Abstract
Autoimmune diseases like rheumatoid arthritis are multifactorial in nature, requiring both genetic and environmental factors for onset. Increased predisposition of females to a wide range of autoimmune diseases points to a gender bias in the multifactorial etiology of these disorders. However, the existing evidence tol date has not provided any conclusive mechanism of gender- bias beyond the role of hormones and sex chromosomes. The gut microbiome, which impacts the innate and adaptive branches of immunity, not only influences the development of autoimmune disorders but may interact with sex-hormones to modulate disease progression and sex-bias. Here, we review the current information on gender bias in autoimmunity and discuss the potential of microbiome-derived biomarkers to help unravel the complex interplay between genes, environment and hormones in rheumatoid arthritis.
1. INTRODUCTION
Autoimmune diseases are characterized by alterations in normal immune function, resulting in hyperactive immune response against self proteins and tissues. Even though the etiology of autoimmune disorders is unknown, extensive clinical research over the past decade has pointed to genetic and environmental factors that interact to trigger disease. The genetic basis of autoimmunity is associated with a complex array of risk loci, the most important being those located in the Major Histocompatibility Complex (MHC), conferring susceptibility or resistance to disease [1]. Different disease outcomes in genetically identical individuals [2] imply that environmental triggers such as diet [3], infections and smoking exacerbate autoimmunity[4–6]. Although, in these studies, environment-derived antigens have been reported to increase (inflammatory reactions), mechanistic insight into how autoimmunity arises remain largely obscure.
Recent advances in “omic”-based approaches (metagenomics, metabolomics and proteomics) and bioinformatics have facilitated our understanding of the mechanisms of a broad range of diseases and have allowed us to identify potential biomarkers for diagnosis and therapeutic intervention [7]. One particular area of research receiving increasing attention over the past 5 years has focused on using omic-based techniques to study how the gut microbiome, the collection of bacteria, viruses, fungi and protozoa lining the gastrointestinal mucosa, significantly impact health and disease [8–10]. These vastly diverse microbial communities not only play a vital role in nutrient synthesis and energy harvest from foods but also tightly regulate the innate and adaptive branches of immunity [11–16]. Recent research about the role of gut microbes in adaptive immune response has substantially changed our understanding of how genes, environmental factors and our “second genome” (the gut microbiome) interact to influence autoimmunity.
In this review we focus on the sex-bias of autoimmune disorders that, although well documented, still lacks mechanistic insight with regards to genetic and gut microbial interactions. Studies in humans and mouse models have revealed that females are 2–10 times more susceptible than males into a wide range of autoimmune disorders, including rheumatoid arthritis (RA), Multiple Sclerosis (MS), systemic lupus erythematosus (SLE), myasthenia gravis (MG), Sjogren’s syndrome and Hashimoto’s thyroiditis [17, 18]. Yet, recent evidence is just beginning to emerge linking sex-specific microbial clades during disease progression, and pointing to complex interactions between gut microbes, genetic factors, environment and sex hormones. This review does not intend to discuss the current knowledge on the genetic or environmental triggers of autoimmune disorders and gender-bias, which have been elegantly reviewed elsewhere [19–22]. Here, we review the current literature relating gut microbes to the sex-based differences observed in various autoimmune disorders and discuss how diverse experimental platforms contribute to developing useful biomarkers for disease progression and for therapeutics.
2. The gut microbiome and autoimmunity
Mucosal surfaces are exposed daily to various environmental factors and therefore require an effective protection that can efficiently eliminate the majority of external agents. The mucosa-associated lymphoid system (MALT), which carries most of the immunologically active cells in the body, is the main barrier against potential insults from gut commensals and external agents. A characteristic feature of mucosal immunity that distinguishes it from systemic immunity is the maintenance of tolerance to non-dangerous antigens in the gut [23–26]. Intestinal bacteria are necessary for the development of competent mucosal immunity. Experiments with germ-free (GF) and specific-pathogen free mice (SPF) have shown that stimuli from intestinal commensals are required for maturation, development and function of important components of humoral and cell-mediated immunity [27, 28]. Bacterial metabolites and metabolic products generated from specific dietary substrates, mainly short chain fatty acids (SCFA), also regulate immune function. For instance butyrate is reported to exert immunomodulatory effects on intestinal macrophages and induce differentiation of T regulatory cells resulting in inhibition of IFN-γ-mediated inflammation [29, 30]. Thus, a homeostatic environment between the host and microbes is maintained by keeping these microorganisms from crossing the intestinal mucosa, yet maintaining tolerance to exploit the beneficial contribution of microbes to host physiology. However, failures in the epithelial integrity and mucosal immunity allow for bacteria to cross this barrier, triggering a pro-inflammatory response systemically. Consequently, diet-host- microbe molecular interactions are critical components of immune-competence as well as autoimmune disease development.
