels to activation by H2O2 in striatal DAergic axons (Avshalumov & Rice, 2003; Patel et al. 2011) as well as SNc DAergic neurons (Avshalumov et al. 2005). These findings are consistent with the greater metabolic sensitivity of SUR1- vs. SUR2-expressing SNc DAergic neurons in slices from neonatal mice (Liss et al. 1999), although adult mice appear to express only SUR1-based KATP channels (Liss et al. 2005). Rat SNc DAergic neurons also show KATP channel-dependent hyperpolarization with exogenous H2O2 application, with equal sensitivity of all DAergic cells, at least under the conditions tested (Geracitano et al. 2005). Interestingly, in these same studies, hypoxia-induced KATP channel activation was reversed by H2O2, which served as a source of molecular oxygen, as shown previously (Walton & Fulton, 1983).
Another species difference in H2O2-dependent modulation of neuronal activity is that H2O2 elevation in mouse midbrain slices leads to inhibition of SNr GABAergic neurons via predominant KATP channel activation, as opposed to the TRPM2-dependent excitation seen with H2O2 elevation in guinea pig SNr neurons (Lee et al. 2011). Differences in the functional activation of H2O2-dependent KATP and TRPM2 channels between guinea pigs and mice suggest divergent roles for this regulatory process across species. The need for neuronal regulation by a metabolic signal like H2O2 might depend on unique behavioural demands across species that require differential patterns of ion channel expression. Other possible factors include species differences in H2O2 generation or metabolism. For example, H2O2 metabolism by the glial antioxidant network differs between species, with stronger control in guinea pig (or human) vs. mouse (or rat), because of higher glia-to-neuron ratio of guinea pig brain (Avshalumov et al. 2004). Regardless of these differences, however, responsiveness to H2O2 across diverse species supports the idea that this molecule is an important regulator of neuronal function.
Opposing effects of H2O2 via KATP and TRPM2 channels in specific neuron populations
A seemingly paradoxical factor in H2O2-mediated signalling is that many target neurons express both KATP and TRPM2 channels that have opposing effects when activated by H2O2. For example, in addition to TRPM2 channels (Lee et al. 2013), GABAergic SNr neurons also express KATP channels (Schwanstecher & Panten, 1993; Stanford & Lacey, 1996; Lutas et al. 2014) that lead to H2O2-activated hyperpolarization of guinea pig SNr GABAergic neurons when TRPM2 channels are blocked (Fig.5C–E; Lee et al. 2011). Conversely, an inward current is observed in SNc DAergic neurons in response to H2O2 as opposed to the usual outward current and hyperpolarization when KATP and other potassium channels are blocked (Avshalumov et al. 2005; Geracitano et al. 2005; Chung et al. 2011). This raises the interesting possibility that the effect of H2O2 on target neurons is defined by the abundance and/or relative activity of these opposing channels and suggests that ATP and H2O2 are involved in a dynamic interplay regulating neuronal excitability, as discussed further below (Fig.8).
Figure 8. Net influence of mitochondrially generated H2O2 on DA release and on the excitability of guinea pig basal ganglia neurons mediated by KATP and TRPM2 channels.
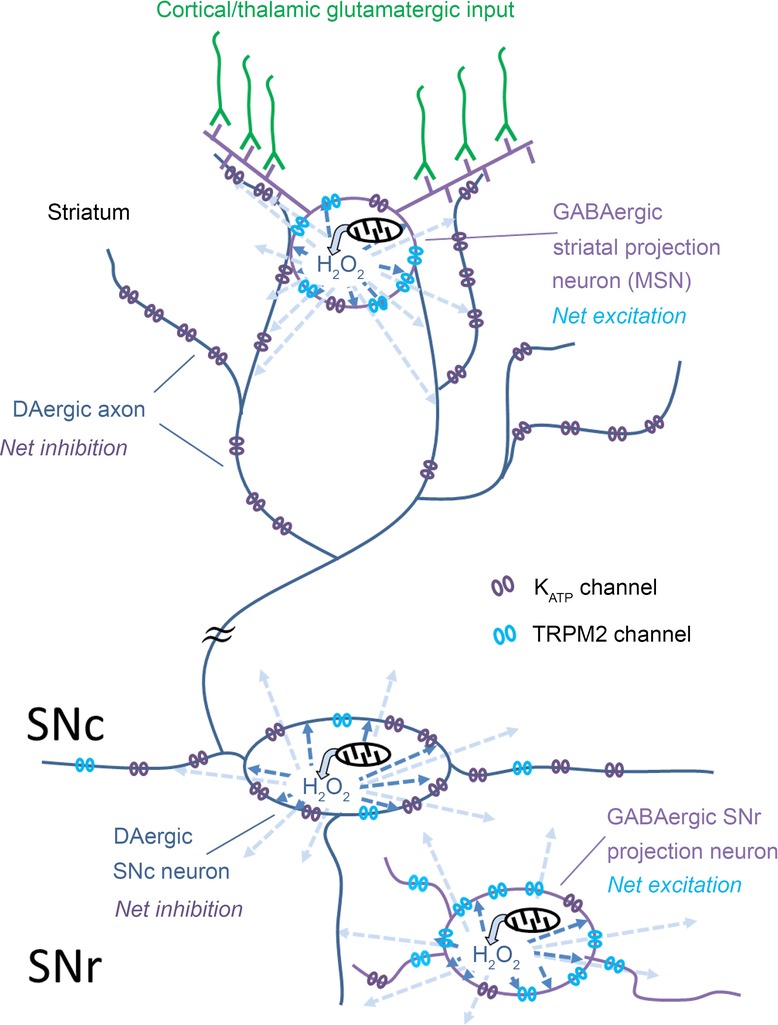
