Abstract
Human medial gastrocnemius (MG) motor units (MUs) are thought to occupy small muscle territories, with low-threshold units preferentially located distally. In this study, subjects (n = 8) performed ramped and sustained isometric contractions (ankle plantar flexion and knee flexion; range: ∼1–40% maximal voluntary contraction) and we measured MU territory size with spike-triggered averages from fine-wire electrodes inserted along the length (seven electrodes) or across the width (five electrodes) of the MG muscle. Of 69 MUs identified along the length of the muscle, 32 spanned at least half the muscle length (≥ 6.9 cm), 11 of which spanned all recording sites (13.6–17.9 cm). Distal fibres had smaller pennation angles (P < 0.05), which were accompanied by larger territories in MUs with fibres located distally (P < 0.05). There was no distal-to-proximal pattern of muscle activation in ramp contraction (P = 0.93). Of 36 MUs identified across the width of the muscle, 24 spanned at least half the muscle width (≥ 4.0 cm), 13 of which spanned all recording sites (8.0–10.8 cm). MUs were not localized (length or width) based on recruitment threshold or contraction type, nor was there a relationship between MU territory size and recruitment threshold (Spearman's rho = −0.20 and 0.13, P > 0.18). MUs in the human MG have larger territories than previously reported and are not localized based on recruitment threshold or joint action. This indicates that the CNS does not have the means to selectively activate regions of the MG muscle based on task requirements.
Key points
Human medial gastrocnemius (MG) motor units (MUs) are thought to occupy small muscle territories or regions, with low-threshold units preferentially located distally.
We used intramuscular recordings to measure the territory of muscle fibres from MG MUs and determine whether these MUs are grouped by recruitment threshold or joint action (ankle plantar flexion and knee flexion).
The territory of MUs from the MG muscle varied from somewhat localized to highly distributed, with approximately half the MUs spanning at least half the length and width of the muscle. There was also no evidence of regional muscle activity based on MU recruitment thresholds or joint action.
The CNS does not have the means to selectively activate regions of the MG muscle based on task requirements.
Introduction
The human medial gastrocnemius muscle (MG) is an ankle plantar flexor that plays an important role in our ability to stand, walk and run. There is evidence from surface electromyography (EMG) studies of regional MG muscle activation (Segal et al. 1991; Staudenmann et al. 2009; Wakeling, 2009; Vieira et al. 2010; von Tscharner et al. 2014). Further support for the presence of regional activation in this muscle comes from surface EMG array recordings where the longitudinal territory of muscle fibres from human MG motor units (MUs) was found to be 2.5 cm on average, with a maximum possible size of 4 cm (Vieira et al. 2011). Such regional muscle activity may allow for more controlled and efficient torque generation (English et al. 1993; Monti et al. 2001; Butler, 2007; Hudson et al. 2009; Wakeling, 2009). For example, regional differences in muscle fibre pennation angles have been linked to the preferential activation of the distal portion of the MG muscle in standing balance and low-level contractions (Viera et al. 2011; Hodson-Tole et al. 2013). However, the presence of regional activation in the MG muscle has been questioned. The lack of clear anatomical or neuromuscular compartments within the muscle and the common distal tendon upon which all MG muscle fibres act seem at odds with regional concentrations of MUs (Wolf & Kim, 1997). Furthermore, Burke & Tsairis (1973) showed that MU territories extended extensively along the length of the cat MG muscle. Previous reports of regional muscle activity and small MU territory size in humans have primarily focused on surface EMG recordings, a technique with limited spatial resolution where large regional variations in the amplitude of recorded action potentials can occur due to differences in muscle fibre pennation angle and subcutaneous adipose tissue thickness as well as proximity to muscle innervation zones and tendinous regions (Roeleveld et al. 1997b; Farina et al. 2004; Rodriguez-Falces et al. 2013). Also, surface EMG electrode arrays cannot determine the size of MU territories when muscle fibres are parallel or nearly parallel to the skin surface, which is the case for the distal portion of the MG muscle (Viera et al. 2011). Fortunately, some of these limitations can be overcome with intramuscular EMG recordings given their more focal recording area and ability to capture activity from a limited number of nearby muscle fibres (Stålberg & Antoni, 1980; Stålberg & Falck, 1997; Harris et al. 2005). Recently, we investigated the discharge behaviour of triceps surae MUs in standing balance with intramuscular single-unit recordings (Héroux et al. 2014) and noted several instances where the same MU (time-locked spike trains) was recorded from intramuscular electrodes located ∼5–6 cm from one another along the length of the muscle. Although anecdotal, this observation contradicted previous reports of small MU territories and regional activation.
In the present study we sought to estimate the size and location of MG MU territories and determine whether they were grouped based on recruitment threshold or joint action. For the purpose of this study, MU territory is defined as the muscle region that contains muscle fibres from a single MU. We inserted a series of intramuscular fine-wire EMG electrodes at regular intervals across and along the MG muscle and computed spike-triggered averages of fine-wire EMG signals from the discharge times of single MUs. Based on our prior observations, our primary hypothesis was that muscle fibres from the majority of individual MUs active in light to moderate intensity contractions would be distributed over a territory (length and width) greater than 4 cm (reported by Vieira et al. 2011). However, muscle fibres in the distal portion of the MG muscle have the smallest pennation angle and therefore span a greater percentage of the total length of the muscle (Vieira et al. 2011), and thus we expected that distally located MUs would have greater longitudinal territories. In the absence of MUs that are spatially localized, we also hypothesized that the onset of EMG activity along the length of the muscle in ramp contractions would not reflect the previously reported higher concentration of low-threshold MUs in the distal portion of the muscle (Viera et al. 2011; Hodson-Tole et al. 2013). However, motoneurones do innervate muscle fibres in a size-dependent manner, with higher threshold MUs possessing more muscle fibres (Rafuse et al. 1997). Thus, we expected the size of MU territories (longitudinal and horizontal distance in cm) to be correlated with MU recruitment thresholds. Finally, given evidence in human bi-articular muscles of greater activation in the portion of the muscle closest to the mobilized joint (Miyamoto et al. 2012; Watanabe et al. 2013), we investigated the possibility that MUs with territories located close to the ankle joint or the knee joint are preferentially recruited based on joint action.
Methods
Eight healthy subjects (two females, six males; age range: 23–35 years) with no known history of neurological disease or injury participated in the study. Seven subjects participated in Experiment 1 and three subjects participated in Experiment 2, two of whom also participated in Experiment 1. The experimental protocol was explained and written informed consent was obtained prior to participation in the study. The study conformed to the Declaration of Helsinki and was approved by the University of British Columbia's Clinical Research Ethics Board.
Experimental set-up
Subject position and torque measurement
Subjects lay prone on a height adjustable bed with their ankle maleoli projecting 10 cm off the end of the bed. Their right foot was securely strapped to a footplate mounted on a six degrees-of-freedom force–torque sensor (MODEL 45E15, JR3 Inc., Woodland, CA, USA) with the centre of rotation of the ankle in line with the centre of the sensor. Their right knee was flexed 10 deg and their right ankle was in a neutral position (i.e. foot perpendicular to shank).
Multi-unit fine-wire recordings
Intramuscular multi-unit EMG signals were recorded in a bipolar configuration with fine-wire electrodes custom-made from two 0.05 mm insulated stainless steel wires (California Fine Wire, Grover Beach, CA, USA) wound together and inserted via a 1.5 inch 25 gauge hypodermic needle (EXEL International Medical Products, St Petersburg, FL, USA). The distal tip of the wires was folded back and cut to create one 1 mm barb and one 10 mm barb. The distal 5 mm of insulation was removed from the longer barb to favour multi-unit EMG recordings. The fine-wire electrodes used to study proximal–distal territories were inserted with the needle angled ∼45 deg medially whereas the electrodes used to study medial–lateral territories were inserted with the needle angled ∼45 deg distally. This method of needle insertion ensured the active recording surface of the fine-wire electrodes was located within a thin slice perpendicular to the investigated muscle axis (i.e. proximal–distal or medial–lateral), and that the distance between recording sites was not influenced by the length of the 10 mm barb. Prior to insertion, the longitudinal and horizontal limits of the right MG muscle were identified with ultrasound (SonoSite Micro Maxx, Bothell, WA, USA) and marked on the skin. A surface electrode (Ag/AgCl surface electrode, Ambu Blue, Sensor M, Ballerup, Denmark) was placed over the lateral malleolus of the right ankle and served as the ground for fine-wire electrode recordings.
Experiment 1 investigated longitudinal (i.e. proximal–distal) MU territory size (see Fig.1). Seven fine-wire electrodes were inserted to an intramuscular depth of ∼2 cm along the midline of the muscle under ultrasound guidance. The most distal and proximal electrodes were inserted 1.5–2.0 cm from the distal and proximal muscle limits; the other five electrodes were evenly spaced between these two electrodes. The distance between insertion sites was 2.0 cm in four subjects, 2.5 cm in two subjects and 3.0 cm in one subject. The difference in insertion site spacing was due to differences in MG muscle length, which ranged from 16 to 20 cm (proximal and distal tendons excluded).
