Abstract
Thermal perception is a fundamental physiological process pertaining to the vast majority of organisms. In vertebrates, environmental temperature is detected by the primary afferents of the somatosensory neurons in the skin, which express a ‘choir’ of ion channels tuned to detect particular temperatures. Nearly two decades of research have revealed a number of receptor ion channels that mediate the perception of several temperature ranges, but most still remain molecularly orphaned. Yet even within this well-researched realm, most of our knowledge largely pertains to two closely related species of rodents, mice and rats. While these are standard biomedical research models, mice and rats provide a limited perspective to elucidate the general principles that drive somatosensory evolution. In recent years, significant advances have been made in understanding the molecular mechanism of temperature adaptation in evolutionarily distant vertebrates and in organisms with acute thermal sensitivity. These studies have revealed the remarkable versatility of the somatosensory system and highlighted adaptations at the molecular level, which often include changes in biophysical properties of ion channels from the transient receptor potential family. Exploiting non-standard animal models has the potential to provide unexpected insights into general principles of thermosensation and thermoregulation, unachievable using the rodent model alone.
Elena Gracheva graduated from Moscow State University and obtained her PhD in neuroscience from the University of Illinois at Chicago. Her postdoctoral work at University of California San Francisco focused on the molecular basis of thermoreception in acutely thermosensitive vertebrates. She is now an Assistant Professor of Cellular and Molecular Physiology at Yale University School of Medicine. Her group works on the molecular basis of thermosensitivity and thermoregulation in hibernating mammals. Sviatoslav (Slav) Bagriantsev graduated from Moscow State University and carried out his PhD research on prions and amyloids at the University of Illinois at Chicago. After his postdoctoral training at University of California San Francisco in biophysics of heat- and mechanosensitive ion channels, he became an Assistant Professor of Cellular and Molecular Physiology at Yale University School of Medicine. His laboratory studies the molecular basis of mechano- and thermosensitivity in the somatosensory system of vertebrates.

Temperature sensitivity: basic principles and experimental approaches
In vertebrates, environmental temperature is perceived in the skin by primary afferents of the somatosensory neurons. Somas of the neurons are housed in the trigeminal ganglia (TG), which innervate the head, or dorsal root ganglia (DRG), which innervate the body. TG and DRG neurons form specialized, but not exclusive, functional groups, tuned to detect a particular range of temperatures. The molecular basis of temperature detection relies on the ability of specialized ion channels in the plasma membrane of primary afferents to initiate and propagate action potentials. Several major classes of ion channels contribute to this process: (i) receptor channels, which detect temperature changes and depolarize neurons via a non-selective cation influx; (ii) voltage-gated sodium and potassium channels, which open in response to receptor-mediated depolarization and propagate action potentials; and (iii) leak channels, which dynamically regulate the membrane potential at rest through voltage-independent potassium efflux (Fig.1). A combination of the three classes of ion channels is thought to constitute the core of the mechanism that fine-tunes neuronal sensitivity to a particular temperature range.
Figure 1. Temperature-gated ion channels known to contribute to thermal sensitivity in somatosensory neurons of vertebrates.
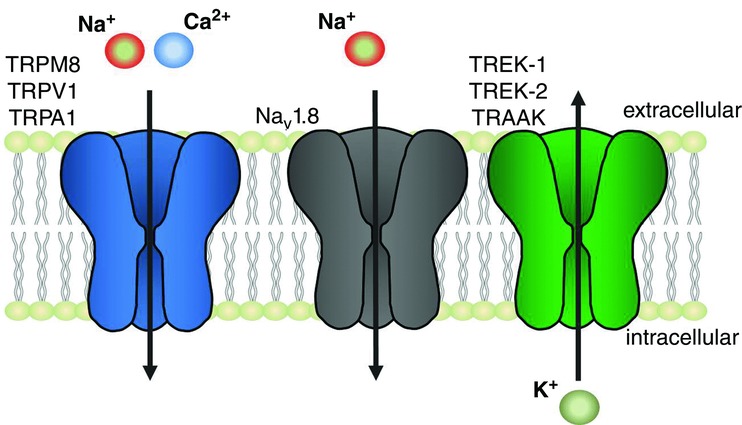
Transient receptor potential channels (TRPV1, TRPA1, TRPM8) sense temperature variations and cause neuronal excitation through sodium and calcium influx. The voltage-gated sodium channel Nav1.8 contributes to action potential propagation at noxiously cold temperatures. The two-pore ‘leak’ channels (TREK-1, TREK-2, TRAAK) mediate temperature-controlled efflux of potassium, thus contributing to the electric potential on the plasma membrane at rest and counterbalancing the excitatory action of the TRPs during temperature-driven excitation.
