Abstract
Objective
To determine if the incidence of viridans group streptococcal infective endocarditis (VGS-IE) had increased following publication of the 2007 American Heart Association's IE prevention guidelines.
Patients and Methods
We performed a population-based survey of all adults (18 years and older) residing in Olmsted County, Minnesota, from January 1, 1999, through December 31, 2013, to identify definite or possible cases of VGS-IE using the Rochester Epidemiology Project. The NIS hospital discharge database was examined to determine the number of VGS-IE cases in the United States between 2000 and 2011.
Results
Rates of incidence (per 100,000 person-years) during time intervals of 1999-2002, 2003-2006, 2007-2010, 2011-2013 were 3.6 (95% confidence interval [CI], 1.3-5.9), 2.7 (95% CI, 0.9-4.4), 0.7 (95% CI, 0.0-1.6), and 1.5 (95% CI, 0.2-2.9), respectively, reflecting an overall significant decline (P=.03 from Poisson regression). Likewise, nationwide estimates of hospital discharges with a VGS-IE diagnosis trended downwards during 2000-2011, with an average number per year of 15,853 and 16,157 for 2000-2003 and 2004-2007, respectively and falling to 14,231 in 2008-2011 (P=.05 from linear regression using weighted least squares method).
Conclusion
Despite major reductions in the number of indications for antibiotic prophylaxis for invasive dental procedures espoused by the 2007 AHA IE prevention guidelines, both local and national data indicate that the incidence of VGS-IE has not increased.
Keywords: Endocarditis, Epidemiology, Guidelines, Prevention
Introduction
Infective endocarditis (IE) is a severe, life-threatening illness with high morbidity and mortality despite advances in diagnostics and therapeutics1,2. Since 1955, the American Heart Association (AHA) has made recommendations for the prevention of IE in patients with certain cardiac conditions prior to invasive dental procedures3. Controversy concerning efficacy and safety of antibiotic prophylaxis has existed for many decades, with progressive reductions in the number of patients for which prophylaxis is recommended, duration of antibiotic prophylaxis, and types of invasive procedures since the AHA guidelines were first published3,4. In 2007, the AHA recommended restriction of antibiotic prophylaxis to only four groups of patients who are at the highest risk of adverse outcomes if they develop endocarditis. This resulted in a major reduction in the number of indications for prophylaxis: if followed, these guidelines could reduce the number of patients who no longer require prophylaxis by approximately 90 percent3. The recommendations were based on an analysis of outcomes among high risk groups if IE developed and the recognition that IE was much more likely to develop secondary to frequent bacteremia associated with daily activities (tooth brushing, chewing food) than from a dental procedure3.
Recognizing the large number of people at risk of IE, there was concern among some medical and dental health care providers that the reduction in the number of at risk patients who were to receive antibiotic prophylaxis could result in a rise in the number of IE cases due to viridans group streptococci (VGS)1. Data were examined over a 12-year period that included a before and after analysis of VGS-IE between 1999 and 2010 in Olmsted County, MN and there was a gradual decline in VGS-IE incidence1. The number of hospital discharges due to VGS-IE using the Nationwide Inpatient Sample (NIS) between 1999 and 2009 was included in our initial analysis and the number of hospital discharges due to VGS-IE appeared to have remained stable1.
Additional investigations that examined the incidence of VGS-IE despite reductions or elimination of antibiotic prophylaxis for dental and other invasive procedures provided consistent results—no increase in VGS IE incidence was observed. Data just published, however, demonstrated a 25% increase in the incidence of IE and an ∼89% reduction in antibiotic prophylaxis use for dental procedures in patients from the United Kingdom5. Nevertheless, this study did not contain organism specific incidence data and the apparent increase in IE incidence may have been driven by an increase in Staphylococcus aureus IE incidence as observed in other recent studies2,6,7. Despite the limitations of this study, we expect that it will generate more controversy about the updated IE prophylaxis guidelines and will create confusion for patients and physicians regarding this issue.
Therefore, we performed a temporal trend analysis of the incidence of VGS-IE in Olmsted County, MN, between 1999 and 2013, which extends our initial evaluation of VGS-IE incidence before and after the 2007 AHA IE prevention guidelines. We also formally evaluated trends in the number of hospital discharges with a primary diagnosis of VGS-IE among adult patients using the NIS database from 2000 to 2011.
