Abstract
Redox processes and radical intermediates are found in many biochemical processes, including deoxyribonucleotide synthesis and oxidative DNA damage1. One of the core principles that underlies DNA biosynthesis is the radical-mediated elimnation of H2O to deoxygenate ribonucleotides, an example of ‘spin-center shift’ (SCS)2, during which an alcohol C–O bond is cleaved, resulting in a carbon-centered radical intermediate. While SCS is a well-understood biochemical process, it is underutilized by the synthetic organic chemistry community. We wondered whether it would be possible to take advantage of this naturally occurring process to accomplish mild, non-traditional alkylations using alcohols as radical precursors. Considering traditional radical-based alkylation methods require the use of stoichiometric oxidants, elevated temperatures, or peroxides3–7, the development of a mild protocol using simple and abundant alkylating agents would have significant utility in the synthesis of diversely functionalized pharmacophores. In this manuscript, we describe the successful execution of this idea via the development of a dual catalytic alkylation of heteroarenes using alcohols as mild alkylating reagents. This method represents the first broadly applicable use of unactivated alcohols as latent alkylating reagents, achieved via the successful merger of photoredox and hydrogen atom transfer (HAT) catalysis. The utility of this multi-catalytic protocol has been demonstrated through the late-stage functionalization of the medicinal agents, fasudil and milrinone.
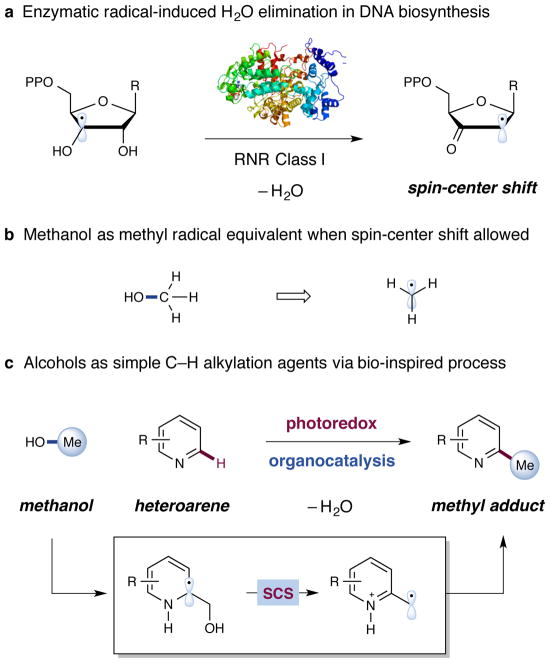
During DNA biosynthesis, ribonucleoside diphosphates are converted into their deoxyribonucleoside equivalents via the enzymatic activity of ribonucleotide reductase (class I–III)8. Crucially, a (3′,2′)-spin center shift occurs, resulting in β-C–O scission and elimination of water (Fig. 1a). Considering the efficiency of this mild enzymatic process to cleave C–O bonds to generate transient radicals, we postulated whether an analogous chemical process could occur with simple alcohols, such as methanol, to access radical intermediates for use in challenging bond constructions (Fig. 1b). In the medicinal chemistry community, there is growing demand for the direct introduction of alkyl groups – especially methyl groups – to heteroarenes, given their influence on drug metabolism and pharmacokinetic profiles9. The open-shell addition of alkyl radical intermediates to heteroarenes, known as the Minisci reaction10, has become a mainstay transformation with broad application within modern drug discovery11. Unfortunately, many current methods are limited in their application to late-stage functionalization of complex molecules due to their dependence upon the use of strong stoichiometric oxidants or elevated temperatures to generate the requisite alkyl radicals3–6. DiRocco and coworkers recently demonstrated a photoredox-catalyzed alkylation protocol using peroxides as the alkyl radical precursors7. Given the state of the art, we recently questioned whether a general alkylation protocol could be devised in which a broad range of substituents could be installed from simple commercial alcohols under mild conditions.
