Abstract
Adaptive immune resistance is a process where the cancer changes its phenotype in response to a cytotoxic or pro-inflammatory immune response, thereby evading it. This adaptive process is triggered by the specific recognition of cancer cells by T cells, which leads to the production of immune-activating cytokines. Cancers then hijack mechanisms developed to limit inflammatory and immune responses and protect themselves from the T cell attack. Inhibiting adaptive immune resistance is the mechanistic basis of responses to PD-1 or PD-L1 blocking antibodies, and may be of relevance for the development of other cancer immunotherapy strategies.
Keywords: Checkpoint inhibitors, programed death-1, adaptive resistance
Introduction
There is clear evidence that the human immune system can mount cytotoxic immune responses that can eradicate cancers. This incriminates that cancers that grow progressively are either not recognized by the immune system or have developed mechanisms to avoid the immune system. Evidence from mouse models of carcinogen-induced cancers led Robert Schreiber and colleagues to postulate the concept of immunoediting, which explains how an otherwise immunogenic cancer can grow progressively (1–4). The demonstration that non-silent point mutations (which lead to antigenic neoepitopes) are more frequently lost in cancers compared to silent point mutations (not recognized by T cells) highlights the relevance of the immunoediting process in human cancers (5). Following this logic, it is reasonable to think that some cancers grow progressively because they are no longer immunogenic. However, this cannot explain the progression of all cancers, as the administration of immune activating cytokines or the release of immune checkpoints such as the cytotoxic T lymphocyte-associated antigen 4 (CTLA4) or the programmed cell death-1 (PD-1) can lead to durable tumor responses in mice and patients (6, 7), incriminating that there are T cells still capable of recognizing and killing cancer cells when adequately activated. Therefore, there have to exist mechanisms that limit immune responses to cancer by actively inhibiting the cytotoxic effects of T cells. But these mechanisms have to be specific for cancer antigens as there is little evidence that most patients with cancer have a state of systemic immune suppression (patients with cancer do not usually get opportunistic infections), other than at terminal stages when the cancer has overwhelmed many body systems.
The concept of adaptive immune resistance is used to describe a process in which tumor antigen-specific T cells attempt to attack the cancer, but then the cancer changes in a reactive fashion to protect itself from this immune attack. It was first used by Drew Pardoll to describe how the production of interferons by T cells upon recognition of their cognate antigen results in the reactive expression of the ligand of PD-1 (PD-L1) by cancer cells and turning off the PD-1 positive T cells (7). This concept can explain how there can be a state of specific lack of recognition of otherwise immunogenic cancers while the immune system continues to be able to protect the body from opportunistic infections. In addition to PD-1:PD-L1 interactions, it is possible that adaptive immune resistance can be mediated by several other mechanisms triggered by the recognition of immune-stimulating proteins by cancer cells that then result in a protective changes. Evidence is available for adaptive cancer cell changes induced by the exposure to interferons and tumor necrosis factor alpha (TNF-alpha) as well as other inflammatory cytokines, which are discussed below. The concept of adaptive resistance used here is different from adaptive resistance when used to describe resistance to targeted therapies for cancer. Adaptive immune resistance is a natural process resultant from the cross-talk between immune cells and cancer cells within the tumor microenviroment, while adaptive resistance to targeted therapies refers to the bypass signaling once a constitutive driver oncogene is blocked by treating with the drug.
