Abstract
Healthy ageing is characterised by deterioration of motor performance. In normal circumstances motor adaptation corrects for movements’ inaccuracies and as such, it is critical in maintaining optimal motor control. However, motor adaptation performance is also known to decline with age. Anodal transcranial direct current stimulation (TDCS) of the cerebellum and the primary motor cortex (M1) have been found to improve visuomotor adaptation in healthy young and older adults. However, no study has directly compared the effect of TDCS on motor adaptation between the two age populations. The aim of our study was to investigate whether the application of anodal TDCS over the lateral cerebellum and M1 affected motor adaptation in young and older adults similarly. Young and older participants performed a visuomotor rotation task and concurrently received TDCS over the left M1, the right cerebellum or received sham stimulation. Our results replicated the finding that older adults are impaired compared to the young adults in visuomotor adaptation. At the end of the adaptation session, older adults displayed a larger error (−17 deg) than the young adults (−10 deg). The stimulation of the lateral cerebellum did not change the adaptation in both age groups. In contrast, anodal TDCS over M1 improved initial adaptation in both age groups by around 30% compared to sham and this improvement lasted up to 40 min after the end of the stimulation. These results demonstrate that TDCS of M1 can enhance visuomotor adaptation, via mechanisms that remain available in the ageing population.
Key points
Healthy ageing in man is associated with a decline in motor adaptation.
Transcranial direct current stimulation (TDCS) over the primary motor cortex (M1) or the lateral cerebellum can improve motor adaptation in young and older adults, but as yet no direct comparisons of TDCS effects exist between the two age groups and the two stimulation sites.
TDCS over M1 enhanced the motor adaptation in both age groups by ∼30% relative to their respective non-stimulated groups and improved the performance of older adults to the extent that it compared with that of young adults without stimulation.
The study suggests that the plastic mechanisms activated by TDCS that underpin improvements in motor behaviour in young adults remain available in older adults.
The results indicate that TDCS may be a useful tool to help combat the normal decline in motor performance seen in normal healthy ageing.
Introduction
When tested with sensitive laboratory-based behavioural tests, healthy ageing has been shown to be associated with a general reduction in motor performance, characterised by decreases in accuracy, coordination and movement speed and increases in movement duration and variability (Seidler et al. 2002, 2010; Krampe, 2002; Sarlegna, 2006; Heuninckx et al. 2008; Dutta et al. 2013). These impairments are linked to change in brain structure such as regional cortical thinning, decreases in the volume of subcortical structures, concordant ventricular enlargement and changes in white matter integrity (Salat et al. 2004; Walhovd et al. 2005, 2011; Fjell et al. 2009; Hafkemeijer et al. 2014; Sexton et al. 2014). Brain physiology is also affected in healthy aging, for example decreased neural plasticity is seen (Rogasch et al. 2009; Freitas et al. 2011; Pascual-Leone et al. 2011). These changes to brain structure and function may explain the decrease in motor performance that can be seen using lab-based behavioural testing.
Moving successfully in a dynamic and ever-changing world relies on continuous calibration of the motor system. Motor adaptation is a form of motor learning that restores accuracy when systematic motor errors are encountered. As such, it is thought to play a critical role in maintaining motor accuracy in the face of changing factors such as muscle fatigue or weakening. Several studies have reported that motor adaptation is impaired in older adults and as such may play a role in the general decline of motor performance in the ageing population (Buch et al. 2003; Bock & Girgenrath, 2006; Seidler, 2006; Anguera et al. 2010; Heuer & Hegele, 2011; Langan & Seidler, 2011; Huang & Ahmed, 2014).
The last 15 years have seen a seemingly exponential rise in the use of transcranial direct current stimulation (TDCS) in both the experimental and clinical settings. TDCS has the attractive property of being capable of modulating neural excitability whilst being painless, non-invasive and well tolerated. It has been repeatedly demonstrated that TDCS can improve some motor behaviour in both healthy subjects (Nitsche et al. 2003; Boggio et al. 2006; Reis et al. 2009) and a number of chronic and acute movement disorders such as stroke (Hummel et al. 2005), spinal cord injury (Fregni et al. 2006a) and Parkinson's disease (Fregni et al. 2006b). In general, it has been shown that the modulation of excitability is polarity dependent with anodal TDCS being excitatory and cathodal TDCS being inhibitory (Nitsche & Paulus, 2000).
A recent study took advantage of anodal TDCS ability to enhance behaviour to explore the respective role of the primary motor cortex (M1) and the lateral cerebellum in the adaptation of upper limb movements in young healthy adults (Galea et al. 2011). The authors found that anodal TDCS over the lateral cerebellum increased the rate of adaptation, while anodal TDCS over M1 increased the amount of retention of the adaptation. Their conclusion was that the lateral cerebellum was involved in the development of the adaptation itself, while M1 was responsible for retention of the adapted state. Following this first study, other studies found that anodal TDCS over the lateral cerebellum improved other forms of adaptation such as force-field adaptation, locomotor adaptation and eyeblink conditioning (Jayaram et al. 2012; Zuchowski et al. 2014; Herzfeld et al. 2014), suggesting that anodal TDCS could be a useful tool to enhance the adaptive process in healthy young adults. More recently, a study demonstrated that the application of anodal TDCS over the lateral cerebellum during the adaptation of reaching movements could compensate for the deficit in adaptation normally seen in older adults when compared to young controls (Hardwick & Celnik, 2014). However, to date, no single study has directly compared the effect of TDCS over M1 and the cerebellum between young and older adults.
Therefore, the aim of this study was to investigate the effect of anodal TDCS over M1 and over the lateral cerebellum on the motor adaptation of both young and older adults. Specifically, we intended to replicate earlier findings that visuomotor adaptation is indeed impaired in the older adults, and then identify whether the beneficial effect of anodal TDCS over M1 and the cerebellum was comparable between the two age groups. Because of the changes in brain structure and function underlying healthy aging, we could expect that the effect of anodal TDCS would quantitatively differ between young and older adults. Moreover, we tested the online effect of anodal TDCS on the adaptation process, but also its off-line short-term effect 50 min later. We used a version of the classic visuomotor rotation task in which participants must adapt to a rotation imposed between a joystick, controlled using small movements of the fingers and wrist, and the cursor presented on a computer screen (Cunningham, 1989; Krakauer et al. 1999; Miall et al. 2004). Participants received anodal TDCS over left M1, right cerebellum or received sham stimulation while adapting to this visuomotor rotation. Participants were subsequently re-tested after a 50 min break on the same adaptation protocol to evaluate short-term retention, before de-adapting back to their natural state.
