Abstract
Background
The success of many neuroscientific studies depends upon adequate head fixation of awake, behaving animals. Typically, this is achieved by surgically affixing a head-restraint prosthesis to the skull.
New Method
Here we report the use of thermoplastic masks to non-invasively restrain monkeys’ heads. Mesh thermoplastic sheets become pliable when heated and can then be molded to an individual monkey’s head. After cooling, the custom mask retains this shape indefinitely for day-to-day use.
Results
We successfully trained rhesus macaques (Macaca mulatta) to perform cognitive tasks while wearing thermoplastic masks. Using these masks, we achieved a level of head stability sufficient for high-resolution eye-tracking and intracranial electrophysiology.
Comparison with Existing Method
Compared with traditional head-posts, we find that thermoplastic masks perform at least as well during infrared eye-tracking and single-neuron recordings, allow for clearer magnetic resonance image acquisition, enable freer placement of a transcranial magnetic stimulation coil, and impose lower financial and time costs on the lab.
Conclusions
We conclude that thermoplastic masks are a viable non-invasive form of primate head restraint that enable a wide range of neuroscientific experiments.
Keywords: Non-human primates, Electrophysiology, Transcranial magnetic stimulation, Eye-tracking, Head-post, Head restraint
1. Introduction
Neuroscientific studies in awake, behaving non-human primates make immense and unique contributions to our understanding of brain function. Intracranial electrophysiological recordings offer unparalleled spatial and temporal precision compared with techniques like functional magnetic resonance imaging (Wurtz and Sommer, 2006), and are of particular value in the monkey due both to structural and functional homologies with human brains and complex cognitive behavior (e.g. Adams et al., 2012; Chang et al., 2013; Goulas et al., 2014, 2014; Grefkes and Fink, 2005; Hutchison et al., 2012; Miranda-Dominguez et al., 2014; Tsao et al., 2008). Such recordings often require the animal’s head to be immobilized to ensure stability of the recording electrode (but see (Roy and Wang, 2012; Schwarz et al., 2014) for examples of wireless recordings in freely-moving primates). Head immobilization further facilitates eye-tracking (Kimmel et al., 2012), a crucial component of many neuroscientific studies. Other techniques facilitated by head fixation include microstimulation (Tehovnik et al., 2006) and drug delivery via intracranial injection (Kurata and Hoffman, 1994; Roy et al., 2014; Sommer and Wurtz, 2006) or a nebulizer (Chang et al., 2012). Recent interest in the neural mechanisms underlying transcranial magnetic stimulation (TMS) has also focused attention on electrophysiological recordings in the monkey before and after TMS (Mueller et al., 2014), and TMS application requires the head to be stationary.
The wide array of neuroscientific methods that depend on primate head fixation has led to the development of several immobilization devices. The majority of these devices involve implantation of screws or bolts into the subject’s skull, as well as the attachment of additional hardware – most commonly a head-post – that protrudes from the head and can be attached to the primate chair during experimental sessions (Adams et al., 2007; Betelak et al., 2001; Evarts, 1968; Foeller and Tychsen, 2002; Porter et al., 1971). An alternative technique is the halo device, in which an aluminum ring surrounds the skull and is attached by several skull pins which require a smaller amount of skin to be removed and shallower penetrations into the skull (Friendlich, 1973; Isoda et al., 2005; Pigarev et al., 1997, 2009).
While the stability and biocompatibility of such implants continues to improve, they still carry five main disadvantages. First, the animal typically needs to be placed under general anesthesia for the initial surgical attachment of the device to the skull. Although routine, anesthesia is a risky procedure, with a wide range of potential harmful side-effects like hypothermia, hemorrhage, aspiration, respiratory insufficiency, cardiovascular emergencies, and death (Thurmon et al., 1996). Second, post-operative recovery can be stressful for the animal since analgesic and antibiotic drugs need to be administered intramuscularly while the animal is awake, and socially-housed animals must be separated from one another. Third, implanted head-restraint devices carry the risk of failure and detachment from the skull, particularly when they are under pressure during repeated head fixation. Implant failure requires an emergency surgical procedure to close the wounds on the animal’s head and/or re-attach the device. Fourth, the protrusion of these devices themselves, as well as the hardware on the primate chair to which they attach, impede the application of TMS. During TMS, the large stimulating coil must lie flush with the scalp, centered over the brain region of interest, and the thick wires attaching the coil to the stimulator must have space to emerge from the set-up. Finally, metal implants on an animal’s head cast shadows during magnetic resonance imaging, thus limiting brain scan utility.
A recent innovation uses a two-piece plastic head mold and a bar clamp holder to restrain monkeys’ heads during experiments, and does not require surgery or metal implants (Amemori et al., 2015). However, the thick plastic mold and large bar clamp create potential barriers to TMS delivery. The design may also limit intracranial recordings or injections in far posterior and temporal regions. Furthermore, the plastic mold covers the animal’s ears, potentially muffling sound and interfering with auditory testing. Finally, while the authors report that they were able to record neuronal activity and gaze position stably using this head-fixation technique, they note that animals do still move their heads during reward delivery, a potential confound for studies of reward-related processing.
Here we describe a non-invasive means of primate head restraint that mitigates the problems described above. Thermoplastic masks are plastic mesh sheets that become soft upon heating and can be molded to any shape, which they retain when cooled. Machado & Nelson (2011) adapted thermoplastic masks, which are used in human radiotherapy to stabilize the head, for eye-tracking in macaques. We expand upon their innovation by using these masks for electrophysiological recordings and TMS in macaques. We present a comprehensive comparison of the performance of thermoplastic masks and traditional head-posts on multiple measures relevant to neurobiology, including single-unit recording stability, eye-tracking accuracy, TMS coil placement, MRI clarity, and financial cost to the lab. We find that thermoplastic masks compare favorably with implanted head-posts on each of these metrics.
2. Materials and Methods
All procedures were approved by Duke University Medical Center’s Institutional Animal Care and Use Committee.
2.1 Subjects
Subjects were 24 adult rhesus macaque monkeys (Macaca mulatta), 10 female and 14 male, housed individually or in pairs at the Duke University Medical Center. 18 monkeys had head-posts, 5 monkeys had thermoplastic masks, and 1 monkey had a head-post that failed and was subsequently given a thermoplastic mask. Information from all monkeys was used in calculating cost to the lab. Eye-tracking data were collected from one mask monkey (Monkey Fe: female, 19 years old, 7.6kg) and one post monkey (Monkey Br: male, 17 years old, 13.4kg). Neuronal recording data were collected from one mask monkey (Monkey Sc: female, 17 years old, 6.8kg) and one post monkey (Monkey Da: male, 17 years old, 10kg). Implant size data were collected from two mask monkeys (Monkey Fr: female, 7 years old, 5.2kg; Monkey Sc: female, 17 years old, 6.8kg) and two post monkeys (Monkey Go: female, 7 years old, 5.4kg; Monkey Br: male, 17 years old, 13.4kg). Magnetic resonance images were collected from one mask monkey (Monkey Sc: female, 17 years old, 6.8kg) and one post monkey (Monkey Br: male, 17 years old, 13.4kg).
