Abstract
Interlimb reflexes contribute to the central neural co-ordination between different limbs in both humans and animals. Although commissural interneurons have only been directly identified in animals, spinally-mediated interlimb reflexes have been discovered in a number of human lower limb muscles, indicating their existence in humans. The present study aimed to investigate whether short-latency crossed-spinal reflexes are present in the contralateral biceps femoris (cBF) muscle following ipsilateral knee (iKnee) joint rotations during a sitting task, where participants maintained a slight pre-contraction in the cBF. Following iKnee extension joint rotations, an inhibitory reflex was observed in the surface electromyographic (EMG) activity of the cBF, whereas a facilitatory reflex was observed in the cBF following iKnee flexion joint rotations. The onset latency of both cBF reflexes was 44 ms, which is too fast for a transcortical pathway to contribute. The cBF inhibitory and facilitatory reflexes followed the automatic gain control principle, with the size of the response increasing as the level of background pre-contraction in the cBF muscle increased. In addition to the surface EMG, both short-latency inhibitory and facilitatory cBF reflexes were recorded directly at the motor unit level by i.m. EMG, and the same population of cBF motor units that were inhibited following iKnee extension joint rotations were facilitated following iKnee flexion joint rotations. Therefore, parallel interneuronal pathways (probably involving commissural interneurons) from ipsilateral afferents to common motoneurons in the contralateral leg can probably explain the perturbation direction-dependent reversal in the sign of the short-latency cBF reflex.
Key points
The present study is the first to show short-latency crossed-spinal reflexes in the human upper leg muscles following mechanical rotations to the ipsilateral knee (iKnee) joint.
The short-latency reflex in the contralateral biceps femoris (cBF) was inhibitory following iKnee extension perturbations, and facilitatory following iKnee flexion perturbations. The onset latency was 44 ms, indicating that purely spinal pathways mediate the cBF reflexes.
The short-latency cBF inhibitory and facilitatory reflexes followed the automatic gain control principle, becoming larger as the level of background contraction in the cBF increased.
The short-latency cBF reflexes were observed at the motor unit level using i.m. electromyography recordings, and the same population of cBF motor units that was inhibited following iKnee extensions was facilitated following iKnee flexions.
Parallel interneuronal pathways from ipsilateral afferents to common motoneurons in the contralateral leg can therefore probably explain the perturbation direction-dependent reversal in the sign of the short-latency cBF reflex.
Introduction
Interlimb reflexes have been proposed to play an important role in the central neural co-ordination of different limbs in both animals and humans (Zehr et al. 2001; Haridas et al. 2006; Stubbs et al. 2011b). Recent studies in humans have identified several interlimb reflexes in the contralateral hamstrings muscles when the ipsilateral leg is perturbed by either peripheral nerve stimulation or mechanical perturbations during walking, with latencies ranging from 62 to 80 ms (Dietz et al. 1986; Nielsen et al. 2008; Mrachacz-Kersting et al. 2011; Stevenson et al. 2013; Stevenson et al. 2015). For example, an unexpected rotation of the ipsilateral ankle or knee joint elicits crossed reflex responses in the contralateral biceps femoris (cBF) muscle during walking (Mrachacz-Kersting et al. 2011; Stevenson et al. 2013). A transcortical pathway contributes to the cBF reflex following ipsilateral knee (iKnee) extension joint rotations, potentially allowing for more adaptable responses following changes in the external environment (Stevenson et al. 2013). Indeed, unexpected increases in treadmill velocity enhanced the cBF reflex, whereas unexpected decreases in treadmill velocity diminished the reflex, supporting the notion that the cBF reflex has a functional role in slowing the forward progression of the body to maintain dynamic stability during gait (Stevenson et al. 2015).
The longer latencies of reflexes observed in the cBF muscle following ipsilateral perturbations are in contrast to the short-latency interlimb pathway identified in the contralateral soleus (cSOL) and gastrocnemius muscle (Stubbs & Mrachacz-Kersting, 2009; Stubbs et al. 2011b; Gervasio et al. 2013; Hanna-Boutros et al. 2014). Following electrical stimulation applied to the ipsilateral tibial nerve, a depression was observed in the ongoing activity of the cSOL muscle (Stubbs & Mrachacz-Kersting, 2009; Stubbs et al. 2011b), along with a facilitation of the contralateral gastrocnemius lateralis (cGL) muscle (Gervasio et al. 2013). The latency of the cSOL inhibition (37–41 ms) suggests that purely spinal pathways mediate the response (Stubbs & Mrachacz-Kersting, 2009; Gervasio et al. 2013).
Commissural interneurons, as characterized in animal studies, are spinal interneurons connecting afferent nerves from one side of the spinal cord to interneurons and motoneurons on the contralateral side (Jankowska, 2008). They are proposed to play a pivotal role in interlimb co-ordination in animals, as well as in humans, in part through the mediation of interlimb reflexes (Jankowska, 2008). Although commissural interneurons have not been directly identified in humans, they are probably candidates for mediating the short-latency crossed spinal inhibitory responses in the cSOL muscle (Stubbs & Mrachacz-Kersting, 2009; Stubbs et al. 2011b).
Given that direct crossed-spinal connections to the contralateral hamstrings muscles exist in animals (Arya et al. 1991) and because spinally-mediated connections arising from ipsilateral afferents to the biceps femoris muscle exist in humans (Pierrot-Deseilligny et al. 1981; Marchand-Pauvert & Nielsen, 2002), it is somewhat surprising that short-latency interlimb reflexes have not been identified in the cBF. Short-latency ipsilateral and contralateral reflexes are often diminished during walking compared to standing or sitting, probbaly as a result of an increase in descending input modulating presynaptic inhibition to maintain stability and balance during walking (Morin et al. 1982; Capaday & Stein, 1986; Hayashi et al. 1992; Hanna-Boutros et al. 2014). Indeed, the group II-mediated component of the short-latency cSOL inhibition was abolished during the stance phase of walking (Hanna-Boutros et al. 2014). Therefore, the present study aimed to investigate whether short-latency crossed reflexes are present in the cBF following iKnee joint rotations during a sitting task, where participants maintained a slight pre-contraction in the cBF. We hypothesized that short-latency crossed-spinal reflexes would be observed in the cBF muscle following iKnee joint rotations, and that these interlimb reflexes would depend on the direction of the rotation applied to the iKnee joint.
Methods
Ethical approval
A total of 32 human participants (20 males and 12 females) aged 18–39 years (mean ± SD, 25.5 ± 4.2 years) took part in the present study. Four experiments were conducted (over 54 experimental sessions) and eight participants took part in at least two experiments. All participants were right-leg dominant. At the time of the study, all participants were free of any known physical or neurological disorders. All participants provided their written, informed consent to the experimental procedures, which were approved by the scientific ethics committee for Nordjylland (Approval No. 20110076). The study was performed in accordance with the Declaration of Helsinki.
Apparatus and instrumentation
Participants were seated in a chair that was fixed to the floor, with their hip and knee joints flexed at 90 deg and 45 deg, respectively (Fig.1). The right (ipsilateral) leg was affixed to a servo-controlled hydraulic actuator (215.35; MTS Systems Corporation, Eden Prairie, MN, USA) (Voigt et al. 1999), such that the anatomical knee axis of rotation was closely aligned with the fulcrum of the actuator. The lower segment of the right leg of the participant was firmly strapped to a custom-made plate that extended from the actuator, thus producing a tight interface between the arm of the motor and the leg of the participant. The lower segment of the left (contralateral) leg was firmly strapped to a custom-made plate that extended from the floor, such that the left leg was in the same start position as the right leg. In addition, the knees of both legs were fixed with a custom-made adjustable bar fixed to the chair and placed over the thigh slightly proximal to the knee joint. This position minimized both hip and ankle movement, ensuring that the movement of the actuator was transmitted solely to the knee joint. The angular position of the actuator was monitored with an angular displacement transducer (DC ADT series 600; Transtek, Beirut, Lebanon).
Figure 1. General experimental set-up.