Somatosensory neurons are intrinsically thermosensi-tive, i.e. they do not require other tissue components to convert temperature changes into excitatory ionic current. Temperature-evoked electrical activity in these cells is usually studied either by an ex vivo skin–nerve preparation, whereby afferent signals are recorded from exposed afferent fibres, or in dissociated neuronal cultures using patch-clamp electrophysiology or ratiometric calcium imaging. A disadvantage of dissociated culture is that functional responses are studied in neuronal soma, whereas in vivo the signal is detected in the distal part of primary afferents. Nevertheless, dissociated neurons appear to provide an accurate description of neuronal specialization in vivo, and the approach is widely used due to its relative simplicity, and the ability to study functional responses of individual neurons or neuronal populations in a quantitative way and in precisely controlled experimental conditions.
A typical experimental protocol aimed at understanding the role of a particular ion channel in thermosensitivity consists of a comparison of the electrophysiological responses of the somatosensory neurons from wild-type and genetically modified strains, and of behavioural experiments, such as the temperature preference test. A good correlation between electrophysiological and behavioural data, coupled with biochemical confirmation of the expression of an ion channel in the nerve endings, provides solid ground for considering a molecular target as pertaining to thermosensitivity. This approach, which has been extensively used over the last 20 years, has identified a number of key ion channels. Even though the overall picture is far from complete, a general molecular framework that underlies thermosensitivity at the level of somatosensory neurons is now well accepted, and the molecular identity of receptors that respond to some temperature ranges have been widely recognized. These include transient receptor potential (TRP) cation channel types V1 (TRPV1) and M8 (TRPM8), the receptors of noxious heat and mild cold, respectively. Other temperature ranges remain molecular orphans, even though a number of candidates have been suggested over the years.
Most of our knowledge about the mechanism of cutaneous thermosensitivity comes from studies in mice and rats. Like many other mammals, including humans, mice perceive temperatures of 15–40°C as innocuous, and above or below this range as noxious. It is generally accepted that the mouse model provides a reliable ‘reference point’ for both molecular and physiological aspects of thermosensitivity, largely due to the availability of a standardized set of animal strains, genetic approaches, electrophysiological techniques and behavioural protocols.
With the advent of advanced and accessible experi-mental techniques, researchers have acquired the oppor-tunity to look beyond the mouse model. Studies of birds, infrared-sensing snakes, bats and other organisms revealed a surprising versatility of the thermoreceptive apparatus, and at the same time confirmed the general molecular framework established in mouse and rat. Studying ‘non-standard’ animals, especially those with unique thermosensory specializations (Fig.2) provides a unique perspective with which to understand the general principles of thermosensitivity. Below we discuss recent advances in the field of cutaneous thermosensitivity in both the standard and non-standard animal models, with a focus on vertebrates.
Figure 2. Molecular adaptations to temperature sensitivity in vertebrate TRP channels.
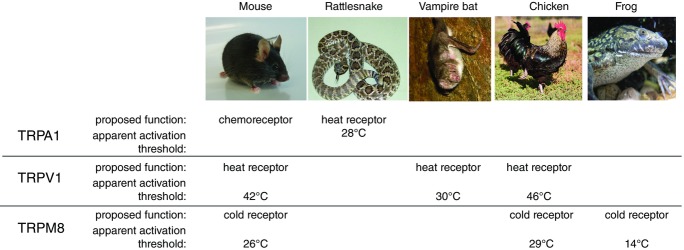
In mice and rats, TRPA1 serves as a receptor for noxious chemicals, TRPV1 detects noxious heat (apparent activation threshold, Tact 42°C), and TRPM8 detects mild cold (Tact 24°C). In infrared-sensing snakes and bats, however, TRPA1 and TRPV1 are both functionally modified and serve as low-intensity heat receptors, which contribute to the detection of infrared radiation. In chicken and frog, TRPV1 and TRPM8 have shifted apparent temperature activation thresholds, probably reflecting differences in the core body temperature between these species and the standard laboratory rodents. Image courtesy of Willem Laursen (Gracheva lab) and Wikimedia Commons.
Sensing cold
TRPM8
The menthol- and cold-activated non-selective cation channel TRPM8 contributes to the detection of environmental cold in vivo and in somatosensory neurons. In heterologous systems, such as mammalian cell lines or Xenopus oocytes, mammalian TRPM8 exhibits detectable current at around 26°C, which steeply increases further upon cooling (McKemy et al. 2002; Peier et al. 2002). Similar to other temperature-gated ion channels, the temperature at which TRPM8 activity exceeds background is often referred to as ‘channel activation threshold’, even though the value of this parameter can change depending on expression level or other experimental conditions. TRPM8 is found in 15–20% of all somatosensory neurons, usually with small (<30 μm) soma diameter (Kobayashi et al. 2005). TRPM8-deficient mice show a significant loss of menthol- and cold-evoked responses (≤26°C) at the behavioural, cellular and nerve fibre levels (Bautista et al. 2007; Colburn et al. 2007; Dhaka et al. 2007). In behavioural tests, temperature deficit is evident only at mildly cooling range, and becomes insignificant at temperatures below 10°C. At the same time, obliteration of the neuronal line which gives rise to TRPM8-positive neurons (Mishra et al. 2011) or targeted ablation of TRPM8 neurons in adult mice (Knowlton et al. 2013; Pogorzala et al. 2013) eliminates cold sensitivity in the full testable range. These data strongly suggest that TRPM8-positive neurons contain an unidentified TRPM8-independent molecular mechanism responsible for the detection of mildly and severely noxious cold.