2.2 Thermoplastic masks
2.2.1 Fitting
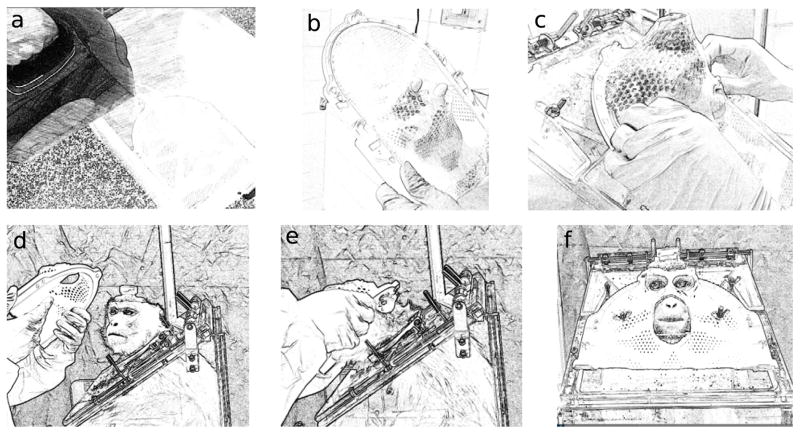
The process of fitting a thermoplastic mask and applying it to an awake monkey is shown in Figure 1. We used a commercially-available reinforced thermoplastic mask (Type-S IMRT Reinforced Style 22 Mask, product number MTAPUID2232, from CIVCO Medical Solutions, Coralville, Iowa, USA). Since the mask would be used while the monkey was seated in a primate chair, it needed to be molded while the monkey was in a chair, since the angle of the monkey’s head relative to the chair neckplate needed to be replicated. For mask fitting, the animal was sedated with ketamine (3mg/kg, intramuscular (IM)) and dexdomitor (0.075–0.15mg/kg, IM), as well as ondansetron (0.15mg/kg, IM) in cases where animals were known to become nauseated due to sedation. The monkey’s head and face were shaved to achieve a closer fit, and petroleum jelly or vitamin E oil was applied to the animal’s head and any existing implant (i.e. a recording chamber), as well as the top of the primate chair, to make the mask easier to remove. Ophthalmic lubricant was applied to the animal’s eyes. The thermoplastic mask was submerged in hot water (at least 165°F) until soft. It was then removed from the water and allowed to cool until it was comfortable to touch with a bare hand. A hole was cut near the center of the mask where the animal’s nose would be to enable breathing. The monkey was then held in place in a primate chair by an experimenter who ensured that the animal’s head and neck stayed in a proper position for normal breathing. Another experimenter placed the thermoplastic mask on the monkey’s head and molded it to fit the contours of the face and head, with an emphasis on the bridge of the nose and the brow ridge. Adequate space was left at the back of the head and sides of the muzzle and neck such that the mask could be removed once it hardened. Locations for eye and mouth holes were marked on the mask. Once the mask had hardened, it was removed and the animal was returned to its cage and sedation was reversed with antisedan at the same dose volume as dexdomitor. Holes were cut in the eye and mouth regions of the mask such that the animal would be able to see and drink while wearing it. This entire procedure typically lasted 20–30 minutes. If any further modifications to the mask were needed, such as widening areas that seem too tight on the animal, a heat gun or boiling water was used to soften and reshape parts of the mask while it was off the animal. Bicycle helmet padding (Helmet Foam Kits, USA) was also attached inside some masks to improve comfort and stability by enabling a tighter fit while protecting the animal’s skin. Screws were placed through the top of the primate chair so that the mask could be attached to it using two to four wing nuts or bolts.
Figure 1. Fitting and applying a thermoplastic mask.
First the mask is submerged in hot water (a). Then a hole is widened for the animal’s nose and mouth to enable breathing (b). Next the mask is stretched and molded over the sedated animal’s head while she is propped up in the chair used during experiments (c). After cutting holes for the monkey’s eyes, nose, and mouth, the mask is placed on the awake animal’s head for day-to-day use as shown in (d), (e), and (f).
2.2.2 Training
We began training monkeys to accept the thermoplastic masks as early as the day after mask fitting. We used positive reinforcement to gradually condition the monkeys to wear the masks in their primate chairs, providing juice and treats as reinforcement during each of the following phases: 1) Presented the mask to the monkey for increasing amounts of time and increasing proximity to its head; 2) Touched the mask to the monkey’s head and face for increasing amounts of time; 3) Placed mask partway over the monkey’s head for increasing amounts of time; 4) Placed mask fully over the monkey’s head for increasing amounts of time; 5) Attached mask to chair neckplate for increasing amounts of time. For some monkeys it was helpful to offer a syringe of juice through the mask’s mouth hole in order to properly position their heads for the mask to be attached to the chair. While this training sequence has been used successfully in our lab, other strategies may be just as effective for habituating different monkeys.
2.3 Head-post implantation
Affixing a head-post to a monkey’s skull required aseptic surgical conditions. The animal was pre-anesthetized with ketamine (10mg/kg, IM) and atropine sulfate (0.015mg/kg, IM) or ketamine (3mg/kg, IM) and dexdomitor (0.075–0.15mg/kg, IM); ondansetron (0.15mg/kg, IM) was also administered in cases of unplanned (emergency) surgeries, or in cases where animals were known to become nauseated due to sedation. When sufficiently sedated, the animal was placed on a mobile treatment table and transported, covered, to the surgical pavilion. Blood samples (3mL) for running blood chemistry panels were taken, and an intravenous catheter was placed in the saphenous vein using aseptic technique. To anesthetize the animal sufficiently for intubation (as determined by corneal blink reflex and pedal reflex), the animal was masked (isofluorane, 2–5%) and/or given propofol at a dose of 2–5mg/kg intravenously to effect, with repeated doses administered as needed for surgical induction. The animal was then intubated and placed on inhalant anesthesia, typically isofluorane at 0.5–5%. Monitors for cardiac (EKG and pulse-oximeter) and respiratory (thermal respiration sensory) function were then placed and temperature was monitored rectally and maintained with hot water pad heaters. If the surgery was expected to require more than one hour to complete, replacement fluids were provided (typically Normosol-R, or similar) at 5–10 ml/kg/hr. The animal was placed sternally in a stereotaxic instrument (David Kopf Instruments, Tujunga, California, USA). Surgeons (typically 2) then scrubbed and gowned while a third person, administering anesthesia, monitored the animal. The site of implantation on the animal’s head was shaved and scrubbed at least three times with a chlorhexadine disinfectant alternating with alcohol. The subsequent procedure varied depending on whether the animal was receiving a footed or non-footed head-post, which depended on the animal’s surgical history.