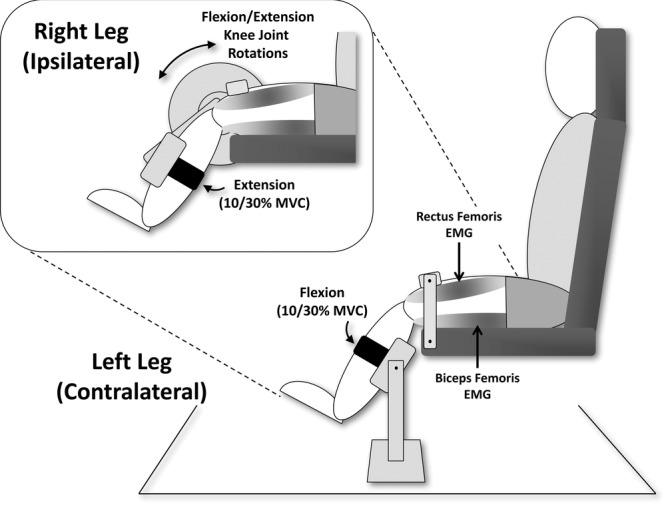
Participants sat comfortably in a chair with their hip and knee joints flexed at 90 deg and 45 deg, respectively. The right (ipsilateral) leg was affixed to a servo-controlled hydraulic actuator such that the anatomical knee axis of rotation was closely aligned with the fulcrum of the actuator. The lower segment of the right leg of the participant was firmly strapped to a custom-made plate that extended from the actuator. The lower segment of the left (contralateral) leg was firmly strapped to a custom-made plate that extended from the floor such that the left leg was in the same start position as the right leg. The knees of both legs were fixed with a custom-made adjustable bar fixed to the chair and placed over the thigh slightly proximal to the knee joint. Surface EMG activity was recorded from the iRF and iBF muscles, along with the cRF and cBF muscles. i.m. EMG activity was also recorded from the cBF in Experiment 3. Participants were asked to isometrically pre-activate the iRF and cBF to a level that produced EMG activity corresponding to 10% or 30% MVC. Knee flexion and extension joint rotations (6–8 deg and 150 deg s–1) were imposed in separate blocks of 30–60 trials every 3–5 s.
Surface electromyographic (EMG) activity was recorded by Ag/AgCl electrodes (720-01-K; Medicotest, Los Angeles, CA, USA) placed over the belly of the ipsilateral (right) rectus femoris (iRF) and biceps femoris (iBF), along with the contralateral (left) RF (cRF) and BF (cBF). In Experiments 1b and 3, surface EMG activity was only collected from the iRF and cBF muscles. The electrodes were placed in accordance with the recommendations of Cram et al. (1998). The EMG signals were amplified and bandpass filtered at 10 Hz to 1 kHz. They were also rectified and low pass filtered at 20 Hz (Butterworth 1st order digital filter). In Experiment 1b, flexible electrogoniometers (XM180 series; Biometrics Ltd, Cwmfelinfach, Newport, UK) were used to measure the left (contralateral) hip (cHip), knee (cKnee) and ankle (cAnkle) joint angles. Surface EMG and kinematic data were collected at a sampling frequency of 2 kHz. In Experiment 3, i.m. EMG was recorded from the cBF muscle using two bipolar wire electrodes. i.m. recordings were adopted primarily to confirm whether the same cBF motoneurons could have different behaviours depending on the inhibitory or facilitatory reflexes, and to confirm that changes in the surface EMG are not interference produced by the mechanical perturbation. i.m. wire electrodes were made of Teflon-coated stainless steel (diameter 50 μm; A-M Systems, Carlsborg, WA, USA) and were inserted with a sterile 25-gauge hypodermic needle. The insulated wires were cut to expose 3 mm of the wire. The needle was inserted into the muscle and then removed to leave the wire electrodes inside the muscle. i.m. signals were analogue bandpass filtered between 0.1 and 4.4 kHz and sampled at 10 kHz.
General experimental set-up
For all four experiments, visual feedback of the rectified surface EMG activity from the iRF and cBF was provided on a computer monitor placed in front of the participants with custom computer software. Initially, the participants were asked to perform a maximal isometric voluntary contraction (MVC) of the iKnee extensors and contralateral knee flexors, simultaneously. This was repeated twice and the best effort in each muscle was deemed the MVC. In all subsequent trials, the participants were asked to tonically pre-activate the iRF and cBF to a level that produced EMG activity corresponding to 10% or 30% of MVC. This was chosen because both the iKnee extensors and contralateral knee flexors are active during the late stance phase of the human gait (Stevenson et al. 2013).
Experiment 1a: Ipsilateral knee flexion and extension joint rotations
Nine participants (mean ± SD age, 27.8 ± 4.2 years) took part in this experiment. Following the set-up described above and the determination of MVC, participants were asked to tonically pre-activate the iRF and cBF to a level that produced EMG activity corresponding to 10% of MVC. Knee flexion and extension joint rotations (±6 deg amplitude with a peak angular velocity of ±150 deg s–1) were imposed in separate blocks every 3–5 s. The new joint position was held for 200 ms and then released, giving a total duration of the perturbation of 300 ms. Thirty trials were collected for each perturbation direction for each participant, for a total of 60 trials. Data collection for each trial began 100 ms prior to the perturbation and lasted for 500 ms. The order of the iKnee joint rotation direction was randomized. The instructions to the participants were to maintain the level of contraction level as displayed on the computer monitor and not to intervene when the knee joint rotation was applied.
Experiment 1b: Measurement of contralateral joint kinematics
To conform that the short-latency reflexes observed in the cBF in Experiment 1a were not a result of movement of the contralateral leg caused by the mechanical linkage between the legs, we measured cHip, cKnee and cAnkle joint angles following iKnee joint rotations. Eight participants (mean ± SD age, 26.82 ± 1.5 years) took part in this experiment. The experimental procedures were identical to the procedures described in Experiment 1a.
Experiment 2: Manipulation of background muscle contraction level
To further confirm that the short-latency reflexes observed in the cBF in Experiment 1a were not simply a result of vibrations caused by the mechanical actuator, we tested the automatic gain control principle (Matthews, 1986) by varying the level of background contraction in the iRF and cBF. Thirteen participants (mean ± SD age, 23.7 ± 4.1 years) took part in this experiment. Participants were asked to tonically pre-activate the iRF and cBF to a level that produced EMG activity corresponding to either 10% or 30% of MVC. Knee flexion or knee extension joint rotations (6 deg and 150 deg s–1) were imposed in separate blocks of 30 trials every 3–5 s, with the iRF and cBF pre-contracting at different levels. The combinations of pre-contraction included: iRF 10% MVC and cBF 10% MVC; iRF 10% MVC and cBF 30% MVC; iRF 30% MVC and cBF 10% MVC; and iRF 30% MVC and cBF 30% MVC. Participants therefore received a total of 240 knee joint rotations separated into eight blocks of 30 trials. The new joint position for all conditions was held for 200 ms and then released, giving a total duration of the perturbation of 300 ms. Data collection for each trial began 100 ms prior to the perturbation and lasted for 500 ms. The order of the blocks varied across participants, and participants were allowed to rest between blocks as required to prevent fatigue. The instructions to the participants were to maintain the level of contraction level as displayed on the computer monitor and not to intervene when the knee joint rotation was applied.
Experiment 3: i.m. EMG recordings in the cBF
In this experiment, i.m. EMG was recorded in the cBF to determine whether the reversal in the sign of the short-latency cBF reflexes measured by surface EMG following either iKnee extension or flexion joint rotations can be quantified at the motor unit (MU) level and involved the same population of MUs (De Serres et al. 1995). Eleven participants (mean ± SD age, 25.5 ± 4.8 years) took part in this experiment. Participants were asked to maintain a comfortable level of activation in the iRF and cBF to a level that produced EMG activity constrained between 5% and 10% of MVC. Knee flexion or knee extension joint rotations (±8 deg amplitude with a peak angular velocity of ±150 deg s–1) were imposed in separate blocks of 60 trials every 3–5 s. This condition was performed because MU activity is more sensitive than surface EMG. Participants therefore received a total of 120 knee joint rotations separated into two blocks of 60 trials. The new joint position for all conditions was held for 200 ms and then released, giving a total duration of the perturbation of 300 ms. Data collection for each trial began 1 s prior to the perturbation and lasted for 3 s. The order of the conditions was randomized. The instructions to the participants were to maintain the level of contraction level as displayed on the computer monitor and not to intervene when the knee joint rotation was applied.
