Abstract
Spinal sensory neurons innervate many large blood vessels throughout the body. Their activation causes the hallmarks of neurogenic inflammation: vasodilatation through the release of the neuropeptide calcitonin gene-related peptide and plasma extravasation via tachykinins. The same vasodilator afferent neurons show mechanical sensitivity, responding to crushing, compression or axial stretch of blood vessels – responses which activate pain pathways and which can be modified by cell damage and inflammation. In the present study, we tested whether spinal afferent axons ending on branching mesenteric arteries (‘vascular afferents’) are sensitive to increased intravascular pressure. From a holding pressure of 5 mmHg, distension to 20, 40, 60 or 80 mmHg caused graded, slowly adapting increases in firing of vascular afferents. Many of the same afferent units showed responses to axial stretch, which summed with responses evoked by raised pressure. Many vascular afferents were also sensitive to raised temperature, capsaicin and/or local compression with von Frey hairs. However, responses to raised pressure in single, isolated vessels were negligible, suggesting that the adequate stimulus is distortion of the arterial arcade rather than distension per se. Increasing arterial pressure often triggered peristaltic contractions in the neighbouring segment of intestine, an effect that was mimicked by acute exposure to capsaicin (1 μm) and which was reduced after desensitisation to capsaicin. These results indicate that sensory fibres with perivascular endings are sensitive to pressure-induced distortion of branched arteries, in addition to compression and axial stretch, and that they contribute functional inputs to enteric motor circuits.
Key points
A major class of mechano-nociceptors to the intestine have mechanotransduction sites on extramural and intramural arteries and arterioles (‘vascular afferents’).
These sensory neurons can be activated by compression or axial stretch of vessels.
Using isolated preparations we showed that increasing intra-arterial pressure, within the physiological range, activated mechano-nociceptors on vessels in intact mesenteric arcades, but not in isolated arteries. This suggests that distortion of the branching vascular tree is the mechanical adequate stimulus for these sensory neurons, rather than simple distension.
The same rises in pressure also activated intestinal peristalsis in a partially capsaicin-sensitive manner indicating that pressure-sensitive vascular afferents influence enteric circuits.
The results identify the mechanical adequate stimulus for a major class of mechano-nociceptors with endings on blood vessels supplying the gut wall; these afferents have similar endings to ones supplying other viscera, striated muscle and dural vessels.
Introduction
Major blood vessels receive prominent innervation by post-ganglionic sympathetic neurons. In addition, many vessels are innervated by sensory axons that originate from dorsal root ganglia (Westcott 2013). While sympathetic inputs cause vasoconstriction, the sensory innervation comprises many axons that contain the neuropeptide calcitonin gene-related peptide (CGRP), a potent vasodilator (Brain et al. 1985; Uddman et al. 1986). GGRP colocalises with a tachykinin in many of these axons (Gibbins et al. 1985), and there is evidence that the same axons may also release a purine, such as ATP (Burnstock, 2007). The sensory nerve endings have a significant effector role; it has long been known that stimulation of dorsal roots can cause vasodilatation in some vascular beds (Bayliss, 1901). The vasodilator effects of stimulation of sensory nerves are mediated, at least in part, by CGRP (Kawasaki et al. 1988).
Sensory vasodilatation is one component of ‘neurogenic inflammation’, which can be triggered via activation of nociceptive nerves, and which underlies the characteristic wheal and flare in skin (Lewis, 1927). Co-released tachykinins mediate a second component, causing a localised increase in plasma extravasation and swelling (Lembeck & Holzer, 1979). Peptidergic sensory nerves are present in many vascular beds (Furness et al. 1982b; Gibbins et al. 1985). The question arises of the mechanisms by which vasodilator sensory neurons are activated. It has been argued that this may occur on their perivascular terminals (so that the same endings play both sensory and effector roles). Alternatively, activation may occur at specialised sensory endings, and is then transmitted via axonal conduction to the effector terminals on blood vessels (Bruce, 1913).
In the gut, the mesenteric blood vessels receive a prominent sensory innervation, which can be activated by noxious distension of the intestinal wall (Meehan & Kreulen, 1992). This was attributed to activation of spinal afferent endings in the gut wall that generated action potentials which propagate into efferent collaterals on the upstream arteries (Meehan & Kreulen, 1992). This upstream dilatation may be an important protective mechanism; activation of capsaicin-sensitive afferent fibres by high intraluminal acidity contributes a protective hyperaemia in the stomach (Holzer, 2006). Sensory vasodilator neurons can also be activated directly from their endings on mesenteric blood vessels. Pressing on mesenteric arteries activates mesenteric afferent neurons (Bessou & Perl, 1966). Similar mechanosensitivity has been reported for endings on blood vessels supplying other viscera including spleen, ovaries, pancreas, bladder, uterus, ureter and prostate (Floyd & Morrison, 1974; Floyd et al. 1976). Mechanosensitive afferent axons are also present on intramural vessels in the gut wall, corresponding to capsaicin-sensitive mechano-nociceptors (Song et al. 2009). In most of these studies, mechanical stimulation was applied via von Frey hairs or blunt glass probes, pressed on the surface of the vessels. Both of these stimuli involve very intense, localised pressure and are unlikely to be replicated by natural mechanical stimuli. Thus the nature of the adequate stimulus for vascular afferents remains uncertain. There is evidence that some vascular afferent endings are sensitive to raised intravascular pressure (Malliani & Pagani, 1976; Bahns et al. 1986). Reductions in flow have also been reported to cause sensory discharge in mesenteric vessels of rat ileum (Brunsden et al. 2002) and this appears to be due to mechanical distortion of the mesenteric fan caused by changes in perfusion (Brunsden et al. 2007). It is also established that traction on mesenteric membranes powerfully activates nociceptive pathways (Holzer-Petsche & Brodacz, 1999).
In the present study we tested whether vascular afferent neurons innervating isolated guinea-pig mesenteric arteries are sensitive to changes in intravascular pressure and respond to external stimuli (compression and axial stretch). During the course of these studies, it became apparent that distension of mesenteric vessels activated motility reflexes in the gut wall: the mechanisms underlying this phenomenon were also investigated.
Methods
Ethical approval and tissue handling
Adult guinea pigs (Duncan–Hartley IMVS coloured, bred in-house, 289–400 g) were killed humanely by stunning and exsanguination, in a manner approved by the Animal Welfare Committee (no. 844/12) of Flinders University and in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes (8th edn 2013, National Health and Medical Research Council of Australia). A total of 53 guinea pigs (37 male) were used in the course of the study. The ileum with attached mesentery was then removed and placed in Krebs solution (in mm: NaCl, 118; KCl, 4.75; NaH2PO4, 1.0; NaHCO3, 25; MgCl2, 1.2; CaCl2, 2.5; glucose, 11; bubbled with 95% O2–5% CO2). The contents were flushed out of the intestine and it was pinned out in a Petri dish lined with Sylgard (Dow Corning, Midland, MI, USA), and superfused with Krebs solution warmed to 37°C.
Extracellular recordings
Several fine mesenteric nerve trunks (2–5 trunks, 20–60 μm in diameter) running close to blood vessels were dissected free of connective tissue over a length of 4–6 mm. Preparations were then transferred to a 20 ml, Sylgard-lined organ bath. The mesenteric membrane was pinned to the bath along the edge furthest from ileum, using pins made of 50 μm tungsten wire. The paravascular nerve trunks were pulled into a small chamber (1 ml), sealed with silicone grease (Ajax Chemicals, Sydney, Australia) and a glass coverslip, and the chamber was filled with paraffin oil. Differential extracellular recordings were made between a single nerve trunk and a fine strand of connective tissue, via two platinum wire electrodes.
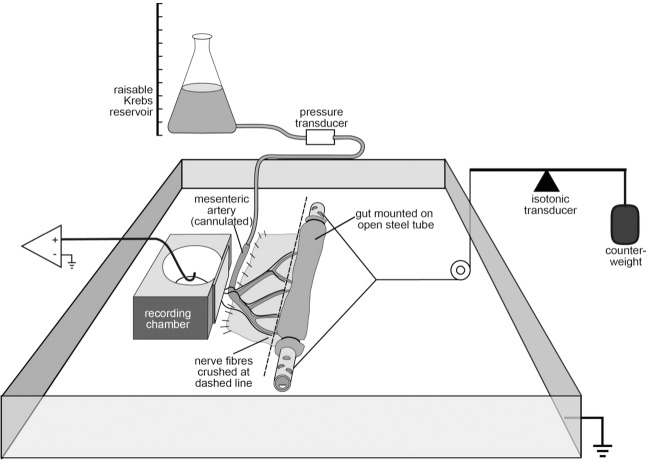
Signals were amplified (DAM 50, WPI, Sarasota, FL, USA), digitised at 20 kHz and recorded (MacLab8sp, Chart v7.2.2 software, ADInstruments, Sydney, Australia). Single units were discriminated by amplitude and duration (Spike Histogram software, ADInstruments). In most experiments, mesenteric nerve trunks were crushed at the point where they entered the gut wall, using the bevelled edge of a microscope slide, so that only fibres with transduction sites in the mesenteries (outside the intestine) contributed to recordings (Fig.2). A perforated stainless steel tube with open ends (∼1 mm diameter, 25 mm long) was passed through the intestinal lumen to allow Krebs solution to circulate freely between the lumen and the organ bath (preventing the build-up of intraluminal pressure). The segment of intestine was tightly ligated to this tube at either end, to ensure that intramural blood vessels, severed at the cut ends of the preparation, were occluded. The perforated tube was also connected via cotton threads to the lever of an isotonic transducer (52-9511, Harvard Bioscience, South Natick, MA, USA) and thus served as an anchor point to pull on the gut and stretch the mesenteric membrane. By adding counterweights to the isotonic lever, the intestine was pulled, thus evenly stretching the mesenteric membranes (Fig.1). Counterweights of 10–15 g were applied to the isotonic lever for 30 s. A minimum rest period of 5 min was allowed between stretches.