A growing body of evidence supports the notion that in addition to its role sensing environmental cold, TRPM8 is involved in body temperature regulation (e.g. see Almeida et al. 2012; Gavva et al. 2012). Correlating with this function are data showing that cold activation parameters for TRPM8 orthologues differ significantly between warm- and cold-blooded animals, and appear to tie in with the value of normal core body temperature. For example, the half-maximal temperature activation value for the TRPM8 of chickens (29°C) is higher than that from rats (24°C), which possibly reflects the difference in core body temperature between these species (37°C and 40–42°C, respectively). In accordance with this trend, the half-maximal temperature activation of two species of frogs, Xenopus laevis and Xenopus tropicalis, is 14°C (Fig.2) (Myers et al. 2009). Cold sensitivity of TRPM8 thus appears to be coupled to body temperature, agreeing with a role for TRPM8 in body temperature regulation, and highlighting evolutionary flexibility of the temperature receptor.
Nav1.8
The tetrodotoxin-resistant voltage-gated sodium channel Nav1.8 (Akopian et al. 1996) is expressed in the majority of somatosensory neurons, including virtually all (90%) nociceptors (Shields et al. 2012). In comparison with tetrodotoxin-sensitive voltage-gated sodium channels from the somatosensory system, such as Nav1.7, Nav1.8 is more resistant to inhibition by cold (Zimmermann et al. 2007). Pharmacological and genetic studies have shown that Nav1.8−/− animals are almost completely insensitive to painful cold (below 10°C), suggesting a key role of this ion channel in cold sensing and/or propagation of action potentials at low temperatures (Zimmermann et al. 2007; Abrahamsen et al. 2008; Minett et al. 2014).
Recently, a gain of function mutation, T790A, has been identified in the mouse Nav1.8 (Blasius et al. 2011). Animals carrying the mutation exhibit a severe neurobehavioural phenotype referred to as ‘Possum’. Electrophysiological studies showed that the Possum mutation causes a dramatic increase in Nav1.8-mediated current density, leading to enhancement of excitability of DRG neurons and peripheral afferent nerve fibres. Notably, Possum mice are hypersensitive to noxious cold, but not to other types of painful stimuli, which supports the notion that Nav1.8 is a key regulator of cold sensitivity (Blasius et al. 2011; Garrison et al. 2014).
A recent study reported cold-induced regulation of Nav1.8 expression in neurons of gastropod molluscs. It has been demonstrated that hibernating snails have a reduced Nav1.8-like current density and significant down-regulation of Nav1.8 protein expression in buccal and cerebral ganglia – the regions responsible for feeding and olfaction, respectively – when compared to the active state (Kiss et al. 2014) (Fig.2). Thus the involvement of Nav1.8 in cold-induced regulation of neuronal excitability may have deep evolutional origin. Whether or not a similar molecular mechanism takes place in mammalian hibernators, is unknown. We surmise that a systematic investigation of molecular adaptations to cold tolerance in the somatosensory system of hibernators from different clades has the potential to reveal new molecular pathways of cold sensitivity, but such an analysis has yet to be performed.
Sensing heat
TRPV1
The capsaicin- and heat-activated non-selective cation channel TRPV1 exhibits robust temperature activation above 42°C in heterologous systems and primary neurons (Caterina et al. 1997; Cao et al. 2013a). TRPV1 is expressed in 40–60% of somatosensory neurons with small (<30 μm) and medium (30–40 μm) soma diameter (Kobayashi et al. 2005). TRPV1-deficient mice show complete loss of capsaicin sensitivity and significant reduction in the detection of noxious (≥50°C) heat stimuli and/or thermal hyperalgesia (Tominaga et al. 1998; Guo et al. 1999; Caterina et al. 2000; Davis et al. 2000; Cavanaugh et al. 2011; Park et al. 2011). The 10°C difference between the detectable TRPV1 activity in vitro (42°C) and a clear physiological effect of its deletion on thermosensitivity in vivo (≥50°C) is puzzling. Even after taking into consideration the difference between the test object temperature and afferent ending in the skin, the gap appears significant, while the underlying mechanism remains elusive. At the neuronal level, the deletion of TRPV1 significantly attenuates heat-evoked discharges in C-fibres (however, see Woodbury et al. 2004) and inhibits heat-stimulated excitation in dissociated DRG neurons (Caterina et al. 2000; Davis et al. 2000; Zimmermann et al. 2005; Vriens et al. 2011). It appears likely that even though TRPV1 marks almost all heat-sensitive neurons it is not the only molecular thermosensor in these cells. Indeed, whereas the deletion of TRPV1 does not affect thermosensitivity below 50°C, selective ablation of TRPV1-positive neurons in adult mice obliterates temperature discrimination in the whole testable range of temperatures above 37°C (Pogorzala et al. 2013), suggesting the existence of a TRPV1-independent mechanism of heat sensation. Over the years, a number of temperature-sensitive ion channels, mostly of the TRP group, have been suggested as novel heat sensors. Some of them, like TRPV2, TRPV3 and TRPV4, were later shown to have a minimal role in heat sensation (Huang et al. 2011; Park et al. 2011), while others, such as TRPM3 remain interesting and in need of further study (Vriens et al. 2011; Straub et al. 2013). As of this writing, temperatures in the non-TRPV1 range do not have a commonly accepted molecular sensor.