Data analysis
For the surface EMG data, the amplitude of the responses in each muscle for each condition was calculated as the area under the mean, filtered, full-wave rectified surface EMG signal. For each condition, the amplitude of the responses was expressed as a percentage of the baseline surface EMG, recorded in the 100 ms preceding the perturbation to the iKnee joint. The onset of the reflex responses was determined for each muscle in each participant using an algorithm in Matlab (MathWorks Inc., Natick, MA, USA) and was defined as the first SD of the mean rectified EMG data above or below two SDs of the mean rectified baseline EMG that lasted for at least 10 ms. The offset of the reflex responses was defined as the point where the mean rectified EMG in the perturbation trials returned to within two SDs of the baseline EMG for at least 10 ms. The reflex onsets and offsets were manually verified for accuracy. When either a depression or facilitation was observed in the EMG, the onset and duration of the response were recorded. To quantify the amplitude of the response to the imposed knee joint rotations for each muscle, the mean integrated EMG activity was calculated from the onset for the duration of the response. As a result of the lack of background EMG in the iBF and cRF, only responses from the iRF and cBF were analysed.
For Experiment 1b, the onsets of cHip, cKnee and cAnkle joint movement were defined as the first SD of the mean joint angles above or below two SDs of the mean baseline joint angle that lasted for at least 10 ms. When deflections in joint angles were observed, the onset, direction and peak amplitude were recorded.
i.m. EMG provides the means to decode the neural drive as the ensemble of the timings of activations of the motoneurons innervating the muscle. Thus, most of the information lies in the number of MU action potentials discharged per unit time. i.m. EMG data were decomposed using EMGLAB (McGill et al. 2005) into constituent MU action potentials and analysed in two ways. First, the total number of action potentials (over all 60 trials) was quantified using a 10 ms window to create a peristimulus time histogram (PSTH) expressed by the number of counts as a percentage of the number of perturbations. A window of 10 ms was used to ensure a reliable resolution when determining the onset and duration of the response. Second, the instantaneous discharge frequencies (discharge rate) for each MU were quantified 100 ms pre- and post-perturbation to investigate how they were affected by the perturbation. This analysis was carried out for all detected MUs; however, signals from the two conditions were concatenated prior to MU decomposition to enable tracking and estimation of the number of common units across conditions using EMGLAB.
Statistical analysis
For Experiment 1a, the amplitude of the responses observed in the cBF for each perturbation direction was tested for significance with respect to baseline levels using single-sample Student's t tests. For Experiment 1b, the onset of the joint angle movements were compared with the cBF reflex onset using a repeated measures ANOVA for each perturbation direction, with mean onset (cBF reflex, cHip, cKnee, cAnkle) as the within-subjects factor. For Experiment 2, investigating the effects of altering the cBF pre-contraction level on the cBF response magnitude, correlation analyses were performed between cBF pre-contraction level and cBF response amplitude (with background activity subtracted). Separate analyses were performed for each level of pre-contraction in the iRF (10% and 30% MVC) and for each perturbation direction (flexion and extension). For each perturbation direction, the cBF reflex amplitudes for the two different iRF contraction level (10% and 30% MVC) were tested for significance using paired samples t tests.
For Experiment 3, mean PSTHs for both extension and flexion perturbations were calculated across all participants. The mean background MU firing counts and firing frequencies were calculated during the 100 ms period immediately prior to perturbation onset. Periods of facilitation and inhibition were determined if the firing counts in three or more adjacent bins were above (or below) the mean background firing plus (or minus) 3 SDs (Mao et al. 1984). The background firing frequencies in the cBF for each perturbation direction were tested for significance using a paired samples t test. The effects of perturbation direction on i.m. EMG firing frequency in the cBF were assessed via a two-way ANOVA with perturbation direction (extension, flexion) and time (background, response window) as within-subject factors.
Greenhouse-Geisser corrected degrees of freedom were used to correct for violations in the assumption of sphericity. P < 0.05 was considered statistically significant.
Results
A summary of the mean ipsilateral and contralateral reflex response data across all participants following iKnee flexion and extension joint rotations in Experiment 1a, including means and SDs, is provided in Table 1. The main result of Experiment 1a was that, following iKnee extension joint rotations, we observed an inhibition response in the cBF muscle, whereas, following iKnee flexion joint rotations, we observed a facilitation response in the cBF muscle. The mean onset latencies of the inhibition and facilitation responses were 44.3 ms and 43.7 ms, respectively.
Table 1.
Mean reflex response data for all participants following iKnee extension and flexion joint rotations
| Perturbation direction |
||||
|---|---|---|---|---|
| Extension |
Flexion |
|||
| Variable | iRF | cBF | iRF | cBF |
| Onset (ms) | 31.5 (6.3) | 44.3 (10.7) | 24.2 (3.2) | 43.7 (7.2) |
| Duration (ms) | 25.9 (14.0) | 45.3 (20.9) | 35.2 (19.7) | 45.8 (18.9) |
| Amplitude (% baseline) | 82.0 (16.1) | 75.2 (7.1) | 411.0 (402.9) | 142.3 (28.1) |
Data are included for each perturbation direction for iRF and cBF muscles, with SDs given in parenthesis.
Experiment 1a: Ipsilateral knee flexion joint rotations
Mean data from one representative participant following both iKnee flexion and extension joint rotations in Experiment 1a are shown in Fig.2 (30 trials). Across all participants, the stretch reflex response in the iRF following iKnee flexion joint rotations had a mean ± SD latency of 24 ± 3 ms (Fig.2B). The mean ± SD amplitude of the iRF stretch reflex response (411 ± 403% of baseline) was significantly greater than mean background EMG activity prior to the perturbation (t8 = 2.32, P = 0.049). Additionally, a facilitatory response occurred in the cBF in all participants with a mean ± SD onset latency of 44 ± 7 ms (Fig.2C). The mean ± SD amplitude of the cBF response (142 ± 28% of baseline) was significantly greater than mean background EMG activity prior to the perturbation (t8 = 4.52, P = 0.002) (Fig.2D).
Figure 2. Mean data from one participant and group mean data following extension (grey lines and grey bars) and flexion (black lines and black bars) joint rotations (6 deg, 150 deg s–1) to the right (ipsilateral) knee joint during a sitting task.