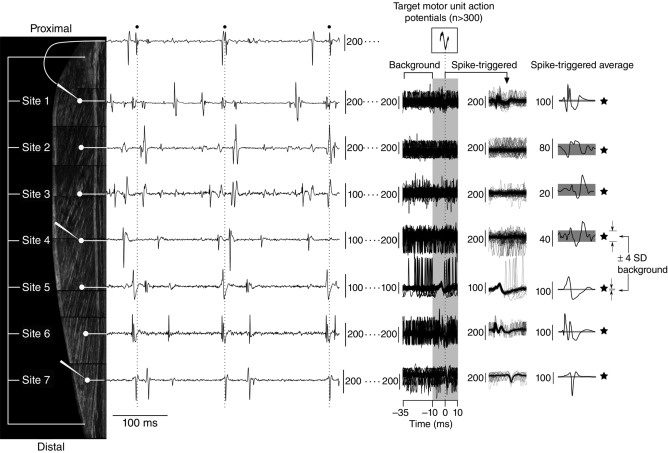
Figure 1. Experimental procedures and signal processing.
Seven multi-unit fine-wire electrodes (white circles) were inserted at even intervals along the medial gastrocnemius (MG) and three microelectrodes (white elongated triangles) were inserted as close as possible to the tips of the fine-wire electrodes located at Sites 1, 4 and 7. When subjects held a steady isometric contraction, the activity of several motor units (MUs) could be seen on the fine-wire recordings. At the same time the three microelectrodes recorded MU discharge activity (only signal from microelectrode at Site 1 included for clarity). The discharge times (black circles and dashed vertical lines) of selected MUs were used to spike-trigger each of the fine-wire recordings. In some instances it was possible to see potentials associated with the selected MU directly on the raw, overlayed multi-unit spike-triggered data (i.e. Sites 1, 5–7). In other instances the presence of an action potential associated with the selected MU was only visible in the spike-triggered average data (i.e. Sites 3 and 4). When a spike-triggered average had an action potential greater than four times the background signal (horizontal grey bands), it was considered that the selected MU had muscle fibres in close vicinity to the fine-wire recording electrode (black stars). Composite ultrasound image (subcutaneous tissue omitted) is from a subject with a 17 cm long MG muscle. The graphical location of fine-wire electrodes and microelectrodes was estimated from an insertion depth of 2 cm and an interelectrode distance of 2.5 cm. All vertical scale bars are in microvolts.
Experiment 2 investigated horizontal (i.e. medial–lateral) MU territory size. Five fine-wire electrodes were inserted under ultrasound guidance to an intramuscular depth of ∼1.0–1.5 cm along the medial–lateral axis half way down the muscle (see muscle inset to Fig.5). These fine-wire electrodes were not inserted as deeply as those in Experiment 1 because the lateral portion of the muscle was thinner and we wanted all the electrodes to be inserted at the same depth for a given subject. The most medial and lateral electrodes were inserted 1.0–1.5 cm from the medial and lateral borders of the MG muscle. The other three electrodes were evenly spaced between these two electrodes; the distance between insertion sites was 2.0 cm in all subjects (muscle width 10.5–11.0 cm).
Figure 5. Fine-wire EMG onset in plantar flexion ramp contractions.
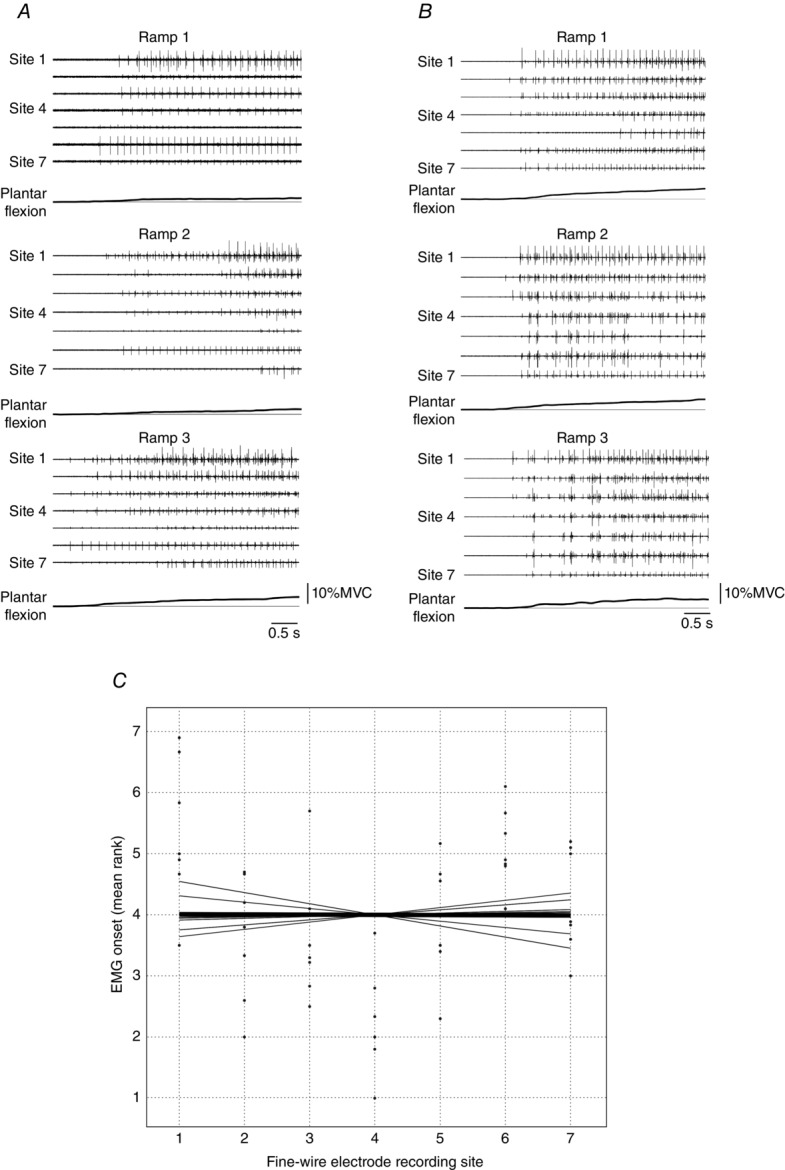
A and B, three ramp contractions from two different subjects. Fine-wire EMG activity recorded from Sites 1–7 and plantar flexor torque are presented. The onset of EMG activity across the seven sites was clearly related to the onset of torque generation, although there were some between-trial differences in the order of this activation. Note the similar number of motor units (MUs) that were recruited along the length of the muscle, which indicates that low threshold MUs were distributed throughout the MG muscle in these two subjects. Scale bars are omitted for clarity. C, onset times of the seven fine-wire electrodes were ranked for each ramp contraction and then averaged. The dots indicate the mean rank for each subject at the seven fine-wire recordings sites. The thin lines represent the line that was fitted to each subject's data and the thick line represents the group average. Across subjects, the slopes of these lines were not statistically different from zero (t6 = −0.094, P = 0.93).
Single-unit microelectrode recordings
Single MU activity was recorded from three insulated tungsten microelectrodes (0.2 mm diameter, 65 mm length, standard profile tip, Fred Haer Inc., Bowdoin, MA, USA) inserted to a depth of ∼2 cm (Experiment 1; see Fig.1) or ∼1.0–1.5 cm (Experiment 2). One microelectrode was inserted in the same location as each of the distal and proximal fine-wire electrodes (Experiment 1) or lateral and medial fine-wire electrodes (Experiment 2). The third microelectrode was inserted in the same location as the middle fine-wire electrode (Experiments 1 and 2). A fourth uninsulated tungsten microelectrode was inserted under the skin ∼4 cm distal to the fibular head and served as the reference. All fine-wire electrodes and microelectrodes were steam sterilized prior to use (PVdry2 Barnstead-Harvey, Dubuque, IA, USA).
Fine-wire and microelectrode signals were filtered (band-pass 30–6000 Hz) and amplified (×5000–10000) with a high impedance GRASS 15CT amplifier (Astromed Inc., West Warwick, RI, USA) and then digitized at 15 kHz with a 16-bit CED DAQ-card and Spike2 software (Cambridge Electronics Design, Cambridge, UK). Torque sensor signals were digitized at 500 Hz with the same system.