In mice, neither the deletion of TRPV1 nor complete elimination of TRPV1-expressing neurons affects core body temperature (Mishra et al. 2011). However, pharmacological blockade of TRPV1 causes hypothermia (Gavva et al. 2007), suggesting a role for TRPV1 in a cross-talk between environmental and body temperature. A capsaicin-insensitive splice variant of TRPV1 is expressed in the neurons of the supraoptic area of hypothalamus (Sharif Naeini et al. 2006) (however, see Cavanaugh et al. 2011). These neurons exhibit temperature-activated firing and vasopressin release and are tuned to detect minute variations in the normal 37°C body temperature. Both these properties become significantly attenuated upon pharmacological inhibition or genetic ablation of TRPV1 (Sharif-Naeini et al. 2008; Sudbury et al. 2010; Sudbury & Bourque, 2013). Even though the capsaicin-insensitive splicing isoform of TRPV1 from hypothalamus still awaits cloning and analysis in heterologous systems, these studies support the notion of a key role of TRPV1 in the regulation of physiological responses to changes in core body temperature.
Similar to TRPM8, TRPV1 function is modified in birds, whose body temperature (40–42°C) is significantly higher than in most mammals. The chicken orthologue of TRPV1 is heat sensitive, but the apparent activation threshold is shifted to around 46°C (Jordt & Julius, 2002), which possibly reflects the need to ‘adjust’ sensitivity in accordance with body temperature. It would be interesting to see if this trend continues in other animals with ‘non-standard’ body temperatures. Such an analysis would not only reveal evolutionary flexibility of temperature sensors, but would also help elucidate molecular determinants in the TRPV1 structure (Cao et al. 2013b; Liao et al. 2013) which fine-tune the temperature sensitivity of the channel.
TRPA1
The non-selective cation channel TRPA1, found in a subset (20–25%) of TRPV1-positive neurons (Story et al. 2003), is perhaps the most versatile of all polymodal sensory ion channels. Different TRPA1 orthologues were reported to mediate the detection of cold, heat and noxious chemicals, such as allyl isothiocyanate (AITC), the pungent agent from wasabi and other mustard plants (Jordt et al. 2004). In rodents, TRPA1 appears to contribute to noxious cold detection in pathological conditions (Bautista et al. 2006; Karashima et al. 2009; del Camino et al. 2010; Knowlton et al. 2010). Somewhat similarly, TRPA1 acts as a cold sensor in Caenorhabditis elegans (Chatzigeorgiou et al. 2010). In striking contrast, TRPA1 serves as a heat sensor in birds (Saito et al. 2014) and in ancestral vertebrates such as snakes (Gracheva et al. 2010), frogs and lizards (Saito et al. 2012; Kurganov et al. 2014). In invertebrates, TRPA1 mediates heat sensitivity in silkworm (Sato et al. 2014), mosquito and fly (Viswanath et al. 2003; Rosenzweig et al. 2005; Hamada et al. 2008; Kang et al. 2012; Zhong et al. 2012), exhibiting robust activation at around 21°C, 25°C and 28°C, respectively. The biophysical origin of temperature responses in TRPA1 is poorly understood and the location of temperature-sensing and -gating elements in the channel structure are obscure. Several reports pointed at the ankyrin repeats in the N-terminal domain of TRPA1 as major regulators, which can either dramatically affect or even reverse the directionality of temperature responses (Cordero-Morales et al. 2011; Wang et al. 2013; Jabba et al. 2014). A recent study, however, suggested that the N-terminal domain could play only a regulatory role, because purified recombinant human TRPA1 without the N-terminus is activated by cold and chemicals, including AITC (Moparthi et al. 2014). Together, these observations suggest that TRPA1 evolved as a temperature sensor several times in different vertebrate and invertebrate species, and that its functional fine-tuning often proceeds through the process of convergent evolution.