Figure 2. Anterograde labelling of axons from a single mesenteric nerve bundle with in vitro biotinamide after electrophysiological recording.

Fibres generally run parallel to mesenteric blood vessels. Dye filling stops abruptly before axons reach the gut wall, indicating that all nerve trunks were crushed successfully and only sensory axons with transduction sites in the mesentery contributed to recordings. Scale bar = 1 mm.
Figure 1. Schematic diagram of the preparation to record firing from afferent nerves innervating blood vessels in the mesentery of guinea pig ileum.
All nerve trunks were crushed before entering the gut wall (dashed line) so that only axons with transduction sites in the mesenteries were recorded. By cannulating the mesenteric artery, intra-arterial pressure could be altered by raising the Krebs reservoir. Applying counterweights to the lever of the isotonic transducer stretched the mesenteric membranes containing the blood vessels and associated nerve fibres. The ileum was tightly ligated at either end to a perforated steel tube with open ends, which ensured that intraluminal pressure could not build up and that perfusate did not leak from severed blood vessels at cut ends of the preparation.
A branch of the main mesenteric artery to the segment of gut was cannulated with a tapered thin-walled glass micropipette (pulled from 1 mm outer diameter glass, inner diameter 0.75 mm), with a tip outer diameter of approximately 50 μm. This was connected to a T-piece, with a pressure transducer on the side-arm to monitor intra-arterial pressure. Because the artery remained patent with the intramural vasculature, Krebs solution flowing into the artery faced a significant resistance. A reservoir, also containing Krebs solution, attached to the other arm of the T-piece could be raised to increase intra-arterial pressure from 0 to 100 mmHg, in a controlled manner. A minimum rest period of 5 min was allowed between each increase in intra-arterial pressure. Physiological intravascular pressure was considered to be 60 mmHg, close to the mean arterial pressure of 63 ± 2 mmHg recorded telemetrically from conscious guinea pigs (Hess et al. 2007).
In some preparations, individual mesenteric arteries were isolated from the mesenteric membranes, together with a paravascular nerve. These preparations were relatively short (6–12 mm and geometrically simpler than preparations of the mesenteric tree. They never contained more than a single arterial branch-point. In these preparations, the artery was cannulated proximally and ligated distally.
Preparations were allowed to equilibrate for 1 h after set-up (Carbone et al. 2012), with the mesentery under a low constant tension of 5 mN (basal load on the isotonic lever) and minimal intra-arterial pressure set to 5 mmHg to ensure continuous slow perfusion of the vasculature (Fig.1). The main organ bath was independently superfused at 3 ml min−1 with Krebs solution warmed to 36°C and maintained at a temperature of 32–34°C.
Focal compression, localised heating and capsaicin application
With the mesentery under 5 mN tension and intra-arterial pressure at 5 mmHg, mechano-transduction sites were identified by probing the mesentery with a 3 mN von Frey hair. In some preparations, Krebs solution warmed to 45°C was applied via a fine-tipped syringe (inner diameter ∼100 μm) onto the preparation to heat small areas of the artery locally (approximately 1 mm2) in order to identify heat-sensitive transduction sites (Adelson et al. 1997). As the jet of warmed Krebs solution moved over a sensitive site, a burst of action potentials could be repeatedly evoked. Krebs solution at 37°C was used as a control. The effects of capsaicin (1 μm in Krebs solution) were measured by ejecting 5 μl of solution directly onto the blood vessel. Capsaicin was made up as a 10−3 m solution in dimethly sulphoxide, stored at 4°C and diluted on the day into Krebs solution.
Anterograde labelling
At the end of the recording period, anterograde labelling (Tassicker et al. 1999) of axons in the mesenteric nerve trunk recorded from was carried out (Zagorodnyuk & Brookes, 2000). A drop of 5% biotinamide (Molecular Probes, Eugene, OR, USA) in ‘artificial intracellular medium’ (150 mm monopotassium l-glutamic acid, 7 mm MgCl2, 5 mm glucose, 1 mm EGTA, 20 mm Hepes, 5 mm disodium ATP, 0.02% saponin, 1% dimethyl sulphoxide) was placed on the nerve trunk. The main chamber, containing the preparation, was filled with sterile supplemented culture medium (Dulbecco's modified Eagle's medium (DMEM)/Ham's F12 nutrient mixture with 10% fetal bovine serum, 1.8 mm CaCl2, 100 i.u. ml−1 penicillin, 100 μg ml−1 streptomycin, 2.5 μg ml−1 amphotericin B, 20 μg ml−1 gentamicin (Cytosystems, NSW, Australia), pH 7.4). The tissue was incubated in a humidified incubator in 5% CO2 in air at 37°C. After 15–20 h, preparations were fixed overnight in modified Zamboni's fixative (0.2% saturated picric acid and 2% paraformaldehyde in a 0.1 m phosphate buffer, pH 7.0), cleared in DMSO for 30 min and rinsed in phosphate buffered saline (PBS: 150 mm NaCl in 10 mm sodium phosphate buffer, pH 7.2). Labelled nerve fibres were visualised with streptavidin Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA; 1:1000, 6 h) on an Olympus IX71 inverted fluorescence microscope. Images of labelled neural structures were captured via a CoolSNAP ES digital camera (Roper Scientific (Photometrics), Tucson, AZ, USA) using analySIS 5 software (Soft Imaging System, Gulfview Heights, South Australia, Australia). Images were combined, cropped, scaled and adjusted for brightness and contrast in Adobe Photoshop.
Recording motility of preparations
To investigate the effects of raising intra-arterial pressure on gut motility, some preparations were set up without extracellular recordings or crushing of the nerve fibres where they entered the gut wall. The mesenteric artery was cannulated and perfused with Krebs solution supplemented with 3% dextran (Mr, 35,000–45,000). Video recordings of gut movements were captured by a camera attached to the dissecting microscope (Dino-Eye AM-4023CT, Dino-Lite, Torrance, CA, USA; Video Capture software, ADInstruments) and used to create spatio-temporal maps of gut movements (Hennig et al. 1999). Video images were synchronised with pressure recordings by matching a small flashing LED in the corner of the video image with recordings of the driving voltage pulses (Chart software).
Statistical analysis
Results are expressed as means ± 95% confidence intervals, with n referring to the number of discriminated afferent units and N referring to the number of animals. Two means were compared using Student's paired or unpaired two-tailed t test with Prism 6 software (GraphPad, San Diego, CA, USA). Differences were considered significant if P < 0.05.
Results
Biotinamide dye fills of the recorded mesenteric nerves labelled numerous axons in nerve trunks that branched extensively in the mesenteric tree, providing both perivascular and paravascular fibres. In preparations in which mesenteric nerve trunks had been crushed adjacent to the gut wall, biotinamide filling consistently stopped at the crush site, confirming that axonal continuity with the gut had been interrupted (Fig.2); however, vascular continuity with the ileum was maintained.
Afferent responses to mesenteric stretch
In another set of preparations, with the mesentery under 0.5 g load and the mesenteric artery at an intra-arterial pressure of 5 mmHg, there was a slow rate spontaneous firing of sensory units at 0.045 ± 0.046 Hz (n = 18, N = 10). When counterweights on the isotonic lever were increased to 10–15 g, the mesentery was lengthened and firing increased significantly to an average of 1.02 ± 0.0.74 Hz (averaged over 20 s of stretch; n = 18, N = 10; Fig.3B; P = 0.02). In each case where stretch sensitivity was apparent, there was an initial burst of firing at a higher frequency (instantaneous frequency of 35.82 ± 9.23 Hz (n = 9, N = 7), then partial adaptation to a steady level, which persisted, if stretch was maintained. Of the 18 units analysed in detail, nine showed a clear sensitivity to mesenteric stretch (Fig.3A), with the other nine showing negligible or no sensitivity.
Figure 3. Afferent responses to mesenteric stretch.