Mechanisms of Adaptive Immune Resistance
1. Interferon-induced adaptive immune resistance
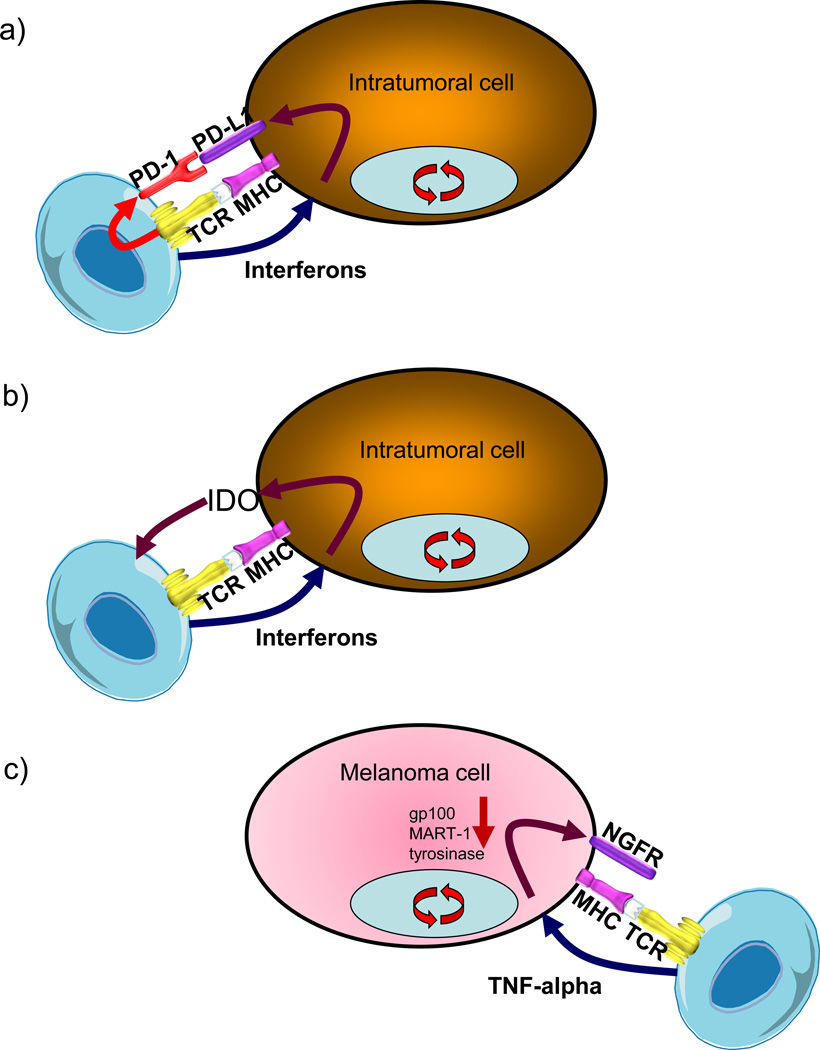
When tumor antigen-specific T cells recognize their cognate antigen expressed by cancer cells, then signaling through the T cell receptor (TCR) leads to the production of interferons and at the same time the expression of activation-induced regulatory receptors including PD-1 (Figure 1A). The interferons are aimed at amplifying the immune response and attracting other leukocytes such as NK cells and macrophages. However, in both mouse models (8, 9) and in humans (5), interferons also lead to the expression of a series of interferon-inducible immune suppressive factors, including PD-L1 and indolamine 2,3 dioxygenase (IDO, Figure 1B) (9). This is an adaptive process that limits immune and inflammatory responses, and cancer uses it to its advantage.
Figure 1. Examples of adaptive immune resistance.
a) Specific recognition of tumor antigen by T cells leads to the production of interferons but also the expression of immune-inhibitory receptors like the programmed cell death-1 (PD-1). Cells within the tumor, including cancer cells and macrophages, react to the presence of interferons by expressing the ligand to PD-1 (PD-L1), which functionally inactivates the tumor-infiltrating T cells. b) T cells producing interferons lead to the expression of immune-suppressive molecules beyond PD-L1, including indolaimine-2,3-deoxygenase (IDO), which is a rate-limiting enzyme in the tryptophan metabolism and essential for adequate T cell functionality. c) The production of tumor-necrosis factor alpha (TNF-alpha) by tumor-specific T cells can result in the de-differentiation of melanoma cells to stop expressing melanosomal antigens such as gp100, and instead express neural crest antigens such as the nerve growth factor receptor (NGFR or CD271).