Methods
Participants
Eighty-four healthy participants took part in the study, but the data of four older adults was excluded from this paper, as they did not follow the instructions to perform the task (i.e. they did not return to the starting position before the beginning of each trial). In total, we report the results for 80 healthy participants: 38 older participants (20 females, mean age: 63.2 ± 7.5 years old, 4 left-handed participants) and 42 young adults (20 females, mean age: 22.5 ± 3.1 years old, 8 left-handed participants). Handedness was self-reported as the dominant hand and participants received monetary compensation for their time (£10 per hour). Participants were assigned randomly to one of three groups as follows.
Motor cortex (M1) stimulation: 14 young (3 left-handers), 13 older adults (1 left-hander).
Cerebellar stimulation: 14 young (2 left-handers), 12 older adults (3 left-handers).
Sham stimulation: 14 young (3 left-handers), 13 older adults (no left-hander).
There was no age difference in the three sub-groups of young participants (F(2,39) < 1, P = 0.81) or the three sub-groups of older participants (F(2,35) < 1, P = 0.73). Young and older participants were screened for personal or familial history of epilepsy, neurological condition, neurosurgery, strokes and depression. Experimental procedures conformed to the Code of Ethics of the World Medical Association (Declaration of Helsinki) and were approved by the National Research Ethics Service (NRES) Committee South Central – Oxford B and C. Written informed consents were obtained from all participants who took part in the study.
TDCS
TDCS was applied via two saline-soaked electrodes (5 cm × 7 cm) using a DC-stimulator Plus (NeuroConn, Ilmenau, Germany). For M1 stimulation, the anodal electrode was placed over the hand area of the left primary motor cortex, identified in each subject with single-pulse transcranial magnetic stimulation (TMS: Magstim 200, Dyfed, UK), with the cathodal electrode positioned on the contralateral supraorbital area (Nitsche & Paulus, 2000). For cerebellar stimulation, the anodal electrode was centred on the right cerebellar cortex, 3 cm lateral to the inion (Galea et al. 2009; Jayaram et al. 2012; Hardwick & Celnik, 2014), while the cathodal electrode was placed on the left superior aspect of the trapezius muscle. For cerebellar TDCS, we used an extra-cephalic reference electrode to avoid the confound arising from placing the cathodal electrode on the participant's head which would influence the activity of the brain beneath. The cathodal electrode was placed over the trapezius muscle as this montage was successfully used to stimulate the cerebellum and cerebral cortex in several published studies (Joundi et al. 2012; Brittain et al. 2013; Mehta et al. 2014, 2015; Panouillères et al. 2015). In both stimulation conditions, anodal stimulation was delivered at 2 mA (Iyer et al. 2005; Ferrucci et al. 2008; Galea et al. 2011) with the stimulation intensity gradually ramped on and off over a 10 s period. TDCS started at the beginning of the baseline phase, continued during the first adaptation phase (7 min) and continued for approximately 10 min into the break (a total of 17 min stimulation: Fig.1A). The stimulation was continued into the break as it has been shown that stimulating M1 for at least 13 min leads to changes in M1 excitability in young adults lasting up to 60 min after stimulation termination (Nitsche & Paulus, 2001). For the sham-stimulation sessions, the electrodes were placed as for M1 stimulation, but stimulation only lasted for 30 s, with 10 s ramping on and off. In this way, all participants thought they were being stimulated. To maintain the blinding regarding the stimulation condition as much as possible, subjects were told that the results of the stimulated group will be compared to a non-stimulated group, but it was not mentioned that the non-stimulated group was in fact a sham group.
Figure 1. Experimental design.
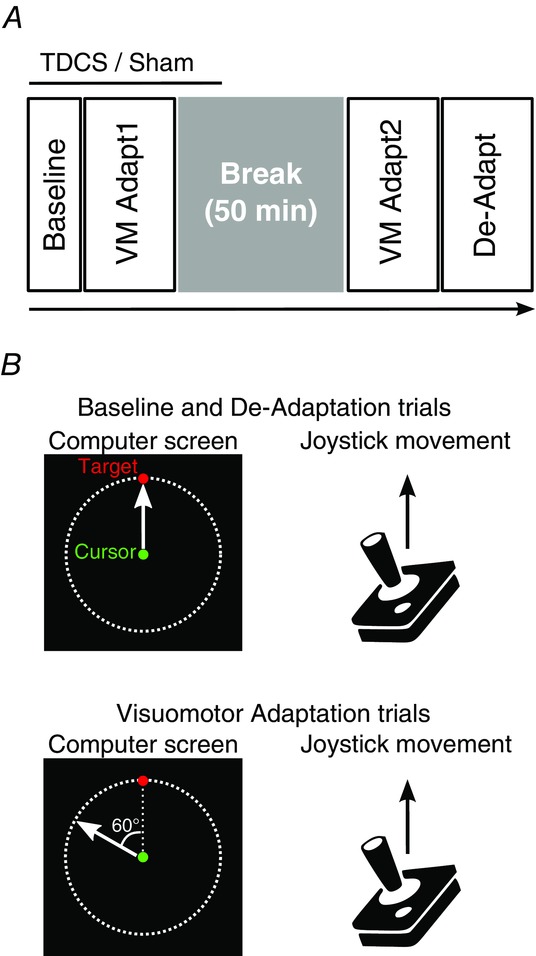
A, time course of the sessions. TDCS or sham was started in baseline, carried on during the first visuomotor adaptation phase (VM Adapt1) and was turned off approximately 10 min into the break (stimulation duration: 17 min). After the 50 min break, participants performed the second visuomotor adaptation phase (VM Adapt2) followed by the de-adaptation (De-Adapt). B, in the baseline and de-adaptation trials, the movements of the green cursor followed the exact path of the joystick movement. For the visuomotor adaptation trials (VM Adapt1 and VM Adapt2), the movement of the green cursor was rotated by 60 deg counterclockwise relative to the joystick movement. Note that the red target was presented randomly to one of 8 equidistant positions located on the dashed line circle.