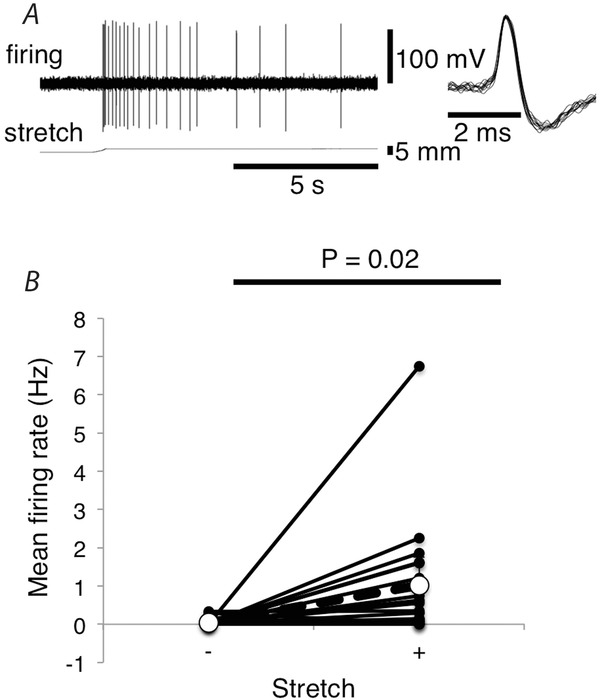
A, mesenteric afferent firing evoked by stretching the mesentery with a load of 15 g. B, mean firing rates (Hz) over the 20 s before stretch vs. the initial 20 s of stretch (10–15 g). Values shown are the average of 18 units from 10 animals. Both stretch-sensitive and stretch-insensitive units are included.
Afferent responses to increased intra-arterial pressure
With the mesentery under a constant tension of 0.5 g, the effect of increasing intra-arterial pressure from 5 mmHg to 20, 40, 60 and 80 mmHg on mesenteric afferent discharge was characterised. Afferents showed a graded response (Fig.4B). Increasing intra-arterial pressure from 5 mmHg to 60 mmHg resulted in afferent discharge changing from 0.054 ± 0.034 Hz to 0.43 ± 0.22 Hz (averaged over a 20 s; n = 34, N =17). As this response was reproducible and 60 mmHg is close to guinea pig mean blood pressure, this distension was used as standard for subsequent experiments. Visually, increasing intra-arterial pressure to 60 mmHg caused a marked distension of vessels and distortion and twisting of the vascular tree (Fig.5). A sharp increase in firing occurred at the onset of the rise in intra-arterial pressure, which then partially adapted. Typically, firing peaked in the first 10 s after the increase in intra-arterial pressure, then plateaued, but did not return to spontaneous levels within the 2 min period analysed (n = 9, N = 6; Fig.4C). Of the 34 units analysed, 12 showed clear responses to an increase in intra-arterial pressure from 5 mmHg to 60 mmHg (Fig.4A). Patterns of firing were quite variable, when examined unit by unit. About half of the afferent units showed an initial burst of firing at the onset of pressurisation. In two units firing started after a delay of 5–10 s (e.g. Fig.11A). Most units showed some slowing of firing from peak rates, during maintained pressure but few showed complete abatement of firing. About half of the units showed bursts of firing (usually 3–10 action potentials) during steady maintained distension (e.g. Fig.7C). These features of firing did not appear to cluster; it was not possible to identify distinct combinations of patterns that could be used to distinguish functional sub-classes of sensory fibres.
Figure 4. Afferent responses to increase in intra-arterial pressure.
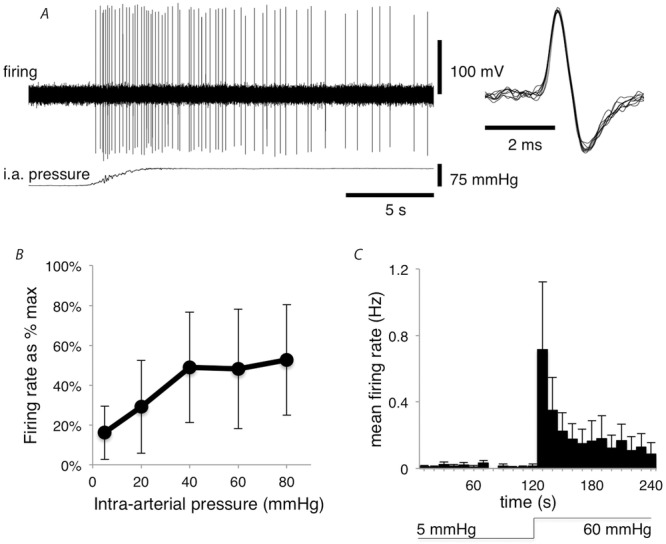
A, mesenteric afferent firing evoked by increasing the intra-arterial pressure from 5 mmHg to 60 mmHg. B, mean firing rates (Hz) over the 20 s before vs. 20 s immediately after intra-arterial pressure was increased from 5 mmHg to 20, 40, 60 or 80 mmHg (n = 9, N = 5, F(1,3) = 14.74, P < 0.05). C, average firing rate, in 10 s bins, at 5 mmHg (2 min) and 60 mmHg (2 min) (n = 9, N = 6).
Figure 5. Visible distortion of the vascular tree.
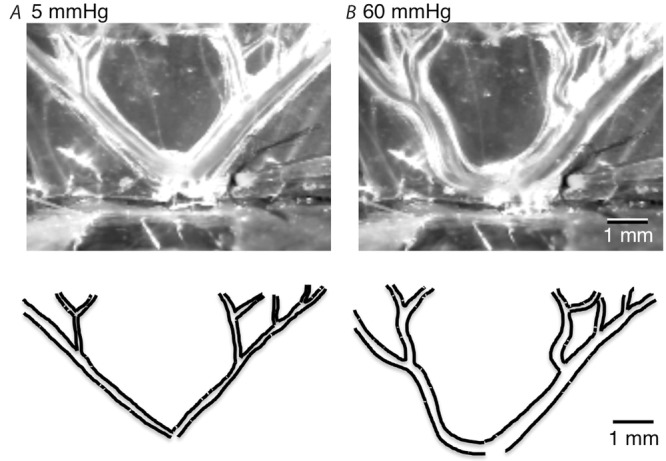
Outline of the arteries at an intra-arterial pressure of 60 mmHg (B) shows clear distension and twisting of the vascular tree compared to at 5 mmHg (A).
Figure 11. Effect of increasing intra-arterial pressure in the presence of the vasoconstrictor phenylephrine.
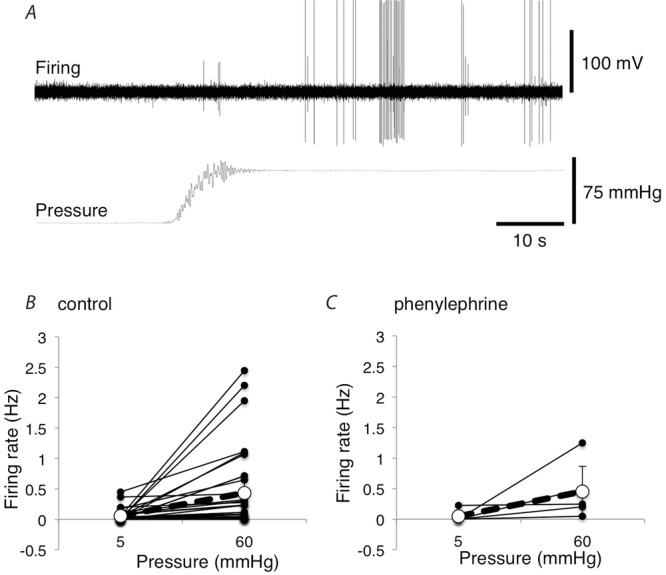
A, mesenteric afferent firing of 2 units activated by increasing the intra-arterial pressure from 5 mmHg to 60 mmHg in the presence of 10 μm phenylephrine. B, mean firing rates (Hz) over the 20 s before vs. 20 s immediately after intra-arterial pressure was increased from 5 mmHg to 60 mmHg in preparations with and without 10 μm phenylephrine in the organ bath (without phenylephrine: n = 34, N = 17; with phenylephrine: n = 5, N = 4).
Figure 7. Afferent responses to mesenteric stretch, increase in intra-arterial pressure, focal compression, localised heating and capsaicin application.