Axonal DA release in striatum and somatodendritic DA release in SNc are suppressed by the net inhibitory effect of elevated H2O2 acting at KATP channels in DAergic neurons. In striatum, H2O2 generated in MSNs acts as a diffusible messenger (light blue arrows) to inhibit DA release by activating KATP channels on neighbouring DAergic axons. The source of dynamically generated H2O2 is mitochondrial respiration. The net effect of H2O2 generated within spontaneously active SNc DAergic neurons (dark blue arrows) is also inhibitory via KATP channel activation. Although striatal MSNs, which are GABAergic projection neurons, and SNr GABAergic projection neurons express both KATP and TRPM2 channels, the predominant effect of H2O2 on these cells is excitatory via TRPM2 channels. It should be noted that glutamatergic drive is required for action potential-dependent generation of H2O2 in MSNs, although partial mitochondrial inhibition by rotenone can lead to unregulated H2O2 generation. By contrast, sufficient H2O2 is generated tonically in spontaneously active SNr and SNc neurons to modulate firing rate independent of synaptic input.
There is a rich literature linking KATP channel activity to metabolic state in basal ganglia structures, with ATP identified as the primary signalling molecule. Factors that influence ATP (and ATP/ADP), such as oxygenation, influence KATP channel status, with anoxic conditions leading to KATP channel opening (Amoroso et al. 1990; Murphy & Greenfield, 1992; Jiang et al. 1994; Guatteo et al. 1998). Glucose concentration can also influence KATP channel activity with lower glucose concentrations favouring KATP channel opening, presumably through effects on intracellular ATP concentration (Amoroso et al. 1990; During et al. 1995; Marinelli et al. 2000). However, we have also found a relationship between glucose concentration and mitochondrial H2O2 production with lower glucose concentrations promoting mitochondrial activity and H2O2 production in striatal MSNs (L. Bao, C. R. Lee & M. E. Rice, unpublished observations), further linking metabolic activity to signalling by H2O2.
Less is known about the physiological processes that regulate TRPM2 channels in SNc and SNr neurons. However, several metabolic and activity-dependent mechanisms have been implicated, including activity-dependent increases in intracellular calcium, as well as conditions that elevate H2O2 and other ROS (Lee & Tepper, 2007; Freestone et al. 2009; Chung et al. 2011; Mrejeru et al. 2011; Lee et al. 2013).
Overall, H2O2 can have an excitatory or inhibitory effect on an individual neuron that is apparently based on the relative activities of KATP and TRPM2 channels in that cell. This working model is supported by evidence from our studies of H2O2-dependent modulation of DA release and basal ganglia neuron excitability in ex vivo guinea pig brain slices, reviewed here (Fig.8). Specifically, the net effect of H2O2 elevation on striatal DA release is inhibitory via KATP channels (Avshalumov & Rice, 2003; Bao et al. 2005; Patel et al. 2011). Similarly, the predominant influence of KATP channels on SNc DAergic neuron excitability is also reflected in the net hyperpolarization seen with H2O2 elevation in these midbrain projection neurons (Avshalumov et al. 2005) (Fig.8). In contrast, the net influence of H2O2 is to increase the excitability of GABAergic striatal MSNs (Bao et al. 2005) and GABAergic SNr neurons (Lee et al. 2011, 2013), which would enhance the output of these inhibitory projection neurons, presumably by the predominant influence of TRPM2 channels in both neuron populations (Fig.8). The source of modulatory H2O2 that can influence transmitter release and neuronal activity on a subsecond time scale is presumed to be mitochondrial respiration, as shown for striatal DA release regulation by H2O2-dependent activation of KATP channels (Bao et al. 2009; Patel et al. 2011). Notably, basal H2O2 levels in midbrain SN DAergic and GABAergic neurons generated during spontaneous firing activity in these cells is sufficient to provide a modulatory tone that is mildly inhibitory in SNc DAergic neurons (Avshalumov et al. 2005) and mildly excitatory in SNr GABAergic cells (Lee et al. 2011). In the striatum, however, dynamically generated H2O2 in MSNs requires activation of AMPARs by glutamatergic input and consequent action potential generation (Avshalumov et al. 2003, 2008).