Experimental procedures
Subjects performed three maximal voluntary isometric contractions (MVCs) for ankle plantar flexion and knee flexion. Verbal encouragement was provided along with a 2 min rest period between contractions. The fine-wire electrodes and the microelectrodes were then inserted. Trials began with a 5 s period of relaxation – used for torque and fine-wire recording background amplitude measurements – followed by an ankle plantar flexion contraction that ramped slowly until regular discharge activity from the first recruited MU(s) appeared in the microelectrode signals. Once this target contraction intensity was identified, subjects performed an additional three ramp contractions followed by a 60–90 s isometric contraction at the target intensity. After this sequence of contractions, subjects performed a higher intensity ramp contraction until one or more new MUs were recruited. Three ramp contractions and a 60–90 s isometric contraction were then performed at this new target intensity. The search for higher threshold MUs was repeated until it was no longer possible to visually distinguish newly recruited MUs. Four to six contraction intensities were assessed per subject and these generally ranged between 1 and 30% MVC, with a few MUs recorded between 30 and 40% MVC. Across subjects, the mean rate for the plantar flexor ramp contraction was 1.06 Nm s−1 (95% confidence interval 0.93–1.19), or ∼1.9% MVC s–1. Next, the entire process was repeated for knee flexion with three ramp and isometric contractions performed for each previously unidentified MU. Although not always successful, subjects were instructed to keep ankle plantar flexion torque below 5% MVC in the knee flexion trials. For higher intensity ramp contractions, this necessarily involved activation of the ankle dorsiflexors. EMG was not recorded from these muscles. The three microelectrodes were then moved to slightly different positions (movement distance ∼4–5 mm) near the location of their respective fine-wire recording sites to record action potentials from new MUs and the process described above was repeated (i.e. ramp and isometric contractions at increasing contraction intensities for both ankle plantar flexion and knee flexion). Microelectrodes were moved 4–5 times per subject to record activity from a minimum of 20 MUs per subject across both ankle plantar flexion and knee flexion contractions (mean = 26 MUs per subject). A 3–5 min rest period was provided between each series of ramp and isometric contractions to avoid muscle fatigue.
Data analysis
Torque and multi-unit fine-wire EMG data were processed with Matlab (Mathworks, Natick, MA, USA). Torque data were low-pass filtered (40 Hz, zero-lag, 8th order Butterworth filter) and mean torque from the 5 s background period was removed from each trial. Fine-wire multi-unit EMG data were band-pass filtered (30–2000 Hz, zero-lag, 8th order Butterworth filter).
MU territory
For each trial, MU action potentials recorded from the microelectrodes were extracted based on their size, shape and timing using Spike2 software (Cambridge Electronic Design). MU action potential shape and instantaneous discharge rate plots were used to manually review spike trains to include actions potentials not properly categorized by the software. This also resolved instances where action potentials from two or more MUs were superimposed and were thus uncategorized by the software. The summation of MU action potentials leads to EMG signal cancellation, which reduces the size of spike-triggered averages (Day & Hulliger, 2001; Keenan et al. 2006; Farina et al. 2008). Thus, only MUs with a minimum of 300 action potentials in the 60–90 s isometric contractions were exported to Matlab to compute fine-wire EMG spike-triggered averages (see Fig.1). To determine whether EMG activity from the target MU was present in the signal of a fine-wire electrode, the standard deviation (SD) of spike-triggered averages activity was calculated in a 25 ms window prior to the target MU spike time (−35 ms to −10 ms). Based on the analyses of pilot data, a threshold value of ± 4SD of the baseline was used to determine whether an action potential associated with the target MU was present in the spike-triggered average at a given fine-wire site.
Action potentials from the same MU could be recorded from the same microelectrode after the electrode was moved or from different microelectrodes if the MU spanned a large portion of the muscle. In both cases, action potential shapes would differ because the microelectrode(s) would record activity from a different intramuscular location. However, fine-wire electrode location did not change over the course of the experiment, which allowed us to use the shape and pattern of spike-triggered averages (from the multi-unit recordings) to identify when the same MU was recorded (Fig.2A). When this occurred, only the first microelectrode occurrence of the MU was used for analysis.
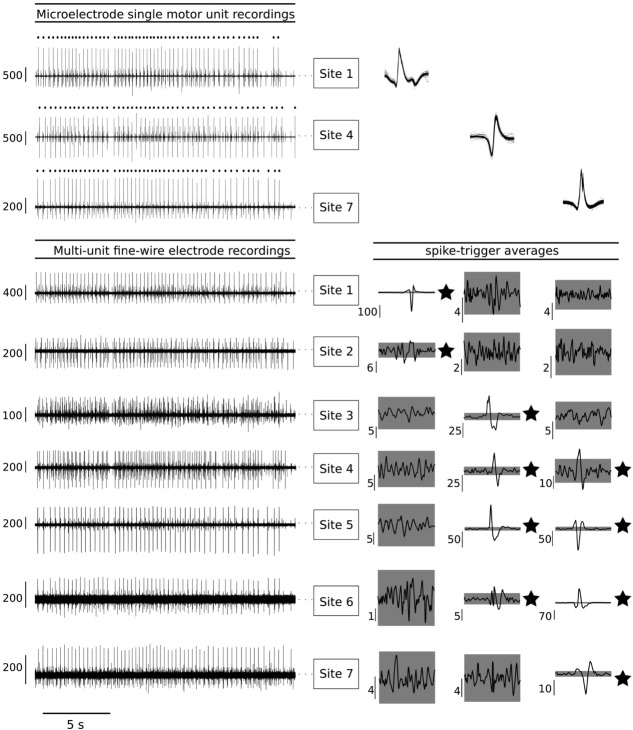
Figure 2. Processing of spike-triggered averages and determination of motor unit distribution size.
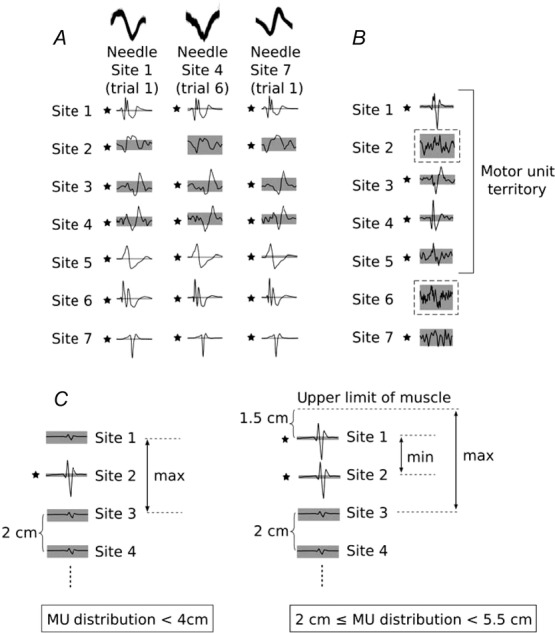
A, the same motor unit (MU) was recorded from all three intramuscular microelectrodes. Note the difference in action potential shape between microelectrode recordings (overlayed top traces). When we consider the spike-triggered averages from the seven fine-wire recording sites, it becomes clear that this is the same MU. B, two gaps (dashed boxes) where action potentials did not cross the ± 4SD threshold are present amongst other spike-triggered averages that crossed threshold. The gap at Site 2 is situated between two spike-triggered averages with clear action potentials associated with the target MU that crossed threshold. The gap at Site 6 is situated between a spike-triggered average with a clear action potential that crossed threshold (Site 5) and another spike-triggered average that crossed threshold, but did not have a clear action potential (Site 7). In this instance, the MU distribution was determined as Site 1 to Site 5. C, schematic representation of an MU with spike-triggered averages that crossed threshold at a single fine-wire electrode site (left) and an MU with spike-triggered averages that crossed threshold at two fine-wire electrode sites (right). Given an interelectrode distance of 2 cm and a distance between Site 1 and the edge of muscle, we can identify the maximum distribution for the MU on the left and the range for the MU on the right. The grey area indicates ± 4SD of baseline; *an action potential crossed the threshold value.
Figure 3. Proximo-distal distribution of muscle fibres from three medial gastrocnemius motor units.
Data from a subject in whom a single motor unit (MU) was identified and decomposed for each microelectrode recording site. Discharge times from these MUs were used to compute spike-triggered averages for fine-wire electrode signals that were simultaneously recorded. Spike-triggered averages of the MU recorded with the microelectrode at Site 1 (left column) show that its muscle fibres were located in a relatively small muscle area (Sites 1 and 2). Inspection of the spike-triggered averages for the MUs recorded from the microelectrodes at Sites 4 and 7 (middle and right columns) reveal that these MUs have spatial distributions that spanned four fine-wire recording sites. The interelectrode distance was 2.5 cm for this subject and Sites 1 and 7 were 2.0 cm from the edge of the muscle. Thus, the estimated distribution for the MUs recorded from the three microelectrodes are: Site 1 = 2.5–7 5 cm; Site 3 = 7.5–12.5 cm; Site 7 = 7.5–10.0 cm. All vertical scale bars are in microvolts.