A growing body of evidence has linked specific signatures of microbial clades to various autoimmune diseases. Additionally, there is a strong association between sex and incidence of disease in a variety of conditions. Thus, a deeper understanding of the gut microbial composition in males and females will be informative for sex-based treatment options. Although very few studies have made an association with the sex-biased nature of diseases, type I diabetes (T1D), an autoimmune disease occurring with male to female ratio of 3:2 in populations of European descent aged 15–40 years [31], is perhaps the most studied disorder for correlations with the gut microbiome. Patients with T1D have shown shifts in ratios of the main phyla within the gut microbiome exhibiting decreased Bacteroidetes: Firmicutes ratios, lower abundance of potential butyrate producers, and lower bacterial diversity [32]. A recent study in children showed that low abundance of bacteria typically associated to lactate and butyrate production was associated with β cell autoimmunity [33]. Additional evidence comes from another study where T1D incidence was associated with an increased abundance of specific taxa such as Clostridium and decreased Bifidobacterium and Lactobacillus compared to healthy subjects [34]. NOD mice develop disease with an increased incidence in SPF compared to GF conditions suggesting a potentially protective role of the gut commensals [35]. This is supported by the observations where segmented filamentous bacteria (SFB) were shown to segregate with protection from diabetes in NOD female mice [36]. Although the mechanism of the gender-bias protection is yet to be elucidated, it can, in part, be explained by an increase in testosterone levels by SFB [37]. Exploratory analyses in female-biased MS,, showed a decrease in abundance of Faecalibacterium Prausnitzii, a taxon with known butyrate producing potential, in affected individuals vs. controls [38]. Mouse models of MS, experimental autoimmune encephalomyelitis (EAE), showed that oral antibiotic treatment prior to EAE induction significantly reduced severity and onset of disease suggesting a role of gut microbes in pathogenesis [39].
3. Gut microbiome and rheumatoid arthritis
Rheumatoid arthritis has a strong genetic predisposition. Genome wide association studies have described multiple genes that are linked with susceptibility to RA; in partcular genes encoded in the class II loci provide the major risk factor. Alleles sharing the 3rd hypervariable region sequences with the DRB1*0401 called the “shared epitope” have been associated with predisposition to RA in most ethnic populations [40]. On the other hand, DRB1*0402 is considered a resistant allele for arthritis development. A role for hormones in pathogenesis of RA is underscored by the remission of arthritis during pregnancy and increased prevalence in women, occurring 2–3 times more often in women than in men. However, not all patients are carry the RA-susceptible alleles, suggesting that besides host genotype, environmental triggers may be important determinants of disease onset. An infectious etiology of RA has been suggested but the evidence is not conclusive [41]. The concept that resident commensal microbes may be a causal factor is not new [42]. An association between gut commensals and RA was inferred by the -discovery that some microbial DNA, likely of the gut origin, -is present in the synovial fluids of patients [43, 44]. The involvement of resident commensals in driving RA progression is also supported by a high recurrence of periodontal inflammatory disorders in RA patients, caused by Porphyromonas gingivalis [45]. The mechanism by which P. gingivalis contributes to RA pathogenesis likely involves citrullination of bacterial and human antigens by this commensal and loss of tolerance to self-proteins in genetically susceptible individuals. An analysis of bacterial community composition through 16S rRNA high throughput sequencing in fecal samples of RA patients indicated that Prevotella copri and Prevotella-like taxa were consistently found as a markers of disease, [46]. The involvement of gut commensals in the pathogenesis of RA is further supported by increased gut permeability, suggesting that compromised intestinal barrier integrity may lead to egress of luminal antigens including bacterial fragments and metabolites resulting in enhanced pro-inflammatory response against specific commensals in RA [47].
An exposure to environmental factors early in life has a role in pathogenesis of RA [48]. The gut is the largest immune organ in the body that is exposed daily to environmental factors - but still maintains a tolerant state. The ability of antigen-specific oral tolerance to modulate arthritis suggests a role for the mucosal immune system in generating tolerance for protection from organ-specific autoimmunity [49]. Other factors associated with development of RA include aging and environmental factors like smoking. There is a growing interest in determining how genotype, hormones and environmental factors may impact the gut microbiome and RA. Recent data has suggested that genotype, age and sex may determine the gut microbiota. In RA patients, particular gut microbial community structure have also been found to be important determinants of disease onset [9, 50].
We have used humanized mice expressing RA-susceptible DRB1*0401 and resistant DRB1*0402 genes to determine if genetic susceptibility is associated with dysbiosis in the gut [47]. 16S sequencing of fecal samples showed differences in the gut microbial composition between susceptible and resistant mice, with significantly lower Bacteroidetes:Firmicutes ratios in the former. Further, data in these mice showed that the arthritis-susceptible mice had lost the dynamic changes that occur with age in the gut microbiota while the arthritis-resistant mice showed age-dependent changes. Dysbiosis in the gut microbiota of the susceptible mice was associated with increased gut permeability suggesting a gut-joint-axis in predisposition to RA.
A more direct evidence of commensal microbe causality in RA has come particularly from the use of germfree animal models [9]. One study showed that while joint inflammation was absent in GF mice, monocolonization of GF mice with a bacterium could lead to the development of T helper17-dependent arthritis [51]. These findings suggest that a dysbiotic intestinal microbiota present in a genetically susceptible host is required to trigger systemic autoimmunity leading to inflammatory arthritis in this animal model. The dependency of the gut microbial composition on the factors known to influence the onset and progression of RA mandate an understanding of the gut dysbiosis and associated metabolites that cause immune dysregulation in RA. Despite associations between specific microbial repertoires and autoimmune diseases, most reports have not considered the impact of sex hormones on the gut microbiome.