Methods
Setting
Olmsted County, Minnesota, allows population-based studies given its geographic isolation from other urban centers, as well as a unique medical records-linkage system that encompasses all residents of Olmsted County1. Our group has previously performed two population-based analyses of IE incidence in Olmsted County between 1970 and 2006, in which a total of 150 cases of IE were identified, with an incidence of VGS-IE ranging from 1.7 to 3.5 cases per 100,000 person-years8,9.
Data Collection
The Endocarditis Registry of the Division of Infectious Diseases at Mayo Clinic and the Rochester Epidemiology Project (REP) database were our primary resources for case ascertainment and data collection. The IE registry at Mayo Clinic has been previously described8. The REP database indexes and links diagnostic and procedure information from all sources of health care in Olmsted County into a single centralized system10. All Olmsted County residents 18 years or older with definite or possible IE caused by VGS, as defined by the modified Duke criteria, between January 1, 1999, and December 31, 2013 were identified using this system1.
The NIS is a stratified probability sample developed as part of the Healthcare Cost and Utilization Project (HCUP) funded by the Agency for Healthcare Research and Quality (AHRQ)11. Between 1999 and 2011, the NIS sampled about 20 percent of hospitals, and all discharges from those hospitals were collected representing more than 95% of the US population. In 2012, the NIS was renamed the “National Inpatient Sample” and refined its survey design to sample 20 percent of discharges from all hospitals that participate in HCUP. Despite this new sampling strategy and its apparent improvement in precision (it is suggested that numbers derived using the previous sample weights may be overestimated by 4.35 percent), these new survey design elements necessary for formal trend analysis have not yet been made readily available in the NIS database for the years prior to 2012. Given the accessibility of the sampling data elements from the previous era, which largely overlaps with our primary study period, we limited the analysis of temporal trends in NIS discharge counts to the years 2000-2011 based on the previous sample weighting scheme. The following ICD-9-CM codes were used to identify potential VGS-IE cases1: acute or sub-acute bacterial endocarditis: 421.0; streptococci unspecified: 041.00; and other streptococci: 041.09. We excluded the following ICD-9-CM diagnostic codes from our search: streptococcus group A: 041.01; streptococcus group B: 041.02; streptococcus group C: 041.03; enterococcus group D: 041.04; streptococcus group G: 041.05.
Statistical Analysis
Incident cases included all adults residing in Olmsted County, Minnesota with a new diagnosis of VGS-IE between the years 1999 and 2013. Rates of incidence were derived based on the counts of these cases per age-, sex-, and calendar time-specific stratum, divided by corresponding numbers of the at-risk population (assumed to be all adults living in the county) obtained from annual census data. Age- and sex-adjusted incidence rates were computed based on direct standardization against the 2010 U.S. Caucasian population, with 95% confidence intervals (CIs) estimated based on the Poisson distribution. For brevity, incidence of VGS-IE is reported across the study period for age categories and calendar year groups (1999-2002, 2003-2006, 2007-2010, and 2011-2013). For formal analyses, however, we used count data expressed in sex-specific strata per single calendar year and per integer age, to model trends in incidence. In particular, multivariable Poisson regression was used to evaluate the association between calendar time and rate of incidence (i.e., test of temporal trend) adjusting for age and sex. Various options for the functional form of calendar time were considered based on graphical impressions of smoothed trends in the observed incidence (as estimated by the non-parametric loess method and weighted according to population counts). In addition, the effect of calendar year on incidence was tested in regression as a piecewise linear function with single breakpoint in 2007 (i.e., test for a difference in slopes for the intervals before and after the change in guidelines). Using a likelihood ratio test, all two-way interactions among age, sex, and time were tested simultaneously as a group to avoid inflating the type I error rate. Nationwide estimates of VGS-IE were derived from the NIS database using a statistical routine customized for survey data (PROC SURVEYFREQ in SAS) that directly accounted for NIS survey design elements relating to stratification, clustering and weighting. To statistically test for a significant linear trend in VGS-related hospitalizations during 2000-2011, survey-estimated annual counts were modeled via linear regression using weighted least squares with each count weighted inversely to its survey-estimated variance. All analyses were carried out with SAS statistical programming (Version 9.3, SAS Institute Inc., Cary, NC). An alpha level of 0.05 was used to define statistical significance.