The relative size of MU territories was defined as the number of consecutive fine-wire recording sites with spike-triggered averages that reached the ± 4SD threshold. This relative threshold criterion was based on pilot testing and analyses establishing that the quality and amplitude of fine-wire EMG signals were influenced by factors difficult to control (e.g. breakdown of the wire insulation during insertion, differences in fine-wire electrode fabrication, final position of recording surfaces relative to one another, slight bleeding when inserting electrodes). These factors and their effect on electrode impedance meant that the detection volume would vary between electrodes, and the absolute amplitude of our fine-wire EMG signals would also vary between electrodes – even if the arrangement of the active muscle fibres about the electrodes were identical. For these reasons, we favoured relative amplitude measures (e.g. ± 4SD) over absolute amplitude measures. We noted a few instances (n = 9) where spike-triggered averages reached threshold, but not from consecutive sites. This is similar to the silent areas observed when indwelling scanning EMG electrodes are used to measure MU territory (Stålberg & Falck, 1997). In these instances, if one of the spike-triggered averages on either side of the ‘gap’ just reached the threshold and the action potential was not clearly associated with the target MU, the smallest number of consecutive sites was used to estimate territory size (n = 5; see Fig.2B bottom for an example). If the spike-triggered averages on either side of the ‘gap’ were large and clearly associated with the target MU, the ‘gap’ was included in the MU territory (n = 4; see Fig.2B top for an example).
The absolute size of MU territories was estimated based on the distance between fine-wire electrodes, the distance between the most extreme recording sites and the edge of the muscle, and the relative size of MU territories. Only the maximum territory size could be estimated when the target MU was present at a single site (Fig.2C, left). In theory, the minimal territory size for these MUs could be as little as a few millimetres; however, this value could not be estimated with the current approach. Both the minimum and the maximum territory size could be estimated when the target MU was present at two or more sites (Fig.2C, right). Absolute MU territory size estimates are presented in Table 1.
Table 1.
Estimates of absolute motor unit (MU) territory size based on the number of multi-unit spike-triggered average sites that crossed the threshold, the distance between insertion sites, and the distance between the most extreme recording sites and the edge of the muscle
| 1 Site | 2 Sites | 3 Sites | 4 Sites | 5 Sites | 6 Sites | 7 Sites | |
|---|---|---|---|---|---|---|---|
| Longitudinal MU territory (cm)* | MU < 4.2 | 2.1 ≤ MU < 6.1 | 4.8 ≤ MU < 9.1 | 6.9 ≤ MU < 11.3 | 9.3 ≤ MU < 13.8 | 12.0 ≤ MU < 14.3 | 13.6 ≤ MU < 17.9† |
| Horizontal MU territory (cm)‡ | MU < 3.5 | 2.0 ≤ MU < 5.8 | 4.0 ≤ MU < 7.3 | 6.0 ≤ MU < 9.4 | 8.0 ≤ MU < 10.8§ |
Distance between insertion sites ranged from 2 to 3 cm; insertion sites 1 and 7 were 1.5–2.0 cm from the edge of the muscle; muscle length ranged from 16 to 20 cm. Territory estimates are weighted averages based on the number of MUs from each subject that spanned the various number of sites and whether the territory included a site at the edge of the muscle.
This value corresponds to mean medial gastrocnemius muscle length across the seven subjects.
Distance between insertion sites was 2.0 cm in all three subjects; insertion sites A and E were 1.0–1.5 cm from the edge of the muscle; muscle width ranged from 10.5 to 11.0 cm. Territory estimates are weighted averages based on the number of MUs from each subject that spanned the various number of sites and whether the territory included a site at the edge of the muscle.
This value corresponds to mean medial gastrocnemius muscle width across the three subjects.
Pennation angle of muscle fibres
To identify potential differences in MU territory size related to muscle architecture, a series of ultrasound images were recorded along the longitudinal axis of the MG muscle where the fine-wire electrodes were inserted. These images were concatenated (Inkscape, v.0.48.3.1) and pennation angles were measured (ImageJ, v.1.48) for the most discernible muscle fascicle that crossed the recording area of Sites 1 and 7. For two of the measures at Site 1 it was not possible to obtain pennation angles relative to the superior aponeurosis, and thus measures were made closer to the location of Site 2. Muscle fascicle pennation angle was defined as the mean angle measured at the fascicle insertion into both the superficial and the deep aponeuroses (Narici et al. 1996).
MU recruitment thresholds
MU discharge times from isometric ramp contractions were used to determine recruitment thresholds. The torque at which each target MU was first recruited (three consecutive MU action potentials with interspike intervals < 250 ms) was identified and normalized to MVC (Héroux et al. 2014). If the target MU was recorded in multiple ankle plantar flexion trials, the first trial was used to determine its recruitment threshold. For approximately half the MUs recorded in knee flexion trials, subjects generated large torques to recruit MG MUs (>50% MVC); however, torque levels could subsequently be reduced to 10–40% MVC without the target MU being derecruited. These high intensity contractions were accompanied by plantar flexor torques above 5% MVC. Given the uncertainty associated with knee flexion MU recruitment thresholds, only ankle plantar flexion recruitment thresholds were analysed.
MU onset from longitudinal fine-wire electrodes in ramp contractions
The onset time of fine-wire EMG activity was determined for all ramp contractions from Experiment 1. Onset was defined as the time when EMG amplitude exceeded ± 2SD of the background EMG activity at each respective site. It was not possible to compare and average onset times across ramp contractions because the rate of torque production was not strictly controlled (mean 1.06 Nm s−1, 95% CI 0.93–1.19) and this resulted in skewed onset time distributions. Therefore, onset times from each ramp contraction were ranked to reflect the relative order of EMG onset activity along the length of the muscle. Across the seven subjects, a total of 12 ramp contractions were excluded because they were excessively fast (> 2 Nm s–1) and associated with the simultaneous onset of EMG activity in all fine-wire recording sites. For each subject, rank values from the retained ramp contractions were averaged for each fine-wire recording site to provide a mean rank onset value.
Statistical analysis
Descriptive parametric statistics were calculated to summarize group results. To determine whether there were regional differences in longitudinal MU territory size, a chi-square test was performed on data classified based on two categorical variables, region and size, each of which had two levels. Region was divided into the number of MUs recorded with the proximal microelectrode (Site 1) and those that were recorded with the distal microelectrode (Site 7), while size was divided into the number of MUs that spanned at most half the muscle (Sites 1–4) and those that spanned more than half the muscle (Sites 5–7). One MU was recorded from the microelectrodes at Site 1 and Site 7 and was excluded from this analysis. To determine whether regional differences in MU territory size were accompanied by regional differences in muscle fibre pennation angle, a paired t-test was performed between the pennation angle of muscle fascicles that crossed the recording region of Sites 1 and 7.
A Spearman's rank correlation was performed to quantify the relationship between the relative size of MU territories (i.e. number of consecutive sites) and MU recruitment thresholds. The territory size of MUs that spanned a single site could not be defined by a range (see Table 1) and therefore were not included in the final rank correlation; however, these data are included in the relevant figures (see Figs4C and 6C) to allow for a comparison. The final rank correlation was carried out only for MUs that had ankle plantar flexion recruitment thresholds (Experiment 1, n = 50 MUs; Experiment 2, n = 23 MUs). To determine if there was a distal-to-proximal (or proximal-to-distal) gradient of muscle activation during ramp contraction across subjects, a regression line was first fit to the mean rank onset values associated with the seven fine-wire recording sites of each subject. A one-sample t-test was then used to determine whether the slopes of these regression lines were significantly different from zero. If slope values were significantly different from zero, a mean slope across subjects > 0 would indicate the presence of a proximal-to-distal gradient whereas a mean slope < 0 would indicate the presence of a distal-to-proximal gradient. The R statistical language (version 3.2.1) was used to perform the statistical analyses. The level of statistical significance was set at α = 0.05.
Figure 4. Proximo-distal distribution of motor unit muscle fibres in the medial gastrocnemius and its relationship with recruitment thresholds.