4. Autoimmunity, sex hormones and gut microbes
The gender-biased nature of autoimmune disease in humans relies on an increase of innate and adaptive immune responses in females compared to males. In mice, antigen challenge can lead to increased autoreactive responses in females versus males [52]. Mechanistically, several fold increases in T cell activation, cytokine production, expression of genes involved in Toll-like receptor pathways and more efficient antigen presenting cell activity[53, 54] (among others) characterize the female autoimmune landscape. The hormonal environment in autoimmunity suggests that androgens and estrogens strongly modulate the Th1/Th2 balance [55]. Androgens such as testosterone are reported to have a down-regulating effect on natural killer (NK) cells, tumor necrosis factor-alpha (TNF-α) production and Toll-like receptor 4, while enhancing the production of anti-inflammatory IL-10. In contrast, estrogens show an enhancing effect on cell-mediated and humoral immune response, NK cell cytotoxicity and the production of pro-inflammatory cytokines IL-1, IL-6 and TNF-α [53, 56]. This explains the enhanced immune reactivity in females, which is associated with more effective resistance to infection compared to males [53] but consequently increases susceptibility to autoimmune diseases [57].
Most animal models for rheumatoid arthritis show development of disease with similar incidence in both sexes. To generate a model that mimics the sex-bias of human disease, we used humanized DRB1*0401 and DRB1*0401/DQ8 mice. Upon immunization with type II collagen, *0401 and *0401/DQ8 mice develop arthritis predominantly in females mimicking human female to male ratio of 3:1 as well as the associated autoantibodies, rheumatoid factor and anti-citrullinated antibodies [52]. The molecular basis of the sex-bias of arthritis in this model was due, in part, to the differential function of antigen presentation cells leading to an enhanced T cell activation resulting in significant differences in cytokine production and regulatory responses between males and females [58, 59]. Thus, an interaction between genetic factors and sex-hormones influences the immune response systemically in RA. This phenomenon may also define the immune responses in the gut and hence determine the microbial composition that is able to maintain the homeostasis in that environment and may have an advantage for colonization.
Although the molecules involved in the complex interplay between genes and sex hormones in gender-based autoimmunity have been long identified, the role that gut microbes play in this scenario is less clear. Studies reporting specific gender associations of the gut microbiome composition in healthy humans are inconsistent [60–63]. In these reports abundance of specific taxa have been correlated with males (Bacteroides (Bacteroidetes), Ruminococcus Eubacterium, Blautia (Firmicutes)) and females (Treponema (Spirochaetes)), however, these differences likely reflect specific lifestyle and cultural gender-related factors rather than hormone-associated gender differences. Since in human studies it is difficult to interpret causality versus consequence of diseases due to a myriad of factors influencing the overall immune response, mouse models have been critical in understanding the role of gut microbes on human health.
Studies in mice have shown strong associations between host genetic background and gut microbiota configuration, regardless of gender [64]. The abundance of taxa within the main gut bacterial phyla (Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria) has been reported to be mainly affected by specific quantitative trait loci (QTL) in mice [65, 66], many of which also control vital immunological functions. Thus, it is likely that, when controlling for the effect of environment, host genotype has the strongest influence on mammalian gut microbiomes. This has critical implications in disease-susceptible genotypes, consistent with the observation that particular microbial community structure could modify disease progression in autoimmune disorders [9, 50]. An important question, nonetheless, is how sex hormones and specific gut microbiome compositions interact in gender-biased autoimmunity.
Members of the gut microbiome are reported to interact with steroids, possibly impacting the steroid balance at the intestinal level and the metabolic activity in the colonic ecosystem [67, 68]. As such, some specific taxa have the capacity to metabolize sex steroid hormones and influence their activity [69]. For instance, the intestinal commensal Clostridium scindens encodes hydroxysteroid dehydrogenases and other enzymes involved in glucocorticoid conversion into androgens [70]. Slackia sp., a common member of the gut microbiome, can exert inter-conversion of B-estradiol and estrogen. Even though some sterols can be re-absorbed from the colonic ecosystem through enterohepatic circulation, it is unclear whether or how microbiome-derived sex steroids have an impact on host physiology and immunity, analogous to that of host-derived hormones.
Recent reports on the influence of gut microbes on gender-based autoimmunity suggest that both bacteria and sex hormones interact directly to regulate disease fate in genetically susceptible individuals. Two elegant studies recently showed that the GF NOD mice do not exhibit a gender bias in diabetes [37, 71]. However, gender-bias in the SPF conditions occurs more often in females and can be reversed via transfer of gut microbiota from males. These data suggest that a feedback loop between sex-hormones and gut microbes determine the expansion of microbial lineages that can likely influence autoimmunity by triggering an inflammatory or tolerogenic effect. Overall, these studies argue that gut microbes drive testosterone-dependent attenuation of T1D via immune regulation, which could be a mechanism characteristic of other gender-biased autoimmune disorders (Figure 1).
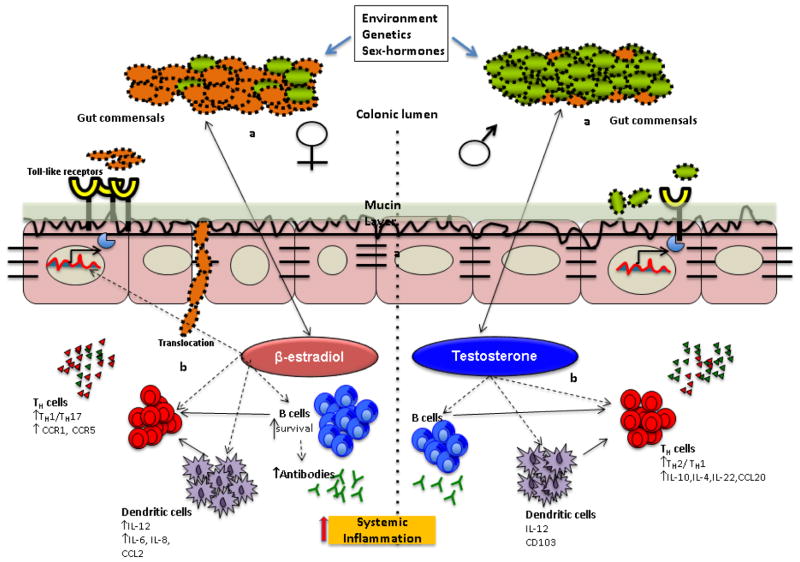
Figure 1. Influence of sex-hormones on the gut microbial composition and immune response.