Figure 1. Bio-inspired alkylation process using alcohols as spin-center shift equivalents via a dual catalytic platform.
(a) DNA biosynthesis occurs via a spin-center shift (SCS) process, catalyzed by ribonucleotide reductase (RNR) class I to generate a carbon-centered radical. (b) Alcohols as radical intermediates when SCS-allowed. (c) Proposed direct installation of alkyl groups using alcohols under mild photoredox organocatalytic conditions.
Visible light-mediated photoredox catalysis has emerged in recent years as a powerful technique in organic synthesis that facilitates single-electron transfer (SET) events with organic substrates12–14. This general strategy allows for the development of bond constructions that are often elusive or currently impossible via classical two-electron pathways. Recently, our laboratory introduced a novel dual photoredox-organocatalytic platform to enable the functionalization of unactivated sp3-C–H bonds15–17. This catalytic manifold provides access to radical intermediates via C–H abstraction, resulting in the construction of challenging C–C bonds via a radical–radical coupling mechanism. With the insight gained from this dual catalytic system and our recent work on the development of a photoredox-catalyzed Minisci reaction18, we questioned whether it would be possible to generate alkyl radicals from alcohols and employ them as alkylating agents in a heteroaromatic C–H functionalization reaction (Fig. 1c). While there are a few early reports of alcohols as alkyl radical precursors formed via high-energy irradiation (UV light and gamma rays)19–21, a general and robust strategy for using alcohols as latent alkylating agents has been elusive. This transformation would represent a direct C–H alkylation of heteroaromatics with alcohols via a spin-center shift pathway, eliminating H2O as the only byproduct. We recognized that this mild alkylating procedure would serve as a powerful and general method in late-stage functionalization, using commercially available and abundant alcohols as latent alkylating agents.
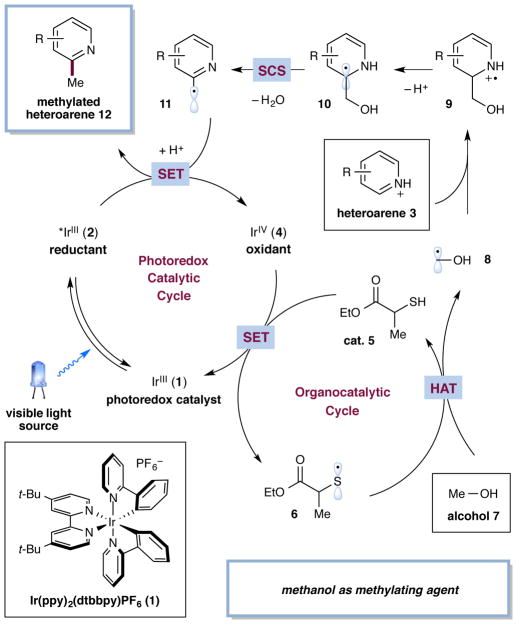
A detailed description of our proposed dual catalytic mechanism for the alkylation of heteroarenes with alcohols is outlined in Fig. 2. Irradiation of Ir(ppy)2(dtbbpy)+ (1) (ppy = 2-phenylpyridine, dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine) will generate the long-lived *Ir(ppy)2(dtbbpy)+ (2) excited state (τ = 557 ns)22. As *Ir(ppy)2(dtbbpy)+ (2) can function as a reductant or an oxidant, we postulated that 2 would undergo a single-electron transfer event with a sacrificial quantity of protonated heteroarene 3 to initiate the first catalytic cycle and provide the oxidizing Ir(ppy)2(dtbbpy)2+ (4). Given the established oxidation potential of Ir(ppy)2(dtbbpy)2+ (4) [E1/2red = +1.21 V vs. saturated calomel electrode (SCE) in CH3CN]22, we anticipated that single-electron transfer (SET) from the thiol catalyst 5 (E1/2red = +0.85 V vs. SCE for cysteine)23 to Ir(ppy)2(dtbbpy)2+ (4) would occur and, after deprotonation, furnish the thiyl radical 6 while returning Ir(ppy)2(dtbbpy)+ (1) to the catalytic cycle. At this stage, we presumed that the thiyl radical 6 would undergo hydrogen atom transfer with the alcohol 7 (a comparable thiol, methyl 2-mercaptoacetate S–H BDE = 87 kcal/mol24, MeOH α-C–H