Conclusions
The findings summarized here reveal an exquisite interaction between mitochondrial respiration and neuronal excitability, bridged by H2O2, which acts as a ‘translational’ substance that communicates the increase in metabolism to neuronal membranes via activation of KATP and TRPM2 channels. In its translational role, H2O2 generated within a given neuron can mediate autoinhibition and/or autoexcitation, but can act as a diffusible messenger to influence the activity of neighbouring cells (Fig.8). Actions of H2O2 at KATP and TRPM2 channels indicate that the net effect of H2O2 on a given cell or transmitter release site will reflect the balance of activity between H2O2-sensitive target channels expressed and thereby provide cell-type-specific patterns of modulation. These patterns of regulation have implications not only for normal regulation of basal ganglia transmitters and neuronal activity, but also for pathological conditions like Parkinson's disease, in which oxidative stress has been identified as a potential underlying factor in SNc DAergic neuron degeneration (e.g. Obeso et al. 2010). It should be noted that even without DAergic neuron loss, elevated levels of H2O2 would be expected to cause a net decrease in DAergic transmission. Increased H2O2 generation (or impaired metabolism) could lead to suppression of axonal DA release via KATP channel activation, which would be compounded by H2O2-dependent inhibition of SNc DAergic neuron excitability, resulting in functional DA denervation of target regions, like dorsal striatum (Bao et al. 2005; Avshalumov et al. 2005) (Fig.8). At the same time, the increased excitability of SNr GABAergic output neurons via H2O2 and TRPM2 channels (as seen in guinea pig SNr) would further exaggerate motor inhibition (Fig.8).
Two final points about H2O2-dependent regulation of neuronal signalling are that: (1) KATP and TRPM2 channels are expressed by many neurons in addition to those discussed here, so that modulation by H2O2 is likely to be widespread; and (2) additional targets for H2O2-dependent regulation are emerging, including GABA receptors that mediate inhibitory synaptic transmission (Accardi et al. 2014; Penna et al. 2014). In this light, it is also likely that dynamic cellular modulation by H2O2 is not limited to the CNS. For example, there are established functional roles for KATP channels in pancreatic β-cells, cardiac myocytes and muscle (McTaggart et al. 2010; Flagg et al. 2010; Coetzee, 2013), with emerging evidence for TRPM2 channels in β-cell function, as well (Uchida & Tominaga, 2014). Moreover, KATP channels in β-cells and cardiac myocytes are sensitive to exogenous H2O2 (Ichinari et al. 1996; Tokube et al. 1998; Krippeit-Drews et al. 1999), supporting the idea that metabolically generated H2O2 may be poised to provide modulatory signals in excitable cells throughout the body.
Acknowledgments
We thank Rijul Asri for recent contributions to studies of H2O2-dependent modulation in mice, and previous members of the Rice laboratory and our collaborators for their contributions to the original studies reviewed in this article.
Glossary
- AMPAR
AMPA receptor
- DA
dopamine
- DCF
dichlorofluorescein
- FFA
flufenamic acid
- FI
fluorescence intensity
- GSH
glutathione
- KATP channel
ATP-sensitive K+ channel
- MAO
monoamine oxidase
- MCS
mercaptosuccinate
- MSN
medium spiny neuron
- NOX
NADPH oxidase
- RF1
Redoxfluor-1
- ROS
reactive oxygen species
- SNc
substantia nigra pars compacta
- SNr
substantia nigra pars reticulata
- TRP channel
transient receptor potential channel
Additional information
Competing interests
The authors declare no competing financial interest.
Funding
The authors gratefully acknowledge support from the National Institutes of Health, grants R01 NS036362 (M.E.R.), F32 NS063656 (C.R.L.) and T32 DA007254 (B.O'N.), and from the Olympia and Attilio Ricciardi Research Fund.
Author's present address
C. R. Lee: Department of Cell Biology and Neuroscience, Rutgers, The State University of New Jersey, 604 Allison Road, Piscataway, NJ 08854, USA.