Environment and genetic factors have a significant effect on gut microbial composition and modulate the abundance of specific taxa within the microbial ecosystem. Gender-bias in - gut microbiome profiles is caused, in part, by the sex hormones, estradiol and testosterone, which may influence gut microbial composition directly (a) or indirectly (b) by shaping the gut mucosal immune environment. β-estradiol promotes differentiation of conventional dendritic cells (DCs) into IL-12, IFNγ-producing DCs which activates pathways for pro-inflammatory cytokines IL-6 and IL-8 and polarization of T cells into Th1/Th17 (red dots) rather than anti-inflammatory cytokines (green dots). Estradiol also enhances the survival of B cells and polyclonal B-cell activation, which could be related to increased autoantibody production. In females, there is an increased expression of genes involved in Toll-like receptor pathways. The pro-inflammatory immune environment compromises gut permeability, causing translocation of gut commensals in to the lamina propria where they can amplify pro- inflammatory responses. In males (right panel), testosterone has a suppressive effect on T cell proliferation, resulting in attenuated immune responses and a balanced immune system. DCs maintain a tolerant environment by generating Th1/Th17 as well as T regulatory cells by production of IL-4, IL-10, IL-22 and CCL20. Testosterone is associated with decreased expression of genes involved in Toll-like receptors pathways and antigen presentation so that integrity of the intestinal barrier is not compromised. Thus sex-hormones modulate local immune response by involving cells of the adaptive immune system which leads to changes in systemic immune responses contributing to pathology.
Using the humanized transgenic mouse model of rheumatoid arthritis, we showed that arthritis-susceptible (DRB1*0401) and -resistant (DRB1*0402) mice exhibit a significantly different gut microbiome composition [47]. In addition, only resistant mice showed gender- driven and age-dependent gut microbiomes. The presence of Allobaculum, a genus related to Clostridiales, in susceptible mice was associated with proinflammatory conditions in the gut, which likely resulted in increased gut permeability in arthritic mice compared to naïve mice. Interestingly, the expansion of Clostridiales showed a significant correlation with increases in gene transcripts of pro-inflammatory cytokines (Th1/Th17) with a concomitant decrease in regulatory cytokines (IL-4, IL-22) in *0401 females as compared to males. The Clostridiales, one of the prevalent clades in the mammalian gut, have been isolated from synovial tissue of RA patients [43] suggesting translocation of gut commensals to peripheral tissues in the setting of increased gut permeability. While the arthritis-susceptible mice displayed high abundances of taxa related to the Clostridiales order, the arthritis-resistant mice showed increased abundance of Bifidobacteria and taxa related to Parabacteroides (Bacteroidales, Porphyromonadaceae family). Interestingly, these protective microbiome traits were always significantly enriched in resistant females compared to resistant males. However, when the microbiomes of genetically susceptible mice were explored, a higher abundance of Bifidobacteria was common to males. Taxa related to Porphyromonadaceae, normal gut commensals, have been associated with protective and balanced microbiomes after antibiotic-associated dysbiosis [72] and Bifidobacteria and Lactobacillaceae are known health-promoting taxa in the gut [73]. These observations point not only to a possible pattern of health and disease biomarkers in gender-biased autoimmunity but also indicate that these pathologies may be further understood by taking into account the role of environmental, dietary and lifestyle factors,, for disease progression. These observations led us to propose that while susceptibility in *0401 mice are determined by HLA-driven control of dysbiotic (unbalanced) microbiomes, the sexual dimorphism of disease may be due to hormonal impact on gut microbes.
Although a tremendous effort has been made -to characterize the gut microbial community structures and microbe-derived metabolites that shape the immune responses in health and disease, several questions still remain. It is still unclear how sex hormones or genotype primarily modulate the colonic ecosystem to impact immunity in gender based autoimmune disorders. The mechanisms involved may differ substantially in different autoimmune pathologies and may be confounded by an interaction between the three axes; genotype, sex steroids and gut microbes (Figure 2). To clarify the complex interactions between these three axes in influencing autoimmunity, one could be looking for specific gut bacterial clades with either potentially protective or pro-inflammatory roles. Most importantly, biomarker characterization based on microbiome studies will further allow us to assess the effect of environmental factors (i.e., diet, lifestyle) on triggering gender-based autoimmunity. However, while these biomarkers may provide clues to associations between the specific microbiome traits and disease and help us understand possible mechanism of pathogenesis, they do not predict the cause or provide details on the involvement of gut bacteria in disease. Nonetheless, despite the existence of certain common taxonomic and functional traits between healthy or disease and specific bacterial markers, they may not always be consistent. This inconsistency may be due to variable conditions including experimental and sequencing platforms and differences in disease models. Table 1 shows bacterial taxa found to be associated with gender-biased autoimmune disease. However, the abundance or detection of specific taxa in diseased or healthy subjects does not illustrate on their specific involvement in disease development.