Results
Overall, 27 definite and possible cases of VGS-IE were identified in the Olmsted County adult population between the years 1999 and 2013. For this period, the average annual incidence rate, age- and sex-adjusted with respect to the 2010 U.S. Caucasian population, was 2.0 (95% confidence interval [CI], 1.2-2.8) per 100,000 person-years. In Table 1, incidence results are presented according to gender along with groupings of age and calendar year. In particular, rates of incidence (per 100,000 person-years) during time intervals of 1999-2002, 2003-2006, 2007-2010, 2011-2013 were 3.6 (95% CI, 1.3-5.9), 2.7 (95% CI, 0.9-4.4), 0.7 (95% CI, 0.0-1.6), and 1.5 (95% CI, 0.2-2.9), respectively (Table 1). Also, as reflected by the average annual rates over the entire 15-year study period, incidence of VGS-IE was higher among males (3.3 per 100,000) compared with females (3.3 vs. 0.8 per 100,000) and increased with older age (from 0.6 per 100,000 for adults 18-39 years to 5.6 per 100,000 for adults ≥ 80 years). From a multivariable Poisson regression model (data not shown), the association of increased VGS-IE incidence with both male gender and increasing age was statistically significant (P<.001 for both effects).
Table 1.
Age- and sex-adjusted incidence rate of infective endocarditis (definite and possible) due to VGS in Olmsted County, Minnesota between 1999 and 2013.
| Groupings | Females | Males | Both Genders |
|---|---|---|---|
| Age | # cases (unadjusted incidence rate, per 100,000 person-years) | ||
| 18-39 | 0 (0.0) | 4 (1.2) | 4 (0.6) |
| 40-59 | 2 (0.7) | 6 (2.2) | 8 (1.4) |
| 60-79 | 2 (1.4) | 9 (7.4) | 11 (4.2) |
| 80+ | 2 (4.3) | 2 (8.1) | 4 (5.6) |
| Calendar Time* | Adjusted incidence rate (95% CI), per 100,000 person-years | ||
| 1999-2002 | 0.6 (0.0, 1.7) | 7.3 (2.3, 12.2) | 3.6 (1.3, 5.9) |
| 2003-2006 | 2.6 (0.3, 5.0) | 2.4 (0.0, 4.8) | 2.7 (0.9, 4.4) |
| 2007-2010 | 0.0 (0.0, 0.0) | 1.5 (0.0, 3.2) | 0.7 (0.0, 1.6) |
| 2011-2013 | 0.0 (0.0, 0.0) | 3.2 (0.4, 6.0) | 1.5 (0.2, 2.9) |
| Overall* | 0.8 (0.2, 1.4) | 3.3 (1.9, 4.7) | 2.0 (1.2, 2.8) |
Time-specific and overall rates were directly adjusted to the 2010 U.S. Caucasian population of adults according to age and sex
To assess the change in incidence of VGS-IE over the study period, several formulations of a time effect were considered using both graphical and analytical methods. In particular, visual inspection of the smoothed trends of incidence in Figure 1 suggested a fairly linear effect of time over the study period. Furthermore, we found no evidence of a shift in incidence or a differential slope in the time trends before and after 2007, and thus a linear effect (one overall slope) of calendar time was adopted in the final Poisson regression model. Controlling for age and sex in this model, incidence of VGS-IE decreased approximately 9% per year over the study timeframe (P=.03). However, from a sensitivity analysis in which the first 4 years were excluded, there was no significant change in incidence from 2003 to 2013 (P=.31), suggesting that a decreasing trend in the overall period was likely driven by the sharp decline observed at the beginning. Regardless, neither analysis showed evidence of a significant increase in the incidence of VGS-IE relating to the 2007 AHA guideline changes.
Figure 1.