Experimental design and procedure
Participants sat in an armless chair about 80 cm away from a computer screen (size: 26.5 cm × 16.5 cm) placed vertically in front of them and manipulated a joystick with their right hand, regardless of handedness, that was fixed to a table at a comfortable height on their right side. The joystick was 6.5 cm in height, 2 cm in width and the maximal centre-out excursion of 17 deg (low profile contactless joystick, APEM 9000 Series, RS Components). Subjects then controlled the joystick by moving their fingers and/or wrist. The joystick (sampling rate: 60 Hz) moved a green cursor (diameter: 0.3 cm) on the computer screen. A shield was used to prevent the participants from seeing their hand or joystick while performing the task. During the experiment, participants had to follow a red target (diameter: 0.3 cm) that jumped from the centre of the screen to one of eight equidistant positions, separated by 45 deg, located at the perimeter of a visible circle (radius: 4.6 cm, Fig.1B). The red target was presented in the centre of the screen for 750 ms and then jumped to a randomly selected peripheral position and stayed in this location for a further 750 ms. Participants started each trial with the green cursor in the centre of the screen (resting position of the joystick) and were instructed to make fast, accurate and ballistic movements with the joystick, in order to ‘shoot’ the red target with the green cursor. Participants had 750 ms to perform this movement; they were asked not to stop on the target but to pass through it and then to release the joystick so that the green cursor could come back to the starting position for the next trial. On average, outward movements for the older participants lasted about 220 ms and those of the young adults lasted about 160 ms, suggesting that both groups were able to elicit fast, ballistic movements.
Behavioural testing was divided into five phases: baseline, first adaptation (VM Adapt1), consolidation period (break), second adaptation (VM Adapt2) and de-adaptation (De-Adapt, Fig.1A). During the baseline, participants performed 50 trials in which the direction of movement of the green cursor matched the movement of the joystick (Fig.1B). After a break of 1 min, the adaptation phase (VM Adapt1) started and lasted for 150 trials. In this phase, the movement of the green cursor was rotated counter-clockwise by 60 deg relative to the joystick movement (Fig.1B). Participants were told the nature of the cursor rotation before the start of the adaptation phase, to give young and older participants similar explicit knowledge about the perturbation. Participants were instructed to keep moving as fast, accurately and straight as in the baseline phase and to avoid making corrective secondary movements despite the large errors initially incurred as a consequence of the rotation. Participants were also told not to use any explicit strategy to overcome the error and that the learning will occur implicitly. The consolidation period consisted of a 50 min break where participants were at rest. This period was followed by the second adaptation phase (VM Adapt2) that was identical to the initial adaptation phase. Finally, participants performed the de-adaptation phase (150 trials) in which the movement of the green cursor once again matched the movement of the joystick (Fig.1B).
Data analysis
Joystick movements were analysed on a trial-by-trial basis using in-house software written in MATLAB (Mathworks Inc., Natick, MA, USA). Our main measure was the angular error between the initial outward movement of the cursor and the target. We calculated the movement error as the angular difference between a straight line from the start position to the target and the position of the cursor at peak velocity. The automated calculation of movement error based on maximal velocity was checked by the operator trial-by-trial. Trials with premeditated or otherwise poorly defined movements were rejected from further analysis (mean ± standard deviation: 0.86 ± 0.97% of trials were rejected per subject). Baseline performance was measured by averaging the last 10 trials of the baseline phase. Adaptation in the different phases (VM Adapt1, VM Adapt2 and De-Adapt) was measured by averaging the movement error across blocks of 10 trials. The late adapted level reached at the end of VM Adapt1, VM Adapt2 and De-Adapt was taken as the average of the last 30 trials of each phase, where adaptation reaches an asymptote. For all individuals in the M1 groups, the percentage improvement in VM Adapt1 relative to sham was calculated as the difference between the late adapted level of each M1 subject and the mean value of the late adapted level in their respective sham group divided by the mean value of the late adapted level in their respective sham group.
Statistical analyses were performed with the SPSS Statistics software package (IBM, Armonk, NY, USA). We ran ANOVAs in the general linear model framework, but for simplicity we will refer to them as ANOVAs. To test for differences in adaptation, blocked error of every 10 trials for the different phases was compared with a three-way ANOVA with the between-subject factors Group (Young and Older) and Stimulation (Sham, M1 and Cerebellum) and the within-subject factor Blocks (1,2, … 15). The effect of stimulation on movement error in baseline and on the late adapted levels was evaluated using two-way ANOVAs with the between-subject factors Group (Young and Older) and Stimulation (Sham, M1 and Cerebellum). ANOVAs were performed separately for the different phases. Greenhouse–Geisser corrections to the degrees of freedom were applied if Mauchly's sphericity test revealed a violation of the assumption of sphericity for any of the factors in the ANOVAs. Significant main effects or interactions in the ANOVAs were followed by Bonferroni post hoc tests.
Results
Baseline performance
Performance of baseline as measured by the mean error across the last 10 baseline trials did not significantly differ between groups or stimulation conditions (two-way ANOVA: Group effect: F(1,74) < 1, P = 0.12; Stimulation effect: F(2,74) = 1.66, P = 0.20). There was a trend for an interaction between group and stimulation conditions (F(2,74) = 3.02, P = 0.06), which is explained by the fact that older participants in the M1 condition had slightly larger positive errors than the other older groups and that young in the cerebellar condition also had slightly larger errors compared to young in the sham condition (Table 1). However, these contrasts were far from being significant (Bonferroni post hoc tests for older M1 vs. older sham: P = 0.26; for older M1 vs. older cerebellum: P = 0.23; for young cerebellum vs. young sham: P = 0.11; for young M1 vs. young sham: P = 1). This result then suggests that TDCS over M1 or the cerebellum did not modify the initial motor performance in this joystick task.
Table 1.
Mean values (± standard deviation) of error (degrees) in baseline for the two groups and the three stimulation conditions
| Older | Young | |
|---|---|---|
| Sham TDCS | 0.09 ± 3.68 | −0.93 ± 2.55 |
| M1 TDCS | 2.21 ± 2.90 | −0.46 ± 2.32 |
| Cerebellar TDCS | −0.04 ± 2.45 | 0.85 ± 1.51 |
Older participants are impaired in motor adaptation
Separate ANOVAs for the VM Adapt1, VM Adapt2 and De-Adapt phases were performed on the mean error averaged across blocks of 10 trials with the between-subject factors Group (Young and Older) and Stimulation (Sham, M1 and Cerebellum) and the within-subject factor Blocks (1,2, … 15). Both the young and older participants demonstrated the ability to adapt to the visuomotor rotation by significantly decreasing their error within VM Adapt1 and VM Adapt2 in all stimulation conditions (Fig.2; main effect of Block: F(8,556) = 331.40, P < 0.001 and F(9,660) = 152.54, P < 0.001, respectively). Both age groups also significantly de-adapted (reduced their error) during the De-Adapt phase (main effect of Block: F(5,378) = 481.41, P < 0.001). However, older participants consistently demonstrated impaired adaptation performance compared with young participants in all three phases (main effect of Group: VM Adapt1: F(1,74) = 16.75, P < 0.001; VM Adapt2: F(1,74) = 17.32, P < 0.001; De-Adapt: F(1,74) = 9.27, P < 0.01). For all three phases, a significant interaction between Group and Block confirmed the differences in adaptation rate between young and older participants (VM Adapt1: F(8,556) = 2.52, P < 0.05; VM Adapt2: F(9,660) = 4.66, P < 0.001; De-Adapt: F(5,378) = 3.32, P < 0.01).