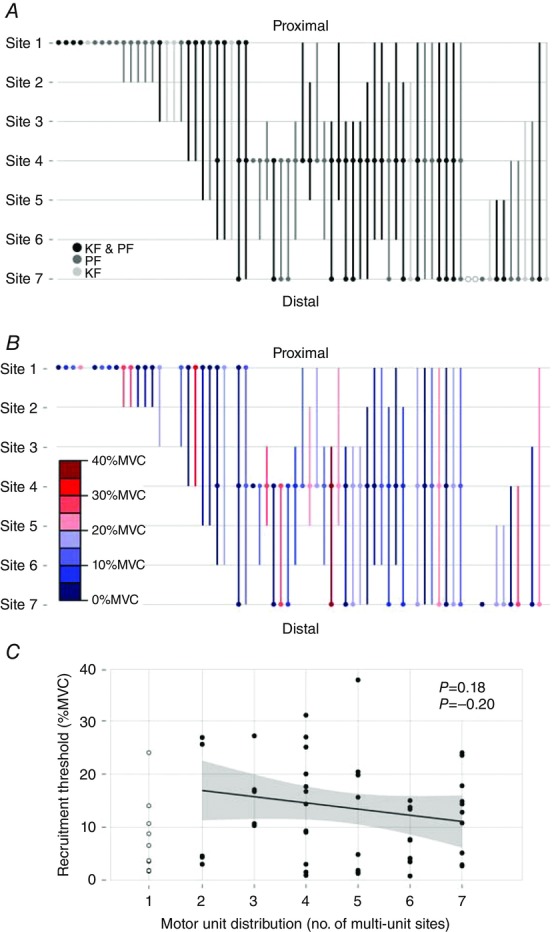
A, spatial distribution of the 69 motor units (MUs) from Experiment 1 is depicted with circles and vertical lines. Open circles (○) indicate MUs that were recorded from the microelectrode at a given site, but were not present on the spike-triggered average at that same site. Shades of gray indicate whether the MU was recorded in an ankle plantar flexion (PF) trial, a knee flexion (KF) trial or both (KF & PF). Filled circles (•) indicate MUs that were recorded from the microelectrode and the fine-wire electrode (i.e. spike-triggered average) at a given site. Filled circles only appear at Sites 1, 4 and 7 because these are where the microelectrodes were located. Vertical lines indicate the spatial distribution of an MU on the spike-triggered averaged traces. B, the spatial distribution of the 61 MUs from Experiment 1 that were recorded in a plantar flexor trial. Colour indicates the normalized plantar flexion torque recruitment thresholds (in 5% MVC bins) for each MU. C, There was no relationship between the plantar flexor torque recruitment threshold and spatial distribution of MUs. Line and shaded area represent linear regression ± 95% Confidence Intervals (CI); MUs present at one multi-unit site were not included in this analysis, but are shown for comparison.
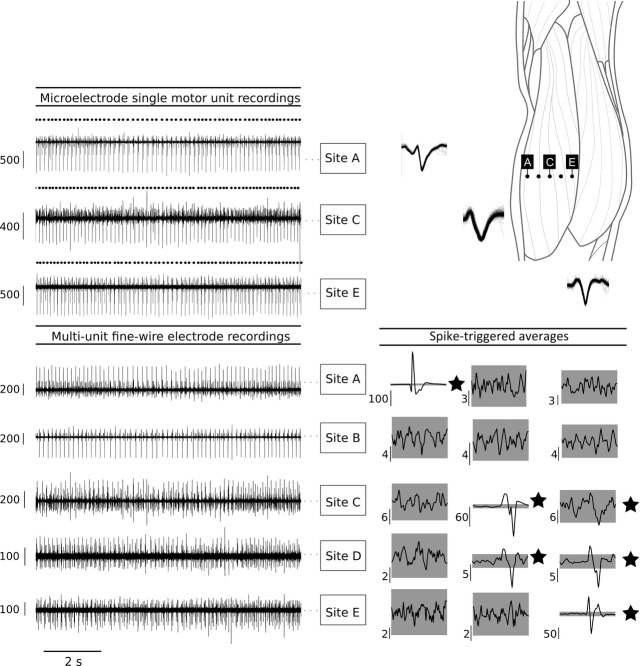
Figure 6. Medio-lateral distribution of muscle fibres from three medial gastrocnemius motor units.
Data from a subject in whom a single motor unit (MU) was identified and decomposed for each microelectrode recording site. Discharge times from these MUs were used to compute spike-triggered averages for fine-wire electrode signals that were simultaneously recorded. Spike-triggered averages of the MU recorded with the microelectrode at Site A (leftmost column) show that its muscle fibres were located in a relatively small muscle area. Inspection of the spike-triggered averages for the MUs recorded from the microelectrodes at Sites C and E (middle and rightmost columns) reveal that these MUs have spatial distributions that span two and three recording Sites. The interelectrode distance was 2.0 cm for this subject. Sites A and E were 1.0 cm from the edge of the muscle. Therefore, the estimated distribution for the MU recorded from the three microelectrodes are: Site A = < 3.0 cm; Site C = 2.0–6.0 cm; Site E = 4.0–7.0 cm. All vertical scale bars are in microvolts.
Results
Experiment 1: longitudinal MU territory
A total of 169 MUs were recorded from the microelectrodes and used to calculate spike-triggered averages. After the removal of MUs recorded from the same needle in separate trials and MUs recorded from different microelectrodes in the same or different trials, 69 MUs were retained for further analysis (5–17 MUs per subject). Twenty-nine MUs were recorded in plantar flexion trials, 8 MUs were recorded in knee flexion trials and 32 MUs were recorded in both ankle plantar flexion and knee flexion trials.
Across the sample of 69 MUs, longitudinal territories ranged from one site to all seven sites. Only 5 MUs were present at two fine-wire recordings sites (2.1–6.1 cm) and another 11 were present at a single site (< 4.2 cm). Comparatively, 32 MUs had territories that spanned at least half the length of the MG muscle (i.e. ≥ 6.9 cm), 11 of which spanned all seven recording sites (13.6–17.9 cm; Fig.4A, B). The proportion of MUs with short (spanning 1–4 sites) versus long (spanning 5–7 sites) territories was statistically different between MUs recorded from the proximal and distal microelectrodes (χ21 = 8.85, P < 0.01). Specifically, the proximal microelectrode recorded activity from 20 MUs that spanned half the muscle or less and 6 MUs that spanned more than half the muscle. In comparison, the distal microelectrode recorded activity from 8 MUs that spanned half the muscle or less compared to 15 MUs that spanned more than half the muscle. The higher proportion of distal MUs with large territories was accompanied by significantly smaller pennation angles for muscle fibres located in this region of the MG muscle (mean 14.3 deg, 95% CI 12.0–15.6) compared to muscle fibres located in the middle region of the muscle (mean 26.9 deg, 95% CI 23.3–30.5, t6 = 10.6, P < 0.0001) (see ultrasound images in Supporting information, Fig. S1).
MUs recorded solely in ankle plantar flexion or knee flexion trials had a wide range of territory sizes that were located throughout the muscle. There was no evidence that muscle fibres of MUs were clustered near the knee joint (proximal sites) or ankle joint (distal sites) based on joint action (Fig.4A).
The recruitment threshold of MUs recorded from plantar flexor contraction trials ranged from 2.0 to 38.5 % MVC. There was no evidence that MUs were preferentially located in either the proximal or the distal portion of the muscle based on their recruitment threshold (Fig.4B), nor was there a relationship between recruitment threshold values and the size of MU longitudinal territories (Pearson's rho = –0.20, P = 0.18, Fig.4C). There was also no proximal-to-distal or distal-to-proximal gradient of muscle activation in ramp contractions across subjects. The lines fitted to each subject's mean rank onset values calculated for each fine-wire recording site had a slope of −0.004 (range –0.101 to 0.093) and were not statistically different from zero (t6 = –0.094, P = 0.93; Fig.5).
Experiment 2: horizontal MU territory
A total of 76 MUs were recorded from the microelectrodes and used to calculate spike-triggered averages. After removal of MUs recorded from the same needle in separate trials and MUs recorded from different microelectrodes in the same or different trials, a total of 36 MUs (10–15 per subject) were retained. Fifteen MUs were recorded in plantar flexion trials, 8 MUs were recorded in knee flexion trials, and 13 MUs were recorded in both plantar flexion and knee flexion trials.
Across the entire sample of 36 MUs, horizontal territories ranged from a single multi-unit site to all five sites. Five MUs were limited to a single multi-unit site (< 3.5 cm). In contrast, there were 24 MUs with horizontal territories that spanned at least half the width of muscle (i.e. ≥ 4.0 cm), 13 of which spanned all five multi-unit recording sites (8.0–10.8 cm, Fig.6A, B).
Figure 7. Medio-lateral distribution of motor unit muscle fibres in the medial gastrocnemius and its relationship with recruitment thresholds.