2.3.1 Non-footed head-post
A portion of the dorsal scalp was removed and the skin bordering the incision was retracted. The periosteum of the exposed bone was removed, and, if necessary, a portion of the temporal muscle was retracted or removed. A sterile drill was then used to cut 4–6 small holes. These holes were then tapped using an orthopedic bone tap, and surgical bone screws (typically surgical-grade titanium) were implanted in these prepared holes (equipment and bone screws typically by Synthes, Westchester, Pennsylvania, USA; Veterinary Orthopedic Implants, St. Augustine, Florida, USA; or Bristol-Myers/Zimmer, Warsaw, Indiana, USA). A sterile titanium head restraint post (Crist Instruments, Damascus, Maryland, USA, or similar) was lowered stereotaxically into contact with the skull. The post and screws were then bonded together with sterile orthopedic bone cement (Cobalt HV or GHV (Biomet, Warsaw, Indiana, USA) or similar), or dental acrylic (Jet Denture Repair Acrylic (Lang Dental, Wheeling, Illinois, USA) or similar). The wound was then checked for tight opposition to the cranial implant. Typically, tight opposition between the wound margin and the implant was achieved solely through the elasticity of the skin. If opposition between the wound margin and the implant was compromised, however, the wound margin was secured to the implant using sterile surgical adhesive (VetBond (3M, Maplewood, Minnesota, USA). If opposition of the wound margin and the implant could not be achieved in this fashion, small incisions with Z-plastic re-anastamoses were performed to reduce any space between the wound margin and the implant. In this procedure, small incisions were made at oblique angles to the wound margin and small lenticular pieces of scalp were removed. The edges of this incision were then stitched together, thereby removing the slack in the skin and bringing the wound margin into tight contact with the cranial implant. Once the wound margin was secured to the implant, the implant margin was treated topically with triple antibiotic ointment (neomycin, polymyxin, bacitracin; Henry Schein Inc., Melville, New York, USA). This entire procedure typically lasted between 1.5 and 4 hours.
2.3.2 Footed head-post
When possible, a “footed” head-post (Adams et al., 2007) was used to reduce the overall size of the implant and wound margin. In this procedure, a sagittal incision was made in the scalp at the desired position of the head-post and the skull was exposed. A sterilized footed titanium head-post (DKW Precision Machining, Manteca, California, USA or similar) was lowered into position and was visually analyzed to determine the degree to which each foot would need to be bent in order to fit snugly against the skull. The post was them removed and the feet were bent to match the curvature of the skull by bending them with sterile “bending forks” and/or tapping them against a sterilized metal rod with a sterile hammer. The head-post was again lowered into position to check the fit; subsequent adjustments to the curvature of the feet were sometimes necessary to ensure the closest fit possible to the skull. Once a close fit was achieved, the screw holes on opposite side of the post were marked on the skull using a surgical pen and drilled and tapped using sterile instruments. Titanium or ceramic bone screws (Synthes; Thomas Recording, Giessen, Germany; or similar) were then driven into each hole. Once all screws were in place and were sufficiently tight, hydroxyapatite bone matrix material (Mimix (Biomet) or similar) was used to fill any gaps that existed between the skull and the feet to facilitate bone growth under and over the titanium prosthetic. Once complete, the area was irrigated with physiological saline, and the incision was closed, first the galea, with absorbable suture, followed by the skin. This entire procedure typically lasted between 1.5 and 4 hours.
2.3.3. Recovery
Following surgery, a course of analgesic (buprenorphine, 0.01mg/kg, every 6–12 hours for 72 hours), antibiotic (enrofloxacin, 5mg/kg, once daily for 7–10 days), and anti-inflammatory (ketorolac tromethamine, 1mg/kg, once daily for 3 days) began. The animal was also observed for abnormal behavior or physical signs indicative of neurological insult and sometimes received additional drugs (including dexamethasone trisodium phosphate, midazolam, lorazepan, diazepam, and cefazolin) as necessary per veterinary consult. Following a first head-post implantation, animals were allowed at least 28 days for recovery. In the case of subsequent head-post surgeries, animals were allowed at least 7 days for recovery.
2.3.4 Training
We began conditioning monkeys to head fixation after the recovery period. As with mask training (Section 2.2.2) we used positive reinforcement, delivering juice or treats when monkeys allowed us to touch their head-post, then to secure their post in the post-holder attached to the chair for increasing amounts of time.
2.4 Eye-tracking
Gaze position was sampled at 1000 Hz using an Eyelink 1000 infrared eye-tracker (SR Research, Mississauga, Ontario, Canada). Monkeys performed a visual orienting task that began when a 1° white square appeared at the center of an otherwise black screen. Monkeys had to fixate within 1.5° of the center of the white square (400 ms). A second white 1.5° square then appeared in one of eight evenly distributed locations 10° in the periphery. As soon as the target appeared, the central fixation point disappeared, and the monkey was required to shift gaze to the target (±2.5°) and maintain fixation (300 milliseconds) to receive a juice reward. Stimulus presentation and data collection were performed using MATLAB (The Mathworks, Natick, Massachusetts, USA) and the Psychophysics Toolbox (Brainard, 1997; http://psychtoolbox.org/).
We compared data from one session with a mask monkey and one session with a post monkey. Analyses were performed using custom-scripts written in MATLAB. We first considered drift in fixation position over the course of the session by fitting linear models separately to the horizontal and vertical gaze positions of each monkey on each trial for the first 450 trials in the session, where a value for each trial was determined by taking the mean over all eye samples during the fixation period on that trial. We used t-tests to compare the slopes (beta coefficients) of the regression lines for the horizontal gaze position between monkeys, and the vertical gaze position between monkeys. Next we considered reliability of saccadic endpoints by taking the mean of the horizontal and vertical gaze positions over all eye samples during the target fixation period, and computing the variances of the horizontal and vertical positions over 40 trials for each target location. We used F-tests to compare the variances at each target location between monkeys.
2.5 Neuronal recording
2.5.1 Recording chamber implantation
Sterile surgeries were performed on several animals in order to implant cylinders permitting electrode/cannula access to the brain. In this procedure, the monkey was anesthetized and prepared for surgery as described above (Section 2.3). The anesthetized animal was placed in a stereotax (David Kopf Instruments). The placement of cylinders was determined by the stereotaxic coordinates of the target brain structure. Sterile surgeons first removed a circular region of scalp over the pre-selected stereotaxic coordinates. The periosteum was removed and, if necessary, a portion of the temporal muscle was retracted or removed. A circular section of skull was then removed using a sterile drill and bits or a sterile trephine. Two to six additional surgical screws were implanted as above (Section 2.3). A stereotax was used to lower the sterile cylinder (titanium or polysulfone, Crist Instruments) over the opening in the skull. A sterile bone replacement material (Mimix (Biomet)) was used to fill any gaps that existed between the skull and the base of the recording chamber (Adams et al., 2007). The cylinder was then bonded to the skull and screws with sterile bone cement (Cobalt GHV or HV (Biomet)) or dental acrylic (Jet Denture Repair Acrylic (Lang Dental)). The interior of the cylinder was then washed with sterile saline and in some cases antibiotic (typically Gentamicin: 0.62cc, 40mg/ml concentration), dried, and closed with a sterile cap which mates onto the cylinder. This entire procedure typically lasted between 1.5 and 4 hours. Following surgery, a course of analgesic (buprenorphine, 0.01mg/kg, every 6–12 hours for 72 hours), antibiotic (enrofloxacin, 5mg/kg, once daily for 7–10 days), and anti-inflammatory (ketorolac tromethamine, 1mg/kg, once daily for 3 days) began. The animal was also observed for abnormal behavior or physical signs indicative of neurological insult and sometimes received additional drugs (including dexamethasone trisodium phosphate, midazolam, lorazepan, diazepam, and cefazolin) as necessary per veterinary consult.