Leak potassium channels: possible regulators of cold and warm perception
The ‘two-pore’ (K2P) potassium channels mediate voltage-independent potassium ‘leak’, a key factor that contributes to establishing the resting potential of the plasma membrane (Fig.1). The K2Ps are expressed in various cell types, including somatosensory neurons, where they are thought to regulate excitation (Kang & Kim, 2006; Dobler et al. 2007; Bautista et al. 2008; Acosta et al. 2014; Guo & Cao, 2014). K2Ps of the TREK group, which includes TREK-1, TREK-2 and TRAAK, are activated by temperature. In heterologous systems, the channels are silent at around 14°C and maximally active at around 40–45°C (Maingret et al. 2000; Kang et al. 2005; Bagriantsev et al. 2011, 2012). The expression pattern of the heat-activated K2Ps overlaps with both TRPV1 and TRPM8 (Maingret et al. 2000; Alloui et al. 2006; Yamamoto et al. 2009), suggesting a role in the regulation of temperature sensitivity. Accordingly, genomic deletion of TREK-1 and/or TRAAK increases the firing rate of heat-sensing C-fibres and stimulates heat and cold avoidance in behavioural tests (Alloui et al. 2006; Noel et al. 2009; Descoeur et al. 2011). It should be noted that the deletion of TREK-1 and TRAAK produces a number of neurological phenotypes, including altered mechanosensation, anaesthetic responses and others (e.g. see Laigle et al. 2012), suggesting that the effects of the channel deletions on mechanosensitivity can be indirect. Moreover, it remains unclear to what extent the temperature-regulated dynamics of the background K+ leak, and not the background leak itself, are responsible for the deletion phenotypes. In support of the latter hypothesis, it was shown that the deletion of TASK-3, a K2P channel known to be expressed in a limited subset of DRG neurons (Talley et al. 2001; Marsh et al. 2012), potentiates cold sensitivity at the level of somatosensory neurons and in behavioural tests (Morenilla-Palao et al. 2014), even though the channel itself lacks robust temperature sensitivity (Maingret et al. 2000; Bagriantsev et al. 2011). All things considered, the temperature phenotypes of the K2P knockout strains are most consistent with a hypothesis that K2P activity counterbalances temperature-evoked depolarization caused by TRPV1 or TRPM8, and that the deletion of the K2Ps favours depolarization and thus potentiates sensitivity to both cold and warmth.
Tuning temperature sensitivity of vertebrates to the extreme
Several vertebrate classes (amphibians, reptiles and birds) evolved TRPA1 as a heat sensor (Saito et al. 2012, 2014; Kurganov et al. 2014). In functionally specialized species of snakes, such as rattlesnakes, pythons and boas, TRPA1 was proposed to serve as a primary infrared sensor playing an essential role in the detection of warm-blooded prey (Gracheva et al. 2010). Interestingly, pythons and boas belong to an ancient group of snakes, whereas rattlesnakes are relatively young in evolutionary terms. Nevertheless, despite the huge evolutionary distance – more than 30 million years – infrared-sensing snakes from the three different families co-opted the same molecular strategy to sense minute amounts of heat emanating from their prey: through functional tuning of TRPA1 (but not TRPV1, for example). Indeed, snake TRPA1 has one of the lowest apparent thermal thresholds identified so far in vertebrates, with a detectable temperature activation in vitro and in somatosensory neurons at around 28°C (Gracheva et al. 2010) (Fig.2). This fascinating molecular adaptation allows the snakes to detect their warm-blooded prey not only using visual or olfactory clues, but also though the perception of heat emitted from the prey.
Intriguingly, in comparison with heat-insensitive TRPA1 orthologues, the snake TRPA1 is poorly sensitive to activation by AITC, suggesting that the enhanced thermal sensitivity of the snake channel comes at the expense of chemical activation by electrophilic compounds (Gracheva et al. 2010; Cordero-Morales et al. 2011). This molecular adaptation makes sense from a physiological point of view. Indeed, fine-tuning of a molecular receptor to the extreme may require cancellation or reduction of ‘noise’ from a non-essential modality, such as sensitivity to electrophiles.
Another group of animals that have the capability to detect infrared radiation emitted by the prey are vampire bats. Vampire bats have unique feeding habits as they consume only blood, which implies the ability to efficiently find a hot spot (a superficial blood vessel) on the body of their endothermic prey. To find such a spot, vampire bats have developed a specialized leaf-pit structure on their face, innervated by primary afferents of the trigeminal nerve. At the molecular level, evolutionary specialization involved the modification of the already existing heat sensor, TRPV1, but in an unexpected way: through the generation of a new isoform through alternative RNA splicing. Trigeminal neurons of vampire bats express two splicing isoforms of TRPV1: a long isoform, which is functionally similar to the human, rat and mouse TRPV1 orthologues, and which is activated at around 40°C, and a short isoform, that differs by 62 amino acids in the C-terminus, and is activated at around 30°C. The short TRPV1 isoform is expressed entirely in the trigeminal somatosensory neurons that innervate the pit organ, head and face, whereas the long isoform is localized primarily in dorsal root ganglia and is probably responsible for ‘normal’ heat sensitivity throughout the body (Gracheva et al. 2011).
Molecular tuning of TRPV1 has also been discovered in species evolutionarily distant from bats. Zebrafish TRPV1 is activated at around 32°C, a temperature close to its favoured habitat conditions (around 33°C). Interestingly, in both species evolution has targeted the same region of TRPV1, but through different molecular strategies. As opposed to the alternative splicing strategy in bat TRPV1, zebrafish have a truncation in exon 15 of the TRPV1 gene (Gracheva et al. 2011). Thus, the two species present an example of a convergent molecular evolution, whereby the same gene is independently targeted to tune temperature sensitivity according to the specific behavioural needs.