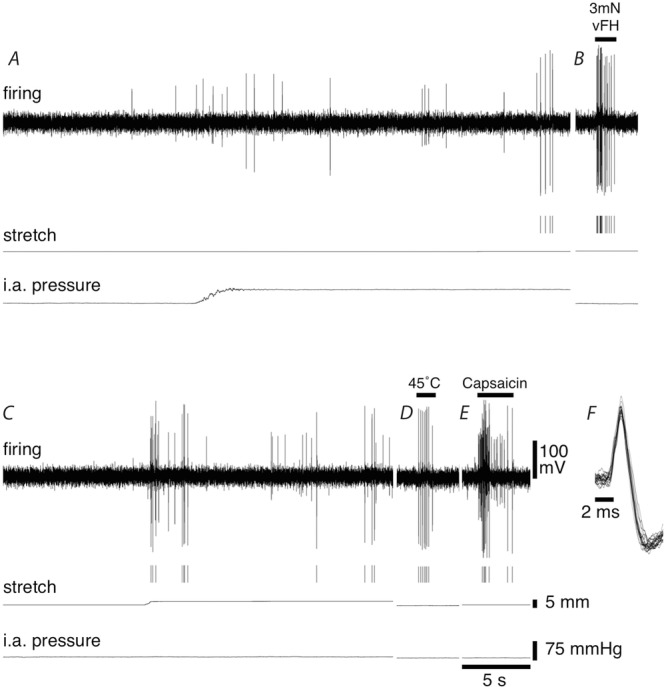
Firing of a single discriminated afferent unit (indicated by markers under firing trace) could be activated by increasing the intra-arterial pressure from 5 mmHg to 60 mmHg (A), probing a transduction site in the mesentery with a 3 mN von Frey hair (B), stretching the mesentery with a load of 15 g (C), heating a transduction site in the mesentery with 45°C Krebs solution (D) and applying 5 μl of 10−6 m capsaicin onto the mesentery (E). F, overlay of 4 action potential from each of the 5 stimuli applied.
Afferent responses to mesenteric stretch at low and high intra-arterial pressure
Many units were activated both by stretching the mesentery and by raising the intra-arterial pressure. Interactions between these stimuli were therefore investigated, by testing whether stretching the mesentery (10–15 g) at high intra-arterial pressure resulted in a larger increase in afferent firing than stretching at low intra-arterial pressure (Fig.6A). When the load was applied with the intra-arterial pressure at 5 mmHg, mean firing rate increased from 0.012 ± 0.013 Hz to 0.44 ± 0.34 Hz (averaged over 20 s). When the same load was applied with the intra-arterial pressure at 60 mmHg, firing rate increased from 0.13 ± 0.18 Hz to 0.80 ± 0.79 Hz (n = 9, N = 6; Fig.6B). Baseline firing was different between holding pressures of 5 mmHg and 60 mmHg, so we calculated the change in firing rate caused by mesenteric stretch at both pressures. At low intra-arterial pressure (5 mmHg), the change in firing rate evoked by addition of a 10–15 g load was 0.43 ± 0.34 Hz, compared to 0.67 ± 0.61 Hz at high intra-arterial pressure (n = 9, N = 6; NS; Fig.6C). This does not support the suggestion that the stretch response interacts synergistically with the effects of raised intra-arterial pressure, under the conditions tested.
Figure 6. Afferent responses to mesenteric stretch at low and high intra-arterial pressure.
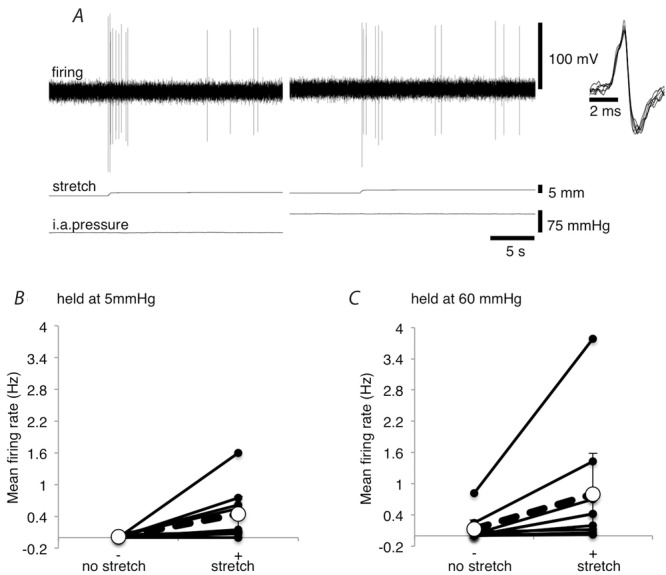
A, mesenteric afferent firing of the same unit evoked by stretching the mesentery with a load of 15 g at intra-arterial pressures of 5 mmHg and 60 mmHg. B, mean firing rates (Hz), averaged over the 20 s directly before the mesentery was stretched and the initial 20 s of stretch (10–15 g) are shown for 5 mmHg and 60 mmHg. Values shown are the average of 9 units from 6 animals. Both stretch sensitive and stretch insensitive units are included. C, mean change in firing rates (Hz) over the 20 s periods described in B.
Comparison of responses to mechanical and chemical stimuli
In a subset of experiments, we tested whether the same unit could respond to stretching the mesentery with loads of 10–15 g, increasing the intra-arterial pressure from 5 mmHg to 60 mmHg, probing the mesentery with a 3 mN von Frey hair, heating the mesentery with warmed Krebs solution (45°C), and application of 10−6 m capsaicin. Overall, 22 unit were analysed from seven animals. The proportion of units that responded to mesenteric stretch and increased intra-arterial pressure was consistent with those reported in previous sections (approximately 50% and 35% respectively), with a higher percentage of units responding to each of the other three stimuli (approximately 75% for each). Seven of the 22 units showed a response to all five stimuli tested and 19 of the 22 units showed a response to at least two of the stimuli (see Fig.7).
Raised intra-arterial pressure affected intestinal motility
When extrinsic neural pathways to the gut wall were left intact (i.e. without crushing the mesentery), we observed that increasing intra-arterial pressure often caused large, propagating contractions of the gut wall. A typical example is shown in Fig.8A. Because the gut was ligated to a perforated, hollow steel tube, an increase in intraluminal pressure (an established stimulus for peristalsis) was excluded. Increasing intra-arterial pressure has already been shown to cause firing of extrinsic sensory neurons, so we tested whether extrinsic sensory neurons contributed to initiation of peristalsis. This was done by examining the effect of a desensitising concentration of capsaicin on contractile responses, compared to control preparations (without prior capsaicin exposure) taken from the same animal (with the order of preparations each day randomised). It has been established that the actions of capsaicin are restricted to a subset of extrinsic sensory neurons in the gut (Bartho & Szolcsanyi, 1978).
Figure 8. Effect of increasing intra-arterial pressure on gut motility in Krebs and capsaicin pre-treated preparations.
For all diameter maps (DMaps), light areas correspond to areas of gut contraction and dark areas correspond to areas of expansion. A, DMap of the motility response to increasing the intra-arterial pressure from 5 mmHg to 60 mmHg in a preparation pre-treated with Krebs solution. B, DMap of the motility response to 2 doses of 1 μm final bath concentration capsaicin. Black lines cover artifacts where capsaicin was applied. Intra-arterial pressure was held constant at 5 mmHg. Capsaicin caused an immediate large contraction that propagated rapidly along the preparation. C, DMap of the motility response to increasing the intra-arterial pressure from 5 mmHg to 60 mmHg in the capsaicin pre-treated preparation shown in B. Preparation is from the same animal shown in A.
Effects of capsaicin on spontaneous and evoked peristalsis
Initial exposure to bath-applied capsaicin (1 μm) consistently induced a large peristaltic contraction of the gut, which typically showed aboral propagation (Fig.8B). This indicates that activation of extrinsic afferent neurons can trigger peristaltic contractions. Subsequent doses of capsaicin were without effect (Fig.8B) confirming that desensitisation had been achieved. As a control, addition of equal volumes of Krebs solution to the organ bath had no effect on spontaneous contractility. In naive preparations (which had not previously been exposed to capsaicin), increasing intra-arterial pressure from 5 mmHg to 60 mmHg triggered large contractions in all six preparations (Fig.8A). However, in capsaicin-desensitised preparations, only 1 in 6 preparations showed a contractile response to the first intra-arterial pressure increase. This suggested that capsaicin pre-treatment inhibited the contractile response activated by increasing intra-arterial pressure. However, when averaged across three trials of increased intra-arterial pressure in each preparation, 4 of 6 capsaicin pre-treated preparations showed at least one contractile response (Fig.8C).
Thus, increases in intra-arterial pressure evoked peristalsis in naive control preparations, with a tendency to produce fewer contractions in capsaicin-pre-treated preparations, but the difference was not significant (Krebs pre-treated: 1.36 ± 0.76 contractions, capsaicin pre-treated: 0.56 ± 0.50 contractions per increase in intra-arterial pressure; N = 6; NS; Fig.9A). However, in capsaicin-pre-treated preparations, the delay between the increase in intra-arterial pressure and the evoked contraction was significantly longer than in control preparations (averaged across all trials). For control preparations (pre-treated with Krebs solution) the average latency was 8.4 ± 0.81 s, compared to 39.3 ± 10.88 s for capsaicin pre-treated preparations (N = 4; Fig.9B). Two other capsaicin pre-treated preparations had no contractile responses to raised intra-arterial pressure. Thus capsaicin pre-treatment reduced, but did not block, the effect of vascular distension on peristalsis.
Figure 9. Effect of capsaicin on number and latency of increased intra-arterial pressure induced gut contractions.