BDE = 96 kcal/mol25) to provide the α-oxy radical 8 and regenerate the thiol catalyst 5, driven by the polar effect in the transition state26. The polar effect is a remarkable property that enables significantly endergonic C–H abstractions that would not be possible otherwise27. The nucleophilic α-oxy radical 8 would then add to the protonated electron-deficient 00heteroarene 3 in a Minisci-type pathway to afford the aminyl radical cation 9. The resulting α-C–H bond of 9 is sufficiently acidic to undergo deprotonation to form the α-amino radical 1028. At this juncture, intermediate 10 is primed to undergo a spin-center shift to eliminate H2O and generate benzylic radical 11. The resulting open-shell species would then undergo protonation followed by a second SET event with the excited photocatalyst to regenerate the active oxidant Ir(ppy)2(dtbbpy)2+ (4) while providing desired alkylation product 12.
Figure 2. Proposed mechanism for the direct alkylation of heteroaromatic C–H bonds via photoredox organocatalysis.
The catalytic cycle is initiated via excitation of photocatalyst 1 to give the excited state 2. A sacrificial amount of heteroarene 3 oxidizes *IrIII 2 to IrIV 4, which then oxidizes thiol catalyst 5 to generate thiyl radical 6 and regenerate catalyst 1. Thiyl radical 6 then abstracts a hydrogen atom from alcohol 7 to form α-oxy radical 8. Radical 8 adds to heteroarene 3, producing radical cation 9, which after deprotonation forms α-amino radical 10. Spin-center shift elimination of H2O forms radical intermediate 11. Protonation and reduction by *IrIII 2 delivers alkylated product 12.
We first examined this new alkylation protocol using isoquinoline and methanol as the coupling partners and evaluated a range of photocatalysts and thiol catalysts. We were pleased to discover that using Ir(ppy)2(dtbbpy)PF6 (1) and ethyl 2-mercaptopropionate (5), along with p-toluenesulfonic acid and blue LEDs as the light source, we were able to achieve the desired C–C coupling to provide 1-methylisoquinoline (15) in 92% yield (see Supplementary Information). Importantly, we observed none of the desired product in the absence of photocatalyst, thiol catalyst, acid, or light, demonstrating the requirement of all components in this dual catalytic protocol. Notably, this method requires only weak visible light and ambient temperature to install methyl substituents using methanol as the alkylating agent.
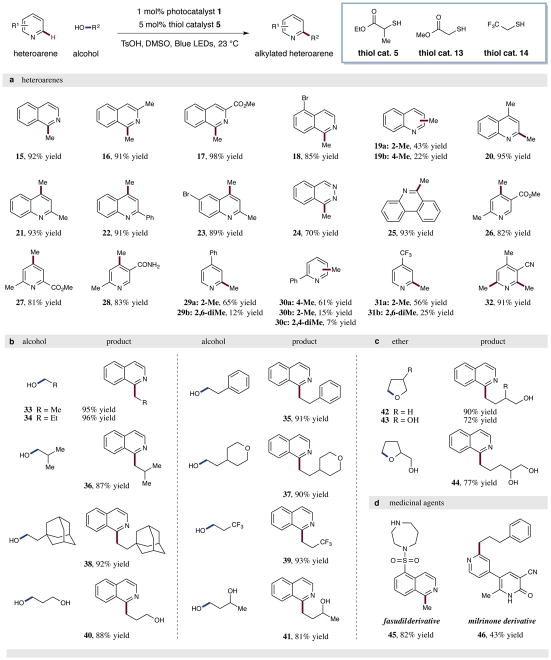
With the optimal conditions in hand, we sought to evaluate the generality of this dual catalytic alkylation transformation. As highlighted in Fig. 3a, a wide range of heteroaromatics are methylated under the reaction conditions. Isoquinolines with electron-donating or -withdrawing substituents (such as methyl substituents, esters, and halides) are functionalized in excellent efficiencies (15–18, 85–98% yield). Quinolines perform effectively, including those that contain non-participating functionality (19–23, 65–95% yield), in addition to phthalazine and phenanthridine coupling partners (24 and 25, 70% and 93% yield). Moreover, a wide range of pyridine derivatives containing diverse functionality (such as esters, amides, arenes, nitriles, and trifluoromethyl groups) can be converted into the desired methylation products in high yield (26–32, 65–91% yield).