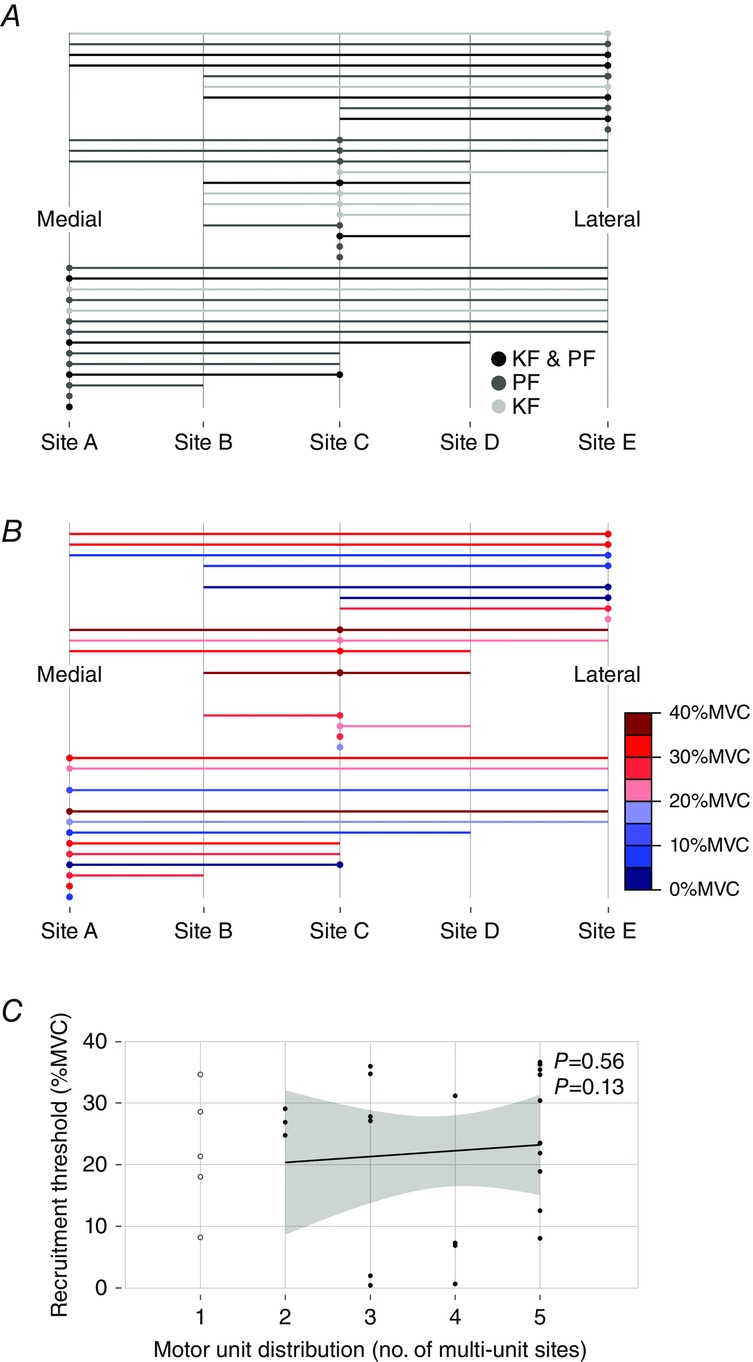
A, the spatial distribution of the 35 motor units (MUs) from Experiment 2 is depicted with filled circles (•) and vertical lines. Shades of gray indicate whether an MU was recorded in an ankle plantar flexion (PF) trial, a knee flexion (KF) trial or both (KF & PF). Filled circles (•) indicate MUs that were recorded from the microelectrode and the fine-wire electrode (i.e. spike-triggered average) at a given site. Filled circles only appear at Sites A, C and E because these are where the microelectrodes were located. Vertical lines indicate the spatial distribution of an MU on the spike-triggered average traces. B, the spatial distribution of the 28 MUs from Experiment 2 that were recorded in a plantar flexor trial. Colour indicates the normalized plantar flexion torque recruitment thresholds (in 5% MVC bins) for each MU. C, there was no relationship between the plantar flexor torque recruitment threshold and spatial distribution of MUs. Line and shaded area represent linear regression ± 95% Cl; MUs present at one multi-unit site were not included in this analysis, but are shown for comparison.
MUs recorded solely in ankle plantar flexion or knee flexion trials had variable territory sizes and were distributed throughout the muscle. The recruitment threshold of MUs recorded from plantar flexor contraction trials ranged from 1 to 37% MVC. There was no evidence that MUs were preferentially located in either the medial or the lateral portion of the muscle based on their recruitment threshold (Fig.4B), nor was there a relationship between recruitment threshold values and the size of horizontal MU territories (ρ = 0.13, P = 0.56, Fig.6C).
Discussion
Based on our prior incidental observations of MU territories that spanned at least 5–6 cm in the triceps surae muscle (Héroux et al. 2014), we hypothesized here that the longitudinal territory of muscle fibres of most MUs in the human MG muscle would be greater than 4 cm. In support of these earlier observations, we measured activity in many instances from the same MG MUs in microelectrodes spaced 8–10 cm (longitudinal) or 5.0–5.5 cm (horizontal) apart. Furthermore, we noted that 43% of the MUs from Experiment 1 and 50% of the MUs from Experiment 2 spanned more than half the length (8–10 cm) or width (5.0–5.5 cm) of the muscle. The large territory of MUs in the human MG muscle was further highlighted by 15% of MUs spanning the full length of the muscle and 36% of MUs spanning the full width of the muscle. These results are in line with findings in the cat where MG MUs span ∼60% of the muscle's length (Burke & Tsairis, 1973). Thus, rather than being spatially localized, results from the present study indicate that low to medium threshold MUs in the human MG muscle have territories that vary widely in size, from a few centimetres to the full length of the muscle.
In contrast to our current results, several studies have reported regional muscle activity in the human MG muscle (Kinugasa et al. 2005, 2011; Staudenmann et al. 2009; Wakeling, 2009; Vieira et al. 2010, 2011; Hodson-Tole et al. 2013; von Tscharner et al. 2014). This type of activity is only possible if MUs possess small spatial territories. In support of this point, Vieira et al. (2011) used an array of surface EMG electrodes to demonstrate that MUs in the human MG muscle span on average 2.5 cm of the muscle length, with a maximum size of 4 cm. These values are considerably smaller than those found in the present study and we believe this reflects important differences between intramuscular and surface EMG recordings. For both intramuscular and surface EMG recordings there is a decrease in signal amplitude as the distance between the recording surface and the active muscle fibres increases (Erkstedt & Stålberg 1973; Roeleveld et al. 1997a,b). However, with surface recordings the electrical signal from muscle fibres must travel through several tissue layers that have different conductivity, which distorts and reduces the amplitude of muscle-related signals (Le & Gevins 1993; Roeleveld et al. 1997a,b). Using surface electrodes, the amplitude of recorded MU action potentials also depends on the density of associated muscle fibres located beneath the electrodes (Buchtal & Schmalbruch 1980; Stålberg & Antoni 1980). This dependency may limit the ability of surface recordings to adequately capture the full range of MU territories because muscle fibres from a MU are arranged in small clusters that decrease in density in the outer region of the territory (Bodine et al 1988; Bodine-Fowler et al. 1990; Edström & Kugelberg, 1968a,b ). Other factors can also lead to regional differences in surface EMG recordings. For example, Rodriguez-Falces et al. (2013) concluded that recordings made close to the innervation zone or the tendinous region of a muscle have variable amplitudes that could account for some of the regional variation reported in surface EMG studies. Regional differences in subcutaneous adipose tissue thickness and muscle pennation angle can also cause similar regional variations in surface recordings (Roeleveld et al 1997b). One or more of these factors may explain the varied patterns of regional activation reported in the MG muscle (Staudenmann et al. 2009; Viera et al. 2010; Hodson-Tole et al. 2013), the lack of regional activation observed in the human lateral gastrocnemius muscle despite this muscle's three distinct compartments (Segal et al. 1991; Wolf et al. 1998; Kinugasa et al. 2005, 2011; Viera et al. 2010), and the failure of regional muscle activity to follow the anatomical borders between the medial gastrocnemius, lateral gastrocnemius and soleus muscles (Staudenmann et al. 2009).
Our finding of smaller pennation angles and thus proportionally longer muscle fibres in the distal portion of the MG muscle is consistent with prior work (Narici et al. 1996; Vieira et al. 2011). In line with our initial hypothesis, MUs located in the distal portion of the muscle had larger longitudinal territories, which may be related to these regional differences in muscle architecture. In the present study fine-wire electrodes with relatively small recording areas were inserted to the same approximate depth to record activity from distinct muscle fibres and resulted in spike-triggered average onset times that were nearly identical across all recording sites. This pattern of onset times reflects the propagation of action potentials along motorneurone axons (40–70 m s–1; Loeb et al. 1987; Troni et al. 2010) rather than muscle fibres (∼4 m s–1; Andreassen & Arendt-Nielsen, 1987) and provides additional validation of our experimental approach to quantify the spatial territory of MUs in the MG muscle. In contrast, surface EMG recordings in the distal portion of the MG muscle are associated with spike-triggered average onset times that reflect the propagation of action potentials along muscle fibres, which limits the interpretability of measures of MU territory size based on these spike-triggered averages in this portion of the muscle (Vieira et al 2011). Thus, the use of intramuscular recordings has shown that MUs in the distal portion of the MG muscle have larger longitudinal territories and this appears to be due to the smaller pennation angle of muscle fibres in this region of the muscle.