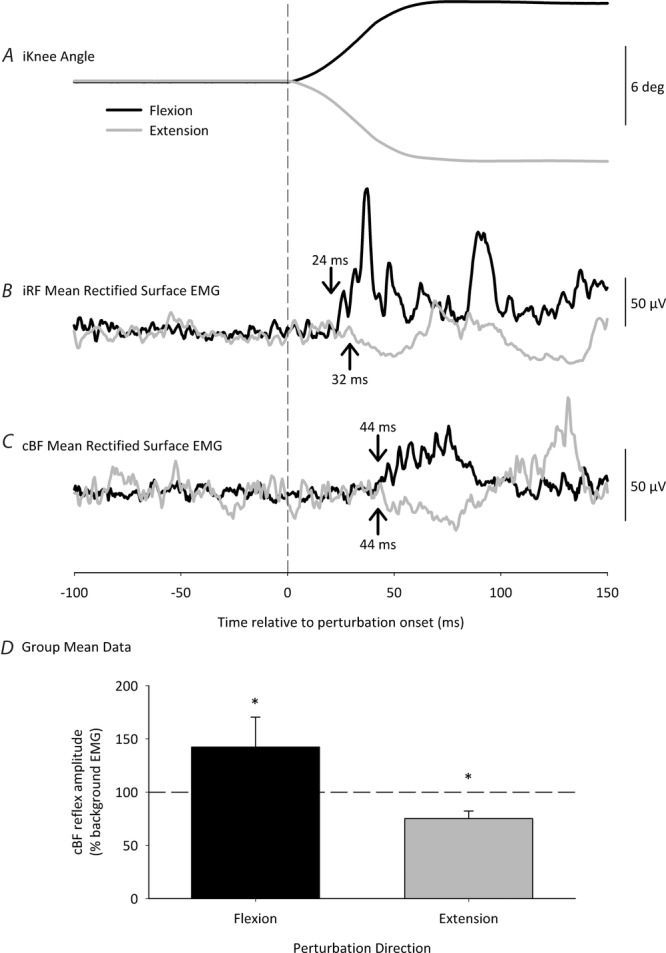
Participants isometrically contracted their iRF and cBF muscles to 10% MVC prior to the perturbation. A, iKnee angle. B, mean rectified iRF EMG. C, mean rectified cBF EMG (30 trials). The vertical dashed line represents perturbation onset. Mean response onsets are denoted by the arrows. Note the period of inhibition in the cBF EMG, beginning at 44 ms following extension perturbation onset, and the period of facilitation beginning 44 ms following flexion perturbation onset. D, group mean data for cBF response amplitudes following iKnee extension and flexion perturbations. The horizontal dashed line represents cBF background EMG levels. Error bars represent the SD. The asterisks denote significant differences from background EMG.
Experiment 1a: Ipsilateral knee extension joint rotations
An inhibition response was observed in all participants in the iRF following iKnee extension joint rotations with a mean ± SD onset latency of 32 ± 6 ms (Fig.2B). The mean ± SD amplitude of the iRF inhibition response (82 ± 16% of baseline) was significantly less than mean background EMG activity prior to the perturbation (t8 = 3.35, P = 0.01). Additionally, an inhibition response occurred in the cBF in all participants with a mean ± SD onset latency of 44 ± 11 ms (Fig.2C). The mean ± SD amplitude of the cBF inhibition response (75 ± 7% of baseline) was significantly less than mean background EMG activity prior to the perturbation (t8 = 10.5, P < 0.001) (Fig.2D).
Experiment 1b: Contralateral joint kinematics
To confirm that the short-latency reflexes observed in the cBF in Experiment 1a were not a result of movement of the contralateral leg caused by the mechanical linkage between the legs, we measured hip, knee and ankle joint angles in the contralateral leg. A summary of the mean cBF reflex and contralateral leg joint movement onset latencies across all participants in Experiment 1b, including the mean ± SD, are provided in Table 2. Overall, the cBF reflex onsets were shorter than the contralateral joint movement onset latencies. Following iKnee extension perturbations, a repeated measures ANOVA revealed significant differences of mean onset latency between the cBF reflex and contralateral joint movement (F3,21 = 5.57, P = 0.006). Post hoc analysis revealed that the cBF reflex onset (mean = 44.5 ms) was significantly shorter than the movement onset of the cKnee (mean = 83.7 ms) and cAnkle (mean = 99.9 ms; both P < 0.02). The cHip movement onset (mean = 82.3 ms) was not significantly different compared to the cBF reflex onset (P = 0.12). Following iKnee flexion perturbations, a repeated measures ANOVA revealed significant differences of mean onset latency between the cBF reflex and contralateral joint movement (F3,21 = 3.81, P = 0.025). Post hoc analysis revealed that the cBF reflex onset (mean = 43.1 ms) was significantly shorter than the movement onset of the cAnkle (mean = 59.2 ms; P = 0.025). The cHip (mean = 67.9 ms) and cKnee (mean = 50.5 ms) movement onsets were not significantly different compared to the cBF reflex onset (both P > 0.2).
Table 2.
Mean cBF reflex onsets and contralateral joint movement onset, direction and amplitude data for all participants following iKnee extension and flexion joint rotations
| Perturbation direction |
||||||||
|---|---|---|---|---|---|---|---|---|
| Extension |
Flexion |
|||||||
| Variable | cBF | cHip | cKnee | cAnkle | cBF | cHip | cKnee | cAnkle |
| Mean onset (ms) | 44.7 (4.9) | 83.3 (42.1) | 83.7 (23.7) | 99.9 (21.8) | 43.1 (5.2) | 67.9 (27.4) | 50.5 (10.5) | 59.2 (9.2) |
| Joint displacement direction | Extension | Flexion | Plantar | Flexion | Extension | Plantar | ||
| Amplitude of joint displacement (deg) | 0.17 (0.28) | −0.12 (0.28) | 0.18 (0.43) | −0.22 (0.33) | 0.50 (0.33) | 0.34 (0.54) | ||
Data are included for each perturbation direction, with SDs given in parenthesis.
Experiment 2: Effect of altering background muscle contraction level on cBF reflexes
To further confirm that the short-latency reflexes observed in the cBF in Experiment 1a were not simply a result of vibrations caused by the mechanical actuator, we tested the automatic gain control principle (Matthews, 1986) by varying the level of background contraction in the iRF and cBF. Figure 3A shows the relationship between cBF pre-contraction level and the short-latency cBF facilitation amplitude above background EMG following iKnee flexion joint rotations. Figure3B shows the relationship between cBF pre-contraction level and the short-latency cBF inhibition amplitude below background EMG following iKnee extension joint rotations. Data are plotted separately for the two different levels of iRF pre-contraction (10% and 30% MVC). Following iKnee flexion joint rotations, there was a significantly strong positive correlation between cBF background activity and the amplitude of the short-latency cBF facilitation response when the iRF was pre-contracted to 10% MVC (r24 = 0.54, P = 0.002) and when the iRF was pre-contracted to 30% MVC (r24 = 0.59, P = 0.001). Thus, as the cBF pre-contraction level increased, the amplitude of the short-latency cBF facilitation also increased. Following iKnee extension joint rotations, there was a significantly strong negative correlation between cBF background activity and the amplitude of the short-latency cBF inhibition response when the iRF was pre-contracted to 10% MVC (r24 = 0.71, P < 0.001) and when the iRF was pre-contracted to 30% MVC (r24 = 0.86, P < 0.001). Thus, as the cBF pre-contraction level increased, the amplitude of the short-latency cBF inhibition also increased. For both perturbation directions, the cBF reflex amplitude was not significantly different when the iRF was contracted at 10% or 30% MVC (both P > 0.12).
Figure 3. Relationship between cBF pre-contraction level and cBF response amplitude following iKnee flexion and extension joint rotations (6 deg, 150 deg s–1) in all participants.
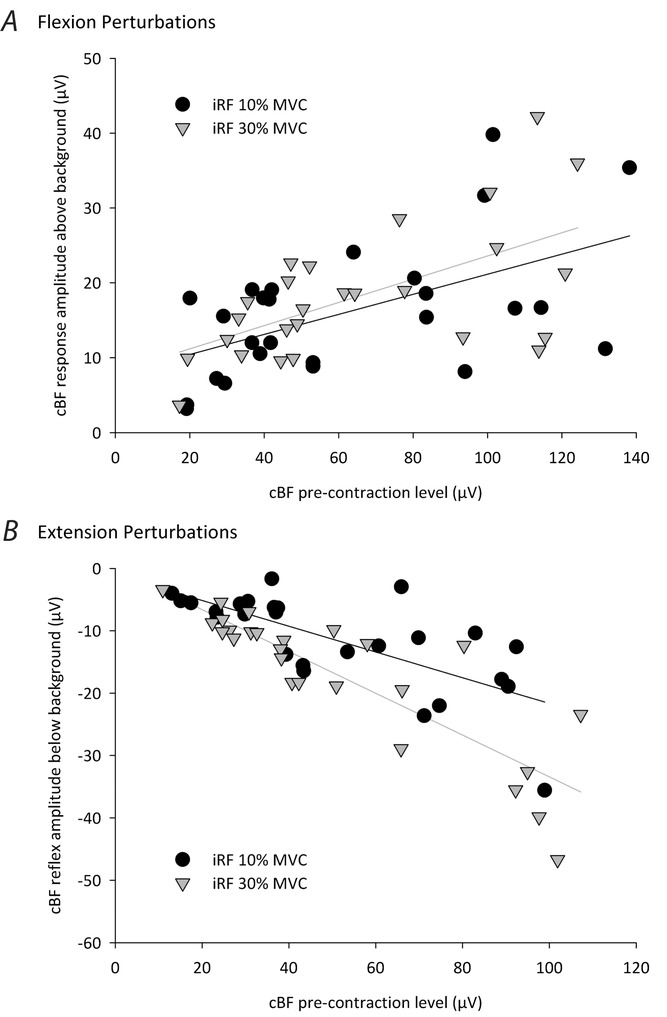
Black circles and black regression lines represent conditions when the pre-contraction level of the iRF muscle was 10% MVC, whereas the grey triangles and grey regression lines represent conditions when the pre-contraction level of the iRF muscle was 30% MVC. Note that the amplitude of the cBF facilitation response and the cBF inhibition response increases as the background cBF activity increases following iKnee flexion (A) and extension (B) joint rotations, respectively.