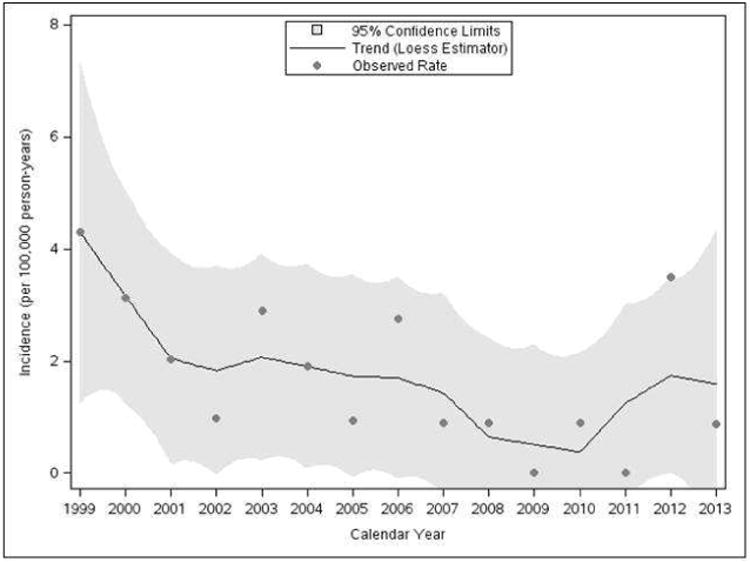
Temporal trends in age- and sex-adjusted incidence rate of infective endocarditis (IE) caused by viridans group streptococci (VGS) from 1999 to 2013 in Olmsted County, Minnesota.
For each year between 2000 and 2011, data on all hospital discharges of adult patients with a primary diagnosis of VGS-IE were extracted from the NIS database. Annual numbers of VGS-IE cases shown in Figure 2 correspond to projected nationwide estimates (along with 95% confidence intervals), which ranged as high as 17,110 in 2003 and as low as 13,334 in 2010. In the test for a linear trend in VGS-IE discharges during 2000-2011, the fitted regression line (displayed in Figure 2 as a black dashed line) showed a marginally significant linear decline over this 12-year period, with an average of 182 fewer VGS-related discharges per year (P=.05). However, since the observed trend appeared to be slightly non-linear (based on the regression line falling outside the 95% confidence limits for a few individual years), the trend analysis was re-tested on years 2003-2011, a period in which the assumption of linearity looked to be more plausible. As there was no evidence of a differential linear trend before and after 2007 during this interval, the final regression model specified a single slope (gray dashed line in Figure 2) and showed a significant downward trend from 2003 to 2011 at an average decrease of 475 VGS-related discharges per year (P<.001).
Figure 2.
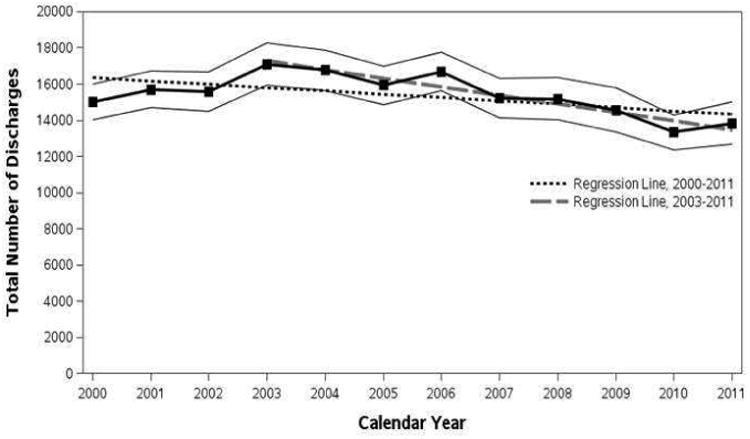
Total number of hospital discharges in adult patients with ICD-9-CM discharge diagnosis of acute and subacute bacterial endocarditis (421.0), streptococcus unspecified (041.00), and other streptococci (041.09) from 2000 to 2011 from the Nationwide Inpatient Sample.