Figure 2. Acquisition of the visuomotor adaptation and de-adaptation.
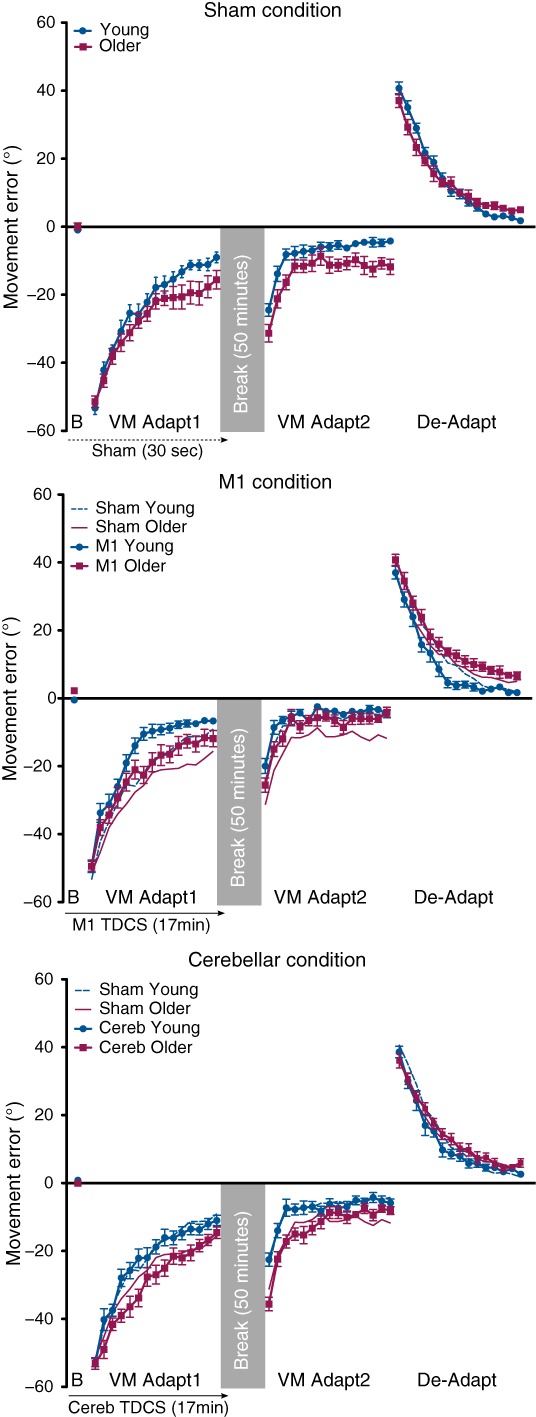
Movement error (degrees) is shown for the blocks (averaged across 10 trials) of the end of baseline (B), the first visuomotor adaptation (VM Adapt1), the second visuomotor adaptation (VM Adapt2) and the de-adaptation (De-Adapt) phases in the sham, M1 and cerebellar TDCS conditions, for the young (blue circles and lines) and older participants (purple squares and lines) groups. Note that for the M1 and cerebellar graphs, the performances of the sham groups are also represented (young participants: dashed thin blue line; older participants: plain thin purple line). Negative (positive) values indicate counterclockwise (clockwise) deviation. Error bars are standard errors of the mean.
To evaluate the level of adaptation and de-adaptation reached at the end of each phase, the last 30 trials of VM Adapt1, VM Adapt2 and De-Adapt were averaged for each subject. Two-way ANOVAs with the between-subject factors Group (young and older adults) and Stimulation (Sham, M1 and Cerebellum) were conducted on these late adapted levels, separately for the three adaptation phases (VM Adapt1, VM Adapt2 and De-Adapt). The reduced adaptation in the older is reflected in the late adapted levels, as measured by the mean error of the last three blocks of each phase (Fig.3). Indeed, in all phases, the young participants consistently made errors of significantly smaller magnitude than the older adults (two-way ANOVAs with main effect of Group: VM Adapt1: F(1,74) = 13.95, P < 0.001; VM Adapt2: F(1,74) = 16.54, P < 0.001; De-Adapt: F(1,74) = 28.60, P < 0.001).
Figure 3. Late adapted levels reached at the end of each phase.
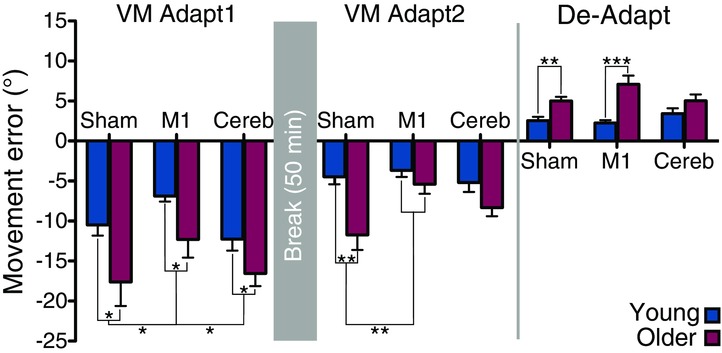
The mean error for the last 30 trials of each phase represents the late adapted level. Error bars are standard errors of the mean. Statistically significant differences are indicated by: *P < 0.05, **P < 0.01 and ***P < 0.001 (Bonferroni post hoc tests following ANOVAs).
M1 stimulation facilitates adaptation in both age groups and aligns the initial adaptation performance of the older participants to that of young participants
Adaptation was facilitated in both the young and older participants by M1 stimulation (Fig.2, M1 condition). With M1 stimulation, error reduction was significantly larger during VM Adapt1 than for the groups that received sham or cerebellar stimulation (main Stimulation effect: F(2,74) = 9.37, P < 0.001; Bonferroni post hoc tests for M1 vs. sham and M1 vs. cerebellum: P < 0.01). Strikingly, adaptation through VM Adapt1 in the older participants receiving M1 stimulation did not differ from the young sham stimulation group (two-way ANOVA comparing the young sham and older with M1 TDCS: main Group effect: F(1,25) < 1, P = 0.85, Block × Group interaction: F(6,151) = 1.21, P = 0.30).