2.5.2 Electrophysiological data collection & analysis
After a post-operative recovery period, neuronal recording studies began. At the beginning of each recording session, the animal was head-restrained and the sterile recording cylinder was opened under aseptic conditions. The cylinder was cleaned with 1% povidone-iodine and then repeatedly lavaged with 0.9% sterile saline. A grid (Crist Instruments) was inserted into the cylinder, and a sterile guide tube was placed through a grid hole and then through the animal’s dura mater. A hydraulic microdive (David Kopf Instruments) was mounted on the recording cylinder, and a tungsten electrode about 0.005 inches in diameter with an impedance between 1 and 15 megaohms (FHC, Bowdoin, Maine, USA) was inserted into the guide tube and attached to the microdrive so it could be moved through the brain microns at a time. As the electrode advanced, voltage at its tip was processed through a series of amplifiers and computer software (Plexon, Dallas, Texas, USA). At the end of the daily session the electrode was withdrawn, the guide tube, grid, and microdrive were removed, and the cylinder was again lavaged with 1% povidone-iodine and sterile saline, then resealed with a sterile cap.
Data from one recording session with a mask monkey and one recording session with a post monkey were analyzed here. Plexon’s Offline Sorter was used to automatically sort waveforms as action potentials from an isolated unit or as background activity. In addition to visually inspecting the voltage traces over time, stability of the recording was quantified using the 2D Pseudo-F metric of sort quality provided by Plexon, including unsorted waveforms as a unit for comparison.
2.6 Measurement of head movement
In order to directly assess how effective the thermoplastic masks were for head restraint, we took measurements of head movement using a gyroscope. Specifically, we attached an iPhone 6 (Apple Inc., Cupertino, California, USA) via an adhesive strip to the recording cylinders of two mask monkeys and two post monkeys. We sampled rotational velocity in each of three axes at near 100Hz using the Sensor Kinetics Pro application (Innoventions Inc., Houston, Texas, USA; http://www.rotoview.com/sensor_kinetics.htm) for ten minutes while juice was periodically delivered to the monkey. We obtained power spectra for each animal’s movement in each axis by using a Fast Fourier Transform in MATLAB.
2.7 Magnetic resonance imaging
To localize brain regions for electrophysiology and TMS studies, high-resolution structural MRI’s of monkeys’ brains were obtained. Since the animal needed to be completely still during scanning, the monkey was sedated using ketamine, dexdomitor, and sometimes ondansetron (see Section 2.2) and then placed on inhalant anesthesia (see section 2.3) for the duration of the scan. Scans were performed with a 3-Tesla (Trio, Siemens Medical Systems, Munich, Bavaria) MR Imaging and Spectroscopy System at Duke University Medical Center’s Center for Advanced Magnetic Resonance Development. During scans, the monkey’s recording chamber was filled with sterile saline, and non-ferrous electrode tips were placed in a grid within the chamber, so that the chamber would be visible in the MR images and recording electrode trajectories could be determined. The scan sequence typically included localizers, coronal T1 MP-RAGE (magnetization-prepared rapid acquisition with gradient echo) 1mm, coronal T2 0.5mm, sagittal T1 MP-RAGE 1mm, and sagittal T2 0.5mm, and could also include a magnetic resonance angiogram, diffusion tensor imaging, and resting-state functional MRI.
2.8 Calculation of cost
Using records of all animals in our lab, we counted how many times each animal had been sedated or undergone surgery for a procedure related to head fixation. We also noted the dates over which the animals had a head-post or thermoplastic mask, so that we could compute the number of sedations and surgeries per year during the relevant periods of their lives. We summed the price of all materials and procedures typically used in post- or mask-related sedations and post-related surgeries, and computed a total cost for each animal based on which type of head-fixation-related procedures s/he had undergone, then divided this by the number of years s/he had a post or mask to produce the cost per year. In averaging these values to compute the cost per animal per year for posts and for masks, each animal was considered either a “post” monkey or a “mask” monkey, except for one animal who was considered a “post” monkey for an initial epoch, and a “mask” monkey for a second epoch after her post failed and she was fitted with a thermoplastic mask.
3. Results
First, we note that conditioning monkeys to wear the thermoplastic masks (see Section 2.2.2) generally took no more than two weeks, equivalent to head-post training (unpublished observations). Monkeys readily performed cognitive tasks for juice rewards requiring various response modalities, including eye movements, joysticks, and touch screens. In the sections that follow, we offer comparisons between the masks and posts on eye-tracking accuracy, neuronal recording stability, head movement, MRI clarity, TMS coil placement, and cost to the lab.
3.1 Eye-tracking accuracy
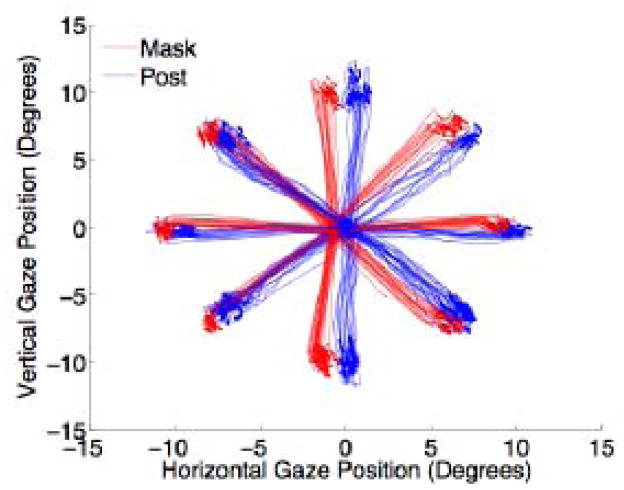
We successfully trained a monkey wearing a thermoplastic mask to perform eye movement tasks with similar precision to that of a monkey with a head-post. Figure 2 shows sample eye traces during the first 120 trials of a visual orienting task (see Section 2.4) for each monkey. At each of the eight target locations, variance in saccade end point was no higher for the mask then the post monkey. Additionally, there was less drift in central fixation position for a fully-trained mask monkey than a fully-trained post monkey over the course of a session (regression slopes for horizontal gaze position, mask: −0.00032, post: −0.00061; for vertical gaze position, mask: −0.00065, post: 0.00118) (Figure 3), indicating that eye tracking with a mask is at least as stable as with a post.
Figure 2. Eye position traces during a saccade task.
Monkeys were required to fixate centrally until a peripheral target appeared at one of eight locations, and then make a saccade to the target. Red and blue curves show the saccade trajectories during the first 120 trials of the session for the mask and post monkey, respectively.
Figure 3. Drift in fixation position.