Discussion
Our investigation extends surveillance of VGS-IE incidence in Olmsted County, MN following publication of the 2007 AHA IE prevention guidelines. We observed an overall decline in the incidence of VGS-IE between 1999 and 2013, though decreases early in the period from 1999 to 2002 appear to largely account for this trend. In particular, there was no significant trend in incident cases of VGS-IE from 2003 to 2013, and no evidence of an increase or decrease coinciding with the 2007 AHA guideline changes. These findings are similar with those from a more limited (in time) survey of this population that extended through 20101 and, until very recently, findings from other population investigations (Table 2) 2,5,7,12,13.
Table 2.
Population-based studies of infective endocarditis before and after guidelines changes.
| Author | Study Years | Country | Population | Results |
|---|---|---|---|---|
| Dayer [5] | 2000-2013 | UK | England Hospital discharge records | Increased incidence of IE (25%) coinciding with approximately 89% reduction in antibiotic prophylaxis use for dental procedures since the 2008 NICE IE prevention guidelines starting within 3 months since 2008 NICE IE prevention guidelines |
| DeSimone [1] | 1999-2010 | US | Adults, Olmsted County, MN NIS database Definite & possible IE | No increase in VGS-IE incidence before and after 2007 AHA IE prevention guidelines |
| Thornhill [2] | 2000-2010 | UK | England Hospital discharge records | No significant change in the upward trend in IE cases due to oral streptococci; 78.6% reduction in antibiotic prophylaxis prescriptions |
| Duval [7] | 1991, 1999, 2008 | France | 11 million patients Age ≥20 Definite, possible, & probable IE | No increase in VGS-IE incidence since 2002 French IE prophylaxis guidelines |
| Bikdeli [12] | 1999-2010 | US | US Medicare beneficiaries Age ≥65 | No increase in rates of hospitalization after 2007 AHA IE prevention guidelines |
| Pasquali [13] | 2003-2010 | US | Pediatric Health Information Systems Database | No increase in IE admission across 37 US children's hospitals following 2007 AHA IE prevention guidelines |
To complement the findings from our local population, data from the NIS database were used to provide a national perspective on the trends of VGS-IE. Over a fairly comparable time period, nationwide estimates of VGS-IE discharges tended to decrease from 2000 to 2011, with a significant linear decline from 2003 to 2011. As seen in Olmsted County, there was no indication of an increase in VGS-IE cases at the national level following the 2007 guideline changes. While findings from our two sets of data were not exactly conflicting, large national databases have distinct strengths and advantages over those derived within a specific region. In contrast to the population of Olmsted County which is relatively small and limited in the number of IE cases per year, the NIS contains approximately 8 million hospital discharges annually11. Furthermore, data from a large national database offers the advantage of increased generalizability to other US populations which may have different characteristics than that of Olmsted County, MN.
Pant et al14, provided data that are consistent with our previous1 and current evaluations of the NIS database. They examined the NIS database for IE incidence between 2000 and 2011 and identified a significant increase in IE. This increase was mainly due to an increase in staphylococcal IE, while the incidence of VGS-IE cases remained stable14.
In contrast, Dayer et al. recently reported a statistically significant increase in IE incidence that started only three months post-2008 NICE guidelines through 20135. This increase in incidence of IE coincided with a significant reduction (∼89%) in antibiotic prophylaxis prescriptions. Although there was a temporal relationship in reduction of antibiotic prophylaxis prescriptions and increase in IE incidence, the data do not establish a causal link. In addition, a major limitation of this study was the lack of detailed microbiological data that defined the causes of IE. It is, therefore, unknown if the observed increase in IE is due to VGS and/or other organisms, in particular S. aureus. In the latter case, there are several contemporary investigations of IE epidemiology that report an increasing burden of disease due to S. aureus and this is, in part, due to the increasing use of invasive devices and procedures in the healthcare setting6,15.
In the current update of the Olmsted County, MN population that includes VGS-IE cases seen between 2011 and 2013, no patients underwent a dental procedure in at least two years prior to the diagnosis of VGS-IE. This suggests that mechanisms other than invasive dental procedures accounted for the development of IE in these cases. This is important as we evaluate the current data, which demonstrated an increased number (n=4) of cases in 2012 (Figure 1). It is conceivable that with a longer period of follow-up subsequent to the changes in the 2007 AHA prevention guidelines that the IE incidence due to VGS could increase if antibiotic prophylaxis for invasive dental procedures is efficacious in patients who were previously included in the “moderate risk” group and no longer receive prophylaxis. As evidenced by the data from the United Kingdom, the use of antibiotic prophylaxis continued to decline with more prolonged follow-up and a similar phenomenon could be anticipated in the US.