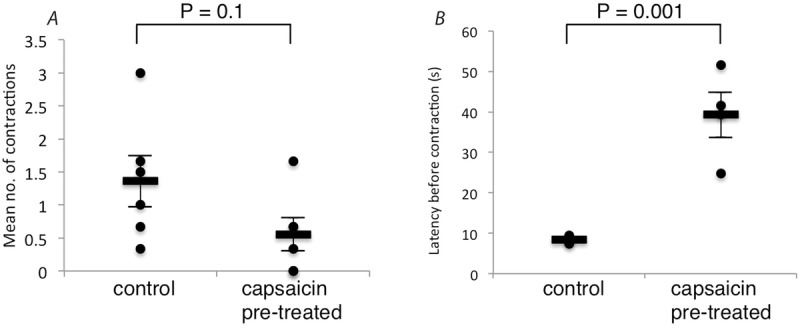
A, mean number of gut contractions caused by increasing the intra-arterial pressure in Krebs vs. capsaicin (1 μm bath concentration) pre-treated preparations. Values shown are the averages of multiple runs from 6 animals. Separate preparations from the same animal were used for the Krebs and capsaicin pre-treated groups. B, time taken from the initiation of the rise in intra-arterial pressure to the first gut contraction in the run where the initial contraction was evoked following Krebs or capsaicin pre-treatment. Values shown are from 4 animals. Separate preparations from the same animal were used for the Krebs and capsaicin pre-treated groups.
Effect of TTX on intra-arterial pressure-induced motility
In a separate set of experiments, control preparations showed at least one peristaltic contraction in each of the three runs when intra-arterial pressure was increased (N = 4; Fig.10). Pre-treating preparations with tetrodotoxin (1 μm) blocked contractions in all three runs in 4 out of 4 preparations.
Figure 10. Effect of TTX treatment on increased intra-arterial pressure induced gut contractions.
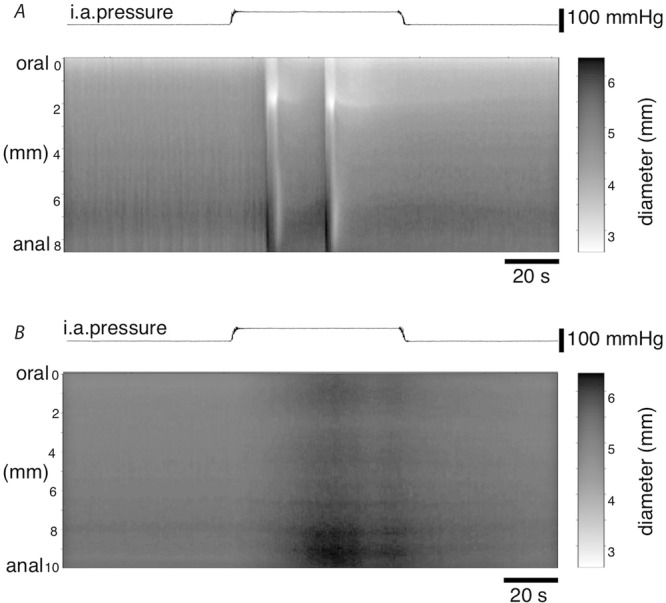
For all diameter maps (DMaps), light areas correspond to areas of gut contraction and dark areas correspond to areas of expansion. Increasing the intra-arterial pressure from 5 mmHg to 60 mmHg induced circumferential contractions in control tissue (A), but not TTX treated tissue (B). DMaps shown are from a control and TTX treated preparation from the same animal.
Effects of phenylephrine pre-contraction
In many vascular studies in vitro, normal sympathetic vasoconstrictor tone is experimentally reinstated by adding phenylephrine to the bathing solution, to pre-constrict vessels. Under resting conditions, addition of 10 μm phenylephrine caused a small, and non-significant, fall in spontaneous afferent firing, from 0.62 ± 0.58 Hz to 0.32 ± 0.20 Hz (n = 6, N = 5; NS). Another set of preparations were tested in the presence of 10 μm phenylephrine to determine whether increases in intra-arterial pressure still evoked sensory firing. On average, raising intraluminal pressure from 5 mmHg to 60 mmHg increased firing from 0.045 ± 0.088 Hz to 0.450 ± 0.420 Hz (averaged over 20 s; n = 5, N = 4), an increase that was comparable to the response in normal Krebs solution (Fig.11). This confirmed that even in the presence of a vasoconstrictor, arterial distension activated vascular afferents.
Increased intra-arterial pressure in isolated vessels
To test whether afferents responded to simple radial distension, 11 mesenteric arteries (15 single units recorded) were isolated from the mesentery, ligated distally and cannulated proximally. The reservoir was used to give a resting pressure of 5 mmHg, then raised to produce an intra-arterial pressure of 60 mmHg. Visually, this caused notable ballooning and lengthening of the isolated artery. About half of the units showed an increase in afferent firing during this stimulus, compared to basal firing at 5 mmHg, but the increases were small. On average firing increased by 0.014 ± 0.072 Hz (n = 15, N = 11). A comparable increase in pressure (from 5 to 60 mmHg) applied to an intact vascular tree caused an average increase in firing of 0.381 ± 0.216 Hz (n = 34, N = 17, P = 0.0351, df = 26). This suggests that the distortion of the mesenteric fan is a major driver of the sensory response, rather than simple radial stretch of vessels (see Fig.12).
Figure 12. Effect of increasing intra-arterial pressure in isolated mesenteric arteries (with 1 or fewer branch-points) compared to intact mesenteric arcades.
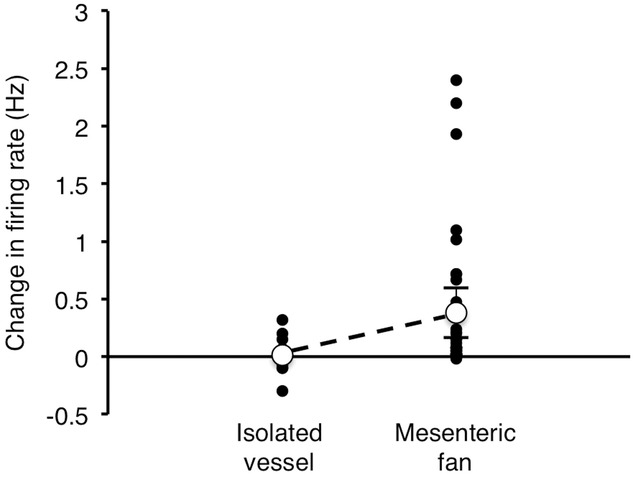
Figure shows the change in firing (averaged over 20 s before and after step to 60 mmHg, in Hz) for isolated arteries (left, n = 15, N = 11) and intact mesenteric fan (n = 34, N = 17). There was a significantly larger increase in firing evoked by raising intra-arterial pressure to 60 mmHg in the intact mesenteric fan, compared to isolated arteries (P = 0.0351).
Discussion
This study focused on the extrinsic sensory nerves to the gut, which have endings associated with blood vessels (‘vascular afferents’). By severing mesenteric nerves before they entered the gut, recordings could be made exclusively from afferent units with transduction sites on mesenteric vessels outside the gut wall, thus excluding many other classes with sensory endings within the intestine (e.g. low threshold vagal mechanoreceptors (Booth et al. 2008), low threshold spinal mechanoreceptors (Lynn et al. 2003) and viscerofugal enteric neurons (Hibberd et al. 2012)). The afferent neurons that were the focus of the present study have been extensively characterised in previous studies. It is well established that sensory endings in the mesentery, predominantly associated with larger blood vessels, show mechanosensory responses to crushing or compression, concentrated at vessel branch points (Bessou & Perl, 1966; Morrison, 1973; Floyd & Morrison, 1974; Song et al. 2009). Typically, very intense compressive stimuli are required to activate them, in terms of force per unit area; these are unlikely to reflect naturally occurring mechanical stimuli experienced by the gut in situ.
Site of endings
Vascular afferents can be activated by stretch of their mesenteric receptive fields, for example via stretch of the mesentery by a pulley system (Morrison, 1973; Song et al. 2009). Consistent with this, traction applied to the mesenteries, evokes a nociceptive-like response in rats (Holzer-Petsche & Brodacz, 1999). It has also been suggested that both gut distension and contraction of the smooth muscle of the muscularis externa can activate mesenteric sensory endings, probably by pulling on the mesenteries (Blumberg et al. 1983). By combining dye filling techniques with recordings from afferent nerve fibres, it was previously shown that the endings of these afferents correspond to varicose branching axons on mesenteric blood vessels, often concentrated near branch-points (Song et al. 2009). There are similar mechanosensory endings on intramural vessels, particularly in the submucosa (Song et al. 2009), which can be directly affected by mechanical deformation of the gut wall. Dye filling has shown that there are few axons (other than axons-of-passage) running in the mesenteric and serosal membranes themselves; the afferents terminals located outside the gut wall are almost exclusively associated with the adventitia of blood vessels. In addition, some afferent axons innervate both mesenteric (extramural) and submucosal (intramural) arteries (Song et al. 2009).