References
- Accardi MV, Daniels BA, Brown PM, Fritschy JM, Tyagarajan SK, Bowie D. Mitochondrial reactive oxygen species regulate the strength of inhibitory GABA-mediated synaptic transmission. Nat Commun. 2014;5:3168. doi: 10.1038/ncomms4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adimora NJ, Jones DP, Kemp ML. A model of redox kinetics implicates the thiol proteome in cellular hydrogen peroxide responses. Antiox Redox Signal. 2010;13:731–743. doi: 10.1089/ars.2009.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoroso S, Schmid-Antomarchi H, Fosset M, Lazdunski M. Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science. 1990;247:852–854. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- Arnaiz SL, Coronel MF, Boveris A. Nitric oxide, superoxide, and hydrogen peroxide production in brain mitochondria after haloperidol treatment. Nitric Oxide. 1999;3:235–243. doi: 10.1006/niox.1999.0229. [DOI] [PubMed] [Google Scholar]
- Avshalumov MV, Bao L, Patel JC, Rice ME. H2O2 signaling in the nigrostriatal dopamine pathway via ATP-sensitive potassium channels: issues and answers. Antioxid Redox Signal. 2007;9:219–231. doi: 10.1089/ars.2007.9.219. [DOI] [PubMed] [Google Scholar]
- Avshalumov MV, Chen BT, Koós T, Tepper JM, Rice ME. Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J Neurosci. 2005;25:4222–4231. doi: 10.1523/JNEUROSCI.4701-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Chen BT, Marshall SP, Peña DM, Rice ME. Glutamate-dependent inhibition of dopamine release in striatum is mediated by a new diffusible messenger, H2O2. J Neurosci. 2003;23:2744–2750. doi: 10.1523/JNEUROSCI.23-07-02744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, MacGregor DG, Sehgal LM, Rice ME. The glial antioxidant network and neuronal ascorbate: protective yet permissive for H2O2 signaling. Neuron Glia Biol. 2004;1:365–376. doi: 10.1017/S1740925X05000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Patel JC, Rice ME. AMPA receptor-dependent H2O2 generation in striatal medium spiny neurons, but not dopamine axons: one source of a retrograde signal that can inhibit dopamine release. J Neurophysiol. 2008;100:1590–1601. doi: 10.1152/jn.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Rice ME. Activation of ATP-sensitive K+ (KATP) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release. Proc Natl Acad Sci U S A. 2003;100:11729–11734. doi: 10.1073/pnas.1834314100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzaro AJ, King J, Kotzuk J, Schoepp DD, Frost J, Schochet S. Guinea pig striatum as a model of human dopamine deamination: the role of monoamine oxidase isozyme ratio, localization, and affinity for substrate in synaptic dopamine metabolism. J Neurochem. 1985;45:949–956. doi: 10.1111/j.1471-4159.1985.tb04086.x. [DOI] [PubMed] [Google Scholar]
- Babior BM. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984;64:959–966. [PubMed] [Google Scholar]
- Bao L, Avshalumov MV, Patel JC, Lee CR, Miller EW, Chang CJ, Rice ME. Mitochondria are the source of hydrogen peroxide for dynamic brain-cell signaling. J Neurosci. 2009;29:9002–9010. doi: 10.1523/JNEUROSCI.1706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Avshalumov MV, Rice ME. Partial mitochondrial inhibition causes suppression of striatal dopamine release and depolarization of medium spiny neuron via H2O2 elevation in the absence of ATP depletion. J Neurosci. 2005;25:10029–10040. doi: 10.1523/JNEUROSCI.2652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Chaumont F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim Biophys Acta. 2014;1840:1596–1604. doi: 10.1016/j.bbagen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Møller AL, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A. Treatment of Parkinson's with L-DOPA. The early discovery phase, and a comment on current problems. J Neural Transm. 2002;109:777–787. doi: 10.1007/s007020200064. [DOI] [PubMed] [Google Scholar]
- Chen BT, Avshalumov MV, Rice ME. H2O2 is a novel, endogenous modulator of synaptic dopamine release. J Neurophysiol. 2001;85:2468–2476. doi: 10.1152/jn.2001.85.6.2468. [DOI] [PubMed] [Google Scholar]
- Chen BT, Avshalumov MV, Rice ME. Modulation of somatodendritic dopamine release by endogenous H2O2: susceptibility in substantia nigra but resistance in VTA. J Neurophysiol. 2002;87:1155–1158. doi: 10.1152/jn.00629.2001. [DOI] [PubMed] [Google Scholar]
- Chung KK, Freestone PS, Lipski J. Expression and functional properties of TRPM2 channels in dopaminergic neurons of the substantia nigra of the rat. J Neurophysiol. 2011;106:2865–2875. doi: 10.1152/jn.00994.2010. [DOI] [PubMed] [Google Scholar]