The somatosensory system evolves rapidly to support different lifestyles and molecular adaptations in the animal kingdom. Different orthologues of TRPM8, TRPV1 and TRPA1 exemplify functional diversifications that help animals adapt to the environmental temperature variations as well as to develop and occupy new ecological niches. Changing the biophysical properties of the receptor channels is probably not the only mechanism of somatosensory fine-tuning. Alternative strategies could involve alterations in the expression level of thermo-TRPs and/or the number of thermosensitive neurons. This strategy is illustrated by the recent findings in the TG of star-nosed moles (Gerhold et al. 2013) and tactile foraging ducks (Schneider et al. 2014), where TRPV1 and TRPM8 expression is significantly down-regulated. The down-regulation of thermoreceptors could be a trade-off for the exceptional mechanosensory ability in these animals, which supports the notion that the development of sensitivity to different modalities is interconnected. These findings underscore evolutional flexibility of the somatosensory system in vertebrates and highlight the need to look beyond the rodent model to explore general and specialized principles of somatosensory system organization, including the ability to sense temperature.
Acknowledgments
We thank Karen Tonsfeldt for insightful comments on the manuscript.
Glossary
- AITC
allyl isothiocyanate
- DRG
dorsal root ganglia
- K2P channel
two-pore-domain potassium channel
- TG, trigeminal ganglia; TRP
transient receptor potential
Additional information
Competing interests
None declared.
Funding
This work was supported by the Arnold and Mabel Beckman Foundation, Rita Allen Foundation and Alfred P. Sloan Foundation to E.O.G. and a grant from the American Heart Association (14SDG17880015) to S.N.B.
References
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- Acosta C, Djouhri L, Watkins R, Berry C, Bromage K, Lawson SN. TREK2 expressed selectively in IB4-binding C-fiber nociceptors hyperpolarizes their membrane potentials and limits spontaneous pain. J Neurosci. 2014;34:1494–1509. doi: 10.1523/JNEUROSCI.4528-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Alloui A, Zimmermann K, Mamet J, Duprat F, Noel J, Chemin J, Guy N, Blondeau N, Voilley N, Rubat-Coudert C, Borsotto M, Romey G, Heurteaux C, Reeh P, Eschalier A, Lazdunski M. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006;25:2368–2376. doi: 10.1038/sj.emboj.7601116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, Garami A, Bautista D, Gavva NR, Romanovsky AA. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci. 2012;32:2086–2099. doi: 10.1523/JNEUROSCI.5606-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagriantsev SN, Clark KA, Minor DL., Jr Metabolic and thermal stimuli control K2P2.1 (TREK-1) through modular sensory and gating domains. EMBO J. 2012;31:3297–3308. doi: 10.1038/emboj.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagriantsev SN, Peyronnet R, Clark KA, Honoré E, Minor DL., Jr Multiple modalities converge on a common gate to control K2P channel function. EMBO J. 2011;30:3594–3606. doi: 10.1038/emboj.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Sigal YM, Milstein AD, Garrison JL, Zorn JA, Tsuruda PR, Nicoll RA, Julius D. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat Neurosci. 2008;11:772–779. doi: 10.1038/nn.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius AL, Dubin AE, Petrus MJ, Lim BK, Narezkina A, Criado JR, Wills DN, Xia Y, Moresco EM, Ehlers C, Knowlton KU, Patapoutian A, Beutler B. Hypermorphic mutation of the voltage-gated sodium channel encoding gene Scn10a causes a dramatic stimulus-dependent neurobehavioral phenotype. Proc Natl Acad Sci USA. 2011;108:19413–19418. doi: 10.1073/pnas.1117020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron. 2013a;77:667–679. doi: 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013b;504:113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O'Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzigeorgiou M, Yoo S, Watson JD, Lee WH, Spencer WC, Kindt KS, Hwang SW, Miller DM, 3rd, Treinin M, Driscoll M, Schafer WR. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat Neurosci. 2010;13:861–868. doi: 10.1038/nn.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Cordero-Morales JF, Gracheva EO, Julius D. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc Natl Acad Sci USA. 2011;108:E1184–1191. doi: 10.1073/pnas.1114124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D'Amours M, Deering N, Brenner GJ, Costigan M, Hayward NJ, Chong JA, Fanger CM, Woolf CJ, Patapoutian A, Moran MM. TRPA1 contributes to cold hypersensitivity. J Neurosci. 2010;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoeur J, Pereira V, Pizzoccaro A, Francois A, Ling B, Maffre V, Couette B, Busserolles J, Courteix C, Noel J, Lazdunski M, Eschalier A, Authier N, Bourinet E. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol Med. 2011;3:266–278. doi: 10.1002/emmm.201100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Dobler T, Springauf A, Tovornik S, Weber M, Schmitt A, Sedlmeier R, Wischmeyer E, Döring F. TRESK two-pore-domain K+ channels constitute a significant component of background potassium currents in murine dorsal root ganglion neurones. J Physiol. 2007;585:867–879. doi: 10.1113/jphysiol.2007.145649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison SR, Weyer AD, Barabas ME, Beutler BA, Stucky CL. A gain-of-function voltage-gated sodium channel 1.8 mutation drives intense hyperexcitability of A- and C-fiber neurons. Pain. 2014;155:896–905. doi: 10.1016/j.pain.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Jr, Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, Kuang R, Le A, Tamir R, Wang J, Youngblood B, Zhu D, Norman MH, Magal E, Treanor JJ, Louis JC. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Davis C, Lehto SG, Rao S, Wang W, Zhu DX. Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation. Mol Pain. 2012;8:36. doi: 10.1186/1744-8069-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhold KA, Pellegrino M, Tsunozaki M, Morita T, Leitch DB, Tsuruda PR, Brem RB, Catania KC, Bautista DM. The star-nosed mole reveals clues to the molecular basis of mammalian touch. PloS One. 2013;8:e55001. doi: 10.1371/journal.pone.0055001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracheva EO, Cordero-Morales JF, Gonzalez-Carcacia JA, Ingolia NT, Manno C, Aranguren CI, Weissman JS, Julius D. Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats. Nature. 2011;476:88–91. doi: 10.1038/nature10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, Sanchez EE, Perez JC, Weissman JS, Julius D. Molecular basis of infrared detection by snakes. Nature. 2010;464:1006–1011. doi: 10.1038/nature08943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Cao YQ. Over-expression of TRESK K+ channels reduces the excitability of trigeminal ganglion nociceptors. PloS One. 2014;9:e87029. doi: 10.1371/journal.pone.0087029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Li X, Yu Y, Wang J, Caterina MJ. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol Pain. 2011;7:37. doi: 10.1186/1744-8069-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabba S, Goyal R, Sosa-Pagan JO, Moldenhauer H, Wu J, Kalmeta B, Bandell M, Latorre R, Patapoutian A, Grandl J. Directionality of temperature activation in mouse TRPA1 ion channel can be inverted by single-point mutations in ankyrin repeat six. Neuron. 2014;82:1017–1031. doi: 10.1016/j.neuron.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Julius D. Molecular basis for species-specific sensitivity to ‘hot’ chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol. 2005;564:103–116. doi: 10.1113/jphysiol.2004.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Kim D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol. 2006;291:C138–C146. doi: 10.1152/ajpcell.00629.2005. [DOI] [PubMed] [Google Scholar]
- Kang K, Panzano VC, Chang EC, Ni L, Dainis AM, Jenkins AM, Regna K, Muskavitch MA, Garrity PA. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature. 2012;481:76–80. doi: 10.1038/nature10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, Battonyai I, Pirger Z. Down regulation of sodium channels in the central nervous system of hibernating snails. Physiol Behav. 2014;131:93–98. doi: 10.1016/j.physbeh.2014.04.026. [DOI] [PubMed] [Google Scholar]
- Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010;150:340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with Aδ/C-fibers and colocalization with Trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Kurganov E, Zhou Y, Saito S, Tominaga M. Heat and AITC activate green anole TRPA1 in a membrane-delimited manner. Pflugers Arch. 2014;466:1873–1884. doi: 10.1007/s00424-013-1420-z. [DOI] [PubMed] [Google Scholar]
- Laigle C, Confort-Gouny S, Le Fur Y, Cozzone PJ, Viola A. Deletion of TRAAK potassium channel affects brain metabolism and protects against ischemia. PloS One. 2012;7:e53266. doi: 10.1371/journal.pone.0053266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honoré E. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh B, Acosta C, Djouhri L, Lawson SN. Leak K+ channel mRNAs in dorsal root ganglia: relation to inflammation and spontaneous pain behaviour. Mol Cell Neurosci. 2012;49:375–386. doi: 10.1016/j.mcn.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minett MS, Eijkelkamp N, Wood JN. Significant determinants of mouse pain behaviour. PloS One. 2014;9:e104458. doi: 10.1371/journal.pone.0104458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 2011;30:582–593. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moparthi L, Survery S, Kreir M, Simonsen C, Kjellbom P, Högestätt ED, Johanson U, Zygmunt PM. Human TRPA1 is intrinsically cold- and chemosensitive with and without its N-terminal ankyrin repeat domain. Proc Natl Acad Sci USA. 2014;111:16901–16906. doi: 10.1073/pnas.1412689111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morenilla-Palao C, Luis E, Fernandez-Pena C, Quintero E, Weaver JL, Bayliss DA, Viana F. Ion channel profile of TRPM8 cold receptors reveals a role of TASK-3 potassium channels in thermosensation. Cell Rep. 2014;8:1571–1582. doi: 10.1016/j.celrep.