PD-L1 can be constitutively expressed through a series of currently incompletely analyzed oncogenic pathways (10–12), which likely converge in the activation of signal transducers and activators of transcription (STAT) proteins or other interferon-receptor downstream effectors, or can be induced in response to both type I and II interferons produced during an active antitumor immune response (13–16). The interferon-inducible expression of PD-L1 seems to be more common than the constitutive expression in most cancer histologies, and results in a restricted PD-L1 expression in T cell-rich areas of tumors, in particular at the invasive margin (17, 18). This pattern of expression suggests that PD-L1 is adaptively induced as a consequence of the presence of tumor antigen-specific T cells that recognized the cancer cells, but these cancer cells (or other tumor microenviroment cells) adapted by expressing PD-L1 and turning off the otherwise specific cytotoxic immune response (17). The signaling pathway through which interferon leads to expression of PD-L1 has not been fully characterized, but current evidence suggests that it follows the canonical type II interferon receptor signaling (16). The adaptive expression of PD-L1 has been noted on the surface of cancer cells, myeloid-lineage cells and other tumor microenviroment stromal cells (18), as well as tumor infiltrating T cells themselves (19), likely a reflection of the presence of tumor-specific T cells producing interferons that can trigger PD-L1 also on T cells. Therefore, the tumor uses the physiological induction of PD-L1, which normally occurs to protect tissues from infection-induced cytotoxic responses, in order to protect itself from an anti-tumor immune response (13, 20).
An alternate hypothesis is that any PD-L1 expression by cancer cells, regardless if it is inducible or constitutive, results in immune evasion. The high response rate to PD-1 blockade in patients with chemotherapy-refractory Hodgkin disease has been explained by the frequent genetic amplification of chromosome 9 including the locus of PD-L1, PD-L2 and the interferon receptor adapter JAK2 (21, 22), which has been termed the PDJ amplicon. Hodgkin disease is notorious for triggering a large lymphocytic infiltrate surrounding the few malignant Reed-Stenberg cells. Therefore, it is possible that the PD-L1 upregulation by gene amplification may also include an adaptive immune resistance mechanism associated with the brisk T cell infiltrate (23). It is interesting that the same PDJ amplicon has been noted in other cancers (head and neck, lung, cervical, stomach, colon) and when it is present it is positively associated with an immune cytolytic activity signature (5). If these other cancers with the PDJ amplicon also respond to PD-1 blockade therapy, then interferon-inducible expression of PD-L1 reflective of adaptive immune resistance may not be the only mechanism that explains cancer responses to anti-PD-1 or anti-PD-L1 antibodies.
It has been recognized that some cancers have a signature of T cell inflammation mediated by interferons that not only leads to the expression of PD-1 and PD-L1, but also to other immune suppressive factors such as IDO and even the active presence of FoxP3+ regulatory T cells (Tregs) (9, 24). The negative feedback through these inhibitory pathways is an adaptive process that follows the T cell infiltration. Data in mice correctly anticipated that checkpoint inhibition might be preferentially beneficial for patients with a preexisting T cell–inflamed tumor microenvironment (8, 9). As IDO is expressed through the same interferon-inducible mechanism, it is possible that the clinical development of specific IDO inhibitors may be able to follow a similar path where the pre-existence of T cells inducing IDO expression could be used to select patients for therapy. Another interferon-inducible checkpoint is the carcinoembryonic antigen cell adhesion molecule-1 (CEACAM1) (25), which has been reported to be a partner of the T-cell immunoglobulin domain and mucin domain-3 (TIM-3) and can be blocked therapeutically using antibodies to result in antitumor activity (26). It is also possible that other interferon-inducible genes that are part of negative immune regulatory loops may be limiting T cell responses to cancer and could provide novel targets for immunotherapy.
2. Inflammatory cytokine-induced adaptive immune resistance
The production of pro-inflammatory cytokines by tumor-infiltrating cells can result in changes in the cancer cells that may lead to immune escape. Conclusive evidence of this mechanism has been provided in a mouse model of adoptive cell transfer (ACT) therapy, where the infusion of T cells that specifically recognize a melanoma differentiation antigen, gp100, resulted in transient tumor responses (27). In this model, during the process of cancer cell killing, the tumor-infiltrating cells released the inflammatory cytokine TNF-alpha, which lead the melanoma cells to adapt by decreasing expression of gp100 and switching to a less differentiated neural crest phenotype (Figure 1C). The melanosomal antigen gp100 is a protein from the pigmentation pathway expressed by normal melanocytes and highly expressed by many melanomas. It is a well-recognized tumor rejection antigen shared by melanomas (28, 29). As with interferon-induced PD-L1 adaptive expression, the exact signaling pathway from TNF-alpha to decreased melanosomal antigen expression has also not been fully characterized. Gp100 is not required for the cancer phenotype, so adaptation by decreasing the expression of this and other lineage-specific immunogenic proteins may be a mechanism of immune evasion. In this model, TNF-alpha produced by tumor-specific T cells triggered a process of de-differentiation of melanoma cells moving back through their embryological development path arising from the neural crest, evidenced by the expression of the nerve growth factor receptor (NGFR, CD271) while losing the expression of several melanosomal antigens (30).