- Coetzee WA. Multiplicity of effectors of the cardioprotective agent, diazoxide. Pharmacol Ther. 2013;140:167–175. doi: 10.1016/j.pharmthera.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. Enzymatic/nonenzymatic sources of oxyradicals and regulation of antioxidant defenses. Ann N Y Acad Sci. 1994;73:8–14. doi: 10.1111/j.1749-6632.1994.tb21784.x. [DOI] [PubMed] [Google Scholar]
- Dienel GA. Astrocytic energetics during excitatory neurotransmission: What are contributions of glutamate oxidation and glycolysis? Neurochem Int. 2013;63:244–258. doi: 10.1016/j.neuint.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Pawlowski PG, Hirrlinger J. Peroxide detoxification by brain cells. J Neurosci Res. 2005;79:157–165. doi: 10.1002/jnr.20280. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-d-aspartate. J Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Leone P, Davis KE, Kerr D, Sherwin RS. Glucose modulates rat substantia nigra GABA release in vivo via ATP-sensitive potassium channels. J Clin Invest. 1995;95:2403–2408. doi: 10.1172/JCI117935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engl E, Attwell D. Non-signalling energy use in the brain. J Physiol. 2015;593:3401–3413. doi: 10.1113/jphysiol.2014.282517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley J, Lees AJ. Aging and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Flagg TP, Enkvetchakul D, Koster JC, Nichols CG. Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol Rev. 2010;90:799–829. doi: 10.1152/physrev.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleig A, Penner R. The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharmacol Sci. 2004;25:633–639. doi: 10.1016/j.tips.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Freestone PS, Chung KK, Guatteo E, Mercuri NB, Nicholson LF, Lipski J. Acute action of rotenone on nigral dopaminergic neurons – involvement of reactive oxygen species and disruption of Ca2+ homeostasis. Eur J Neurosci. 2009;30:1849–1859. doi: 10.1111/j.1460-9568.2009.06990.x. [DOI] [PubMed] [Google Scholar]
- Geracitano R, Tozzi A, Berretta N, Florenzano F, Guatteo E, Viscomi MT, Chiolo B, Molinari M, Bernardi G, Mercuri NB. Protective role of hydrogen peroxide in oxygen-deprived dopaminergic neurones of the rat substantia nigra. J Physiol. 2005;568:97–110. doi: 10.1113/jphysiol.2005.092510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich FJ, Funke F, Hildebrandt B, Fasshauer M, Müller M. H2O2-mediated modulation of cytosolic signaling and organelle function in rat hippocampus. Pflugers Arch. 2009;458:937–952. doi: 10.1007/s00424-009-0672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeger G, Quiney C, Cotter TG. Hydrogen peroxide as a cell-survival signaling molecule. Antioxid Redox Signal. 2009;11:2655–2671. doi: 10.1089/ars.2009.2728. [DOI] [PubMed] [Google Scholar]
- Guatteo E, Federici M, Siniscalchi A, Knöpfel T, Mercuri NB, Bernardi G. Whole cell patch-clamp recordings of rat midbrain dopaminergic neurons isolate a sulphonylurea- and ATP-sensitive component of potassium currents activated by hypoxia. J Neurophysiol. 1998;79:1239–1245. doi: 10.1152/jn.1998.79.3.1239. [DOI] [PubMed] [Google Scholar]
- Guinamard R, Simard C, Del Negro C. Flufenamic acid as an ion channel modulator. Pharmacol Ther. 2013;138:272–284. doi: 10.1016/j.pharmthera.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyulkhandanyan AV, Pennefather PS. Shift in the localization of sites of hydrogen peroxide production in brain mitochondria by mitochondrial stress. J Neurochem. 2004;90:405–421. doi: 10.1111/j.1471-4159.2004.02489.x. [DOI] [PubMed] [Google Scholar]
- Häusser MA, de Weille JR, Lazdunski M. Activation by cromakalim of pre- and post-synaptic ATP-sensitive K+ channels in substantia nigra. Biochem Biophys Res Comm. 1991;174:909–914. doi: 10.1016/0006-291x(91)91504-6. [DOI] [PubMed] [Google Scholar]
- Hill K, Benham CD, McNulty S, Randall AD. Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology. 2004;47:450–460. doi: 10.1016/j.neuropharm.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Hill K, Tigue NJ, Kelsell RE, Benham CD, McNulty S, Schaefer M, Randall AD. Characterisation of recombinant rat TRPM2 and a TRPM2-like conductance in cultured rat striatal neurones. Neuropharmacology. 2006;50:89–97. doi: 10.1016/j.neuropharm.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Ichinari K, Kakei M, Matsuoka T, Nakashima H, Tanaka H. Direct activation of the ATP-sensitive potassium channel by oxygen free radicals in guinea-pig ventricular cells: its potentiation by MgADP. J Mol Cell Cardiol. 1996;28:1867–1877. doi: 10.1006/jmcc.1996.0179. [DOI] [PubMed] [Google Scholar]
- Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- Jiang C, Sigworth FJ, Haddad GG. Oxygen deprivation activates an ATP-inhibitable K+ channel in substantia nigra neurons. J Neurosci. 1994;14:5590–5602. doi: 10.1523/JNEUROSCI.14-09-05590.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W1, Bae SH, Toledano MB, Rhee SG. Role of sulfiredoxin as a regulator of peroxiredoxin function and regulation of its expression. Free Radic Biol Med. 2012;53:447–456. doi: 10.1016/j.freeradbiomed.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Kamsler A, Segal M. Hydrogen peroxide as a diffusible signal molecule in synaptic plasticity. Mol Neurobiol. 2004;29:167–178. doi: 10.1385/MN:29:2:167. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Jones SR, Wightman RM. Simultaneous measurement of oxygen and dopamine: coupling of oxygen consumption and neurotransmission. Neuroscience. 1992;47:603–612. doi: 10.1016/0306-4522(92)90169-3. [DOI] [PubMed] [Google Scholar]
- Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antiox Redox Signal. 2007;9:233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann E, Thiels E. Modulation of protein kinases and protein phosphatases by reactive oxygen species: implications for hippocampal synaptic plasticity. Prog Neuropsychopharm Biol Psychiatry. 1999;23:359–376. doi: 10.1016/s0278-5846(99)00002-0. [DOI] [PubMed] [Google Scholar]
- Krippeit-Drews P, Kramer C, Welker S, Lang F, Ammon HP, Drews G. Interference of H2O2 with stimulus–secretion coupling in mouse pancreatic β-cells. J Physiol. 1999;514:471–481. doi: 10.1111/j.1469-7793.1999.471ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD( NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lange I, Penner R, Fleig A, Beck A. Synergistic regulation of endogenous TRPM2 channels by adenine dinucleotides in primary human neutrophils. Cell Calcium. 2008;44:604–615. doi: 10.1016/j.ceca.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Machold RP, Witkovsky P, Rice ME. TRPM2 channels are required for NMDA-induced burst firing and contribute to H2O2-dependent modulation in substantia nigra pars reticulata GABAergic neurons. J Neurosci. 2013;33:1157–1168. doi: 10.1523/JNEUROSCI.2832-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Tepper JM. A calcium-activated nonselective cation conductance underlies the plateau potential in rat substantia nigra GABAergic neurons. J Neurosci. 2007;27:6531–6541. doi: 10.1523/JNEUROSCI.1678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Witkovsky P, Rice ME. Regulation of substantia nigra pars reticulata GABAergic neuron activity by H2O2 via flufenamic acid-sensitive channels and K-ATP channels. Front Syst Neurosci. 2011;5:14. doi: 10.3389/fnsys.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss B, Bruns R, Roeper J. Alternative sulfonylurea receptor expression defines metabolic sensitivity of K-ATP channels in dopaminergic midbrain neurons. EMBO J. 1999;18:833–846. doi: 10.1093/emboj/18.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss B, Haeckel O, Wildmann J, Miki T, Seino S, Roeper J. K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat Neurosci. 2005;8:1742–1751. doi: 10.1038/nn1570. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- Lutas A, Birnbaumer L, Yellen G. Metabolism regulates the spontaneous firing of substantia nigra pars reticulata neurons via KATP and nonselective cation channels. J Neurosci. 2014;34:16336–16347. doi: 10.1523/JNEUROSCI.1357-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTaggart JS, Clark RH, Ashcroft FM. The role of the KATP channel in glucose homeostasis in health and disease: more than meets the islet. J Physiol. 2010;588:3201–3209. doi: 10.1113/jphysiol.2010.191767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailloux RJ, McBride SL, Harper ME. Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics. Trends Biochem Sci. 2013;38:592–602. doi: 10.1016/j.tibs.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Maker HS, Weiss C, Silides DJ, Cohen G. Coupling of dopamine oxidation (monoamine oxidase activity) to glutathione oxidation via the generation of hydrogen peroxide in rat brain homogenates. J Neurochem. 1981;36:589–593. doi: 10.1111/j.1471-4159.1981.tb01631.x. [DOI] [PubMed] [Google Scholar]
- Makino N, Sasaki K, Hashida K, Sakakura Y. A metabolic model describing the H2O2 elimination by mammalian cells including H2O2 permeation through cytoplasmic and peroxisomal membranes: comparison with experimental data. Biochim Biophys Acta. 2004;1673:149–159. doi: 10.1016/j.bbagen.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Bernardi G, Giacomini P, Mercuri NB. Pharmacological identification of the K+ currents mediating the hypoglycemic hyperpolarization of rat midbrain dopaminergic neurones. Neuropharmacology. 2000;39:1021–1028. doi: 10.1016/s0028-3908(99)00186-0. [DOI] [PubMed] [Google Scholar]