Experiment 3: i.m. EMG recordings in the cBF
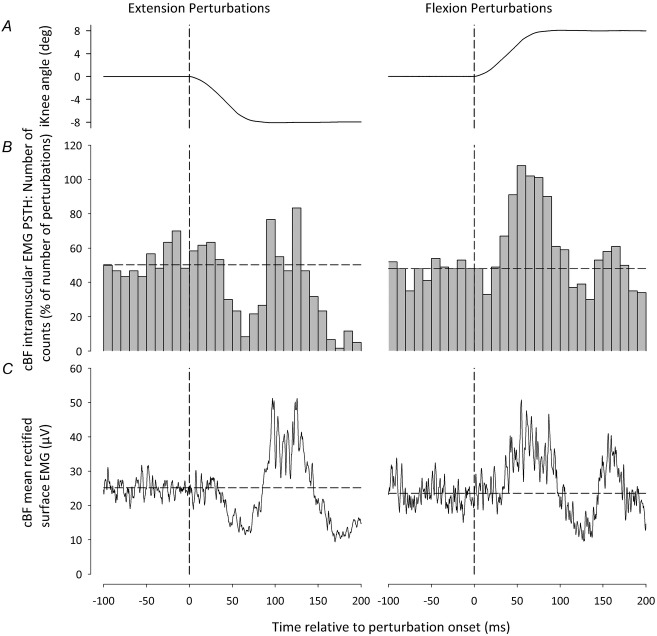
i.m. EMG was recorded in the cBF to determine whether the reversal in the sign of the short-latency cBF reflexes following either iKnee extension or flexion joint rotations can be quantified at the MU level and involved the same population of MUs (De Serres et al. 1995). Figure 4 shows raw i.m. EMG data following one flexion and one extension iKnee joint rotation trial from one representative participant, along with the firing pattern and templates of the five MUs that were identified during decomposition for the corresponding raw data. For all participants, the median number of active MUs during both iKnee flexion and extension conditions was eight and the median number of active MUs in only one or the other condition was two. Mean data from the same representative participant following both iKnee flexion and extension joint rotations in Experiment 3 are shown in Fig.5 (60 trials). The probability of firing of single MUs before and after perturbation onset is represented in the PSTHs of Fig.5C, whereas the mean surface EMG is shown in Fig.5B. For this participant, the period of inhibition in the cBF surface EMG began at 41 ms following extension perturbation onset, and the period of facilitation in the cBF surface EMG began at 41 ms following flexion perturbation onset. The total number of MUs making up the PSTH for this participant was five, and the mean background firing frequency in the cBF was 10.5 pulses s–1 and 9.6 pulses s–1 during extension and flexion perturbations, respectively. Periods of inhibition and facilitation can also be observed in the PSTHs for this participant beginning at 40 ms following iKnee extension and flexion joint rotations, respectively.
Figure 4. Motor unit decomposition.
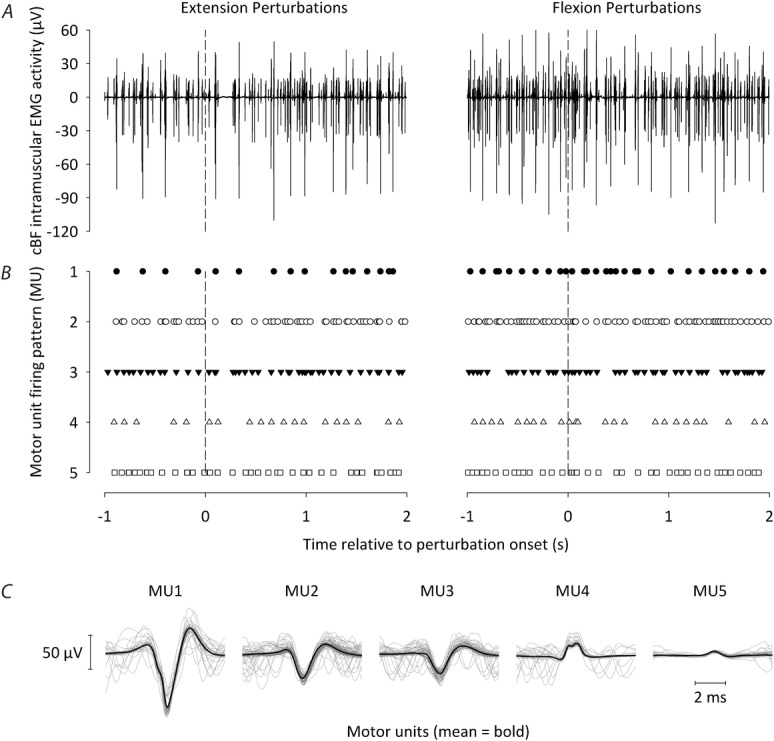
A, raw i.m. EMG data recorded from the cBF following one flexion (left) and one extension (right) iKnee joint rotation trial from one participant. B, firing pattern of the five different motor units that were identified during decomposition for the corresponding raw data in (A). The vertical dashed lines indicate iKnee flexion or extension perturbation onset. C, mean templates of the five different motor units that were identified during decomposition (thick black lines), together with each of the separate instances or each motor unit firing (thin grey lines). Note that the same five motor units were identified during decomposition following both iKnee flexion and extension joint rotations.
Figure 5. Mean cBF i.m. and surface EMG data from one participant following extension (left) and flexion (right) joint rotations (8 deg, 150 deg s–1) to the iKnee joint during a sitting task.
Participants isometrically contracted their iRF and cBF muscles to 10% MVC prior to the perturbation. A, iKnee angle. B, peristimulus time histogram of motor unit firing counts in the cBF normalized to the number of perturbations in bins of 10 ms. C, mean rectified cBF surface EMG (60 trials). The vertical dashed line represents perturbation onset and the horizontal dashed line represents mean cBF background levels. Note the period of inhibition in the cBF surface EMG beginning at 41 ms following extension perturbation onset, and the period of facilitation in the cBF surface EMG beginning 41 ms following flexion perturbation onset.