To date, there have been no studies that have included a review of dental records to determine if the 2007 AHA prevention guidelines that exclude prior “moderate risk” patients for antibiotic prophylaxis, are being followed. Such a determination is important as the lack of increase in VGS-IE incidence could be explained by a lack of compliance with the 2007 AHA IE prevention guidelines. In this regard, Lockhart et al surveyed a random sample of 5,500 dentists in the U.S. following the 2007 AHA prevention guidelines16. Strikingly, 70% of dentists who responded cared for patients who continue to receive antibiotics prior to a dental procedure, although the 2007 guidelines no longer recommend it in these risk groups of patients16. In a similar study, Shah et al. performed a web-based survey among U.S. pediatric cardiologists in 2013 to evaluate the current practice of IE prophylaxis17. Fifty-seven percent of 302 respondents did not follow the 2007 IE prevention guidelines exclusively for various reasons, citing conservative approach (20%), patient/family preference for prophylaxis (13%), and lack of clarity in the 2007 guidelines (12%)17. Studies that include a review of dental records to determine the percentage of moderate-risk patients who continue to receive antibiotic prophylaxis will be imperative as we attempt to evaluate the VGS IE incidence and whether dental procedures contribute to it without clinical trial data available.
A major strength of our study is that we have microbiological identification in every case of IE in the Olmsted County population. Limitations of our study include the small number of annual cases of VGS-IE given that the adult population of Olmsted County is less than 120,000 people. Therefore, we used the NIS database to examine a large nationwide sample for trends in VGS-IE over time and, similar to the Olmsted County population, found no significant increases following the guideline revisions. The NIS contains discharge-level records, however, not patient-level records11 and data from individual patients hospitalized more than once per year may be included in the NIS multiple times. Moreover, diagnosis codes may not be completely accurate in defining disease syndromes. In addition, the predominance of Caucasians in this setting may limit applicability to other places with more racial diversity.
As cited above, local dental office data are not available in the REP database and thus, we are unable to determine the compliance with the 2007 AHA prevention guidelines, which is important as we consider an examination of factors that could influence the incidence of VGS-IE. Moreover, local pharmacy data are not available in the database.
Conclusion
The incidence of VGS-IE in both local and national databases has not increased subsequent to the publication of the 2007 AHA IE prevention guidelines. Efforts are currently underway to obtain dental records in the Olmsted County, MN population to determine if practice changes have occurred in response to the guidelines. Ultimately, a placebo-controlled randomized clinical trial is needed to attempt to define the efficacy and safety of antibiotic prophylaxis in the prevention of IE.
Acknowledgments
Funding/Support: This study was supported by research grants from the Baddour Family Fund, Mayo Foundation for Medical Education and Research. This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of the Sponsor: The funding organizations had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Abbreviations
- VGS
viridans group streptococci
- IE
infective endocarditis
- AHA
American Heart Association
- NIS
Nationwide Inpatient Sample
- REP
Rochester Epidemiology Project
Footnotes
Author contributions: Dr. DeSimone had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Design and conduct of the study: DeSimone, Tleyjeh, Correa de Sa, Wilson, Baddour.
Acquisition of data: DeSimone, Steckelberg, Anavekar, Tleyjeh, Correa de Sa, Baddour.
Analysis and interpretation of data: DeSimone, Sohail, Tleyjeh, Correa de Sa, Lahr, Wilson, Baddour.
Critical revisions of the manuscript for important intellectual content: DeSimone, Sohail, Tleyjeh, Correa de Sa, Lahr, Steckelberg, Anavekar, Wilson, Baddour.
Drafting of the manuscript: DeSimone, Sohail, Tleyjeh, Correa de Sa, Baddour.
Statistical analysis: DeSimone, Lahr, Baddour.
Obtained funding: DeSimone, Wilson, Baddour.
Administrative, technical, or material support: Wilson, Baddour.