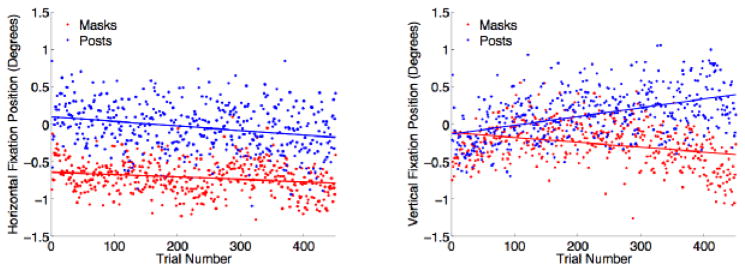
Each point shows the average position of gaze on each trial in the horizontal (left) and vertical (right) direction for the mask monkey (red) and the post monkey (blue). Lines fit by linear regression.
3.2 Neuronal recording stability
Thermoplastic masks also provided sufficient stability to perform intracranial single-unit electrophysiology. Example action potential voltage traces recorded from the parietal cortex of a mask and a post monkey at the beginning and 50 minutes into the session are shown in Figure 4. The similarity in waveform between these two time points illustrates the stability of our recordings. The Pseudo-F value provided by Plexon’s Offline Sorter, which would be maximal if all waveforms over the course of the session had the same shape and amplitude, was 32020 for the post monkey and 36290 for the mask monkey, indicating that the quality of isolation was at least as high for the mask monkey as the post monkey.
Figure 4. Neuronal recording stability.
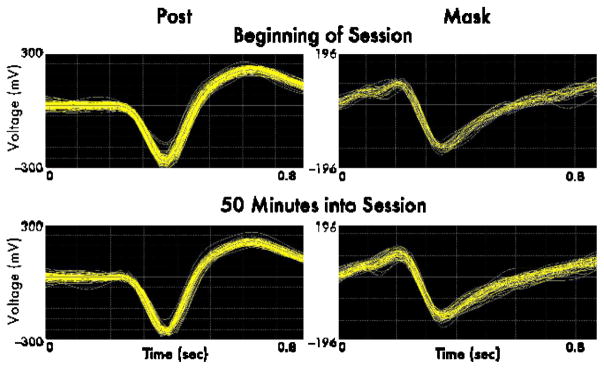
Sample voltage traces of action potentials recorded from a post monkey (left) and a mask monkey (right) are shown over 10 seconds at the beginning of a session (top), and 50 minutes into the session (bottom). Data were collected and spikes were sorted in Plexon.
3.3 Head movement
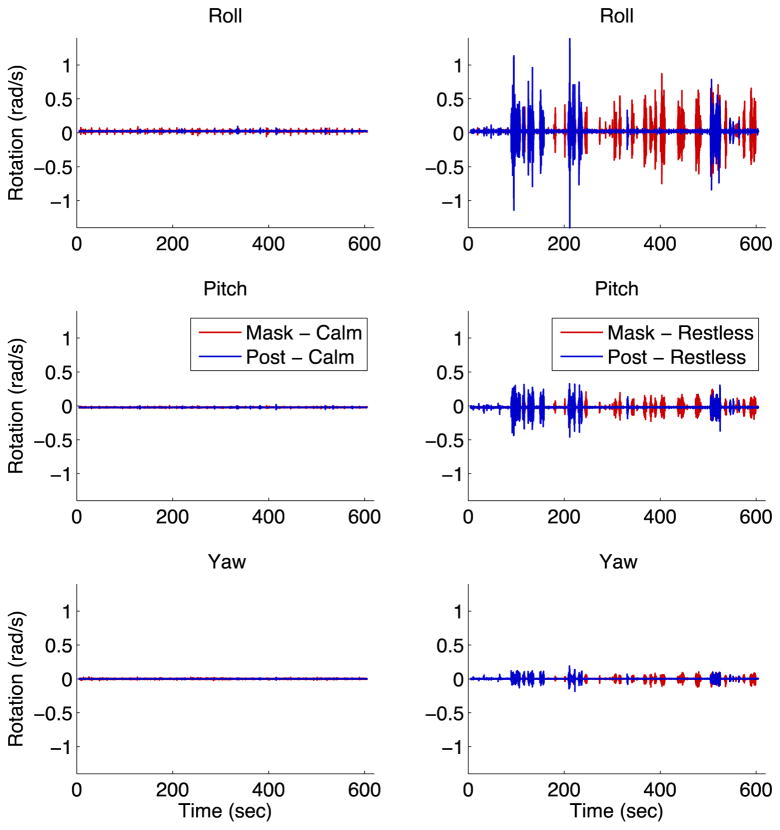
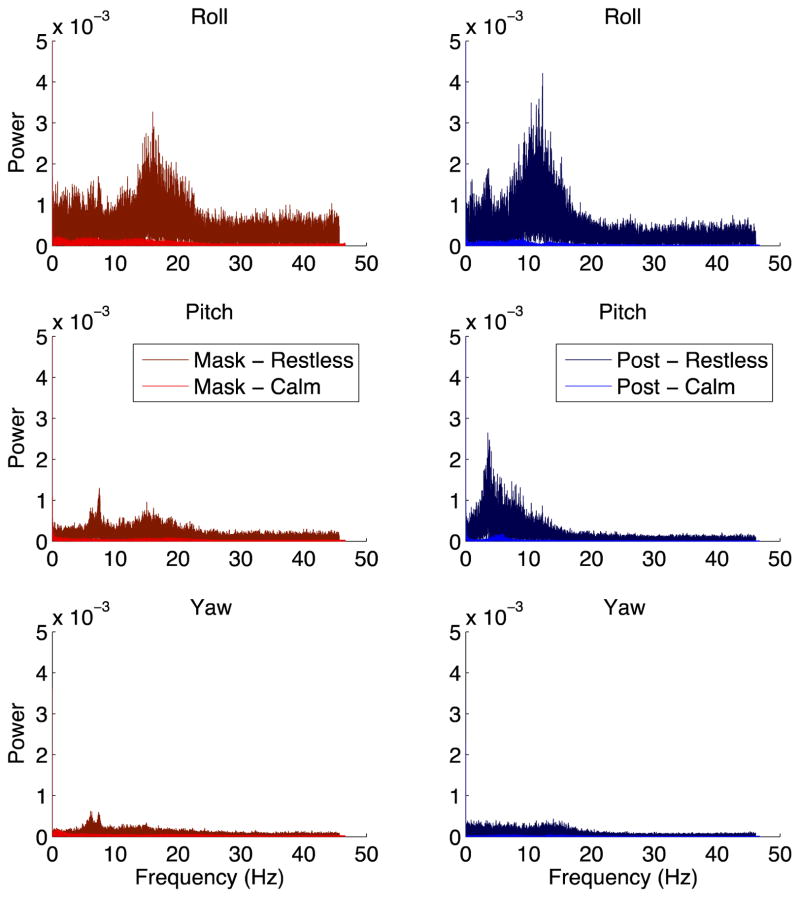
To further test whether thermoplastic masks truly achieve head fixation comparable to head posts, we directly measured head movement at the location of animals’ recording cylinders using a gyroscope. We collected data from two mask and two post monkeys, including one calm and one restless monkey in each condition. We found that the raw movement traces were similar between mask and post monkeys (Figure 5), and the power spectra of these movement traces were also similar (Figure 6). These results demonstrate that thermoplastic masks restrain the head just as well as head posts.
Figure 5. Head movement traces.