Figure 3. Substrate scope for the alkylation of heteroaromatic C–H bonds with alcohols via the dual photoredox organocata-lytic platform.
A broad range of heteroaromatics and alcohols are efficiently coupled to produce alkylated heterocycles under the standard reaction conditions (top, generalized reaction). (a) A variety of isoquinolines, quinolines, phthalazines, phenanthridines, and pyridines are efficiently methylated using methanol as the alkylating reagent. (b) A diverse selection of alcohols serve as effective alkylating agents in this dual catalytic protocol. (c) Ethers are also amenable to the transformation – the products are the corresponding ring opened alcohols. (d) Two pharmaceuticals, fasudil and milrinone, can be alkylated using this protocol, demonstrating its utility in late-stage functionalization. Isolated yields are indicated below each entry. See Supplementary Information for experimental details.
Next, we sought to investigate the nature of the alcohol coupling partner, as demonstrated in Fig. 3b. A broad array of primary alcohols can effectively serve as alkylating agents in this new alkylation reaction. In contrast to the methylation conditions highlighted above, alcohols in Fig. 3b typically employ methyl thioglycolate 13 as the C–H abstraction catalyst. Importantly, simple aliphatic alcohols such as ethanol and propanol deliver the alkylated isoquinoline product in high yields (33 and 34, 95% and 96% yield). Steric bulk proximal to the alcohol functionality is tolerated, as exemplified by the presence of isopropyl, β-tetrahydropyran, β-aryl, and β-adamantyl substituents (35–38, 87–92% yield). The presence of an electron-withdrawing trifluoromethyl (CF3) group distal to the alcohol decreases the rate of the reaction; however, employing the more electrophilic thiol catalyst, 2,2,2-trifluoroethanethiol (14) can promote the transformation more efficiently, possibly due to the polar effect on the HAT transition state (39, 93% yield)26. To our delight, diols also participate readily in this alkylation protocol (40 and 41, 88% and 81% yield). It should be noted that 1,3-butanediol demonstrates exceptional chemoselectivity and undergoes alkylation exclusively at the primary alcohol site. We speculate that the corresponding α-oxy radical at the secondary alcohol position does not attack the protonated heteroarene due to its increased steric hindrance. For these alkylating agents with multiple reactive sites (41, 43, and 44), thiol catalyst 5 is the most effective HAT catalyst – mechanistic studies are ongoing to elucidate the origin of these differences in catalyst reactivity. Ethers, in the form of differentially substituted tetrahydrofurans, are also competent alkylating agents in this dual catalytic platform (42–44, 72–90% yield). In the elimination step, the tetrahydrofuran ring opens to reveal a pendent hydroxyl group. Interestingly, 3-hydroxytetrahydrofuran and tetrahydrofurfuryl alcohol react regioselectively at the ether α-oxy site distal to the alcohol to afford alkylation products with terminal pinacol motifs. We attribute this exclusive regioselectivity to a subtle influence on C–H BDE due to the inductive influence of the oxygen atoms. The application of these substrates represents an effective method to install vicinal diol motifs that would be inaccessible using traditional oxidative alkylation methods. Finally, the utility of this mild alkylation protocol has been demonstrated by the late-stage functionalization of several pharmaceutical compounds. Using methanol as a simple methylating agent, fasudil, a potent Rho-associated protein kinase inhibitor and vasodilator, can be methylated in 82% yield (product 45). Additionally, milrinone, a phosphodiesterase 3 inhibitor and vasodilator, can be alkylated with 3-phenylpropanol in 43% yield (product 46).