Mechanical adequate stimulus
The mechanical adequate stimulus for vascular afferents has not been clear from previous studies. It is well established that their nerve endings are mechanically sensitised by a variety of endogenous substances, including those released during ischaemia (Pan & Longhurst, 1996), bradykinin (Haupt et al. 1983), prostaglandins (Longhurst & Dittman, 1987), TNFα, IL-1β, IL-6 and IL-10 (Hughes et al. 2013), and proteases acting at PAR-2 receptors (Kirkup et al. 2003), as well as by capsaicin (Longhurst et al. 1984). They are also sensitised during experimental inflammation (Hughes et al. 2009; Ibeakanma et al. 2011) and post-inflammation. However, the question remains about the nature of mechanical stimulation that most effectively activates vascular afferents in naive tissue.
The present study has confirmed that extramural vascular afferents can be activated by axial stretch, as reported previously, but has extended this by demonstrating that they are also activated by changes in intravascular pressure in the arterial tree, with threshold responses ranging from 20 to 80 mmHg. This compares to mean arterial pressure of 63 ± 2 mmHg and systolic pressure averaging 72 ± 2 mmHg during telemetric recordings from conscious guinea pigs (Hess et al. 2007), suggesting that the afferents might be constitutively active. However, it seems unlikely that spinal afferent vasodilators contribute in an ongoing fashion to the control of peripheral resistance. CGRP antagonists, at concentrations which block CGRP-mediated transmission, do not have measurable effects on systemic blood pressure (Shen et al. 2001; Petersen et al. 2005).
In theory, these afferents could contribute to the baroreceptor reflex control of arterial pressure. However, baroreceptors in the aortic arch and carotid artery, with cell bodies in nodose, jugular and petrosal ganglia and central projections in the brainstem, are the primary afferents that effect these functions (Neil, 1962; Pilowsky & Goodchild, 2002). In addition, depleting vascular afferent nerves by repeated exposure to capsaicin in vivo does not disrupt baroreceptor reflexes (Furness et al. 1982a). It was striking that afferents ending on single isolated arteries did not show similar robust responses to distension. This suggests that it is not the increase in diameter per se that activates these axons, but rather the overall distortion of the mesenteric arcade. In several published studies, it has been reported that vascular afferent endings are concentrated near vessel branch-points (Bessou & Perl, 1966; Morrison, 1973; Floyd & Morrison, 1974; Song et al. 2009); these are the regions most distorted during raised intra-arterial pressure. When intravascular pressure was raised in the present study, vessels clearly dilated (see Fig.5). Myogenic contractile responses to increased pressure were not recorded, but it has previously been reported that myogenic responses are less pronounced in mesenteric vessels than in other vascular beds such as cerebral vessels (Coombes et al. 1999; Lagaud et al. 1999).
Falls in vascular perfusion rate can also evoke firing of mesenteric vascular afferents (Brunsden et al. 2002). There are several experimental differences which may explain this apparent contradiction with the present study. In the study of Brunsden et al., vessels were routinely pre-contracted with 10 μm phenylephrine, which may reduce the extent of pressure-induced increases in diameter. However, in the present study, pre-constriction of vessels with phenylephrine did not prevent pressure-evoked firing. In addition, in the studies of Brunsden et al. (2002, 2007), the mesenteric vessels were not attached to the resistance vessels in the gut wall that largely determine upstream vascular pressure. In both studies, changes of firing are best explained as being due to mechanical distortion of mesenteric vessels, particularly at branch points, that occurs when perfusion rate is either increased or decreased from a steady state.
Roles of vascular afferents
Vascular afferents, with endings associated with extramural or intramural arteries have previously been described as ‘mesenteric’ or ‘serosal’, depending on the site at which blunt probing activates their axons (Leek, 1977). They comprise a major part of the thoraco-lumbar spinal sensory innervation of the bowel, accounting for 86% of recorded splanchnic nerve fibres innervating the colorectum but accounting for a smaller proportion (about one-third) of pelvic afferents of the mouse (Brierley et al. 2004), indicating regional variability in their density. In addition to strong compression, they are also mechanically activated by traction on the mesenteries (Bessou & Perl, 1966) and by gut distension (Song et al. 2009). They are powerfully modulated by ischaemia, hypoxia and by the TRPv1 agonist, capsaicin (Haupt et al. 1983; Longhurst et al. 1984; Longhurst & Dittman, 1987), and are sensitised by a wide range of endogenous mediators released from damaged or inflamed tissue (Brookes et al. 2013). They are recognised as a major class of gut nociceptors.
The present study has shown that raising intravascular pressure also evokes firing in vascular afferents, which sums with their responses to other mechanical stimuli. This raises the intriguing possibility that vascular afferent responses to mechanical stimuli might be partially entrained with the cardiac cycle. As far as we know this has not yet been tested empirically with sensitive methods such as electrocardiogram-triggered averaging.
An important question arises about the contribution of vascular afferents to sensation. Their mechanical adequate stimulus appears to be distortion of the vascular tree. Vascular afferent endings thus may be using blood vessels as a mechanical substrate (analogous to mounting strain gauges on a beam). However, specific distortion of mesenteric arterial branch-points may be caused by high amplitude distension or contraction of the intestinal wall, especially if this displaces the gut in the abdominal cavity (Morrison, 1973; Holzer-Petsche & Brodacz, 1999). Localised vascular distortion may occur when an endoscope locally presses against the gut wall – a known cause of endoscopic pain (Shah et al. 2002). Furthermore, we speculate that during strong contractions of the muscularis externa (‘spasm’), the reduction in blood flow, which is visible as blanching of the gut (Bayliss & Starling, 1899), may lead to upstream distortion of the feed arteries and thereby augment vascular afferent firing (in addition to activation via ischaemia and via tension on the mesenteries). Vascular afferents are more profoundly sensitised by inflammation than several other classes of lower threshold gut sensory neurons (Lynn et al. 2008; Hughes et al. 2009) suggesting that they make a major contribution to the chronic pain associated with inflammation, post-inflammation and ischaemia. Whether sensitised vascular afferents become responsive to arterial pressure or to the pulse remains to be determined.
Effects on gut motility
Distension of the lumen of the isolated guinea pig intestine reliably evokes propagating peristaltic contractions (Trendelenburg, 1917; Crema et al. 1970; Hennig et al. 1999). The present study has shown that increasing intra-arterial pressure is also an effective stimulus to evoke peristalsis, when vessels’ connections with the gut are preserved. This is not caused by vascular perfusate leaking into the lumen and raising intraluminal pressure, because both ends of the gut were attached to a catheter which was open to the organ bath. When vascular afferent neurons were desensitised (by high concentration of capsaicin), the effects of raised perfusion pressure on peristalsis were reduced. This is consistent with previous reports that antidromic activation of spinal afferents elicits cholinergic contraction (Bartho & Szolcsanyi, 1980), as does an initial application of capsaicin (Bartho et al. 1999). Capsaicin (Bartho & Holzer, 1995) releases tachykinins from the spinal afferent axons in the gut, as does mesenteric nerve stimulation, causing slow depolarisations of enteric neurons, similar to those mediated by tachykinins (Takaki & Nakayama, 1988, 1989).
However, even after capsaicin tachyphylaxis, raised intra-arterial pressure still triggered peristalsis, without raising intraluminal pressure. Close examination of the spatiotemporal maps (Figs8 and 10) revealed a general darkening (intestinal dilatation) associated with raised intra-arterial pressure. This is likely to reflect thickening of the gut wall due to distension of the intramural vasculature. Desensitising concentrations of capsaicin abolish the effects of subsequent applications of capsaicin (Bartho & Holzer, 1995) and reduce or block much afferent activity. When capsaicin desensitisation was carried out in the present study, peristaltic contractions evoked by step increases in intra-arterial pressure were partially inhibited. This suggests that vascular pressurisation triggered peristalsis, at least in part, by activating capsaicin-sensitive spinal afferent neurons, whose axon collaterals then activated enteric neurons. However, peristalsis still occurred, after high concentrations of capsaicin, albeit with a longer delay. There are several possible explanations for the persistence of capsaicin-insensitive reflex activity. Distortion of the gut wall itself, caused by increased intramural vascular volume, may activate enteric mechanosensitive neurons; it has been established that enteric motor reflexes can be elicited in guinea pig small intestine after total extrinsic denervation (Furness et al. 1995). In addition, in our hands, some capsaicin-sensitive vascular afferents remain mechanosensitive, after exposure to capsaicin. Furthermore, the present study confirmed previous reports that a significant minority of distension-sensitive vascular afferents are insensitive to capsaicin (Brierley et al. 2005; Song et al. 2009); activity in these neurons may have been sufficient to trigger peristalsis. Lastly, TRPv1 itself may not be involved in the response to arterial pressurisation.