The movement errors reached at the end of VM Adapt1 (Fig.3) were significantly lower in young and older participants who received TDCS over M1 compared to participants receiving sham or cerebellar TDCS (main Stimulation effect: F(2,74) = 4.29, P < 0.05; Bonferroni post hoc tests for M1 vs. sham and M1 vs. cerebellum: P < 0.05). Indeed, young adults who received sham TDCS reached an error of −10.5 ± 1.3 deg at the end of VM Adapt1 while those who received M1 TDCS had a final mean error of only −6.9 ± 0.7 deg. For older participants, the mean error reached at the end of VM Adapt1 was of −17.6 ± 3.0 deg with sham TDCS and of −12.3 ± 2.3 deg with M1 TDCS. In relative terms, the improvement in late adaptation with M1 stimulation compared to sham was surprisingly similar between the young and older groups: 34.3 ± 6.4% and 30.2 ± 12.9% reduction in error, respectively (t(25) = 0.29, P = 0.77).
Lasting effects of M1 stimulation after the 50 min break
Stimulation of M1 during VM Adapt1 led to a significantly improved performance in VM Adapt2 compared to the sham and cerebellar conditions (Fig.2; three-way ANOVA with Blocks, Group and Stimulation factors: Stimulation effect: F(1,50) = 6.24, P < 0.05; Bonferroni post hoc tests for M1 vs. sham and M1 vs. cerebellum: P < 0.05). The effect was mainly due to the larger decrease in error in the older participants with M1 stimulation for all VM Adapt2 while young participants were mainly improved at the beginning of this phase (Block × Group × Stimulation interaction: F(18,660) = 1.88, P < 0.05). Indeed, the profile of the error reduction during the second adaptation phase for the older participants who had received M1 TDCS was again very similar to that of the young participants in the sham condition (two-way ANOVA: main Group effect: F(1,25) < 1, P = 0.67, Block × Group interaction: F(7,718) < 1, P = 0.46).
The adaptation levels reached at the end of VM Adapt2 were significantly better following M1 TDCS than after sham TDCS in both age groups, as subjects made smaller errors after M1 TDCS than after sham TDCS (Fig.3; two-way ANOVA with Group and Stimulation factors: Stimulation effect: F(2,74) = 4.64, P < 0.05; Bonferroni post hoc tests: P < 0.05).
M1 stimulation speeds up the de-adaptation of young participants
M1 stimulation influenced the performance of young during de-adaptation and this was not seen following cerebellar or sham stimulation (Fig.2; three-way ANOVA with Blocks, Group and Stimulation factors: Group × Stimulation interaction: F(2,74) = 4.43, P < 0.05). Indeed the young group with M1 TDCS de-adapted more quickly than the young group who had received sham (Bonferroni post hoc test: P < 0.05), while the older groups de-adapted similarly in all the stimulation conditions (Bonferroni post hoc tests: P > 0.30). The measure of late adapted levels (Fig.3) shows that all the young participants reached the same level of de-adaptation and that the same was true for older participants (two-way ANOVA with Group and Stimulation factors: main Stimulation effect: F(2,74) < 1, P = 0.42; Group × Stimulation interaction: F(2,74) = 3.07, P = 0.052). Note that there was a small trend for older adults in the M1 condition to de-adapt less than sham and cerebellar conditions, but this was far from being significant (Bonferroni post hoc test: P > 0.25).
Online corrections were not affected by stimulation
It is possible that the increased accuracy described above is due to more rapid online corrections following TDCS, i.e. corrections before peak velocity is attained. To test this, we re-analysed the data to find the initial movement error calculated as the difference between the target angle and the angle of direction described by the initial straight line of the joystick movement (as opposed to that calculated using peak velocity, see Methods). For VM Adapt1, VM Adapt2 and De-Adapt, ANOVAs with the within-subject factor Block and the between-subject factors Group and Stimulation were conducted on initial movement errors. We again found that VM Adapt1 was facilitated with M1 stimulation relative to sham and cerebellar stimulation (main Stimulation effect: F(2,74) = 8.87, P < 0.001; Bonferroni post hoc tests: M1 vs. sham: P < 0.01; M1 vs. cerebellum: P < 0.001) and that adaptation in VM Adapt1 in the older participants receiving M1 stimulation did not differ from the young sham stimulation group (two-way ANOVA: main Group effect: F(1,25) = 0.34, P = 0.57, Block × Group interaction: F(7,184) = 1.39, p = 0.21). In VM Adapt2, the older participants who had received M1 stimulation adapted more than the ones who had received sham (Block × Group × Stimulation interaction: F(21,763) = 1.93, P < 0.01). Finally, M1 stimulation influenced the de-adaptation only in young participants relative to sham stimulation (Group × Stimulation interaction: F(2,74) = 5.57, P < 0.001). The lack of difference between these analyses and those using data where the initial movements’ errors are measured at the peak velocity suggest that TDCS did not influence online corrections, but really affected the adaptation process. This analysis also reveals that the use of peak velocity is an accurate method to calculate initial direction.
No effect of handedness on the results
All participants used their right hand to perform the task regardless of handedness as we wanted to stimulate the same brain sites in all the participants: the left primary motor cortex and the right lateral cerebellum. The left-handed participants were relatively well distributed across the different groups and stimulation conditions (see Methods). However, to be sure that the presence of left-handed participants did not alter the results, we performed ANOVAs on the movements’ error separately for VM Adapt1, VM Adapt2 and De-Adapt after excluding the data of all the 12 left-handers. These ANOVAs showed that older adults adapted at a significantly slower rate than young adults (Group effect: F(1,62) > 11.48, P < 0.01; Group × Block interaction: F(5,290) > 2.50, P < 0.05). Moreover, we also found that anodal TDCS over M1 facilitated the adaptation during VM Adapt1 compared to sham and cerebellar stimulation (Stimulation effect: F(2,62) = 6.25, P < 0.01; Bonferroni post hoc tests: P < 0.05). M1 stimulation was also improving the error reduction during VM Adapt2, mostly for older adults (Stimulation effect: F(2,62) = 2.90, P = 0.06; Stimulation × Block × Group interaction: F(17,533) = 2.02, P < 0.01). Finally, M1 stimulation also affected the performance of young during the de-adaptation phase (Group × Stimulation interaction: F(2,62) = 5.32, P < 0.01). Because these results are qualitatively and statistically similar to the ones presented above, we conclude that the handedness of our subjects did not impact the effect of the stimulation.
Discussion
The main finding of this study is that anodal TDCS over M1 similarly improved the acquisition of motor adaptation in both young and older adults. This facilitation of motor adaptation in older adults made their performance similar to that of young participants who did not receive any stimulation and the effect of the stimulation continued beyond the 50 min break. Surprisingly, we did not find any effect of the stimulation of the lateral cerebellum on the adaptive process.