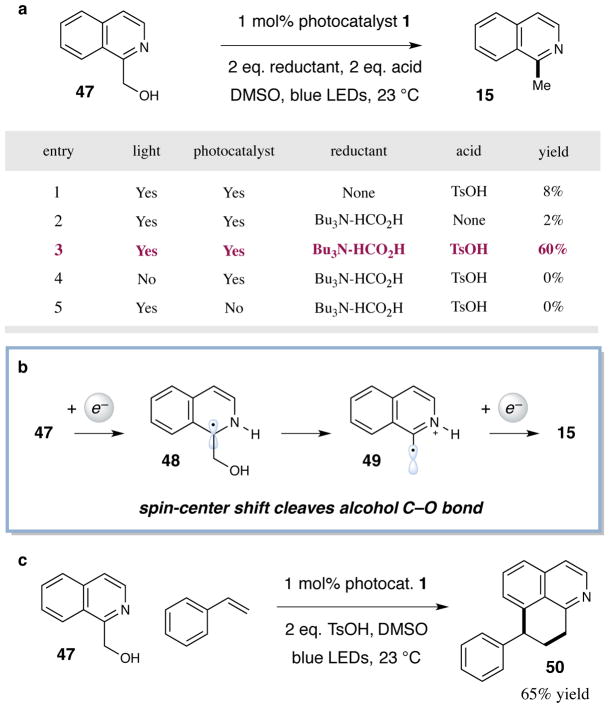
Mechanistic studies have been conducted to support the proposed pathway outlined in Fig. 2. Stern–Volmer fluorescence quenching experiments have demonstrated that the *IrIII excited state 2 is quenched in the presence of protonated heteroarene 3, but not in the presence of the unprotonated heteroarene or thiol catalyst 5, implying an oxidative quenching pathway (see Supplementary Information). Additionally, a series of experiments were conducted to investigate the proposed spin-center shift elimination. Upon exposing hydroxylated intermediate 47 to the reaction conditions, only a modest amount of the methylated isoquinoline 15 is observed (8% yield, entry 1, Fig. 4a). In the absence of an acid additive, only trace yields of the desired product is formed (2% yield, entry 2, Fig. 4a). However, in the presence of a stoichiometric reductant and p-toluenesulfonic acid, the elimination of oxygen can be achieved in good efficiency (60% yield, entry 3, Fig. 4a). Crucially, this elimination pathway is shut down in the absence of either light or photo-catalyst (entries 4 and 5, Fig. 4a). Therefore, this net reductive process supports the proposed generation of α-amino radical 48, which could readily form deoxygenated product 15 via a spin-center shift pathway to β-amino radical 49 (Fig. 4b). This elimination pathway is further corroborated by a series of radical trapping experiments (Fig. 4c and SI). In the presence of styrene, hydroxymethyl arene 47 is transformed to adduct 50 (Fig. 4c, 65% yield), presumably via the intermediacy of β-amino radical 49. Finally, while we support the mechanism outlined in Figure 2, we cannot rule out the possibility of a radical chain pathway in which radical 11 abstracts an H-atom from alcohol 7 or thiol catalyst 5.
Figure 4. Mechanistic studies support spin-center shift elimination pathway.
(a) Hydroxymethyl intermediate 47 can be converted to methylated 15 under net reductive conditions upon addition of formic acid-tributylamine and p-toluenesulfonic acid. (b) Deoxygenation of 47 likely proceeds via a spin-center shift pathway to cleave the alcohol C–O bond. (c) In the presence of styrene, 47 is converted to 50, presumably by trapping of radical 49.