In summary, vascular afferents show mechanosensitivity concentrated at branch points on arteries inside and outside the gut. They are activated by distortion of the vascular tree by intraluminal pressurisation, by applied compression and by axial tension. They fire when there is distension of the gut wall, and during strong contractions of the gut musculature (Song et al. 2009). They can be sensitised by a wide variety of endogenous mediators. In terms of outputs, the present study has shown that they have functional outputs in the enteric nervous system, through which they can excite gut motility. They also activate central pathways that lead to sensation (including pain) and reflex activity. Furthermore their collaterals in prevertebral sympathetic ganglia (Matthews & Cuello, 1984) activate sympathetic pathways (Parkman et al. 1993) which contribute to intestino-intestinal inhibitory reflexes (Peters & Kreulen, 1986), an action which may oppose their excitatory effects on intestinal motility. Lastly, collaterals of vascular afferent neurons probably mediate axon-reflex vasodilatation of blood vessels evoked by gut distension (Meehan & Kreulen, 1992). Vascular afferents contribute to sensation and gastrointestinal reflexes, in a complex, multi-tiered fashion.
Acknowledgments
We would like to thank the staff of Biomedical Engineering at Flinders University/Flinders Medical Centre for assistance with building and refining the experimental setups used in this study.
Glossary
- CGRP
calcitonin gene-related peptide
- DMSO
dimethyl sulphoxide
- PBS
phosphate-buffered saline
- TTX
tetrodotoxin
Additional information
Competing interests
None of the authors of this paper has a conflict of interest to declare.
Author contributions
Conception and design of the experiments was carried out by A.H., B.N.C. and S.J.H.B. Collection, assembly, analysis and interpretation of data was by A.H., B.N.C., L.W., N.J.S., V.P.Z., P.G.D., M.C. and S.J.H.B. Drafting the article or revising it critically for important intellectual content was by A.H., B.N.C., L.W., N.J.S., V.P.Z., P.G.D., M.C., S.J.H.B. All authors have approved a final version of the manuscript.
Funding
This project was funded by project grant no. 1048195 of the National Health and Medical Research Council of Australia to S.J.H.B., P.G.D., N.J.S. and V.P.Z.
References
- Adelson DW, Wei JY, Kruger L. Warm-sensitive afferent splanchnic C-fiber units in vitro. J Neurophysiol. 1997;77:2989–3002. doi: 10.1152/jn.1997.77.6.2989. [DOI] [PubMed] [Google Scholar]
- Bahns E, Ernsberger U, Janig W, Nelke A. Discharge properties of mechanosensitive afferents supplying the retroperitoneal space. Pflugers Arch. 1986;407:519–525. doi: 10.1007/BF00657510. [DOI] [PubMed] [Google Scholar]
- Bartho L, Holzer P. The inhibitory modulation of guinea-pig intestinal peristalsis caused by capsaicin involves calcitonin gene-related peptide and nitric oxide. Naunyn Schmiedebergs Arch Pharmacol. 1995;353:102–109. doi: 10.1007/BF00168922. [DOI] [PubMed] [Google Scholar]
- Bartho L, Lenard L, Jr, Patacchini R, Halmai V, Wilhelm M, Holzer P, Maggi CA. Tachykinin receptors are involved in the "local efferent" motor response to capsaicin in the guinea-pig small intestine and oesophagus. Neuroscience. 1999;90:221–228. doi: 10.1016/s0306-4522(98)00459-x. [DOI] [PubMed] [Google Scholar]
- Bartho L, Szolcsanyi J. The site of action of capsaicin on the guinea-pig isolated ileum. Naunyn Schmiedebergs Arch Pharmacol. 1978;305:75–81. doi: 10.1007/BF00497008. [DOI] [PubMed] [Google Scholar]
- Bartho L, Szolcsanyi J. The mechanism of the motor response to periarterial nerve stimulation in the small intestine of the rabbit. Br J Pharmacol. 1980;70:193–195. doi: 10.1111/j.1476-5381.1980.tb07924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss WM. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. J Physiol. 1901;26:173–209. doi: 10.1113/jphysiol.1901.sp000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P, Perl ER. A movement receptor of the small intestine. J Physiol. 1966;182:404–426. doi: 10.1113/jphysiol.1966.sp007829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg H, Haupt P, Janig W, Kohler W. Encoding of visceral noxious stimuli in the discharge patterns of visceral afferent fibres from the colon. Pflugers Archiv. 1983;398:33–40. doi: 10.1007/BF00584710. [DOI] [PubMed] [Google Scholar]
- Booth CE, Shaw J, Hicks GA, Kirkup AJ, Winchester W, Grundy D. Influence of the pattern of jejunal distension on mesenteric afferent sensitivity in the anaesthetized rat. Neurogastroenterol Motil. 2008;20:149–158. doi: 10.1111/j.1365-2982.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Carter R, Jones W, 3rd, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol. 2005;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–178. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Spencer NJ, Costa M, Zagorodnyuk VP. Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol. 2013;10:286–296. doi: 10.1038/nrgastro.2013.29. [DOI] [PubMed] [Google Scholar]
- Bruce AN. Vaso-dilator axon reflexes. Q J Exp Physiol. 1913;6:339–354. [Google Scholar]
- Brunsden AM, Brookes SJ, Bardhan KD, Grundy D. Mechanisms underlying mechanosensitivity of mesenteric afferent fibers to vascular flow. Am J Physiol Gastrointest Liver Physiol. 2007;293:G422–428. doi: 10.1152/ajpgi.00083.2007. [DOI] [PubMed] [Google Scholar]
- Brunsden AM, Jacob S, Bardhan KD, Grundy D. Mesenteric afferent nerves are sensitive to vascular perfusion in a novel preparation of rat ileum in vitro. Am J Physiol Gastrointest Liver Physiol. 2002;283:G656–665. doi: 10.1152/ajpgi.00343.2001. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Carbone SE, Wattchow DA, Spencer NJ, Brookes SJ. Loss of responsiveness of circular smooth muscle cells from the guinea pig ileum is associated with changes in gap junction coupling. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1434–1444. doi: 10.1152/ajpgi.00376.2011. [DOI] [PubMed] [Google Scholar]
- Coombes JE, Hughes AD, Thom SA. Intravascular pressure-evoked changes in intracellular calcium [Ca2+]i and tone in rat mesenteric and rabbit cerebral arteries in vitro. J Hum Hypertens. 1999;13:855–858. doi: 10.1038/sj.jhh.1000902. [DOI] [PubMed] [Google Scholar]
- Crema A, Frigo GM, Lecchini S. A pharmacological analysis of the peristaltic reflex in the isolated colon of the guinea-pig or cat. Br J Pharmacol. 1970;39:334–345. doi: 10.1111/j.1476-5381.1970.tb12897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd K, Hick VE, Morrison JF. Mechanosensitive afferent units in the hypogastric nerve of the cat. J Physiol. 1976;259:457–471. doi: 10.1113/jphysiol.1976.sp011476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd K, Morrison JF. Splanchnic mechanoreceptors in the dog. Q J Exp Physiol Cognate Med Sci. 1974;59:361–366. doi: 10.1113/expphysiol.1974.sp002279. [DOI] [PubMed] [Google Scholar]
- Furness JB, Elliott JM, Murphy R, Costa M, Chalmers JP. Baroreceptor reflexes in conscious guinea-pigs are unaffected by depletion of cardiovascular substance P nerves. Neurosci Lett. 1982a;32:285–290. doi: 10.1016/0304-3940(82)90308-1. [DOI] [PubMed] [Google Scholar]
- Furness JB, Johnson PJ, Pompolo S, Bornstein JC. Evidence that enteric motility reflexes can be initiated through entirely intrinsic mechanisms in the guinea-pig small intestine. Neurogastroenterol Motil. 1995;7:89–96. doi: 10.1111/j.1365-2982.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Furness JB, Papka RE, Della NG, Costa M, Eskay RL. Substance P-like immunoreactivity in nerves associated with the vascular system of guinea-pigs. Neuroscience. 1982b;7:447–459. doi: 10.1016/0306-4522(82)90279-2. [DOI] [PubMed] [Google Scholar]
- Gibbins IL, Furness JB, Costa M, MacIntyre I, Hillyard CJ, Girgis S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci Lett. 1985;57:125–130. doi: 10.1016/0304-3940(85)90050-3. [DOI] [PubMed] [Google Scholar]
- Haupt P, Janig W, Kohler W. Response pattern of visceral afferent fibres, supplying the colon, upon chemical and mechanical stimuli. Pflugers Archiv. 1983;398:41–47. doi: 10.1007/BF00584711. [DOI] [PubMed] [Google Scholar]