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers BR, Sigal YM, Julius D. Evolution of thermal response properties in a cold-activated TRP channel. PloS One. 2009;4:e5741. doi: 10.1371/journal.pone.0005741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009;28:1308–1318. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park U, Vastani N, Guan Y, Raja SN, Koltzenburg M, Caterina MJ. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J Neurosci. 2011;31:11425–11436. doi: 10.1523/JNEUROSCI.1384-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Pogorzala LA, Mishra SK, Hoon MA. The cellular code for mammalian thermosensation. J Neurosci. 2013;33:5533–5541. doi: 10.1523/JNEUROSCI.5788-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Banzawa N, Fukuta N, Saito CT, Takahashi K, Imagawa T, Ohta T, Tominaga M. Heat and noxious chemical sensor, chicken TRPA1, as a target of bird repellents and identification of its structural determinants by multispecies functional comparison. Mol Biol Evol. 2014;31:708–722. doi: 10.1093/molbev/msu001. [DOI] [PubMed] [Google Scholar]
- Saito S, Nakatsuka K, Takahashi K, Fukuta N, Imagawa T, Ohta T, Tominaga M. Analysis of transient receptor potential ankyrin 1 (TRPA1) in frogs and lizards illuminates both nociceptive heat and chemical sensitivities and coexpression with TRP vanilloid 1 (TRPV1) in ancestral vertebrates. J Biol Chem. 2012;287:30743–30754. doi: 10.1074/jbc.M112.362194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Sokabe T, Kashio M, Yasukochi Y, Tominaga M, Shiomi K. Embryonic thermosensitive TRPA1 determines transgenerational diapause phenotype of the silkworm, Bombyx mori. Proc Natl Acad Sci USA. 2014;111:E1249–1255. doi: 10.1073/pnas.1322134111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ER, Mastrotto M, Laursen WJ, Schulz VP, Goodman JB, Funk OH, Gallagher PG, Gracheva EO, Bagriantsev SN. Neuronal mechanism for acute mechanosensitivity in tactile-foraging waterfowl. Proc Natl Acad Sci USA. 2014;111:14941–14946. doi: 10.1073/pnas.1413656111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif-Naeini R, Ciura S, Bourque CW. TRPV1 gene required for thermosensory transduction and anticipatory secretion from vasopressin neurons during hyperthermia. Neuron. 2008;58:179–185. doi: 10.1016/j.neuron.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Sharif Naeini R, Witty MF, Séguéla P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci. 2006;9:93–98. doi: 10.1038/nn1614. [DOI] [PubMed] [Google Scholar]
- Shields SD, Ahn HS, Yang Y, Han C, Seal RP, Wood JN, Waxman SG, Dib-Hajj SD. Nav1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain. 2012;153:2017–2030. doi: 10.1016/j.pain.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Straub I, Krügel U, Mohr F, Teichert J, Rizun O, Konrad M, Oberwinkler J, Schaefer M. Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo. Mol Pharmacol. 2013;84:736–750. doi: 10.1124/mol.113.086843. [DOI] [PubMed] [Google Scholar]
- Sudbury JR, Bourque CW. Dynamic and permissive roles of TRPV1 and TRPV4 channels for thermosensation in mouse supraoptic magnocellular neurosecretory neurons. J Neurosci. 2013;33:17160–17165. doi: 10.1523/JNEUROSCI.1048-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbury JR, Ciura S, Sharif-Naeini R, Bourque CW. Osmotic and thermal control of magnocellular neurosecretory neurons – role of an N-terminal variant of trpv1. Eur J Neurosci. 2010;32:2022–2030. doi: 10.1111/j.1460-9568.2010.07512.x. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, Patapoutian A, Jegla T. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, Oberwinkler J, Vennekens R, Gudermann T, Nilius B, Voets T. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron. 2011;70:482–494. doi: 10.1016/j.neuron.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Wang H, Schupp M, Zurborg S, Heppenstall PA. Residues in the pore region of Drosophila transient receptor potential A1 dictate sensitivity to thermal stimuli. J Physiol. 2013;591:185–201. doi: 10.1113/jphysiol.2012.242842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Hatakeyama T, Taniguchi K. Immunohistochemical colocalization of TREK-1, TREK-2 and TRAAK with TRP channels in the trigeminal ganglion cells. Neurosci Lett. 2009;454:129–133. doi: 10.1016/j.neulet.2009.02.069. [DOI] [PubMed] [Google Scholar]
- Zhong L, Bellemer A, Yan H, Ken H, Jessica R, Hwang RY, Pitt GS, Tracey WD. Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP channel. Cell Rep. 2012;1:43–55. doi: 10.1016/j.celrep.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature. 2007;447:855–858. doi: 10.1038/nature05880. [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Leffler A, Fischer MM, Messlinger K, Nau C, Reeh PW. The TRPV1/2/3 activator 2-aminoethoxydiphenyl borate sensitizes native nociceptive neurons to heat in wildtype but not TRPV1 deficient mice. Neuroscience. 2005;135:1277–1284. doi: 10.1016/j.neuroscience.2005.07.018. [DOI] [PubMed] [Google Scholar]