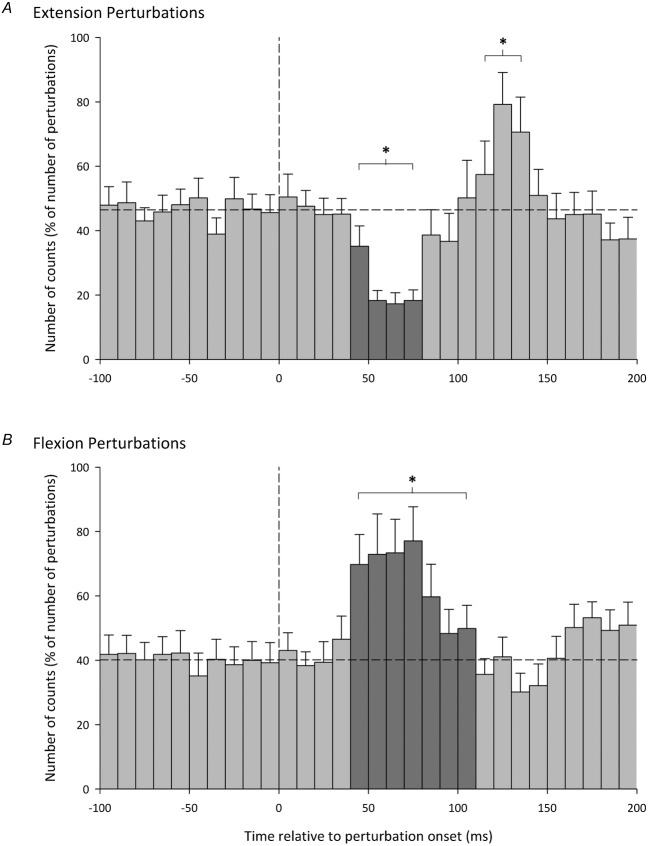
The overall mean responses of the cBF MU activity following iKnee extension and flexion joint rotations for all participants are shown in Fig.6. Responses in the mean cBF PSTHs were considered significant if differences greater than 3 SDs from the mean cBF background levels occurred that lasted for at least three consecutive bins (Mao et al. 1984). The horizontal dashed line represents the mean cBF background level and was calculated during the 100 ms period immediately prior to perturbation onset. Across all participants, an inhibitory reflex in the cBF was observed at a latency of 40–50 ms after the onset of iKnee extension joint rotations, lasting for 30–40 ms, whereas a facilitatory response was also observed at an onset latency of 110–120 ms (Fig.6A). Following iKnee flexion joint rotations, a facilitatory reflex was observed in the cBF with an onset latency of 40–50 ms, lasting for 60–70 ms.
Figure 6. Group mean cBF i.m. EMG data showing peristimulus time histograms following extension and flexion joint rotations (8 deg, 150 deg s–1) to the iKnee joint during a sitting task.
Peristimulus time histogram data show group mean motor unit firing counts in the cBF normalized to the number of perturbations in bins of 10 ms. The vertical dashed line represents perturbation onset and the horizontal dashed line represents mean cBF background levels. Error bars represent the SEM. The asterisks and dark grey bars denote differences greater than 3 SDs from the mean cBF background levels lasting for at least three consecutive bins. Note the period of inhibition following extension perturbations (A) lasting from 40–80 ms and the period of facilitation following flexion perturbations (B) lasting from 40–110 ms.
The total number of MUs making up the PSTHs across all participants was 93, and their background firing frequencies in the cBF were 12.4 ± 3.2 pulses s–1 and 13.5 ± 4.3 pulses s–1 during flexion and extension perturbations, respectively. The mean background instantaneous firing frequency was not significantly different between perturbation directions (t10 = 1.36, P = 0.2). Participants were able to reproduce approximately the same discharge rate across trials as a result of the visual feedback provided. A two-way repeated measures ANOVA revealed a significant interaction between perturbation direction and time window (background, response) (F1,10 = 48.8, P < 0.001). Post hoc analysis revealed that, following iKnee extension joint rotations, the cBF instantaneous firing frequency during the response window (mean = 11.4 ± 2.5 pulses s–1) was significantly lower than during background activity (P = 0.017). Conversely, following iKnee flexion joint rotations, the cBF instantaneous firing frequency during the response window (mean = 16.2 ± 4.6 pulses s–1) was significantly higher than during background activity (P < 0.001). All participants showed the same general trend.
Discussion
To our best knowledge, the present study is the first to demonstrate short-latency crossed-spinal responses in human upper leg muscles following mechanical rotations to the iKnee joint. Following iKnee extension joint rotations, an inhibitory response was observed in the cBF muscle with a mean onset latency of 44 ms. Following iKnee flexion joint rotations, a facilitatory response with a mean onset latency of 44 ms was observed in the cBF. Both the short-latency cBF inhibition and facilitation responses followed the automatic gain control principle (Matthews, 1986), with the size of the response increasing as the level of background pre-contraction in the cBF muscle increased. Furthermore, both short-latency inhibitory and facilitatory responses in the cBF were observed at the MU level using i.m. EMG recordings. The same population of cBF MUs that were inhibited following iKnee extension joint rotations were facilitated following iKnee flexion joint rotations, suggesting that the perturbation direction-dependent reversal in the sign of the short-latency cBF reflex can be explained by parallel interneuronal pathways from ipsilateral afferents to common motoneurons in the contralateral leg (De Serres et al. 1995).
Short-latency crossed responses in the cBF
In the present study, an inhibition of the cBF occurred 44.3 ± 10.7 ms after iKnee extension joint rotations and a facilitation of the cBF occurred 43.7 ± 7.2 ms after iKnee flexion joint rotations. These latencies are much shorter than previous studies in which crossed responses with latencies in the range 62–80 ms after ipsilateral nerve stimulation or mechanical perturbations have been shown in the contralateral hamstrings during walking (Dietz et al. 1986; Nielsen et al. 2008; Mrachacz-Kersting et al. 2011; Stevenson et al. 2013; Stevenson et al. 2015). However, the latencies are similar to recent experiments showing short-latency crossed responses in the cSOL muscle following ipsilateral tibial nerve stimulation with onsets of 37–41 ms during sitting and walking (Stubbs & Mrachacz-Kersting, 2009; Stubbs et al. 2011a,b; Stubbs et al. 2012; Gervasio et al. 2013; Hanna-Boutros et al. 2014). Despite the distance of the neural pathway to the cSOL being further than to the cBF, the slightly longer cBF reflex latency may be a result of the asynchronous activation of afferents following the iKnee joint rotation compared to the synchronous activation of electrical stimulation to the ipsilateral tibial nerve.
Stubbs and Mrachacz-Kersting (2009) suggested that the observed crossed responses in the cSOL could be spinally-mediated because of the short latency of the onset. Because ischaemia to the ipsilateral thigh delayed but did not abolish the cSOL response, both group I and II muscle afferents probably contribute to the response (Stubbs & Mrachacz-Kersting, 2009). The contribution of cutaneous afferents can be ruled out because stimulation of the ipsilateral sural and medial plantar nerves did not produce the same response (Stubbs & Mrachacz-Kersting, 2009; Gervasio et al. 2013). Even if occurring in a muscle more proximal than the soleus, the crossed response in the cBF may also be purely spinally-mediated. Indeed, Mrachacz-Kersting et al. (2006) found that the minimum latency for a transcortical pathway in the iRF following mechanical flexion of the knee was 54 ms by summing the mean onset of sensory evoked potentials (24 ms) together with mean motor evoked potential (MEP) onset latencies (20 ms) in addition to a 10 ms central processing delay. Because the BF muscle under investigation is also an upper leg muscle, the minimum time for a transcortical pathway to the cBF in the present study is probably similar.
The short-latency (44 ms) of the cBF responses observed during sitting in the present study are in contrast to the longer-latency (76-80 ms) facilitatory responses elicited in the cBF by iKnee extension joint rotations during the late stance phase of the gait cycle (Stevenson et al. 2013; Stevenson et al. 2015). A transcortical pathway has indeed been shown to contribute to the cBF response during walking because MEPs elicited by transcranial magnetic stimulation were facilitated when timed to coincide with cBF response onset, whereas MEPs elicited by transcranial electrical stimulation were not (Stevenson et al. 2013; Stevenson et al. 2015). Many ipsilateral and contralateral reflexes are diminished or absent during walking, probably as a result of an increase in descending input modulating presynaptic inhibition to maintain stability and balance (Morin et al. 1982; Capaday & Stein, 1986; Hayashi et al. 1992; Hanna-Boutros et al. 2014), which may account for the lack of short-latency reflexes in the cBF during walking. For example, transcranial magnetic stimulation diminished the short-latency crossed cSOL inhibition elicited by ipsilateral tibial nerve stimulation, whereas the group II afferent component of the cSOL inhibition was completely abolished during the stance phase of walking compared to sitting, highlighting the importance of descending input on regulating short-latency crossed-spinal reflexes (Hanna-Boutros et al. 2014). Although crossed spinal interneurons have not been directly identified in humans, the results of the present study contribute to the growing evidence that commissural interneurons may contribute to interlimb co-ordination in humans, as well as in cats (Arya et al. 1991; Jankowska et al. 2009; Stubbs & Mrachacz-Kersting, 2009).