Comparison of the two age groups who received sham stimulation replicates findings from previous studies that demonstrate that ageing is associated with a decrement in motor adaptation (Buch et al. 2003; Bock, 2005; Bock & Girgenrath, 2006; Seidler, 2006; Anguera et al. 2010; Fernandez-Ruiz et al. 2011; Heuer & Hegele, 2011; Langan & Seidler, 2011; Huang & Ahmed, 2014; Hardwick & Celnik, 2014). For visuomotor rotation, the deficits have been reported for sudden perturbations of both small (30 deg, Hardwick & Celnik, 2014, but see Heuer & Hegele, 2008) and large amplitude (60 deg, Bock, 2005; Heuer & Hegele, 2008, 2011), but not for gradual perturbations (Buch et al. 2003; Cressman et al. 2010). Several mechanisms have been posited for this deficit in adaptation in older people. The impairment could be cognitive and underpinned by a deficit in spatial working memory (Anguera et al. 2010) or by the inability to use explicit strategies to compensate for the rotation (Heuer & Hegele, 2008). More recently, it has been suggested that the decline in adaptation may also be due to a deficit in reinforcement learning in the older adults (Heuer & Hegele, 2014) and/or a deficit of the slow process of motor adaptation (Trewartha et al. 2014). Although our study aimed at replicating the findings of the studies above, further research will be needed to disentangle the mechanisms behind the deficit.
Previous studies have found that anodal TDCS over the lateral cerebellum enhances the acquisition of different forms of motor adaptation in young adults (Galea et al. 2011; Jayaram et al. 2012; Block & Celnik, 2013; Zuchowski et al. 2014; Herzfeld et al. 2014) and in older adults (Hardwick & Celnik, 2014). Our results are directly at odds with these reports as we do not find any effect of the anodal TDCS over the lateral cerebellum. While the studies above placed the cathode over the buccinator muscle (i.e. cheek), we placed it on the shoulder to avoid the confound arising from placing the cathodal electrode on the participant's head. Current density modelling suggests that this montage with the reference on the shoulder provides maximal current flow within the cerebellar hemispheres (Parazzini et al. 2014; Rahman et al. 2014). Moreover, electrode montages with reference on the shoulder have been used successfully in a few stimulation studies (Joundi et al. 2012; Brittain et al. 2013; Mehta et al. 2014, 2015). Finally, in a recent study using a similar montage, we found that TDCS over the cerebellum affected saccadic adaptation in a polarity-dependent manner (Panouillères et al. 2015). All these reasons make it unlikely that the different electrode montage for cerebellar stimulation is the reason for our lack of effect.
Our main finding that TDCS of M1 improves adaptation is also at odds with the study by Galea et al. (2011) who found that anodal stimulation of M1 increased the retention of adaptation, but not its initial acquisition. However, at least one other study has found M1 TDCS effective in improving adaptation (Hunter et al. 2009), while three other studies did not find any effect of M1 stimulation on force-field and visuomotor adaptation (Baraduc et al. 2004; Block & Celnik, 2013; Herzfeld et al. 2014). Our results are then consistent with growing evidence that the behavioural response to TDCS is sensitive to small variations in protocol. Several differences in protocol can be highlighted between our study and others already in the literature. For example, compared to Galea et al.’s study (2011), differences include the number of trials in baseline (50 vs. 196), the explicit knowledge of the perturbation, the type of movement and the effectors used (wrist/finger vs. arm) exist. However, it might be that the most important difference is the size of the visuomotor rotation (60 deg vs. 30 deg). It has been suggested that adapting to larger rotations involves more explicit learning strategies compared with adapting to smaller rotations and it has been hypothesised that these explicit processes are specifically prone to age-related changes (Heuer & Hegele, 2008; Hegele & Heuer, 2013). Furthermore, the processes that underpin the explicit components of visuomotor adaptation are thought to be cortical whereas implicit visuomotor adaptation is thought to be cerebellar. Therefore, it might be that the efficacy of M1 stimulation that we see in this study – and that is not seen in other studies using smaller rotations – is due to the cortical locus of the explicit processes engaged during adaptation to larger visuomotor rotations. It should also be noted that the level of control exerted by M1 over fine finger and wrist movements is far higher than that over reaching movements of the whole arm (see Lawrence & Kuypers, 1968). Therefore, it may be that M1 is more involved in the processes that adapt movements of the hand (as used in the current study) than those of movements of the arm. In agreement with our current result, facilitation of implicit and explicit motor learning of finger movements with M1 TDCS have been demonstrated in serial-reaction time tasks (Nitsche et al. 2003; Kantak et al. 2012) and motor skill learning tasks (Reis et al. 2009; Stagg et al. 2011; Schambra et al. 2011).
Our study shows that online M1 stimulation is beneficial to adaptation performance on our motor adaptation task irrespective of age. This effect could not be attributed to a placebo effect, as it was only present for M1 but not cerebellar TDCS. In relative terms, M1 stimulation improves the final reduction of error compared to sham stimulation (see Methods) by around 30% in both age groups. Our findings are certainly in line with previous studies showing that TDCS over M1 in the older adults could increase M1 plasticity and facilitate skill acquisitions in hand tasks, e.g. the Jebsen-Taylor hand function test and a finger-tapping task (Hummel et al. 2010; Goodwill et al. 2013; Zimerman et al. 2013). Moreover, M1 TDCS has a lasting effect in both age groups as about 40 min after the end of M1 stimulation young and older participants performed better in VM Adapt2 than in the sham condition. These data highlight that in both young and older adults, TDCS can have a similar behavioural impact lasting up to 40 min after stimulation termination. This is in agreement with a previous study that has shown that initial improvements in motor performance brought about using TDCS lead to improved retention 24 h later in older participants (Zimerman et al. 2013). Thus, our results show that, despite the functional and structural brain changes associated with healthy ageing, the mechanisms ‘activated’ by TDCS that result in improved performance in visuomotor adaptation in young adults remain available in older participants.
In conclusion, we confirmed that ageing is associated with a decline in visuomotor adaptation. Anodal TDCS over the motor cortex similarly enhanced the adaptation of both young and older adults and the improvement lasted in both age groups up to 40 min after the stimulation termination. This effect of the stimulation restored the performance of older adults to the one of young adults (without stimulation). These results demonstrate that TDCS of M1 can enhance visuomotor adaptation via mechanisms that remain available in the ageing population. Our findings indicate that TDCS may be a useful tool to help combat the normal decline in motor performance seen in normal healthy ageing.