- Miller EW, Bian SX, Chang CJ. A fluorescent sensor for imaging reversible redox cycles in living cells. J Am Chem Soc. 2007a;129:3458–3459. doi: 10.1021/ja0668973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EW, Dickinson BC, Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci U S A. 2010;107:15681–15686. doi: 10.1073/pnas.1005776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EW, Tulyathan O, Isacoff EY, Chang CJ. Molecular imaging of hydrogen peroxide produced for cell signaling. Nat Chem Biol. 2007b;3:263–267. doi: 10.1038/nchembio871. [DOI] [PubMed] [Google Scholar]
- Mishina NM, Tyurin-Kuzmin PA, Markvicheva KN, Vorotnikov AV, Tkachuk VA, Laketa V, Schultz C, Lukyanov S, Belousov VV. Does cellular hydrogen peroxide diffuse or act locally? Antioxid Redox Signal. 2011;14:1–7. doi: 10.1089/ars.2010.3539. [DOI] [PubMed] [Google Scholar]
- Mrejeru A, Wei A, Ramirez JM. Calcium-activated non-selective cation currents are involved in generation of tonic and bursting activity in dopamine neurons of the substantia nigra pars compacta. J Physiol. 2011;589:2497–2514. doi: 10.1113/jphysiol.2011.206631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KP, Greenfield SA. Neuronal selectivity of ATP-sensitive potassium channels in guinea-pig substantia nigra revealed by responses to anoxia. J Physiol. 1992;453:167–183. doi: 10.1113/jphysiol.1992.sp019222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gems D, Nyström T, Belousov V, Schumacker PT, Winterbourn CC. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011;13:361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, Hirsch EC, Farrer M, Schapira AH, Halliday G. Missing pieces in the Parkinson's disease puzzle. Nat Med. 2010;16:653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- Patel JC, Rice ME. Classification of H2O2 as a neuromodulator that regulates striatal dopamine release on a subsecond time scale. ACS Chem Neurosci. 2012;3:991–1001. doi: 10.1021/cn300130b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JC, Rice ME. Monitoring axonal and somatodendritic dopamine release using fast-scan cyclic voltammetry in brain slices. Methods Mol Biol. 2013;964:243–273. doi: 10.1007/978-1-62703-251-3_15. [DOI] [PubMed] [Google Scholar]
- Patel JC, Witkovsky P, Coetzee WA, Rice ME. Subsecond regulation of striatal dopamine release by presynaptic KATP channels. J Neurochem. 2011;118:721–736. doi: 10.1111/j.1471-4159.2011.07358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna A, Wang DS, Yu J, Lecker I, Brown PM, Bowie D, Orser BA. Hydrogen peroxide increases GABAA receptor-mediated tonic current in hippocampal neurons. J Neurosci. 2014;34:10624–10634. doi: 10.1523/JNEUROSCI.0335-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, Knowles HM, Ferraris D, Li W, Zhang J, Stoddard BL, Scharenberg AM. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem. 2005;280:6138–6148. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- Peuchen S, Bolanos JP, Heales SJ, Almeida A, Duchen MR, Clark JB. Interrelationships between astrocyte function, oxidative stress and antioxidant status within the central nervous system. Prog Neurobiol. 1997;52:261–281. doi: 10.1016/s0301-0082(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Ramasarma T. Generation of H2O2 in biomembranes. Biochim Biophys Acta. 1982;694:69–93. doi: 10.1016/0304-4157(82)90014-4. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Vautrelle N, Reynolds JN. Functional properties of the basal ganglia's re-entrant loop architecture: selection and reinforcement. Neuroscience. 2011;198:138–151. doi: 10.1016/j.neuroscience.2011.07.060. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Rhee SG. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Woo HA. Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxid Redox Signal. 2011;15:781–794. doi: 10.1089/ars.2010.3393. [DOI] [PubMed] [Google Scholar]
- Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- Rice ME. H2O2: a dynamic neuromodulator. Neuroscientist. 2011;17:389–406. doi: 10.1177/1073858411404531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME. Brain ascorbate: protective, yet permissive for redox signaling. In: Choi I-Y, Gruetter R, editors. Neural Metabolism In Vivo; Advances in Neurobiology, vol. 4. New York: Springer; 2012. pp. 1051–1073. [Google Scholar]