Methodological considerations
Two possible explanations for the short-latency cBF reflexes are that vibrations transferred from the mechanical actuator to the seat of the participants caused the responses or that movement of the contralateral leg as a result of a mechanical linkage between the legs caused the cBF reflexes. The present study provides evidence arguing against these possibilities. First, the sign of the cBF responses depended on the direction of the iKnee perturbation. Second, any movement observed in the contralateral leg occurred after the onset of the cBF reflexes (Experiment 1b). Therefore, the cBF reflexes were not caused by movement of the contralateral leg. Third, the onset of the cBF reflexes occur much earlier than the termination of the movement of the mechanical actuator (Figs 2 and 5). Finally, if mechanical vibrations transferred from the mechanical actuator caused the cBF reflexes, increasing the cBF contraction level (and stiffness around the cKnee joint) would result in no changes to the amplitude of the reflexes. However, the amplitude of the cBF reflexes increased in magnitude with increasing levels of background pre-contraction in the cBF, following the automatic gain control principle (Matthews, 1986). Based on experiments on animals or humans at rest, this principle states that the amplitude of reflex responses increases with increments in background contraction of the target muscle as a result of an increase in excitability within the motoneuron pool (e.g. because of an increased central drive) (Duysens & Tax, 1994). In the present study, both the short-latency cBF facilitation elicited by iKnee flexion joint rotations and the cBF inhibition following iKnee extension joint rotations increased in magnitude with increases in cBF background pre-contraction level (Fig.3). Furthermore, increasing the stiffness of the cKnee joint would also dampen the oscillations provided by the perturbation, and Experiment 1b showed that any movement of the contralateral joints occurred after the onset of the cBF reflexes.
Perturbation direction-dependent reversal in sign of the short-latency cBF reflex
The functional relevance of reflex reversals has been highlighted by their phase dependence during walking in both animals and humans (Duysens et al. 1980; Yang & Stein, 1990; Marchand-Pauvert & Nielsen, 2002). By stimulating cutaneous and muscle afferents in humans, several studies have demonstrated functional, phase-dependent reflex reversals in the ipsilateral limb during walking in humans (Duysens et al. 1990; Yang & Stein, 1990; Duysens et al. 1992; De Serres et al. 1995; Marchand-Pauvert & Nielsen, 2002). Phase-dependent reflex reversals have also been identified in the contralateral limb in animals (Duysens et al. 1980; Rossignol & Gauthier, 1980) and more recently in humans (Gervasio et al. 2013).
In the present study, we observed that the perturbation direction-dependent reversal in sign of the short-latency cBF reflex during sitting was present in the same population of MUs recorded with i.m. EMG. De Serres et al. (1995) observed that the same MUs in the ipsilateral tibialis anterior (TA) muscle were facilitated during swing and inhibited during the transition from swing to stance during human walking following electrical stimuli to the ipsilateral posterior tibial nerve (PTN). It was suggested that there are parallel excitatory and inhibitory pathways from cutaneous afferents to single motoneurons of the TA muscle, and that a shift in balance between the two pathways throughout the gait cycle probably generated the reflex reversal. We hypothesize that a similar parallel pathway mediates the observed reversal in sign of the cBF reflex given that the same population of MUs was implicated in both inhibitory and facilitatory reflexes. Although the phase-dependent reflex reversal observed in the TA is mediated by the same cutaneous afferents (De Serres et al. 1995), different afferent populations from the perturbed ipsilateral leg probably mediate the cBF reflexes. Based on the present study, it is not possible to determine the source of the afferent inputs mediating the inhibitory and facilitatory cBF reflexes. Because the iRF is pre-contracted prior to the perturbation, afferents from the iRF probably play a more dominant role than afferents from the iBF. For example, during a knee extension perturbation, the hamstrings muscle group is stretched and the pre-activated quadriceps muscles are unloaded (Fig.2B) and, following a knee flexion perturbation, the quadriceps are stretched and the hamstrings muscles are shortened. However, it is also possible that cutaneous afferents contribute to the cBF reflexes, and further studies are required to determine the source of afferent input mediating the short-latency crossed reflexes in the cBF. Based on the slower onset latency of the cBF reflexes (44 ms in the surface EMG) relative to the shortest responses in the iRF following iKnee flexion perturbations, which are predominantly mediated by fast conducting group Ia afferents (24 ms) (Table 1), we speculate that slower conducting group II afferents probably mediate the cBF reflexes. This is also supported by interlimb reflex data in animals models, where it has been shown that group II afferents play a major role in mediating interlimb reflexes and have direct projections onto commissural interneurons (Arya et al. 1991; Jankowska et al. 2005; Jankowska, 2008; Jankowska et al. 2009).
According to the parallel pathways hypothesis by De Serres et al. (1995), the phase-dependent reflex reversal in the TA could result from an alternate gating or weighting of two parallel interneuronal pathways from afferents innervating the same motoneurons (De Serres et al. 1995). Applied to the short-latency cBF reflexes in the present study, one of these pathways is excitatory and activated following iKnee flexion joint rotations, whereas the other is inhibitory and is activated following iKnee extension joint rotations. It is not known how many interneurons (including commissural interneurons) exist between the afferent fibres and the motoneurons. However, evidence from animal work suggests that interneurons are a probable site for this type of reversal (De Serres et al. 1995).
An interlimb reflex reversal was demonstrated for the first time in humans in the cGL muscle following electrical stimulation to the PTN during normal walking or hybrid walking (Gervasio et al. 2013). A facilitation occurred in the cGL in response to PTN stimulation during normal walking at ipsilateral push off and contralateral touchdown, whereas a reversal in the cGL response was observed at a similar phase of hybrid walking, when both legs are walking in opposite directions. Although this finding underlies the functional significance and task dependence of short-latency interlimb reflexes, the present study is the first to show a perturbation direction-dependent reversal in sign of short-latency reflexes in the contralateral limb in humans. Further studies will be necessary to investigate the functional role in interlimb co-ordination of the short-latency interlimb reflexes in the cBF in humans because interlimb reflexes are impaired following stroke and probably play a role in maintaining a symmetrical gait pattern (Stubbs et al. 2012).
Acknowledgments
The work was carried out at the Center for Sensory-Motor Interaction, Aalborg University. We thank B. D. Ebbesen for assistance with data collection and K. Larsen for programming assistance. We also thank T. Sinkjær and J. B. Nielsen for their valuable comments on earlier versions of the manuscript.
Glossary
- cAnkle
contralateral ankle
- cBF
contralateral biceps femoris
- cGL
contralateral gastrocnemius lateralis
- cHip
contralateral hip
- cKnee
contralateral knee
- cRF
contralateral rectus femoris
- cSOL
contralateral soleus
- EMG
electromyography
- iBF
ipsilateral biceps femoris
- iKnee
ipsilateral knee
- iRF
ipsilateral rectus femoris
- MEP
motor evoked potential
- MU
motor unit
- MVC
maximal voluntary contraction
- PSTH
peristimulus time histogram
- PTN
posterior tibial nerve
- TA
tibialis anterior
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
All authors contributed to the concept and design of the experiment, as well as to the collection, analysis and interpretation of the data. AJTS, ENK and NMK drafted the manuscript and all authors critically revised the manuscript and approved the final version submitted for publication.
Funding
The study was supported by grants from Det Obelske Familiefond and SparNord Fonden of Denmark, as well as the European Research Council Advanced Grant DEMOVE (contract #267888).