Ten minutes of rotational velocity data recorded from the heads of mask and post monkeys are shown. The left column shows a calm mask monkey (red) and a calm post monkey (blue), while the right column shows a restless mask money (red) and a restless post monkey (blue). The top row shows rotation around the “roll” axis, the middle row shows rotation around the “pitch” axis, and the bottom row shows rotation around the “yaw” axis.
Figure 6. Power spectra of head movement.
Following a fast Fourier transformation, the power spectra of head rotational velocity in each of the three axes – roll (top), pitch (middle), and yaw (bottom) – is shown for two mask monkeys (left column), one calm (brighter color) and one restless (darker color), and two post monkeys (right column), one calm and one restless. These plots represent the same data as in Figure 5.
3.4 MRI clarity
For neuronal recording and stimulation studies, it is useful to obtain high-resolution structural brain scans to localize target regions. While our monkeys’ head-posts and bone screws are nonferrous and therefore safe to introduce into the MRI scanning environment, they are still metal and cast a “shadow” (that is, create local distortions) on the resulting image, due largely to metal-induced field inhomogeneities which interfere with the imaging gradient fields (Lu et al., 2009; Lüdeke et al., 1985). Thus it has been our experience that MRIs of monkeys without a head-post – i.e. monkeys that wear thermoplastic masks – are clearer and easier to interpret than those of monkeys with a head-post. Sample MR images from mask and post monkeys are shown in Figure 7 to demonstrate this point.
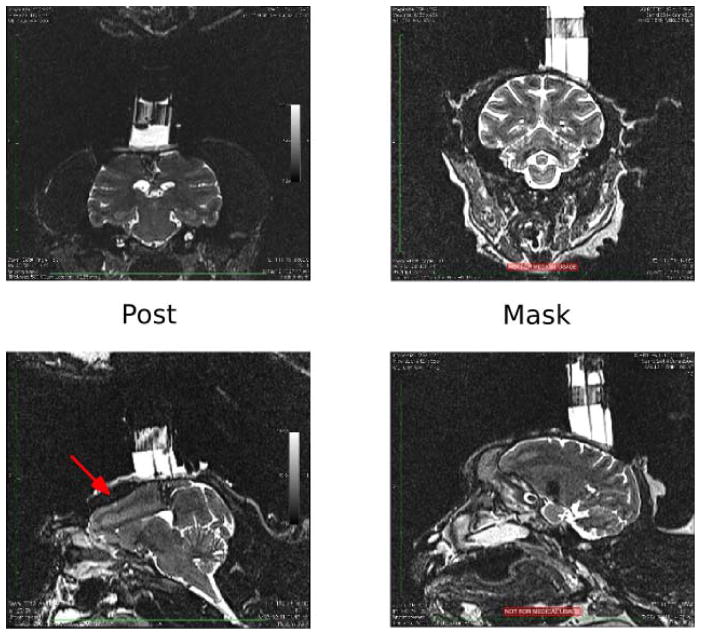
Figure 7. Magnetic resonance images.
Sample MRI images from head-post monkey (left), and a mask monkey (right). Coronal sections (top) and sagittal sections (bottom) are shown through the center of each monkey’s recording cylinder over parietal cortex. Red arrow in bottom-left image indicates shadow cast by post.
3.5 TMS coil placement
To effectively stimulate a particular brain region with TMS, the stimulating coil must lie as close to the scalp as possible over the region of interest. The larger the cranial implant an animal has, the fewer brain regions can be reached by TMS. Animals without head-posts either do not need an implant at all or need an implant only large enough to support a recording cylinder. We measured the implant sizes of our two monkeys who have thermoplastic masks and recording cylinders, as well as two monkeys with head-posts and recording cylinders in similar locations to the mask monkeys. As the irregular shape of these implants makes it difficult to estimate their volume, we report the length (anterior-posterior), width (medial-lateral), and height (dorsal-ventral) of each implant at its longest point in each direction. Implants were smaller for mask than post monkeys. For the two monkeys with frontal pole cylinders, sizes were 4.7cm long, 4.4cm wide, 1.3cm high (mask), and 8.5cm long, 6.5cm wide, 1.5cm high (post); and for the two monkeys with parietal cylinders, sizes were 6.0cm long, 4.5cm wide, 0.9cm high (mask), and 7.5cm long, 5.0cm wide, 0.7cm high (post).
We further note that lack of a head-post allows the stimulating coil more freedom to be moved around the head. There are two reasons for this: first, the head-post itself can obstruct the coil’s placement. Second, stabilizing a monkey’s head via a head-post requires a post-holder above the chair. This is typically attached to the chair via two side panels to the left and right of the monkey’s head. These panels further block the stimulating coil, and they are unnecessary with thermoplastic masks (see Figure 8). The only disadvantage of the mask for TMS coil placement is that it is 0.3cm thick, which could increase the distance between the coil and the brain. However, in our applications, we stimulate around the recording cylinder, where we have cut away part of the mask and where distance between coil and scalp is determined by the thickness of the bone cement.
Figure 8. TMS coil placement.
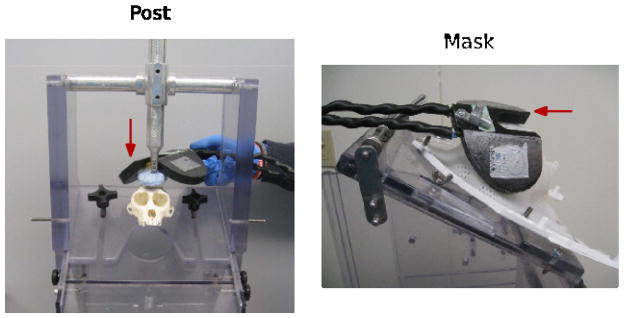
The chair set-up for a head-post (left) partially blocks the movement of the TMS coil around the head, whereas the chair set-up for a mask (right) enables much more latitude in coil placement. Red arrows indicate TMS coil.
3.6 Cost to lab
We first computed the total cost of all the resources required for a typical head-post surgery ($1522.93), a typical non-surgical head-post-related procedure requiring sedation ($63.86), and a typical thermoplastic mask-fitting procedure ($133.87). We then used these values to determine the cost per year of each animal’s head-fixation-related procedures (see Section 2.8). We found that thermoplastic masks cost substantially less than head-posts (mean cost per animal per year: masks, $238, standard error of the mean (SEM) = $233; posts, $1166, SEM = $65; two-sample t-test: t(23) = 2.193, p = 0.0387).
Additionally, we considered the number of research days lost due to head-fixation-related procedures as an important non-monetary cost. Head-post surgeries require several days of recovery time before the animal can be used in an experiment (see Section 2.3.3), whereas the short-term sedations required for mask-fitting enable the animal to be used the next day. We computed the number of research days lost due to surgeries and procedures requiring short-term sedations related to head fixation for each animal, and divided this number by the number of years the animal spent in the lab with a head fixation device, to determine the number of research days lost per animal per year. We found that thermoplastic masks resulted in substantially fewer research days lost than head-posts (mean number of days lost per animal per year: masks, 1.78, SEM = 0.49; posts, 13.55, SEM = 2.24; two-sample t-test: t(23) = 2.897, p = 0.0081).