Phenotype switching from a differentiated melanosomal state to a more undifferentiated state by melanoma cells can be induced not only by inflammatory cytokines but also by other stress-related changes (31, 32). This process is akin to epithelial-to-mesenchymal (EMT) transition in epithelial cancers (33), where cells switch from a more differentiated and proliferative state to a less differentiated and invasive state allowing the process of metastases (34, 35). It is possible that a similar process may mediate the neuroendocrine differentiation of several cancers, such as lung and prostate, and may be related to immune escape. The EMT de-differentiation changes in several cancers have been related to inflammatory cytokines like TNF-a, interleukin-6 (IL-6) and transforming growth factor beta (TGF-b) produced by an antitumor immune response (27, 36, 37). Therefore, it is likely that adaptive immune resistance induced by inflammatory cytokines may be an immune escape and even cancer-promoting mechanism in several cancers (38).
Clinical Decision Making Based on Diagnosing Adaptive Immune Resistance
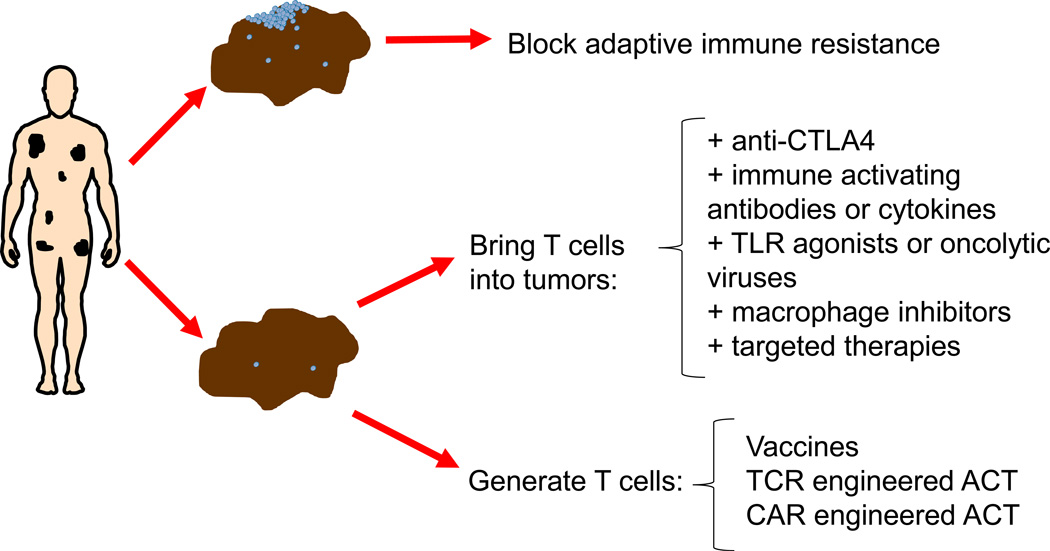
The recognition of the specific mechanism through which cancer adapts to evade an antitumor immune response may lead to rational immunoncology drug development and personalized cancer immunotherapy. Based on the detection of an ongoing adaptive immune resistance, cancers may be classified into two main groups, the ones that have active intratumoral immune responses blocked by adaptive immune resistance and the ones lacking intratumoral T cells (Figure 2). If there is a sufficient density of T cells in tumors, in particular at the invasive margin, these are likely turned off by inducing adaptive immune resistance, and PD-1:PD-L1 may be dominant in this setting at least in some cancer histologies (17, 18, 39). If T cells have not made it into the tumor, then it may be envisioned that combination with another immunotherapy able to bring T cells into tumors would be a rational choice, such as the successful clinical development of anti-CTLA4 combined with anti-PD-1 therapy (40, 41). CTLA4 blockade has a preferential effect in the activation step of an antitumor immune responses, broadening the diversity of the immune response and bringing T cells into peripheral tissues in mouse models and in humans (6, 42–44). Other potential approaches include means to change the tumor microenviroment by direct injection of interferon-inducing molecules such as toll-like receptor agonists or oncolytic viruses, blocking T cell-excluding proteins like IDO or arginase, or inhibiting immune suppressive cells like Treg or macrophages. In the remaining cases there may not be T cells capable of differentially recognizing tumor antigens, so there would be no hope in unleashing an endogenous antitumor immune response. For these patients, immunotherapy would require creating an immune response by gene-engineered adoptive cell transfer (ACT) using TCR or chimeric antigen receptors (CAR). Therefore, analysis of baseline tumor biopsies to detect adaptive immune resistance may guide treatment of cancer in the future (45).