In the present study, the size and location of horizontal and longitudinal spatial territories were determined for MUs with recruitment thresholds of 1–40% MVC and we found no evidence that MUs were physically grouped based on their recruitment thresholds. We also considered the order in which EMG activity first appears on fine-wire recordings inserted along the length of the muscle and found no evidence of either a proximal-to-distal or distal-to-proximal gradient of activation in slow ankle plantar flexion ramp contractions. This lack of regional difference in part reflects the large territory size of the majority of MUs studied here as this limits the possibility of having small clusters of MUs with similar characteristics. Furthermore, MU territory size was not correlated with MU recruitment thresholds, which indicates similar spatial territories for smaller motoneurones that innervate fewer muscle fibres and larger motoneurones that innervate a greater number of muscle fibres (Rafuse et al. 1997). Overall, the present study provides strong evidence that low to moderate threshold MUs in the human MG muscle possess similarly sized spatial territories that are not grouped together based on recruitment thresholds.
MUs that were preferentially activated by knee flexion or ankle plantar flexion contractions were not grouped in the proximal or distal regions of the MG muscle. Similar to our results on MU recruitment thresholds, the lack of joint-specific specialization may reflect the relatively large longitudinal territories of MG MUs, which directly limits the possibility of having functionally different muscle sub-regions. Furthermore, muscle fibres in the human MG muscle attach to the superior and inferior aponeuroses, which transmit force through proximal and distal tendons. In the absence of a separate bony attachment that would confer a mechanical advantage to muscle fibres in the proximal (knee flexion) or distal (ankle plantar flexion) portion of the MG muscle, there is no benefit for MUs with small spatial territories to be grouped in close proximity to either joint given that MU activity will generate tension at the proximal and distal muscle insertions. Although there is evidence from surface EMG studies that voluntary hip flexion activates the portion of the rectus femoris muscle closest to the hip joint, whereas voluntary knee extension seems to activate the muscle more globally (Miyamoto et al. 2012; Watanabe et al. 2013), the proximal portion of this muscle is quite narrow and voluntary hip flexion will necessarily activate other hip flexor muscles that can cause ‘cross-talk’ in surface EMG recordings (Farina et al. 2004). In humans, there is at present no clear evidence that muscle fibres in lower-extremity muscles are organized and activated to favour torque generation at a specific joint.
Compared to surface electrodes, intramuscular electrodes have small, higher impedance recording surfaces and MU action potentials recorded by intramuscular electrodes reflect the activity of only a few muscle fibres located close to this recording surface (Ekstedt & Stålberg, 1973; Stålberg & Falk 1997). While the focal nature of intramuscular recordings was key to our ability to measure the size of MU territories, it also created some limitations. As was presented in Fig.2 and Table 1, our measure of MU territory size is associated with some uncertainty because its resolution depends on the distance between fine-wire electrodes rather than the resolution of the fine-wires themselves. Also, nine MUs had ‘gaps’ in their spike-triggered averages and another two MUs were recorded by the microelectrode at Site 7, but were not associated with spike-triggered average activity (open circles, Fig.4A). While these results may in part reflect the uneven territory of MU muscle fibres, they highlight a level of uncertainty when action potentials are not present in spike-triggered averages that could lead to an underestimation of MU territory size. Finally, it is experimentally difficult to record and reliably discriminate individual MU action potentials in high contraction intensities. Thus, the results and conclusions from the present study are limited to MUs with recruitment thresholds below ∼40% MVC.
The MG muscle transmits force through a common distal tendon (Windhorst, 1989) and activation of its muscle fibres leads to a common mechanical action (Nichols et al. 1993; Vieira et al. 2013). Analysis of the innervation pattern and homogeneous unipennate organization of its muscles fibres has led to the conclusion that localized activation of the MG muscle is unlikely (Wolf & Kim 1997). In line with these observations and similar to what has been reported in the cat (Burke & Tsairis, 1973), we found that many MUs in the human MG muscle have territories that extend over a large portion of the muscle, with no evidence that these MUs are grouped by recruitment threshold or joint action. Together, these results lead us to propose that the human MG muscle is uniformly activated by the CNS and, more cautiously, that previous reports of regional muscle activation may reflect methodological limitations and between- and within-subject variability.
Glossary
- CI
confidence interval
- EMG
electromyography
- MG
medial gastrocnemius
- MU
motor unit
- MVC
maximal voluntary isometric contraction
Additional information
Competing interests
GPS owns shares in a forensic consulting company, and both he and the company may derive benefit from being associated with this work.
Author contributions
M.E.H., H.J.B. and J.S.B. conceived and designed the experiment. H.J.B. and M.E.H. undertook the experimental work and analysis with guidance/input from the other authors. M.E.H. prepared the initial draft of the paper. All authors reviewed, contributed to and approved the final manuscript.
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC: J.S.B., G.P.S. and J.T.I.). J.S.B. received support from the Canadian Chiropractic Research Foundation and the Michael Smith Foundation for Health Research (MSFHR). M.E.H. received support from an MSFHR post-doctoral fellowship and the Canadian Institute for Health Research (CIHR) and partial salary funding from an NSERC Discovery Acceleration Grant to J.T.I. H.J.B. was supported by an NSERC Industrial Postgraduate Scholarship (partnered with MEA Forensic Engineers & Scientists). The ultrasound was purchased with a Research Tools and Instruments grant from NSERC (J.T.I., M. G. Carpenter & J.S.B.).
Supporting information
The following supporting information is available in the online version of this article.
Figure S1 Sagital ultrasound images of the right medial gastrocnemius muscle of the 7 subjects from Experiment 1. For each subject, several images were recorded and concatenated to obtain an composite image of the entire muscle length.
References
- Andreassen S, Arendt-Nielsen L. Muscle fibre conduction velocity in motor units of the human anterior tibial muscle: a new size principle parameter. J Physiol. 1987;391:561–571. doi: 10.1113/jphysiol.1987.sp016756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine-Fowler S, Garfinkel A, Roy RR, Edgerton VR. Spatial territory of muscle fibers within the territory of a motor unit. Muscle Nerve. 1990;13:1133–1145. doi: 10.1002/mus.880131208. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Garfinkel A, Roy RR, Edgerton VR. Spatial distribution of motor unit fibers in the cat soleus and tibialis anterior muscles: local interactions. J Neurosci. 1988;8:2142–2152. doi: 10.1523/JNEUROSCI.08-06-02142.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchtal F, Guld C, Rosenfalk F. Multielectrode study of the territory of a motor unit. Acta Physiol Scand. 1957a;39:83–104. doi: 10.1111/j.1748-1716.1957.tb01411.x. [DOI] [PubMed] [Google Scholar]
- Buchtal F, Guld C, Rosenfalk P. Volume conduction of the spike of the motor unit potential investigated with a new type of multielectrode. Acta Physiol Scand. 1957b;38:331–354. doi: 10.1111/j.1748-1716.1957.tb01396.x. [DOI] [PubMed] [Google Scholar]
- Buchtal F, Schmalbruch H. Motor unit of mammalian muscle. Physiol Rev. 1980;60:90–142. doi: 10.1152/physrev.1980.60.1.90. [DOI] [PubMed] [Google Scholar]
- Burke RE, Tsairis P. Anatomy and innervation ratios in motor units of cat gastrocnemius. J Physiol. 1973;234:749–765. doi: 10.1113/jphysiol.1973.sp010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE. Drive to the human respiratory muscles. Respir Physiol Neurobiol. 2007;159:115–126. doi: 10.1016/j.resp.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Day SJ, Hulliger M. Experimental simulation of cat electromyogram: evidence for algebraic summation of motor-unit action-potential trains. J Neurophysiol. 2001;86:2144–2158. doi: 10.1152/jn.2001.86.5.2144. [DOI] [PubMed] [Google Scholar]
- Edström L, Kugelberg E. Properties of motor units in the rat anterior tibial muscle. Acta Physiol Scand. 1968a;73:543–544. doi: 10.1111/j.1365-201x.1968.tb10894.x. [DOI] [PubMed] [Google Scholar]
- Edström L, Kugelberg E. Histochemical composition, territory of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968b;31:424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstedt J, Stålberg E. How the size of the needle electrode leading off surface influences the shape of the single muscle fibre action potential in electromyography. Comput Programs Biomed. 1973;3:204–212. doi: 10.1016/0010-468x(73)90006-8. [DOI] [PubMed] [Google Scholar]