4. Discussion
Here we show that thermoplastic masks can be used effectively to restrain the head in non-human primates for neurobiological studies. Fitting masks to individual rhesus macaques is a brief, simple procedure that is easy to learn. Once created, these non-invasive masks can be used day after day to restrain monkeys’ heads during experiments. Although monkeys were initially wary of these novel objects, they quickly became conditioned to wearing them through positive reinforcement, and performed cognitive tasks using eye movements, joysticks, and touch screens. Moreover, we were able to record intracranial neuronal activity from animals wearing these masks.
Thermoplastic masks yield a degree of head fixation comparable to traditional head-restraint prostheses. We assessed this directly by recording rotational head movement using a gyroscope affixed to monkeys’ recording cylinders. Similar amounts of movement were observed in monkeys wearing masks and those with head-posts. Additionally, we examined gaze position data during a saccade task, a common paradigm in neuroscientific studies. Drift in eye-tracker accuracy over the course of a behavioral session, as well as variability in saccade endpoint, were at least as low for a monkey wearing a mask as for one with a head-post. Neuronal recording stability was also similar between mask and post subjects. Thus thermoplastic masks may be used instead of head-posts in experiments involving intracranial electrophysiology or high-resolution eye-tracking.
Another neuroscientific method requiring head fixation is TMS. TMS is an increasingly popular method for noninvasive neuromodulation in humans (Hovington et al., 2013; Luber and Lisanby, 2014; Sandrini et al., 2011). Some labs have begun to use TMS in monkeys in order to explore the contribution of various brain regions to cognitive tasks (Gerits et al., 2011; Gu and Corneil, 2014; Valero-Cabre et al., 2012) and which could be used to study the connectivity between regions. Other labs – including ours (Mueller et al., 2014) – are applying TMS to monkeys in combination with intracranial electrophysiological recordings in order to explore the neural basis of its effects. Subjects’ heads need to be still during TMS application. By using thermoplastic masks rather than head-posts, more of the monkey’s head is accessible, enabling better targeting of TMS to particular brain regions.
Thermoplastic masks offer other advantages over head-posts. For example, the reduced amount of metal on monkeys’ heads results in clearer magnetic resonance images, which are useful for targeting brain regions of interest during neuronal recordings or stimulation. Additionally, fitting a thermoplastic mask results in fewer research days lost and lower monetary costs to the lab than implanting a head-post. Finally, masks are preferable from an animal welfare perspective since they are completely non-invasive and do not require subjects to undergo general anesthesia.
Although we did not directly compare thermoplastic masks to the recently-developed head mold technique (Amemori et al., 2015), we note some ways in which masks might be preferable. First, as previously mentioned, the head mold is quite thick and covers the far posterior and temporal regions of the animal’s head, making TMS coil placement and intracranial recordings in those regions difficult. With thermoplastic masks, a TMS coil can be moved freely around the head, and an opening for a recording chamber could be cut from any part of the mask. The masks also should not muffle sound as the head molds might. Furthermore, thermoplastic masks may provide better head stability: Amemori and colleagues note that animals’ heads did move during reward delivery when restrained with a head mold, which we did not observe in our subjects (although note that we did not directly measure head movement as Amemori and colleagues did), and the gaze fixation window in their ocular task was 8° compared with 1.5° for the central fixation point and 2.5° for the peripheral targets in our task. Nonetheless, Amemori and colleagues were able to perform single-unit electrophysiology using a head mold. One potential advantage of the head mold design over masks is that the head molds might be more comfortable for an animal, since its head can move slightly and nothing touches its face. Also, the masks largely prevent delivery of an air-puff stimulus to the animal’s face, which the head mold design appears to allow. Finally, the masks obscure facial movements, which could preclude visual communication in social tasks; the head mold design leaves most of the animal’s face visible.
5. Conclusion
Head fixation of awake, behaving monkeys can be achieved non-invasively using thermoplastic masks. Various types of experiments may be performed with monkeys wearing masks, including those involving cognitive tasks, eye-tracking, intracranial electrophysiology, and transcranial magnetic stimulation. These masks compare favorably to traditional head-posts in terms of experimental outcomes, cost to the lab, and animal welfare. Thus thermoplastic masks may be added to the toolkit of options available that stabilize monkeys’ heads for neuroscientific studies.
Highlights.
Individualized thermoplastic masks restrain awake monkeys’ heads non-invasively.
Masks suppress movement sufficiently for electrophysiology and eye-tracking.
Compared to head-posts, masks cost less and better enable MRI and TMS.
Acknowledgments
We would like to thank Dr. Chris Machado for helpful advice on fitting thermoplastic masks to monkeys; Benjamin Africk, Gregory Gedman, Andrew Luo, K. M. Sharika, Amanda Utevsky, Nicole Wayne, and all members of the Platt Lab for assistance with data collection, mask fitting, and head-post & recording chamber implantation; and Dr. Kyha Williams for discussion of anesthetic techniques. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 1106401 to CBD, the JSPS Postdoctoral Fellowship for Research Abroad to KT, and NIH Grants 1R21-NS-078687-01, 1R01-NS088674-01, and 1R01-MH-095894-01 and the Simons Foundation Autism Research Award number 304935 to MLP.
Footnotes
Conflict of Interest Statement
The authors declare that they have no personal, financial, or other conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Monica L. Carlson, Email: monica.carlson@duke.edu.
Koji Toda, Email: kt131@duke.edu.
Nicholas K. DeWind, Email: nicholas.dewind@duke.edu.
Michael L. Platt, Email: platt@neuro.duke.edu.