Figure 2. Treatment selection based on detecting adaptive immune resistance.
Tumor biopsies from patients with advanced cancers may contain T cell infiltrates that trigger an adaptive immune resistant response. Defining the specific mechanism of this reactive tumor protection would allow tailoring the treatment of the patient to block that particular escape mechanism. For example, if T cells in tumors are turned off by PD-1:PD-L1 interactions then single agent anti-PD-1 or anti-PD-L1 would be the most appropriate therapy with high likelihood of success and avoiding additional toxicities from combinations. But if there are no T cells in tumor biopsies, then combination immunotherapies could be designed to bring T cells into tumors, or the immune system would need to be turned on by vaccination or genetically engineered using an adoptive cell transfer (ACT) approach with T cell receptors (TCR) or chimeric antigen receptors (CAR).
Conclusions
Adaptive immune resistance may be a generalized phenomenon where cancer cells evade otherwise functional tumor-specific T cell responses and foster the cancer’s progressive growth. It gives the advantage to the cancer to be able to specifically escape from T cells while the host’s immune system continues to function correctly for any other antigens. This process has allowed the successful clinical development of checkpoint inhibitors such as anti-PD-1 and anti-PD-L1 antibodies (44, 46), where intratumoral pre-existing T cells specific for the cancer are actively turned off by adaptive immune resistance and therapeutic blocking antibodies to PD-1 or PD-L1 could reverse this situation (18, 19). Identifying similar processes that lead to the expression of other immune checkpoints, or immune suppressive factors through which cancers protect themselves from an active T cell cytotoxic response, may lead to the rational and personalized development of additional cancer immunotherapies. Furthermore, therapeutic interventions with small molecule inhibitors or cytokine-blocking antibodies aimed at inhibiting cancer phenotype switching, de-differentiation and EMT may be rationally combined with immunotherapies for cancer. Clinical decision-making may be guided by the detailed analysis of how the immune system is interacting with cancers in tumor biopsies, which would allow defining if there is an ongoing adaptive process limiting an immune response or if this one is absent within the tumor.
Significance.
Several new immunotherapy strategies to treat cancer are based on inhibiting processes through which cancer adapts and evades from an immune response. Recognizing the specific adaptive resistance mechanisms in each case is likely to allow the personalized development of immunotherapies tailored to block how a particular cancer protects itself from the immune system.
Key Concepts and Relevance.
Adaptive immune resistance is a process through which cancer reactively expresses molecules that actively turn off an otherwise effective antitumor immune response.
The antitumor activity of PD-1 blockade therapy is explained by blocking adaptive immune resistance through the expression of PD-L1.
Recognizing adaptive immune resistance in baseline biopsies may lead to precision immunotherapy.
Acknowledgments
Grant Support: A.R. is funded by NIH grants R01 CA199205, P01 CA168585, U54 CA119347, R01 CA170689, the Ressler Family Fund, the Grimaldi Family Fund, the Dr. Robert Vigen Memorial Fund, and Stand Up To Cancer – Cancer Research Institute (SU2C-CRI) Cancer Immunology Dream Team Translational Research Grant (SU2C-AACR-DT1012). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research (AACR).