- Hennig GW, Costa M, Chen BN, Brookes SJ. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol. 1999;517:575–590. doi: 10.1111/j.1469-7793.1999.0575t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P, Rey M, Wanner D, Steiner B, Clozel M. Measurements of blood pressure and electrocardiogram in conscious freely moving guineapigs: a model for screening QT interval prolongation effects. Lab Anim. 2007;41:470–480. doi: 10.1258/002367707782314337. [DOI] [PubMed] [Google Scholar]
- Hibberd TJ, Zagorodnyuk VP, Spencer NJ, Brookes SJH. Identification and mechanosensitivity of viscerofugal neurons. Neuroscience. 2012;225:118–129. doi: 10.1016/j.neuroscience.2012.08.040. [DOI] [PubMed] [Google Scholar]
- Holzer P. Efferent-like roles of afferent neurons in the gut: Blood flow regulation and tissue protection. Auton Neurosci. 2006;125:70–75. doi: 10.1016/j.autneu.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer-Petsche U, Brodacz B. Traction on the mesentery as a model of visceral nociception. Pain. 1999;80:319–328. doi: 10.1016/s0304-3959(98)00233-4. [DOI] [PubMed] [Google Scholar]
- Hughes PA, Brierley SM, Martin CM, Brookes SJH, Linden DR, Blackshaw LA. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut. 2009;58:1333–1341. doi: 10.1136/gut.2008.170811. [DOI] [PubMed] [Google Scholar]
- Hughes PA, Harrington AM, Castro J, Liebregts T, Adam B, Grasby DJ, Isaacs NJ, Maldeniya L, Martin CM, Persson J, Andrews JM, Holtmann G, Blackshaw LA, Brierley SM. Sensory neuro-immune interactions differ between irritable bowel syndrome subtypes. Gut. 2013;62:1456–1465. doi: 10.1136/gutjnl-2011-301856. [DOI] [PubMed] [Google Scholar]
- Ibeakanma C, Ochoa-Cortes F, Miranda-Morales M, McDonald T, Spreadbury I, Cenac N, Cattaruzza F, Hurlbut D, Vanner S, Bunnett N, Vergnolle N, Vanner S. Brain-gut interactions increase peripheral nociceptive signaling in mice with postinfectious irritable bowel syndrome. Gastroenterology. 2011;141:2098–2108. doi: 10.1053/j.gastro.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Takasaki K, Saito A, Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- Kirkup AJ, Jiang W, Bunnett NW, Grundy D. Stimulation of proteinase-activated receptor 2 excites jejunal afferent nerves in anaesthetised rats. J Physiol. 2003;552:589–601. doi: 10.1113/jphysiol.2003.049387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagaud GJ, Skarsgard PL, Laher I, van Breemen C. Heterogeneity of endothelium-dependent vasodilation in pressurized cerebral and small mesenteric resistance arteries of the rat. J Pharmacol Exp Ther. 1999;290:832–839. [PubMed] [Google Scholar]
- Leek BF. Abdominal and pelvic visceral receptors. Br Med Bull. 1977;33:163–168. doi: 10.1093/oxfordjournals.bmb.a071417. [DOI] [PubMed] [Google Scholar]
- Lembeck F, Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979;310:175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- Lewis T. The Blood Vessels of the Human skin and their Responses. London: Shaw & Sons; 1927. [Google Scholar]
- Longhurst JC, Dittman LE. Hypoxia, bradykinin, and prostaglandins stimulate ischemically sensitive visceral afferents. Am J Physiol Heart Circ Physiol. 1987;253:H556–567. doi: 10.1152/ajpheart.1987.253.3.H556. [DOI] [PubMed] [Google Scholar]
- Longhurst JC, Kaufman MP, Ordway GA, Musch TI. Effects of bradykinin and capsaicin on endings of afferent fibers from abdominal visceral organs. Am J Physiol Regul Integr Comp Physiol. 1984;247:R552–559. doi: 10.1152/ajpregu.1984.247.3.R552. [DOI] [PubMed] [Google Scholar]
- Lynn PA, Chen BN, Zagorodnyuk VP, Costa M, Brookes SJH. TNBS-induced inflammation modulates the function of one class of low-threshold rectal mechanoreceptors in the guinea pig. Am J Physiol Gastrointest Liver Physiol. 2008;295:G862–871. doi: 10.1152/ajpgi.00585.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn PA, Olsson C, Zagorodnyuk V, Costa M, Brookes SJ. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology. 2003;125:786–794. doi: 10.1016/s0016-5085(03)01050-3. [DOI] [PubMed] [Google Scholar]
- Malliani A, Pagani M. Afferent sympathetic nerve fibres with aortic endings. J Physiol. 1976;263:157–169. doi: 10.1113/jphysiol.1976.sp011626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews MR, Cuello AC. The origin and possible significance of substance P immunoreactive networks in the prevertebral ganglia and related structures in the guinea-pig. Philos Trans R Soc Lond B Biol Sci. 1984;306:247–276. doi: 10.1098/rstb.1984.0087. [DOI] [PubMed] [Google Scholar]
- Meehan AG, Kreulen DL. A capsaicin-sensitive inhibitory reflex from the colon to mesenteric arteries in the guinea-pig. J Physiol. 1992;448:153–159. doi: 10.1113/jphysiol.1992.sp019034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JF. Splanchnic slowly adapting mechanoreceptors with punctate receptive fields in the mesentery and gastrointestinal tract of the cat. J Physiol. 1973;233:349–361. doi: 10.1113/jphysiol.1973.sp010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil E. Neural factors responsible for cardiovascular regulation. Circ Res. 1962;11:137–143. doi: 10.1161/01.res.11.1.137. [DOI] [PubMed] [Google Scholar]
- Pan HL, Longhurst JC. Ischaemia-sensitive sympathetic afferents innervating the gastrointestinal tract function as nociceptors in cats. J Physiol. 1996;492:841–850. doi: 10.1113/jphysiol.1996.sp021350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkman HP, Ma RC, Stapelfeldt WH, Szurszewski JH. Direct and indirect mechanosensory pathways from the colon to the inferior mesenteric ganglion. Am J Physiol Gastrointest Liver Physiol. 1993;265:G499–505. doi: 10.1152/ajpgi.1993.265.3.G499. [DOI] [PubMed] [Google Scholar]
- Peters S, Kreulen DL. Fast and slow synaptic potentials produced in a mammalian sympathetic ganglion by colon distension. Proc Natl Acad Sci U S A. 1986;83:1941–1944. doi: 10.1073/pnas.83.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KA, Birk S, Lassen LH, Kruuse C, Jonassen O, Lesko L, Olesen J. The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers. Cephalalgia. 2005;25:139–147. doi: 10.1111/j.1468-2982.2004.00830.x. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens. 2002;20:1675–1688. doi: 10.1097/00004872-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Shah SG, Brooker JC, Thapar C, Williams CB, Saunders BP. Patient pain during colonoscopy: an analysis using real-time magnetic endoscope imaging. Endoscopy. 2002;34:435–440. doi: 10.1055/s-2002-31995. [DOI] [PubMed] [Google Scholar]
- Shen YT, Pittman TJ, Buie PS, Bolduc DL, Kane SA, Koblan KS, Gould RJ, Lynch JJ., Jr Functional role of α-calcitonin gene-related peptide in the regulation of the cardiovascular system. J Pharmacol Exp Ther. 2001;298:551–558. [PubMed] [Google Scholar]
- Song X, Chen BN, Zagorodnyuk VP, Lynn PA, Blackshaw LA, Grundy D, Brunsden AM, Costa M, Brookes SJH. Identification of medium/high-threshold extrinsic mechanosensitive afferent nerves to the gastrointestinal tract. Gastroenterology. 2009;137:274–284. doi: 10.1053/j.gastro.2009.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki M, Nakayama S. Effects of mesenteric nerve stimulation on the electrical activity of myenteric neurons in the guinea pig ileum. Brain Res. 1988;442:351–353. doi: 10.1016/0006-8993(88)91524-7. [DOI] [PubMed] [Google Scholar]
- Takaki M, Nakayama S. Effects of capsaicin on myenteric neurons of the guinea pig ileum. Neurosci Lett. 1989;105:125–130. doi: 10.1016/0304-3940(89)90023-2. [DOI] [PubMed] [Google Scholar]
- Tassicker BC, Hennig GW, Costa M, Brookes SJH. Rapid anterograde and retrograde tracing from mesenteric nerve trunks to the guinea-pig small intestine in vitro. Cell Tiss Res. 1999;295:437–452. doi: 10.1007/s004410051250. [DOI] [PubMed] [Google Scholar]
- Trendelenburg P. Physiologische und pharmakologische Versuche Uber die Dunndarmperistaltik. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1917;81:55–129. doi: 10.1007/s00210-006-0052-7. [DOI] [PubMed] [Google Scholar]
- Uddman R, Edvinsson L, Ekblad E, Hakanson R, Sundler F. Calcitonin gene-related peptide (CGRP): perivascular distribution and vasodilatory effects. Reg Pept. 1986;15:1–23. doi: 10.1016/0167-0115(86)90071-6. [DOI] [PubMed] [Google Scholar]
- Westcott EB, Segal SS. Perivascular innervation: a multiplicity of roles in vasomotor control and myoendothelial signaling. Microcirculation. 2013;20:217–238. doi: 10.1111/micc.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Brookes SJ. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J Neurosci. 2000;20:6249–6255. doi: 10.1523/JNEUROSCI.20-16-06249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]