References
- Adams DL, Economides JR, Jocson CM, Horton JC. A Biocompatible Titanium Headpost for Stabilizing Behaving Monkeys. J Neurophysiol. 2007;98:993–1001. doi: 10.1152/jn.00102.2007. [DOI] [PubMed] [Google Scholar]
- Adams GK, Watson KK, Pearson J, Platt ML. Neuroethology of decision-making. Curr Opin Neurobiol. 2012;22:982–989. doi: 10.1016/j.conb.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amemori S, Amemori K, Cantor ML, Graybiel AM. A non-invasive head-holding device for chronic neural recordings in awake behaving monkeys. J Neurosci Methods. 2015;240:154–160. doi: 10.1016/j.jneumeth.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betelak KF, Margiotti EA, Wohlford ME, Suzuki DA. The use of titanium implants and prosthodontic techniques in the preparation of non-human primates for long-term neuronal recording studies. J Neurosci Methods. 2001;112:9–20. doi: 10.1016/s0165-0270(01)00442-3. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Chang SWC, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci. 2012;109:959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Brent LJN, Adams GK, Klein JT, Pearson JM, Watson KK, Platt ML. Neuroethology of primate social behavior. Proc Natl Acad Sci. 2013;110:10387–10394. doi: 10.1073/pnas.1301213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol. 1968;31:14–27. doi: 10.1152/jn.1968.31.1.14. [DOI] [PubMed] [Google Scholar]
- Foeller P, Tychsen L. Eye movement training and recording in alert macaque monkeys: 1. Operant visual conditioning; 2 Magnetic search coil and head restraint surgical implantation; 3 Calibration and recording. Strabismus. 2002;10:5–22. doi: 10.1076/stra.10.1.5.8154. [DOI] [PubMed] [Google Scholar]
- Friendlich AR. Primate head restrainer using a nonsurgical technique. J Appl Physiol. 1973;35:934–935. doi: 10.1152/jappl.1973.35.6.934. [DOI] [PubMed] [Google Scholar]
- Gerits A, Ruff CC, Guipponi O, Wenderoth N, Driver J, Vanduffel W. Transcranial magnetic stimulation of macaque frontal eye fields decreases saccadic reaction time. Exp Brain Res. 2011;212:143–152. doi: 10.1007/s00221-011-2710-3. [DOI] [PubMed] [Google Scholar]
- Goulas A, Bastiani M, Bezgin G, Uylings HBM, Roebroeck A, Stiers P. Comparative Analysis of the Macroscale Structural Connectivity in the Macaque and Human Brain. PLoS Comput Biol. 2014;10:e1003529. doi: 10.1371/journal.pcbi.1003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. REVIEW: The functional organization of the intraparietal sulcus in humans and monkeys. J Anat. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Corneil BD. Transcranial Magnetic Stimulation of the Prefrontal Cortex in Awake Nonhuman Primates Evokes a Polysynaptic Neck Muscle Response That Reflects Oculomotor Activity at the Time of Stimulation. J Neurosci. 2014;34:14803–14815. doi: 10.1523/JNEUROSCI.2907-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovington CL, McGirr A, Lepage M, Berlim MT. Repetitive transcranial magnetic stimulation (rTMS) for treating major depression and schizophrenia: a systematic review of recent meta-analyses. Ann Med. 2013;45:308–321. doi: 10.3109/07853890.2013.783993. [DOI] [PubMed] [Google Scholar]
- Hutchison RM, Gallivan JP, Culham JC, Gati JS, Menon RS, Everling S. Functional connectivity of the frontal eye fields in humans and macaque monkeys investigated with resting-state fMRI. J Neurophysiol. 2012;107:2463–2474. doi: 10.1152/jn.00891.2011. [DOI] [PubMed] [Google Scholar]
- Isoda M, Tsutsui KI, Katsuyama N, Naganuma T, Saito N, Furusawa Y, Mushiake H, Taira M, Tanji J. Design of a head fixation device for experiments in behaving monkeys. J Neurosci Methods. 2005;141:277–282. doi: 10.1016/j.jneumeth.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Kimmel DL, Mammo D, Newsome WT. Tracking the eye non-invasively: simultaneous comparison of the scleral search coil and optical tracking techniques in the macaque monkey. Front Behav Neurosci. 2012;6:49, 1–17. doi: 10.3389/fnbeh.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata K, Hoffman DS. Differential effects of muscimol microinjection into dorsal and ventral aspects of the premotor cortex of monkeys. J Neurophysiol. 1994;71:1151–1164. doi: 10.1152/jn.1994.71.3.1151. [DOI] [PubMed] [Google Scholar]
- Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. SEMAC: Slice encoding for metal artifact correction in MRI. Magn Reson Med. 2009;62:66–76. doi: 10.1002/mrm.21967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luber B, Lisanby SH. Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS) Neuro Image. 2014;85(Part 3):961–970. doi: 10.1016/j.neuroimage.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdeke KM, Röschmann P, Tischler R. Susceptibility artefacts in NMR imaging. Magn Reson Imaging. 1985;3:329–343. doi: 10.1016/0730-725x(85)90397-2. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Nelson EE. Eye-tracking with nonhuman primates is now more accessible than ever before. Am J Primatol. 2011;73:562–569. doi: 10.1002/ajp.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Dominguez O, Mills BD, Grayson D, Woodall A, Grant KA, Kroenke CD, Fair DA. Bridging the Gap between the Human and Macaque Connectome: A Quantitative Comparison of Global Interspecies Structure-Function Relationships and Network Topology. J Neurosci. 2014;34:5552–5563. doi: 10.1523/JNEUROSCI.4229-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JK, Grigsby EM, Prevosto V, Petraglia FW, Rao H, Deng ZD, Peterchev AV, Sommer MA, Egner T, Platt ML, et al. Simultaneous transcranial magnetic stimulation and single-neuron recording in alert non-human primates. Nat Neurosci. 2014;17:1130–1136. doi: 10.1038/nn.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigarev IN, Nothdurft HC, Kastner S. A reversible system for chronic recordings in macaque monkeys. J Neurosci Methods. 1997;77:157–162. doi: 10.1016/s0165-0270(97)00124-6. [DOI] [PubMed] [Google Scholar]
- Pigarev IN, Saalmann YB, Vidyasagar TR. A minimally invasive and reversible system for chronic recordings from multiple brain sites in macaque monkeys. J Neurosci Methods. 2009;181:151–158. doi: 10.1016/j.jneumeth.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Porter R, Lewis MM, Linklater GF. A headpiece for recording discharges of neurons in unrestrained monkeys. Electroencephalogr Clin Neurophysiol. 1971;30:91–93. doi: 10.1016/0013-4694(71)90210-0. [DOI] [PubMed] [Google Scholar]
- Roy S, Wang X. Wireless multi-channel single unit recording in freely moving and vocalizing primates. J Neurosci Methods. 2012;203:28–40. doi: 10.1016/j.jneumeth.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Shepherd SV, Platt ML. Reversible inactivation of pSTS suppresses social gaze following in the macaque (Macaca mulatta) Soc Cogn Affect Neurosci. 2014;9:209–217. doi: 10.1093/scan/nss123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini M, Umiltà C, Rusconi E. The use of transcranial magnetic stimulation in cognitive neuroscience: A new synthesis of methodological issues. Neurosci Biobehav Rev. 2011;35:516–536. doi: 10.1016/j.neubiorev.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Schwarz DA, Lebedev MA, Hanson TL, Dimitrov DF, Lehew G, Meloy J, Rajangam S, Subramanian V, Ifft PJ, Li Z, et al. Chronic, wireless recordings of large-scale brain activity in freely moving rhesus monkeys. Nat Methods. 2014;11:670–676. doi: 10.1038/nmeth.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature. 2006;444:374–377. doi: 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Tolias AS, Sultan F, Slocum WM, Logothetis NK. Direct and Indirect Activation of Cortical Neurons by Electrical Microstimulation. J Neurophysiol. 2006;96:512–521. doi: 10.1152/jn.00126.2006. [DOI] [PubMed] [Google Scholar]
- Thurmon JC, Tranquilli WJ, Benson GJ. Lumb & Jones’ Veterinary Anesthesia. Baltimore, MD: Williams & Wilkins; 1996. [Google Scholar]
- Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proc Natl Acad Sci. 2008;105:19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cabre A, Wattiez N, Monfort M, François C, Rivaud-Péchoux S, Gaymard B, Pouget P. Frontal Non-Invasive Neurostimulation Modulates Antisaccade Preparation in Non-Human Primates. PLoS ONE. 2012;7:e38674. doi: 10.1371/journal.pone.0038674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz RH, Sommer MA. Single Neurons and Primate Behavior. In: Senior C, Russell T, Gazzaniga MS, editors. Methods in Mind. Cambridge: MIT Press; 2006. pp. 123–139. [Google Scholar]