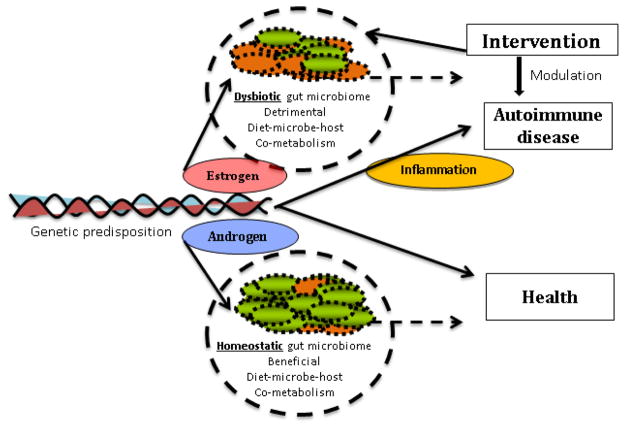
Figure 2. Genetic factors, sex hormones and environment modulate the outcome of gut microbial composition and disease phenotype.
In genetically susceptible individuals, a dysbiotic gut microbiome exacerbates a detrimental diet-microbe-host co-metabolism resulting in an unbalanced or pro-inflammatory mucosal immune environment conducive to autoimmunity. A protective phenotype in healthy individuals is maintained by both genomic and dietary control of a homeostatic colonic ecosystem. Sex hormones can modulate the local immune environment. The impact of estrogen on various immune cells contributes to a hyperactive immune environment while androgens (testosterone) maintain an anti-inflammatory mucosal immune environment. Thus, dietary interventions have the potential to modulate the microbiome composition in susceptible individuals and change disease outcome, especially in affected females.
Table 1.
Bacterial taxa reported in autoimmune disease and their association with sex-biased autoimmunity
| Taxa or bacterial feature | Background | Sex-bias | Ref. |
|---|---|---|---|
| Bacteroidetes:Firmicutes ratio | Low ratios associated with T1D in affected children. Low ratio in genetically arthritis-susceptible humanized mice | NR | [32, 47] |
| Lactobacillus | Potentially protective role in T1D, low abundances in affected children. Also enriched in male mice showing resistance against T1D. Also low abundances in affected female mice in a SLE model. | SLE, T1D | [34, 78, 79] |
| Clostridium, Bacteroides, Veillonella, Allistipes | High abundances associated with high T1D incidence and increased acetate and propionate in distal gut | NR | [79, 80] |
| Bifidobacterium | Low abundance in T1D affected children. High abundance in disease susceptible males (which exhibit less severe RA symptoms). High abundance in arthritis- resistant humanized female mice | RA | [34, 47] |
| Faecalibacterium and Subdoligranulum | Low abundance associated with low butyrate concentrations in distal gut and high T1D incidence. Decreased abundance in (F. prausnitzi) in Multiple Sclerosis | NR | [38, 80] |
| Prevotella and Akkermansia | Low abundance associated with low mucin degradation potential and high T1D incidence | NR | [80] |
| Prevotella copri and Prevotella related taxa | High abundance in RA | NR | [46] |
| Lachnospiraceae | High abundance and prevalence in affected mice in a SLE model. | SLE | ([79] |
| Clostridiales | Increased in collagen induced model of RA | NR | [47] |
| Parabacteroides and Barnesiella | High abundances in disease resistant females (compared to males) in a collagen induced model of RA. Also increased in males (Parabacteroides), with resistant phenotypes for T1D | RA | (6, 10)[47, 71] |
| Roseburia, Blautia, Coprococcus Bilophila | High abundances in susceptible females in a murine T1D model | T1D | [71] |
| Porphyromonadaceae | High abundance in males resistant to T1D in murine model | T1D | [37] |
| Enterobacteriaceae, Peptostreptococcaceae and segmented filamentous bacteria | High abundance in males resistant to T1D in murine model | T1D | [36] |
RA-rheumatoid arthritis, SLE- Systemic lupus erythematosus, T1D- type1diabetes, NR- not reported
5. Conclusions and Future perspectives
The reviewed evidence suggests that specific gut microbiome patterns are associated with autoimmunity. However, the etiology of sex-biased autoimmune disease with regards to, the role of gut microbes and their interactions with hormones and other environmental factors (Figure 2) is still poorly characterized. There is a need to clearly separate the effect of sex background when analyzing the gut microbial ecology of autoimmune disease, an approach that few studies have adopted. Also, it is interesting to see that in many cases in which the effect of sex background on microbial composition has been clearly stated, the bacteria involved are known to have specific dietary roles in the colonic ecosystem (i.e. Clostridium and sugar fermentation) or potential probiotic effects (i.e. Lactobacillus). These observations open the door for exploiting the usability of other “OMIC” based technologies to further unravel the interactions between diet, hosts and gut microbes in sex-biased autoimmunity. Metabolomics surfaces as an attractive tool to identify specific biomarkers that profile the diet-host-microbe co-metabolic environment in stool, urine and plasma in autoimmunity. This approach has been widely implemented in T1D, where disturbance of glucose, lipid and amino acid metabolism as well as of the tricarboxylic acid cycle influenced by gut microbes (bile acids, p-cresol sulfate) are common[74]. In RA, the available reports using metabolomics as an exploratory tool do not specifically address the role of gut microbes in sexual dimorphism and disease etiology [75], but rather, have focused on the effect of specific therapeutic agents on disease progression [76, 77]. As meta-transcriptomics and metagenomics are also incorporated to unravel the role of gut microbes in sex-biased autoimmune disorders, a system-level understanding of disease mechanisms can be achieved. Adopting a systems-biology approach by exploring metabolomics-microbiome-metagenomics and meta-transcriptomics in the colonic ecosystems will make it easier to delineate how diet- microbe co-metabolism converges in sex-biased autoimmunity. This might open new avenues for dietary interventions and design of pre and pro biotics that can be used as therapeutic agents to modulate disease progression.