- Rice ME, Forman RE, Chen BT, Avshalumov MV, Cragg SJ, Drew KL. Brain antioxidant regulation in mammals and anoxia-tolerant reptiles: balanced for neuroprotection and neuromodulation. Comp Biochem Physiol C Toxicol Pharmacol. 2002;133:515–525. doi: 10.1016/s1532-0456(02)00116-3. [DOI] [PubMed] [Google Scholar]
- Rice ME, Patel JC, Cragg SJ. Dopamine release in the basal ganglia. Neuroscience. 2011;198:112–137. doi: 10.1016/j.neuroscience.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- Rigoulet M1, Yoboue ED, Devin A. Mitochondrial ROS generation and its regulation: mechanisms involved in H2O2 signaling. Antioxid Redox Signal. 2011;14:459–468. doi: 10.1089/ars.2010.3363. [DOI] [PubMed] [Google Scholar]
- Routh VH. Glucose sensing neurons in the ventromedial hypothalamus. Sensors. 2010;10:9002–9025. doi: 10.3390/s101009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanstecher C, Panten U. Tolbutamide- and diazoxide-sensitive K+ channel in neurons of substantia nigra pars reticulata. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:113–117. doi: 10.1007/BF00168546. [DOI] [PubMed] [Google Scholar]
- Schmitt FO. Molecular regulators of brain function: a new view. Neuroscience. 1984;13:991–1001. doi: 10.1016/0306-4522(84)90283-5. [DOI] [PubMed] [Google Scholar]
- Schmitt FO. Adventures in molecular biology. Annu Rev Biophys Biophys Chem. 1985;14:1–22. doi: 10.1146/annurev.bb.14.060185.000245. [DOI] [PubMed] [Google Scholar]
- Seutin V, Scuvee-Moreau J, Masotte L, Dresse A. Hydrogen peroxide hyperpolarizes rat CA1 pyramidal neurons by inducing an increase in potassium conductance. Brain Res. 1995;683:275–278. doi: 10.1016/0006-8993(95)00436-t. [DOI] [PubMed] [Google Scholar]
- Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem. 2014;289:8735–8741. doi: 10.1074/jbc.R113.544635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos M, Gras-Najjar J, Letchworth JM, Sanford AL, Toups JV, Sombers LA. Quantitation of hydrogen peroxide fluctuations and their modulation of dopamine dynamics in the rat dorsal striatum using fast-scan cyclic voltammetry. ACS Chem Neurosci. 2013;4:782–789. doi: 10.1021/cn4000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford IM, Lacey MG. Electrophysiological investigation of adenosine trisphosphate-sensitive potassium channels in the rat substantia nigra pars reticulata. Neuroscience. 1996;74:499–509. doi: 10.1016/0306-4522(96)00151-0. [DOI] [PubMed] [Google Scholar]
- Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- Stults FH, Forstrom JW, Chiu DTY, Tappel AL. Rat liver glutathione peroxidase: purification and study of multiple forms. Arch Biochem Biophys. 1977;183:490–497. doi: 10.1016/0003-9861(77)90384-8. [DOI] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- Thorens B. Sensing of glucose in the brain. Handb Exp Pharmacol. 2012;209:277–294. doi: 10.1007/978-3-642-24716-3_12. [DOI] [PubMed] [Google Scholar]
- Tokube K, Kiyosue T, Arita M. Effects of hydroxyl radicals on KATP channels in guinea-pig ventricular myocytes. Pflugers Arch. 1998;437:155–157. doi: 10.1007/s004240050760. [DOI] [PubMed] [Google Scholar]
- Tóth B, Csanády L. Identification of direct and indirect effectors of the transient receptor potential melastatin 2 (TRPM2) cation channel. J Biol Chem. 2010;285:30091–30102. doi: 10.1074/jbc.M109.066464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Tominaga M. The role of TRPM2 in pancreatic β-cells and the development of diabetes. Cell Calcium. 2014;56:332–339. doi: 10.1016/j.ceca.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Votyakova TV, Reynolds IJ. Δψ m-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem. 2001;9:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- Walton K, Fulton B. Hydrogen peroxide as a source of molecular oxygen for in vitro mammalian CNS preparations. Brain Res. 1983;278:387–393. doi: 10.1016/0006-8993(83)90280-9. [DOI] [PubMed] [Google Scholar]
- Wehage E, Eisfeld J, Heiner I, Jüngling E, Zitt C, Lückhoff A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem. 2002;277:23150–23156. doi: 10.1074/jbc.M112096200. [DOI] [PubMed] [Google Scholar]
- Woolley JF, Corcoran A, Groeger G, Landry WD, Cotter TG. Redox-regulated growth factor survival signaling. Antioxid Redox Signal. 2013;19:1815–1827. doi: 10.1089/ars.2012.5028. [DOI] [PubMed] [Google Scholar]
- Yamada T, McGeer PL, Baimbridge KG, McGeer EG. Relative sparing in Parkinson's disease of substantia nigra dopamine neurons containing calbindin-D28K. Brain Res. 1990;526:303–307. doi: 10.1016/0006-8993(90)91236-a. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Lee CR. Intrinsic and integrative properties of substantia nigra pars reticulata neurons. Neuroscience. 2011;198:69–94. doi: 10.1016/j.neuroscience.2011.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]