Study supervision: DeSimone, Steckelberg, Wilson, Baddour.
Institutional review board: Mayo Clinic IRB approved; study ID: 10-007212
Olmsted Medical Center IRB approved; study ID: 053-OMC-10
Financial Disclosures: Sohail: TyRx Inc. (moderate, $ <10,000)
Baddour: Royalty payments-UpToDate, Inc.; Editor-in-Chief payments-Massachusetts
Medial Society (Journal Watch Infectious Diseases); Co-editorship payments-American College of Physicians (PIER)
Conflict of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Desimone DC, Tleyjeh IM, Correa de Sa DD, et al. Incidence of infective endocarditis caused by viridans group streptococci before and after publication of the 2007 American Heart Association's endocarditis prevention guidelines. Circulation. 2012;126(1):60–64. doi: 10.1161/CIRCULATIONAHA.112.095281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thornhill MH, Dayer MJ, Forde JM, et al. Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: before and after study. BMJ. 2011;342:d2392. doi: 10.1136/bmj.d2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 4.Lockhart PB. Antibiotic prophylaxis for dental procedures: are we drilling in the wrong direction? Circulation. 2012;126(1):11–12. doi: 10.1161/CIRCULATIONAHA.112.115204. [DOI] [PubMed] [Google Scholar]
- 5.Dayer MJ, Jones S, Prendergast B, Baddour LM, Lockhart PB, Thornhill MH. Incidence of infective endocarditis in England, 2000-13: a secular trend, interrupted time-series analysis. Lancet. 2014 Nov 18; doi: 10.1016/S0140-6736-(14)62007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slipczuk L, Codolosa JN, Davila CD, et al. Infective endocarditis epidemiology over five decades: a systematic review. PLoS One. 2013;8(12):e82665. doi: 10.1371/journal.pone.0082665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duval X, Delahaye F, Alla F, et al. Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: three successive population-based surveys. Journal of the American College of Cardiology. 2012;59(22):1968–1976. doi: 10.1016/j.jacc.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Tleyjeh IM, Steckelberg JM, Murad HS, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA. 2005;293(24):3022–3028. doi: 10.1001/jama.293.24.3022. [DOI] [PubMed] [Google Scholar]
- 9.Correa de Sa DD, Tleyjeh IM, Anavekar NS, et al. Epidemiological trends of infective endocarditis: a population-based study in Olmsted County, Minnesota. Mayo Clin proceedings. 2010;8(5):422–426. doi: 10.4065/mcp.2009.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic proceedings. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: 2011. [Access date: December 15, 2014]. www.hcup-us.ahrq.gov/nisoverview.jsp. [Google Scholar]
- 12.Bikdeli B, Wang Y, Kim N, Desai MM, Quagliarello V, Krumholz HM. Trends in hospitalization rates and outcomes of endocarditis among medicare beneficiaries. Journal of the American College of Cardiology. 2013;62(23):2217–2226. doi: 10.1016/j.jacc.2013.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasquali SK, He X, Mohamad Z, et al. Trends in endocarditis hospitalizations at US children's hospitals: impact of the 2007 American Heart Association Antibiotic Prophylaxis Guidelines. American heart journal. 2012;163(5):894–899. doi: 10.1016/j.ahj.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pant S, Deshmuch A, Mehta K, et al. Trends in infective endocarditis incidence and microbiology before and after 2007 IDSA/ACC/AHA IE prophylaxis guidelines change. JACC. 2014;63(12):A1933. [Google Scholar]
- 15.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Archives of internal medicine. 2009;169(5):463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockhart PB, Hanson NB, Ristic H, Menezes AR, Baddour L. Acceptance among and impact on dental practitioners and patients of American Heart Association recommendations for antibiotic prophylaxis. Jounral of the American Dental Association. 2013;144(9):1030–1035. doi: 10.14219/jada.archive.2013.0230. [DOI] [PubMed] [Google Scholar]
- 17.Shah NC, Patel N, Naik R. Infective endocarditis prophylaxis-current practice amongst pediatric cardiologists: are we following 2007 guidelines? JACC. 2014;63(12):A602. doi: 10.1017/S1047951115002176. [DOI] [PubMed] [Google Scholar]