Highlights.
Interaction between environmental and genetic factors with gut microbiota influences gut immunity
Sex hormones influence autoimmunity via gut microbial composition
Modulation of gut microbial composition may provide a novel target for treatment for autoimmune diseases
Acknowledgments
The authors would like to thank Chella David for providing the transgenic mice used in studies cited in this review. This publication was supported, in part, by the Center of Individualized Medicine and NIH grants AR30752 and AI075262 to VT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogdanos DP, Smyk DS, Rigopoulou EI, Mytilinaiou MG, Heneghan MA, Selmi C, Gershwin ME. Twin studies in autoimmune disease: genetics, gender and environment. Journal of autoimmunity. 2012;38:J156–169. doi: 10.1016/j.jaut.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Lu H, Huang Z, Lin H, Lei Z, Chen X, Tang M, Gao F, Dong M, Li R, Lin L. Apolipoprotein E-knockout mice on high-fat diet show autoimmune injury on kidney and aorta. Biochem Biophys Res Commun. 2014;450:788–793. doi: 10.1016/j.bbrc.2014.06.060. [DOI] [PubMed] [Google Scholar]
- 4.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. Journal of autoimmunity. 2010;34:J258–265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Klareskog L, Padyukov L, Lorentzen J, Alfredsson L. Mechanisms of disease: Genetic susceptibility and environmental triggers in the development of rheumatoid arthritis. Nature clinical practice. 2006;2:425–433. doi: 10.1038/ncprheum0249. [DOI] [PubMed] [Google Scholar]
- 6.Vassallo R, Luckey D, Behrens M, Madden B, Luthra H, David C, Taneja V. Cellular and humoral immunity in arthritis are profoundly influenced by the interaction between cigarette smoke effects and host HLA-DR and DQ genes. Clin Immunol. 2014;152:25–35. doi: 10.1016/j.clim.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu ZZ, Huang H, Wu CH, Jung M, Dritschilo A, Riegel AT, Wellstein A. Omics-based molecular target and biomarker identification. Methods Mol Biol. 2011;719:547–571. doi: 10.1007/978-1-61779-027-0_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taneja V. Arthritis susceptibility and the gut microbiome. FEBS Lett. 2014 doi: 10.1016/j.febslet.2014.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segata N, Boernigen D, Tickle TL, Morgan XC, Garrett WS, Huttenhower C. Computational meta’omics for microbial community studies. Mol Syst Biol. 2013;9:666. doi: 10.1038/msb.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 12.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sommer F, Backhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 15.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nature immunology. 2013;14:676–684. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belkaid Y, Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nature immunology. 2013;14:646–653. doi: 10.1038/ni.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zandman-Goddard G, Peeva E, Shoenfeld Y. Gender and autoimmunity. Autoimmun Rev. 2007;6:366–372. doi: 10.1016/j.autrev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Goodnow CC. Multistep pathogenesis of autoimmune disease. Cell. 2007;130:25–35. doi: 10.1016/j.cell.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 20.Klareskog L, Padyukov L, Ronnelid J, Alfredsson L. Genes, environment and immunity in the development of rheumatoid arthritis. Current opinion in immunology. 2006;18:650–655. doi: 10.1016/j.coi.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nature reviews Rheumatology. 2012;8:573–586. doi: 10.1038/nrrheum.2012.134. [DOI] [PubMed] [Google Scholar]
- 22.Luckey D, Medina K, Taneja V. B cells as effectors and regulators of sex-biased arthritis. Autoimmunity. 2012;45:364–376. doi: 10.3109/08916934.2012.665528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mestecky RM, Jackson J, Michalek S, SMT-HH, Sterzl J. Advances in Mucosal Immunology. Plenum Press; New York: 1995. [Google Scholar]
- 24.Ogra MJ, Lamm PL, Strober ME, Bienenstock WMGJ. J Muocosal Immunology. Academic Press; New York: 1999. [Google Scholar]
- 25.Sansonetti PJ. War and peace at mucosal surfaces. Nature reviews. 2004;4:953–964. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- 26.Tlaskalova-Hogenova H, Tuckova L, Lodinova-Zadnikova R, Stepankova R, Cukrowska B, Funda DP, Striz I, Kozakova H, Trebichavsky I, Sokol D, Rehakova Z, Sinkora J, Fundova P, Horakova D, Jelinkova L, Sanchez D. Mucosal immunity: its role in defense and allergy. International archives of allergy and immunology. 2002;128:77–89. doi: 10.1159/000059397. [DOI] [PubMed] [Google Scholar]
- 27.Mathis D, Benoist C. Microbiota and autoimmune disease: the hosted self. Cell Host Microbe. 2011;10:297–301. doi: 10.1016/j.chom.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Molloy MJ, Bouladoux N, Belkaid Y. Intestinal microbiota: shaping local and systemic immune responses. Seminars in immunology. 2012;24:58–66. doi: 10.1016/j.smim.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 30.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gale EA, Gillespie KM. Diabetes and gender. Diabetologia. 2001;44:3–15. doi: 10.1007/s001250051573. [DOI] [PubMed] [Google Scholar]