Acknowledgments
The authors are grateful to the participants for contributing with their time and effort to this study and to DeNDRoN for recruiting some of the participants. The authors would also like to thank Professor Heidi Johansen-Berg for her insightful comments on the manuscript. The authors are extremely grateful to Professor Kate Watkins for her considerable help with the statistics and with writing the paper.
Glossary
- M1
primary motor cortex
- TDCS
transcranial direct current stimulation
- TMS
transcranial magnetic stimulation
Additional information
Competing interests
The authors declare that there are no competing interests.
Author contributions
All authors contributed to the conception and design of the experiments. M.T.N.P., R.A.J. and N.J. contributed to the collection, analysis and interpretation of data. All authors contributed to the drafting of the article or revising it critically for important intellectual content, and all authors approved the final version of the manuscript. The experiments were carried out at the Charles Wolfson Clinical Neuroscience Facility, John Radcliffe Hospital, Oxford.
Funding
This study was funded by a research grant from Parkinson's UK/Oddfellows Trust (G-1108). The authors wish to acknowledge support from the Medical Research Council UK (MR/J004588/1), the Oxford Biomedical Research Centre and the NIHR Oxford cognitive health Clinical Research Facility.
References
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Failure to engage spatial working memory contributes to age-related declines in visuomotor learning. J Cogn Neurosci. 2010;23:11–25. doi: 10.1162/jocn.2010.21451. [DOI] [PubMed] [Google Scholar]
- Baraduc P, Lang N, Rothwell JC, Wolpert DM. Consolidation of dynamic motor learning is not disrupted by rTMS of primary motor cortex. Curr Biol. 2004;14:252–256. doi: 10.1016/j.cub.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Block H, Celnik P. Stimulating the cerebellum affects visuomotor adaptation but not intermanual transfer of learning. Cerebellum. 2013;12:781–793. doi: 10.1007/s12311-013-0486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock O. Components of sensorimotor adaptation in young and elderly subjects. Exp Brain Res. 2005;160:259–263. doi: 10.1007/s00221-004-2133-5. [DOI] [PubMed] [Google Scholar]
- Bock O, Girgenrath M. Relationship between sensorimotor adaptation and cognitive functions in younger and older subjects. Exp Brain Res. 2006;169:400–406. doi: 10.1007/s00221-005-0153-4. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Castro LO, Savagim EA, Braite R, Cruz VC, Rocha RR, Rigonatti SP, Silva MTA, Fregni F. Enhancement of non-dominant hand motor function by anodal transcranial direct current stimulation. Neurosci Lett. 2006;404:232–236. doi: 10.1016/j.neulet.2006.05.051. [DOI] [PubMed] [Google Scholar]
- Brittain J-S, Probert-Smith P, Aziz TZ, Brown P. Tremor suppression by rhythmic transcranial current stimulation. Curr Biol. 2013;23:436–440. doi: 10.1016/j.cub.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch ER, Young S, Contreras-Vidal JL. Visuomotor adaptation in normal aging. Learn Mem. 2003;10:55–63. doi: 10.1101/lm.50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman EK, Salomonczyk D, Henriques DYP. Visuomotor adaptation and proprioceptive recalibration in older adults. Exp Brain Res. 2010;205:533–544. doi: 10.1007/s00221-010-2392-2. [DOI] [PubMed] [Google Scholar]
- Cunningham HA. Aiming error under transformed spatial mappings suggests a structure for visual-motor maps. J Exp Psychol Hum Percept Perform. 1989;15:493–506. doi: 10.1037//0096-1523.15.3.493. [DOI] [PubMed] [Google Scholar]
- Dutta GG, Freitas SMSF, Scholz JP. Diminished joint coordination with aging leads to more variable hand paths. Hum Mov Sci. 2013;32:768–784. doi: 10.1016/j.humov.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Wong W, Armstrong IT, Flanagan JR. Relation between reaction time and reach errors during visuomotor adaptation. Behav Brain Res. 2011;219:8–14. doi: 10.1016/j.bbr.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Marceglia S, Vergari M, Cogiamanian F, Mrakic-Sposta S, Mameli F, Zago S, Barbieri S, Priori A. Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. J Cogn Neurosci. 2008;20:1687–1697. doi: 10.1162/jocn.2008.20112. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29:15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Lima MC, Ferreira MJL, Wagner T, Rigonatti SP, Castro AW, Souza DR, Riberto M, Freedman SD, Nitsche MA, Pascual-Leone A. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006a;122:197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti SP, Silva MTA, Barbosa ER, Nitsche MA, Pascual-Leone A. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson's disease. Mov Disord. 2006b;21:1693–1702. doi: 10.1002/mds.21012. [DOI] [PubMed] [Google Scholar]
- Freitas C, Oberman L, Bashir S, Vernet M, Pascual-Leone A. Changes in cortical plasticity across the lifespan. Front Aging Neurosci. 2011;3:5. doi: 10.3389/fnagi.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29:9115–9122. doi: 10.1523/JNEUROSCI.2184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Vazquez A, Pasricha N, de Xivry JJO, Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: The motor cortex retains what the cerebellum learns. Cereb Cortex. 2011;21:1761–1770. doi: 10.1093/cercor/bhq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwill AM, Reynolds J, Daly RM, Kidgell DJ. Formation of cortical plasticity in older adults following tDCS and motor training. Front Aging Neurosci. 2013;5:87. doi: 10.3389/fnagi.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafkemeijer A, Altmann-Schneider I, de Craen AJM, Slagboom PE, van der Grond J, Rombouts SARB. Associations between age and gray matter volume in anatomical brain networks in middle-aged to older adults. Aging Cell. 2014;13:1068–1074. doi: 10.1111/acel.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Celnik PA. Cerebellar direct current stimulation enhances motor learning in older adults. Neurobiol Aging. 2014;35:2217–2221. doi: 10.1016/j.neurobiolaging.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegele M, Heuer H. Age-related variations of visuomotor adaptation result from both the acquisition and the application of explicit knowledge. Psychol Aging. 2013;28:333–339. doi: 10.1037/a0031914. [DOI] [PubMed] [Google Scholar]
- Herzfeld DJ, Pastor D, Haith AM, Rossetti Y, Shadmehr R, O'Shea J. Contributions of the cerebellum and the motor cortex to acquisition and retention of motor memories. Neuroimage. 2014;98:147–158. doi: 10.1016/j.neuroimage.2014.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer H, Hegele M. Adaptation to visuomotor rotations in younger and older adults. Psychol Aging. 2008;23:190–202. doi: 10.1037/0882-7974.23.1.190. [DOI] [PubMed] [Google Scholar]
- Heuer H, Hegele M. Generalization of implicit and explicit adjustments to visuomotor rotations across the workspace in younger and older adults. J Neurophysiol. 2011;106:2078–2085. doi: 10.1152/jn.00043.2011. [DOI] [PubMed] [Google Scholar]