In summary, this alkylation strategy represents the first general use of alcohols as simple alkylating agents and enables rapid late-stage derivatization of medicinally relevant molecules. Given the influence on drug pharmacokinetics and ADME properties, this method of installing inert alkyl groups will likely find wide application in the medicinal chemistry community. We have developed a mild and operationally simple alkylation reaction via the synergistic merger of photoredox and thiol HAT organocatalysis to forge challenging heteroaryl C–C bonds using alcohols as latent nucleophiles. This bio-inspired strategy mimics the key step in enzyme-catalyzed DNA biosynthesis via a novel spin-center shift elimination of H2O to generate radical intermediates from simple alcohols.
Supplementary Material
Acknowledgments
Financial support was provided by NIHGMS (R01 GM103558-03) and kind gifts from Merck and Amgen. J.J. thanks Jack A. Terrett for assistance in preparing this manuscript.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions J.J. performed and analyzed experiments. J.J. and D.W.C.M. designed experiments to develop this reaction and probe its utility, and also prepared this manuscript.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of this article at www.nature.com/nature.
References
- 1.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 4. Oxford University Press; New York: 2007. [Google Scholar]
- 2.Wessig P, Muehling O. Spin-center shift (SCS) – a versatile concept in biological and synthetic chemistry. Eur J Org Chem. 2007:2219–2232. [Google Scholar]
- 3.Minisci F, Vismara E, Fontana F. Homolytic alkylation of protonated heteroaromatic bases by alkyl iodides, hydrogen peroxide, and dimethyl sulfoxide. J Org Chem. 1989;54:5224–5227. [Google Scholar]
- 4.Molander GA, Colombel V, Braz VA. Direct alkylation of heteroaryls using potassium alkyl- and alkoxymethyl-trifluoroborates. Org Lett. 2011;13:1852–1855. doi: 10.1021/ol2003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji Y, Brueckl T, Baxter RD, Fujiwara Y, Seiple IB, Su S, Blackmond DG, Baran PS. Innate C–H trifluoromethylation of heterocycles. Proc Natl Acad Sci USA. 2011;108:14411–14415. doi: 10.1073/pnas.1109059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonchick AP, Burgmann L. Direct selective oxidative cross-coupling of simple alkanes with heteroarenes. Angew Chem Int Ed. 2013;52:3267–3271. doi: 10.1002/anie.201209584. [DOI] [PubMed] [Google Scholar]
- 7.DiRocco DA, Dykstra K, Krska S, Vachal P, Conway DV, Tudge M. Late-stage functionalization of biologically active heterocycles through photoredox catalysis. Angew Chem Int Ed. 2014;53:4802–4806. doi: 10.1002/anie.201402023. [DOI] [PubMed] [Google Scholar]
- 8.Eklund H, Uhlin U, Färnegårdh M, Logan DT, Nordlund P. Structure and function of the radical enzyme ribonucleotide reductase. Prog Biophys Mol Biol. 2001;77:177–268. doi: 10.1016/s0079-6107(01)00014-1. [DOI] [PubMed] [Google Scholar]
- 9.Schönherr H, Cernak T. Profound methyl effects in drug discovery and a call for new C–H methylation reactions. Angew Chem Int Ed. 2013;52:12256–12267. doi: 10.1002/anie.201303207. [DOI] [PubMed] [Google Scholar]
- 10.Minisci F, Bernardi R, Bertini F, Galli R, Perchinunno M. Nucleophilic character of alkyl radicals–VI: A new convenient selective alkylation of heteroaromatic bases. Tetrahedron. 1971;27:3575–3579. [Google Scholar]
- 11.Duncton MAJ. Minisci reactions: versatile CH-functionalizations for medicinal chemists. Med Chem Commun. 2011;2:1135–1161. [Google Scholar]