Footnotes
Disclosure of Potential Conflicts of Interest: A.R. has consulted for Amgen, Genentech, Merck and Novartis with honoraria paid to UCLA. A.R. is in the scientific advisory board of Acteris, Adaptive Biosciences, Compugen, cCAM-Bio, FLX-Bio and Kite Pharma, and holds stock from these companies.
References
- 1.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 2.Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annual review of immunology. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 5.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 7.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bald T, Landsberg J, Lopez-Ramos D, Renn M, Glodde N, Jansen P, et al. Immune cell-poor melanomas benefit from PD-1 blockade after targeted type I IFN activation. Cancer Discov. 2014;4:674–687. doi: 10.1158/2159-8290.CD-13-0458. [DOI] [PubMed] [Google Scholar]
- 9.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nature medicine. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 11.Atefi M, Avramis E, Lassen A, Wong DJ, Robert L, Foulad D, et al. Effects of MAPK and PI3K Pathways on PD-L1 Expression in Melanoma. Clin Cancer Res. 2014;20:3446–3457. doi: 10.1158/1078-0432.CCR-13-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 14.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer research. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 15.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274) FEBS letters. 2006;580:755–762. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 17.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Myers AC, Chen L, Pardoll DM, Truong-Tran QA, Lane AP, et al. Constitutive and inducible expression of b7 family of ligands by human airway epithelial cells. American journal of respiratory cell and molecular biology. 2005;33:280–289. doi: 10.1165/rcmb.2004-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juszczynski P, Ouyang J, Monti S, Rodig SJ, Takeyama K, Abramson J, et al. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc Natl Acad Sci U S A. 2007;104:13134–13139. doi: 10.1073/pnas.0706017104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer journal. 2010;16:399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 25.Markel G, Seidman R, Cohen Y, Besser MJ, Sinai TC, Treves AJ, et al. Dynamic expression of protective CEACAM1 on melanoma cells during specific immune attack. Immunology. 2009;126:186–200. doi: 10.1111/j.1365-2567.2008.02888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012;490:412–416. doi: 10.1038/nature11538. [DOI] [PubMed] [Google Scholar]
- 28.Bakker AB, Schreurs MW, de Boer AJ, Kawakami Y, Rosenberg SA, Adema GJ, et al. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med. 1994;179:1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribas A, Tumeh PC. Cancer therapy: Tumours switch to resist. Nature. 2012;490:347–348. doi: 10.1038/nature11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holzel M, Bovier A, Tuting T. Plasticity of tumour and immune cells: a source of heterogeneity and a cause for therapy resistance? Nat Rev Cancer. 2013;13:365–376. doi: 10.1038/nrc3498. [DOI] [PubMed] [Google Scholar]
- 32.Hoek KS, Eichhoff OM, Schlegel NC, Dobbeling U, Kobert N, Schaerer L, et al. In vivo switching of human melanoma cells between proliferative and invasive states. Cancer Res. 2008;68:650–656. doi: 10.1158/0008-5472.CAN-07-2491. [DOI] [PubMed] [Google Scholar]
- 33.Li FZ, Dhillon AS, Anderson RL, McArthur G, Ferrao PT. Phenotype switching in melanoma: implications for progression and therapy. Frontiers in oncology. 2015;5:31. doi: 10.3389/fonc.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Knutson KL, Lu H, Stone B, Reiman JM, Behrens MD, Prosperi CM, et al. Immunoediting of cancers may lead to epithelial to mesenchymal transition. J Immunol. 2006;177:1526–1533. doi: 10.4049/jimmunol.177.3.1526. [DOI] [PubMed] [Google Scholar]
- 37.Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69:2887–2895. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang RR, Jalil J, Economou JS, Chmielowski B, Koya RC, Mok S, et al. CTLA4 Blockade Induces Frequent Tumor Infiltration by Activated Lymphocytes Regardless of Clinical Responses in Humans. Clin Cancer Res. 2011;17:4101–4109. doi: 10.1158/1078-0432.CCR-11-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 44.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 45.Ribas A, Tumeh PC. The Future of Cancer Therapy: Selecting Patients Likely to Respond to PD1/L1 Blockade. Clin Cancer Res. 2014;20:4982–4984. doi: 10.1158/1078-0432.CCR-14-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–230. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]