- English A, Wolf S, Segal R. Compartmentalization of muscles and their motor nuclei: the partitioning hypothesis. Phys Ther. 1993;73:857–867. doi: 10.1093/ptj/73.12.857. [DOI] [PubMed] [Google Scholar]
- Farina D, Cescon C, Negro F, Enoka RM. Amplitude cancellation of motor-unit action potentials in the surface electromyogram can be estimated with spike-triggered averaging. J Neurophysiol. 2008;100:431–440. doi: 10.1152/jn.90365.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- Harris AJ, Duxson MJ, Butler JE, Hodges PW, Taylor JL, Gandevia SC. Muscle fiber and motor unit behavior in the longest human skeletal muscle. J Neurosci. 2005;25:8528–8533. doi: 10.1523/JNEUROSCI.0923-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Héroux ME, Dakin CJ, Luu BL, Inglis JT, Blouin J-S. Absence of lateral gastrocnemius activity and differential motor unit behavior in soleus and medial gastrocnemius during standing balance. J Appl Physiol. 2014;116:140–148. doi: 10.1152/japplphysiol.00906.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson-Tole EF, Loram ID, Vieira TMM. Myoelectric activity along human gastrocnemius medialis: Different spatial territories of postural and electrically elicited surface potentials. J Electromyogr Kinesiol. 2013;23:43–50. doi: 10.1016/j.jelekin.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A, Taylor J, Gandevia S, Butler J. Coupling between mechanical and neural behaviour in the human first dorsal interosseous muscle. J Physiol. 2009;587:917–926. doi: 10.1113/jphysiol.2008.165043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda K, Hashizume K. Factors causing difference in force output among motor units in the rat medial gastrocnemius muscle. J Physiol. 1992;448:677–695. doi: 10.1113/jphysiol.1992.sp019064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan KG, Farina D, Merletti R, Enoka RM. Amplitude cancellation reduces the size of motor unit potentials averaged from the surface EMG. J Appl Physiol. 2006;100:1928–1937. doi: 10.1152/japplphysiol.01282.2005. [DOI] [PubMed] [Google Scholar]
- Kinugasa R, Kawakami Y, Fukunaga T. Muscle activation and its territory within human triceps surae muscles. Muscle activation and its territory within human triceps surae muscles. J Appl Physiol. 2005;99:1149–1156. doi: 10.1152/japplphysiol.01160.2004. [DOI] [PubMed] [Google Scholar]
- Kinugasa R, Kawakami Y, Sinha S, Fukunaga T. Unique spatial territory of in vivo human muscle activation. Exp Physiol. 2011;96:938–948. doi: 10.1113/expphysiol.2011.057562. [DOI] [PubMed] [Google Scholar]
- Le J, Gevins A. Method to reduce blur distortion from EEG's using a realistic head model. IEEE Trans Biomed Eng. 1993;40:517–528. doi: 10.1109/10.237671. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Pratt CA, Chanaud CM, Richmond FJ. Territory and innervation of short, interdigitated muscle fibers in parallel-fibered muscles of the cat hindlimb. J Morphol. 1987;191:1–15. doi: 10.1002/jmor.1051910102. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Wakahara T, Kawakami Y. Task-dependent inhomogeneous muscle activities within the bi-articular human rectus femoris muscle. PLoS One. 2012;7:e34269. doi: 10.1371/journal.pone.0034269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti RJ, Roy RR, Edgerton RV. Role of motor unit structure in defining function. Muscle Nerve. 2001;24:848–866. doi: 10.1002/mus.1083. [DOI] [PubMed] [Google Scholar]
- Narici MV, Binzoni T, Hiltbrand E, Fasel J, Terrier F, Cerretelli P. In vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. J Physiol. 1996;496:287–297. doi: 10.1113/jphysiol.1996.sp021685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TR, Lawrence JH, Bonasera SJ. Control of torque direction by spinal pathways at the cat ankle joint. Exp Brain Res. 1993;97:366–371. doi: 10.1007/BF00228708. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Pattullo MC, Gordon T. Innervation ratio and motor unit force in large muscles: a study of chronically stimulated cat medial gastrocnemius. J Physiol. 1997;499:809–823. doi: 10.1113/jphysiol.1997.sp021970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Falces J, Negro F, Gonzalez-Izal M, Farina D. Spatial territory of surface action potentials generated by individual motor units in the human biceps brachii muscle. J Electromyogr Kinesiol. 2013;23:766–777. doi: 10.1016/j.jelekin.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Roeleveld K, Blok JH, Stegeman DF, Van Oosterom A. Volume conduction models for surface EMG; confrontation with measurements. J Electromyogr Kinesiol. 1997a;7:221–232. doi: 10.1016/s1050-6411(97)00009-6. [DOI] [PubMed] [Google Scholar]
- Roeleveld K, Stegeman DF, Vingerhoets HM, Van Oosterom A. The motor unit potential territory over the skin surface and its use in estimating the motor unit location. Acta Physiol Scand. 1997b;161:465–472. doi: 10.1046/j.1365-201X.1997.00247.x. [DOI] [PubMed] [Google Scholar]
- Segal RL, Wolf SL, DeCamp MJ, Chopp MT, English AW. Anatomical partitioning of three multiarticular human muscles. Acta Anat (Basel) 1991;142:261–266. doi: 10.1159/000147199. [DOI] [PubMed] [Google Scholar]
- Stålberg E, Antoni L. Electrophysiological cross section of the motor unit. J Neurol Neurosurg Psychiatry. 1980;43:469–474. doi: 10.1136/jnnp.43.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stålberg E, Falck B. The role of electromyography in neurology. Electroencephalogr Clin Neurophysiol. 1997;103:579–598. doi: 10.1016/s0013-4694(97)00138-7. [DOI] [PubMed] [Google Scholar]
- Staudenmann D, Kingma I, Daffertshofer A, Stegeman DF, van Dieën JH. Heterogeneity of muscle activation in relation to force direction: a multi-channel surface electromyography study on the triceps surae muscle. J Electromyogr Kinesiol. 2009;19:882–895. doi: 10.1016/j.jelekin.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Tonndorf ML, Hannam AG. Motor unit territory in relation to tendons in the human masseter muscle. Muscle Nerve. 1994;17:436–443. doi: 10.1002/mus.880170412. [DOI] [PubMed] [Google Scholar]
- Tonndorf ML, Sasaki K, Hannam AG. Single-wire recording of regional activity in the human masseter muscle. Brain Res Bull. 1989;23:155–159. doi: 10.1016/0361-9230(89)90175-5. [DOI] [PubMed] [Google Scholar]
- Troni W, Parino E, Pisani PC, Pisani G. Segmental analysis of motor conduction velocity in distal tracts of tibial nerve: a coaxial needle electrode study. Clin Neurophysiol. 2010;121:221–227. doi: 10.1016/j.clinph.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Von Tscharner V, Maurer C, Nigg MM. Correlations and coherence of monopolar EMG-currents of the medial gastrocnemius muscle in proximal and distal compartments. Front Physiol. 2014;5:223. doi: 10.3389/fphys.2014.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira T, Loram I, Muceli S, Merletti R, Farina D. Postural activation of the human medial gastrocnemius muscle: are the muscle units spatially localised? J Physiol. 2011;589:431–443. doi: 10.1113/jphysiol.2010.201806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira TMM, Loram ID, Muceli S, Merletti R, Farina D. Recruitment of motor units in the medial gastrocnemius muscle during human quiet standing: is recruitment intermittent? What triggers recruitment? J Neurophysiol. 2012;107:666–676. doi: 10.1152/jn.00659.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira TMM, Minetto MA, Hodson-Tole EF, Botter A. How much does the human medial gastrocnemius muscle contribute to ankle torques outside the sagittal plane? Hum Mov Sci. 2013;32:753–767. doi: 10.1016/j.humov.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira TMM, Windhorst U, Merletti R. Is the stabilization of quiet upright stance in humans driven by synchronized modulations of the activity of medial and lateral gastrocnemius muscles? J Appl Physiol. 2010;108:85–97. doi: 10.1152/japplphysiol.00070.2009. [DOI] [PubMed] [Google Scholar]
- Wakeling JM. The recruitment of different compartments within a muscle depends on the mechanics of the movement. Biol Lett. 2009;5:30–34. doi: 10.1098/rsbl.2008.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Kouzaki M, Moritani T. Region-specific myoelectric manifestations of fatigue in human rectus femoris muscle. Muscle Nerve. 2013;48:226–234. doi: 10.1002/mus.23739. [DOI] [PubMed] [Google Scholar]
- Weijs WA, Jüch PJ, Kwa SH, Korfage JA. Motor unit territories and fiber types in rabbit masseter muscle. J Dent Res. 1993;72:1491–1498. doi: 10.1177/00220345930720110601. [DOI] [PubMed] [Google Scholar]
- Windhorst U, Hamm T, Stuart D. On the function of muscle and reflex partitioning. Behavavioral brain Sci. 1989;12:629–681. [Google Scholar]
- Wolf S, Ammerman J, Jann B. Organization of responses in human lateral gastrocnemius muscle to specified body perturbations. J Electromyogr Kinesiol. 1998;8:11–21. doi: 10.1016/s1050-6411(97)00001-1. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Kim JH. Morphological analysis of the human tibialis anterior and medial gastrocnemius muscles. Acta Anat (Basel) 1997;158:287–295. doi: 10.1159/000147942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Sagital ultrasound images of the right medial gastrocnemius muscle of the 7 subjects from Experiment 1. For each subject, several images were recorded and concatenated to obtain an composite image of the entire muscle length.