References
- Arya T, Bajwa S, Edgley SA. Crossed reflex actions from group II muscle afferents in the lumbar spinal cord of the anaesthetized cat. J Physiol. 1991;444:117–131. doi: 10.1113/jphysiol.1991.sp018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci. 1986;6:1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram J, Kasman G, Holtz J. Aspen Publishers, Gaithersburg, MD. Introduction to Surface Electromyography. 1998 [Google Scholar]
- De Serres SJ, Yang JF, Patrick SK. Mechanism for reflex reversal during walking in human tibialis anterior muscle revealed by single motor unit recording. J Physiol. 1995;488:249–258. doi: 10.1113/jphysiol.1995.sp020963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Boos G, Berger W. Obstruction of the swing phase during gait: phase-dependent bilateral leg muscle coordination. Brain Res. 1986;384:166–169. doi: 10.1016/0006-8993(86)91233-3. [DOI] [PubMed] [Google Scholar]
- Duysens J, Loeb GE, Weston BJ. Crossed flexor reflex responses and their reversal in freely walking cats. Brain Res. 1980;197:538–542. doi: 10.1016/0006-8993(80)91143-9. [DOI] [PubMed] [Google Scholar]
- Duysens J, Tax AA, Trippel M, Dietz V. Phase-dependent reversal of reflexly induced movements during human gait. Exp Brain Res. 1992;90:404–414. doi: 10.1007/BF00227255. [DOI] [PubMed] [Google Scholar]
- Duysens J, Trippel M, Horstmann GA, Dietz V. Gating and reversal of reflexes in ankle muscles during human walking. Exp Brain Res. 1990;82:351–358. doi: 10.1007/BF00231254. [DOI] [PubMed] [Google Scholar]
- Duysens JEJ. Interlimb reflexes during gait in cat and human. In: Swinnen SP, Tax AAM, editors. Interlimb coordination: neural, dynamical, and cognitive constraints. San Diego, CA: Academic Press; 1994. pp. 97–126. [Google Scholar]
- Gervasio S, Farina D, Sinkjær T, Mrachacz-Kersting N. Crossed reflex reversal during human locomotion. J Neurophysiol. 2013;109:2335–2344. doi: 10.1152/jn.01086.2012. [DOI] [PubMed] [Google Scholar]
- Hanna-Boutros B, Sangari S, Karasu A, Giboin LS, Marchand-Pauvert V. Task-related modulation of crossed spinal inhibition between human lower limbs. J Neurophysiol. 2014;111:1865–1876. doi: 10.1152/jn.00838.2013. [DOI] [PubMed] [Google Scholar]
- Haridas C, Zehr EP, Misiaszek JE. Context-dependent modulation of interlimb cutaneous reflexes in arm muscles as a function of stability threat during walking. J Neurophysiol. 2006;96:3096–3103. doi: 10.1152/jn.00746.2006. [DOI] [PubMed] [Google Scholar]
- Hayashi R, Tako K, Tokuda T, Yanagisawa N. Comparison of amplitude of human soleus H-reflex during sitting and standing. Neurosci Res. 1992;13:227–233. doi: 10.1016/0168-0102(92)90062-h. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Rev. 2008;57:46–55. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Bannatyne BA, Stecina K, Hammar I, Cabaj A, Maxwell DJ. Commissural interneurons with input from group I and II muscle afferents in feline lumbar segments: neurotransmitters, projections and target cells. J Physiol. 2009;587:401–418. doi: 10.1113/jphysiol.2008.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA, Krutki P, Hammar I. Functional differentiation and organization of feline midlumbar commissural interneurones. J Physiol. 2005;565:645–658. doi: 10.1113/jphysiol.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao CC, Ashby P, Wang M, McCrea D. Synaptic connections from large muscle afferents to the motoneurons of various leg muscles in man. Exp Brain Res. 1984;56:341–350. doi: 10.1007/BF00236290. [DOI] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Nielsen JB. Modulation of heteronymous reflexes from ankle dorsiflexors to hamstring muscles during human walking. Exp Brain Res. 2002;142:402–408. doi: 10.1007/s00221-001-0942-3. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol. 1986;374:73–90. doi: 10.1113/jphysiol.1986.sp016066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill KC, Lateva ZC, Marateb HR. EMGLAB: an interactive EMG decomposition program. J Neurosci Methods. 2005;149:121–133. doi: 10.1016/j.jneumeth.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Morin C, Katz R, Mazieres L, Pierrot-Deseilligny E. Comparison of soleus H reflex facilitation at the onset of soleus contractions produced voluntarily and during the stance phase of human gait. Neurosci Lett. 1982;33:47–53. doi: 10.1016/0304-3940(82)90128-8. [DOI] [PubMed] [Google Scholar]
- Mrachacz-Kersting N, Grey MJ, Sinkjær T. Evidence for a supraspinal contribution to the human quadriceps long-latency stretch reflex. Exp Brain Res. 2006;168:529–540. doi: 10.1007/s00221-005-0120-0. [DOI] [PubMed] [Google Scholar]
- Mrachacz-Kersting N, Nielsen JB, Sinkjær T. The role of muscle generated afferent feedback in human interlimb coordination. Washington, DC: Society for Neuroscience Abstracts; 2011. [Google Scholar]
- Nielsen JB, Stecina K, Barthelemy D. Contralateral effects of femoral nerve stimulation during walking in healthy humans. Washington, DC: Society for Neuroscience Abstracts; 2008. [Google Scholar]
- Pierrot-Deseilligny E, Morin C, Bergego C, Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res. 1981;42:337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Gauthier L. An analysis of mechanisms controlling the reversal of crossed spinal reflexes. Brain Res. 1980;182:31–45. doi: 10.1016/0006-8993(80)90828-8. [DOI] [PubMed] [Google Scholar]
- Stevenson AJT, Geertsen SS, Andersen JB, Sinkjær T, Nielsen JB, Mrachacz-Kersting N. Interlimb communication to the knee flexors during walking in humans. J Physiol. 2013;591:4921–4935. doi: 10.1113/jphysiol.2013.257949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson AJT, Geertsen SS, Sinkjær T, Nielsen JB, Mrachacz-Kersting N. Interlimb communication following unexpected changes in treadmill velocity during human walking. J Neurophysiol. 2015 doi: 10.1152/jn.00794.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs PW, Mrachacz-Kersting N. Short-latency crossed inhibitory responses in the human soleus muscle. J Neurophysiol. 2009;102:3596–3605. doi: 10.1152/jn.00667.2009. [DOI] [PubMed] [Google Scholar]
- Stubbs PW, Nielsen JF, Sinkjær T, Mrachacz-Kersting N. Crossed spinal soleus muscle communication demonstrated by H-reflex conditioning. Muscle Nerve. 2011a;43:845–850. doi: 10.1002/mus.21964. [DOI] [PubMed] [Google Scholar]
- Stubbs PW, Nielsen JF, Sinkjær T, Mrachacz-Kersting N. Phase modulation of the short-latency crossed spinal response in the human soleus muscle. J Neurophysiol. 2011b;105:503–511. doi: 10.1152/jn.00786.2010. [DOI] [PubMed] [Google Scholar]
- Stubbs PW, Nielsen JF, Sinkjær T, Mrachacz-Kersting N. Short-latency crossed spinal responses are impaired differently in sub-acute and chronic stroke patients. Clin Neurophysiol. 2012;123:541–549. doi: 10.1016/j.clinph.2011.07.033. [DOI] [PubMed] [Google Scholar]
- Voigt M, de Zee M, Sinkjær T. A fast servo-controlled hydraulic device for the study of muscle mechanical and reflex properties in humans. Calgary: International Society of Biomechanics; 1999. [Google Scholar]
- Yang JF, Stein RB. Phase-dependent reflex reversal in human leg muscles during walking. J Neurophysiol. 1990;63:1109–1117. doi: 10.1152/jn.1990.63.5.1109. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Collins DF, Chua R. Human interlimb reflexes evoked by electrical stimulation of cutaneous nerves innervating the hand and foot. Exp Brain Res. 2001;140:495–504. doi: 10.1007/s002210100857. [DOI] [PubMed] [Google Scholar]