- 32.Giongo A, Gano KA, Crabb DB, Mukherjee N, Novelo LL, Casella G, Drew JC, Ilonen J, Knip M, Hyoty H, Veijola R, Simell T, Simell O, Neu J, Wasserfall CH, Schatz D, Atkinson MA, Triplett EW. Toward defining the autoimmune microbiome for type 1 diabetes. The ISME journal. 2011;5:82–91. doi: 10.1038/ismej.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Goffau MC, Luopajarvi K, Knip M, Ilonen J, Ruohtula T, Harkonen T, Orivuori L, Hakala S, Welling GW, Harmsen HJ, Vaarala O. Fecal microbiota composition differs between children with beta-cell autoimmunity and those without. Diabetes. 2013;62:1238–1244. doi: 10.2337/db12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murri M, Leiva I, Gomez-Zumaquero JM, Tinahones FJ, Cardona F, Soriguer F, Queipo-Ortuno MI. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 2013;11:46. doi: 10.1186/1741-7015-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King C, Sarvetnick N. The incidence of type-1 diabetes in NOD mice is modulated by restricted flora not germ-free conditions. PloS one. 2011;6:e17049. doi: 10.1371/journal.pone.0017049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11548–11553. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhargava P, Mowry EM. Gut microbiome and multiple sclerosis. Curr Neurol Neurosci Rep. 2014;14:492. doi: 10.1007/s11910-014-0492-2. [DOI] [PubMed] [Google Scholar]
- 39.Ochoa-Reparaz J, Mielcarz DW, Haque-Begum S, Kasper LH. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut microbes. 2010;1:103–108. doi: 10.4161/gmic.1.2.11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis and rheumatism. 1987;30:1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 41.Ebringer A, Rashid T, Wilson C. Rheumatoid arthritis, Proteus, anti-CCP antibodies and Karl Popper. Autoimmun Rev. 2010;9:216–223. doi: 10.1016/j.autrev.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Warden CC. The Toxemic Factor in Rheumatoid Arthritis. Cal State J Med. 1909;7:299–301. [PMC free article] [PubMed] [Google Scholar]
- 43.Kempsell KE, Cox CJ, Hurle M, Wong A, Wilkie S, Zanders ED, Gaston JS, Crowe JS. Reverse transcriptase-PCR analysis of bacterial rRNA for detection and characterization of bacterial species in arthritis synovial tissue. Infection and immunity. 2000;68:6012–6026. doi: 10.1128/iai.68.10.6012-6026.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moen K, Brun JG, Valen M, Skartveit L, Eribe EK, Olsen I, Jonsson R. Synovial inflammation in active rheumatoid arthritis and psoriatic arthritis facilitates trapping of a variety of oral bacterial DNAs. Clinical and experimental rheumatology. 2006;24:656–663. [PubMed] [Google Scholar]
- 45.Scher JU, Abramson SB. Periodontal disease, Porphyromonas gingivalis, and rheumatoid arthritis: what triggers autoimmunity and clinical disease? Arthritis Res Ther. 2013;15:122. doi: 10.1186/ar4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB, Huttenhower C, Littman DR. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez A, Luckey D, Yeoman CJ, Marietta EV, Berg Miller ME, Murray JA, White BA, Taneja V. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PloS one. 2012;7:e36095. doi: 10.1371/journal.pone.0036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colebatch AN, Edwards CJ. The influence of early life factors on the risk of developing rheumatoid arthritis. Clin Exp Immunol. 2011;163:11–16. doi: 10.1111/j.1365-2249.2010.04263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faria AM, Weiner HL. Oral tolerance: therapeutic implications for autoimmune diseases. Clin Dev Immunol. 2006;13:143–157. doi: 10.1080/17402520600876804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luckey D, Gomez A, Murray J, White B, Taneja V. Bugs & us: The role of the gut in autoimmunity. Indian J Med Res. 2013;138:732–743. [PMC free article] [PubMed] [Google Scholar]
- 51.Wu HJ, Ivanov, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taneja V, Behrens M, Mangalam A, Griffiths MM, Luthra HS, David CS. New humanized HLA-DR4-transgenic mice that mimic the sex bias of rheumatoid arthritis. Arthritis and rheumatism. 2007;56:69–78. doi: 10.1002/art.22213. [DOI] [PubMed] [Google Scholar]
- 53.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eidinger D, Garrett TJ. Studies of the regulatory effects of the sex hormones on antibody formation and stem cell differentiation. The Journal of experimental medicine. 1972;136:1098–1116. doi: 10.1084/jem.136.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez DA, Diaz BB, del Rodriguez Perez MC, Hernandez AG, Chico BN, de Leon AC. Sex hormones and autoimmunity. Immunol Lett. 2010;133:6–13. doi: 10.1016/j.imlet.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Chervonsky AV. Influence of microbial environment on autoimmunity. Nature immunology. 2010;11:28–35. doi: 10.1038/ni.1801. [DOI] [PubMed] [Google Scholar]