- Heuer H, Hegele M. Age-related variations of visuo-motor adaptation beyond explicit knowledge. Front Aging Neurosci. 2014;6:152. doi: 10.3389/fnagi.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 2008;28:91–99. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HJ, Ahmed AA. Older adults learn less, but still reduce metabolic cost, during motor adaptation. J Neurophysiol. 2014;111:135–144. doi: 10.1152/jn.00401.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu W-H, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Heise K, Celnik P, Floel A, Gerloff C, Cohen LG. Facilitating skilled right hand motor function in older subjects by anodal polarization over the left primary motor cortex. Neurobiol Aging. 2010;31:2160–2168. doi: 10.1016/j.neurobiolaging.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Sacco P, Nitsche MA, Turner DL. Modulation of internal model formation during force field-induced motor learning by anodal transcranial direct current stimulation of primary motor cortex. J Physiol. 2009;587:2949–2961. doi: 10.1113/jphysiol.2009.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64:872–875. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- Jayaram G, Tang B, Pallegadda R, Vasudevan EVL, Celnik P, Bastian A. Modulating locomotor adaptation with cerebellar stimulation. J Neurophysiol. 2012;107:2950–2957. doi: 10.1152/jn.00645.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joundi RA, Jenkinson N, Brittain J-S, Aziz TZ, Brown P. Driving oscillatory activity in the human cortex enhances motor performance. Curr Biol. 2012;22:403–407. doi: 10.1016/j.cub.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak SS, Mummidisetty CK, Stinear JW. Primary motor and premotor cortex in implicit sequence learning – evidence for competition between implicit and explicit human motor memory systems. Eur J Neurosci. 2012;36:2710–2715. doi: 10.1111/j.1460-9568.2012.08175.x. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi M-F, Ghez C. Independent learning of internal models for kinematic and dynamic control of reaching. Nat Neurosci. 1999;2:1026–1031. doi: 10.1038/14826. [DOI] [PubMed] [Google Scholar]
- Krampe RT. Aging, expertise and fine motor movement. Neurosci Biobehav Rev. 2002;26:769–776. doi: 10.1016/s0149-7634(02)00064-7. [DOI] [PubMed] [Google Scholar]
- Langan J, Seidler RD. Age differences in spatial working memory contributions to visuomotor adaptation and transfer. Behav Brain Res. 2011;225:160–168. doi: 10.1016/j.bbr.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HGJM. The functional organization of the motor system in the monkey. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Mehta AR, Brittain J-S, Brown P. The selective influence of rhythmic cortical versus cerebellar transcranial stimulation on human physiological tremor. J Neurosci. 2014;34:7501–7508. doi: 10.1523/JNEUROSCI.0510-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AR, Pogosyan A, Brown P, Brittain J-S. Montage matters: The influence of transcranial alternating current stimulation on human physiological tremor. Brain Stimul. 2015;8:260–268. doi: 10.1016/j.brs.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Jenkinson N, Kulkarni K. Adaptation to rotated visual feedback: a re-examination of motor interference. Exp Brain Res. 2004;154:201–210. doi: 10.1007/s00221-003-1630-2. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003;15:619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- Panouillères MTN, Miall RC, Jenkinson N. The role of the posterior cerebellum in saccadic adaptation: A transcranial direct current stimulation study. J Neurosci. 2015;35:5471–5479. doi: 10.1523/JNEUROSCI.4064-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parazzini M, Rossi E, Ferrucci R, Liorni I, Priori A, Ravazzani P. Modelling the electric field and the current density generated by cerebellar transcranial DC stimulation in humans. Clin Neurophysiol. 2014;125:577–584. doi: 10.1016/j.clinph.2013.09.039. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, Bashir S, Vernet M, Shafi M, Westover B, Vahabzadeh-Hagh AM, Rotenberg A. Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topogr. 2011;24:302–315. doi: 10.1007/s10548-011-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Toshev PK, Bikson M. Polarizing cerebellar neurons with transcranial direct current stimulation. Clin Neurophysiol. 2014;125:435–438. doi: 10.1016/j.clinph.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch NC, Dartnall TJ, Cirillo J, Nordstrom MA, Semmler JG. Corticomotor plasticity and learning of a ballistic thumb training task are diminished in older adults. J Appl Physiol. 2009;107:1874–1883. doi: 10.1152/japplphysiol.00443.2009. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sarlegna FR. Impairment of online control of reaching movements with aging: A double-step study. Neurosci Lett. 2006;403:309–314. doi: 10.1016/j.neulet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Schambra HM, Abe M, Luckenbaugh DA, Reis J, Krakauer JW, Cohen LG. Probing for hemispheric specialization for motor skill learning: a transcranial direct current stimulation study. J Neurophysiol. 2011;106:652–661. doi: 10.1152/jn.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD. Differential effects of age on sequence learning and sensorimotor adaptation. Brain Res Bull. 2006;70:337–346. doi: 10.1016/j.brainresbull.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Alberts JL, Stelmach GE. Changes in multi-joint performance with age. Motor Control. 2002;6:19–31. doi: 10.1123/mcj.6.1.19. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Walhovd KB, Storsve AB, Tamnes CK, Westlye LT, Johansen-Berg H, Fjell AM. Accelerated changes in white matter microstructure during aging: A longitudinal diffusion tensor imaging study. J Neurosci. 2014;34:15425–15436. doi: 10.1523/JNEUROSCI.0203-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Jayaram G, Pastor D, Kincses ZT, Matthews PM, Johansen-Berg H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia. 2011;49:800–804. doi: 10.1016/j.neuropsychologia.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewartha KM, Garcia A, Wolpert DM, Flanagan JR. Fast but fleeting: adaptive motor learning processes associated with aging and cognitive decline. J Neurosci. 2014;34:13411–13421. doi: 10.1523/JNEUROSCI.1489-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Fjell AM. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. 2011;32:916–932. doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimerman M, Nitsch M, Giraux P, Gerloff C, Cohen LG, Hummel FC. Neuroenhancement of the aging brain: Restoring skill acquisition in old subjects. Ann Neurol. 2013;73:10–15. doi: 10.1002/ana.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchowski ML, Timmann D, Gerwig M. Acquisition of conditioned eyeblink responses is modulated by cerebellar tDCS. Brain Stimul. 2014;7:525–531. doi: 10.1016/j.brs.2014.03.010. [DOI] [PubMed] [Google Scholar]