- 12.Narayanam JMR, Stephenson CRJ. Visible light photoredox catalysis: applications in organic synthesis. Chem Soc Rev. 2011;40:102–113. doi: 10.1039/b913880n. [DOI] [PubMed] [Google Scholar]
- 13.Prier CK, Rankic DA, MacMillan DWC. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz DM, Yoon TP. Solar synthesis: prospects in visible light photocatalysis. Science. 2014;343:1239176. doi: 10.1126/science.1239176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qvortrup K, Rankic DA, MacMillan DWC. A general strategy for organocatalytic activation of C–H bonds via photoredox catalysis: direct arylation of benzylic ethers. J Am Chem Soc. 2014;136:626–629. doi: 10.1021/ja411596q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hager D, MacMillan DWC. Activation of C–H bonds via the merger of photoredox and organocatalysis: a coupling of benzylic ethers with Schiff bases. J Am Chem Soc. 2014;136:16986–16989. doi: 10.1021/ja5102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuthbertson JD, MacMillan DWC. The direct arylation of allylic sp3 C–H bonds via organic and photoredox catalysis. Nature. 2015;519:74–77. doi: 10.1038/nature14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin J, MacMillan DWC. Direct α-arylation of ethers through the combination of photoredox-mediated C–H functionalization and the Minisci reaction. Angew Chem Int Ed. 2014;54:1565–1569. doi: 10.1002/anie.201410432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochiai M, Morita K. A novel photo-induced methylation of pyrimidines and condensed pyrimidine compounds. Tetrahedron Lett. 1967;8:2349–2351. doi: 10.1016/s0040-4039(01)97851-7. [DOI] [PubMed] [Google Scholar]
- 20.Stermitz FR, Wei CC, Huang WH. Imine photoalkylations: quinolone and isoquinoline. Chem Commun (London) 1968:482–483. [Google Scholar]
- 21.Sugimori A, Yamada T, Ishida H, Nose M, Terashima K, Oohata N. Radiation-induced alkylation of quinoline derivatives with alcohol. Bull Chem Soc Jpn. 1986;59:3905–3909. [Google Scholar]
- 22.Slinker JD, Gorodetsky AA, Lowry MS, Wang J, Parker S, Rohl R, Bernhard S, Malliaras GG. Efficient yellow electroluminescence from a single layer of a cyclometalated iridium complex. J Am Chem Soc. 2004;126:2763–2767. doi: 10.1021/ja0345221. [DOI] [PubMed] [Google Scholar]
- 23.Shaidarova LG, Ziganshina SA, Budnikov GK. Electrocatalytic oxidation of cysteine and cystine at a carbon-paste electrode modified with ruthenium(IV) oxide. J Anal Chem. 2003;58:577–582. [Google Scholar]
- 24.Escoubet S, Gastaldi S, Vanthuyne N, Gil G, Siri D, Bertrand MP. Thiyl radical mediated racemization of nonactivated aliphatic amines. J Org Chem. 2006;71:7288–7292. doi: 10.1021/jo061033l. [DOI] [PubMed] [Google Scholar]
- 25.Berkowitz J, Ellison GB, Gutman D. Three methods to measure RH bond energies. J Phys Chem. 1994;98:2744–2765. [Google Scholar]
- 26.Roberts BP. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem Soc Rev. 1999;28:25–35. [Google Scholar]
- 27.Cai Y, Roberts BP. Radical-chain racemization of tetrahydrofurfuryl acetate under conditions of polarity-reversal catalysis: possible implications for the radical-induced strand cleavage of DNA. Chem Commun. 1998:1145–1146. [Google Scholar]
- 28.McNally A, Prier CK, MacMillan DWC. Discovery of an α-amino C–H arylation reaction using the strategy of accelerated serendipity. Science. 2011;334:1114–1117. doi: 10.1126/science.1213920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.