- 57.Mangalam AK, Taneja V, David CS. HLA class II molecules influence susceptibility versus protection in inflammatory diseases by determining the cytokine profile. Journal of immunology. 2013;190:513–518. doi: 10.4049/jimmunol.1201891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Behrens M, Trejo T, Luthra H, Griffiths M, David CS, Taneja V. Mechanism by which HLA-DR4 regulates sex-bias of arthritis in humanized mice. Journal of autoimmunity. 2010;35:1–9. doi: 10.1016/j.jaut.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taneja V, Behrens M, Basal E, Sparks J, Griffiths MM, Luthra H, David CS. Delineating the role of the HLA-DR4 “shared epitope” in susceptibility versus resistance to develop arthritis. J Immunol. 2008;181:2869–2877. doi: 10.4049/jimmunol.181.4.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Dore J, Antolin M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, vande Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Merieux A, Melo Minardi R, M’Rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC, Clavel T, Koebnick C, Zunft HJ, Dore J, Blaut M. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Applied and environmental microbiology. 2006;72:1027– 1033. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G, Turroni S, Biagi E, Peano C, Severgnini M, Fiori J, Gotti R, De Bellis G, Luiselli D, Brigidi P, Mabulla A, Marlowe F, Henry AG, Crittenden AN. Gut microbiome of the Hadza hunter- gatherers. Nat Commun. 2014;5:3654. doi: 10.1038/ncomms4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509:357–360. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kovacs A, Ben-Jacob N, Tayem H, Halperin E, Iraqi FA, Gophna U. Genotype is a stronger determinant than sex of the mouse gut microbiota. Microbial ecology. 2011;61:423–428. doi: 10.1007/s00248-010-9787-2. [DOI] [PubMed] [Google Scholar]
- 65.McKnite AM, Perez-Munoz ME, Lu L, Williams EG, Brewer S, Andreux PA, Bastiaansen JW, Wang X, Kachman SD, Auwerx J, Williams RW, Benson AK, Peterson DA, Ciobanu DC. Murine gut microbiota is defined by host genetics and modulates variation of metabolic traits. PloS one. 2012;7:e39191. doi: 10.1371/journal.pone.0039191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, Zhang M, Oh PL, Nehrenberg D, Hua K, Kachman SD, Moriyama EN, Walter J, Peterson DA, Pomp D. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18933–18938. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hughes DT, Sperandio V. Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol. 2008;6:111–120. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adlercreutz H, Pulkkinen MO, Hamalainen EK, Korpela JT. Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. J Steroid Biochem. 1984;20:217–229. doi: 10.1016/0022-4731(84)90208-5. [DOI] [PubMed] [Google Scholar]
- 69.Lombardi P, Goldin B, Boutin E, Gorbach SL. Metabolism of androgens and estrogens by human fecal microorganisms. J Steroid Biochem. 1978;9:795–801. doi: 10.1016/0022-4731(78)90203-0. [DOI] [PubMed] [Google Scholar]
- 70.Ridlon JM, Ikegawa S, Alves JM, Zhou B, Kobayashi A, Iida T, Mitamura K, Tanabe G, Serrano M, De Guzman A, Cooper P, Buck GA, Hylemon PB. Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J Lipid Res. 2013;54:2437–2449. doi: 10.1194/jlr.M038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science (New York, NY. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 72.Ubeda C, Bucci V, Caballero S, Djukovic A, Toussaint NC, Equinda M, Lipuma L, Ling L, Gobourne A, No D, Taur Y, Jenq RR, van den Brink MR, Xavier JB, Pamer EG. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infection and immunity. 2013;81:965–973. doi: 10.1128/IAI.01197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nicholson JK, Holmes E, Wilson ID. Gut microorganisms, mammalian metabolism and personalized health care. Nat Rev Microbiol. 2005;3:431–438. doi: 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- 74.Balderas C, Ruperez FJ, Ibanez E, Senorans J, Guerrero-Fernandez J, Casado IG, Gracia-Bouthelier R, Garcia A, Barbas C. Plasma and urine metabolic fingerprinting of type 1 diabetic children. Electrophoresis. 2013;34:2882–2890. doi: 10.1002/elps.201300062. [DOI] [PubMed] [Google Scholar]
- 75.van Wietmarschen HA, Dai W, van der Kooij AJ, Reijmers TH, Schroen Y, Wang M, Xu Z, Wang X, Kong H, Xu G, Hankemeier T, Meulman JJ, van der Greef J. Characterization of rheumatoid arthritis subtypes using symptom profiles, clinical chemistry and metabolomics measurements. PloS one. 2012;7:e44331. doi: 10.1371/journal.pone.0044331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Madsen R, Rantapaa-Dahlqvist S, Lundstedt T, Moritz T, Trygg J. Metabolic responses to change in disease activity during tumor necrosis factor inhibition in patients with rheumatoid arthritis. J Proteome Res. 2012;11:3796–3804. doi: 10.1021/pr300296v. [DOI] [PubMed] [Google Scholar]
- 77.Wang Z, Chen Z, Yang S, Wang Y, Yu L, Zhang B, Rao Z, Gao J, Tu S. (1)H NMR-based metabolomic analysis for identifying serum biomarkers to evaluate methotrexate treatment in patients with early rheumatoid arthritis. Exp Ther Med. 2012;4:165–171. doi: 10.3892/etm.2012.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lau K, Benitez P, Ardissone A, Wilson TD, Collins EL, Lorca G, Li N, Sankar D, Wasserfall C, Neu J, Atkinson MA, Shatz D, Triplett EW, Larkin J., 3rd Inhibition of type 1 diabetes correlated to a Lactobacillus johnsonii N6.2-mediated Th17 bias. Journal of immunology. 2011;186:3538–3546. doi: 10.4049/jimmunol.1001864. [DOI] [PubMed] [Google Scholar]
- 79.Zhang H, Liao X, Sparks JB, Luo XM. Dynamics of gut microbiota in autoimmune lupus. Applied and environmental microbiology. 2014;80:7551–7560. doi: 10.1128/AEM.02676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, Casella G, Drew JC, Ilonen J, Knip M, Hyoty H, Veijola R, Simell T, Simell O, Neu J, Wasserfall CH, Schatz D, Atkinson MA, Triplett EW. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PloS one. 2011;6:e25792. doi: 10.1371/journal.pone.0025792. [DOI] [PMC free article] [PubMed] [Google Scholar]