Abstract
BACKGROUND
Irritable bowel syndrome (IBS) is one of the common gastrointestinal disorders with unknown etiology. In experimental models, it is proposed that soy isoflavones may suppress the clinical and psychological symptoms of IBS by alteration of gut barrier tight junctions.
METHODS
We conducted this study to evaluate the effects of soy isoflavones on IBS symptoms and patients’ quality of life. In a randomized double blind placebo-controlled clinical trial, 67 patients with IBS were allocated to consume either soy isoflavones capsules or a placebo for 6 weeks. The primary outcome was a significant reduction in symptoms severity score and the secondary outcome was a significant improvement in quality of life.
RESULTS
45 participants completed the study. There was no significant changes in mean differences of symptoms severity score between the two groups; however soy isoflavone supplementation could significantly improve the quality of life scores (p=0.009).
CONCLUSION
Soy isoflavones supplementation could improve the quality of life in patients with IBS; however it did not suppress the symptoms severity in 6 weeks. Further research with a longer duration is needed to determine the sustained clinical efficacy. This study was registered at clinicaltrials.gov as NCT02026518
Keywords: Irritable Bowel Syndrome, randomized clinical trial, quality of life, Soy Isoflavone
INTRODUCTION
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disease.1 The etiology of this frequent disorder has not yet been well elucidated. The proposed mechanisms involved in the pathophysiology of IBS are microbial overgrowth, high intestinal permeability, microinflammation, psychological factors, dietary habits, and visceral hypersensitivity. Current treatments are insufficient and unconvincing.2,3
Owing to more susceptibility to develop emotional psychological disorders, women are more susceptible to IBS and their symptoms are more severe compared with men.4,5 In female patients with IBS, ovarian steroids protect against aggravation of IBS symptoms ahead of menstrual cycles.6
Soy isoflavones are defined as phytoestrogens that are structural analogs of 17- β estradiol. Their affinity for ERβ (Esterogen Receptor β) in intestinal mucosa is high.7 There is evidence supporting the role of estradiol and estradiol like molecules in a healthy epithelial line through their receptors in colonic mucosa.8 The safety of soy isoflavones supplementation has been shown previously.9 Thus, we hypothesized that soy isoflavones might alter IBS symptoms and quality of life in affected patients. We designed this randomized clinical trial to determine the effects of soy isoflavones supplementation on IBS symptoms severity score (SSS) and the quality of life (QOL) in women with IBS.
MATERIALS AND METHODS
Women with IBS were recruited at Shariati Hospital in Tehran, Iran from September 2013 to June 2014. The diagnosis of IBS was determined on the basis of the Rome III criteria.10 The inclusion criteria were: age 18-75 years, no organic intestinal diseases, no intestinal infection, no history of chronic colorectal diseases, no major surgery, no pregnancy and lactation, not being athletes or on bed rest, no history of breast cancer in herself or her family, and no severe psychosis. Patients with history of regular use of antibiotics, anti-constipation and anti-diarrhea drugs, immune suppressors, metoclopramide, cisapride, diphenoxylate, opium, and non-steroidal anti-inflammatory drugs were not included in the study either.
The exclusion criteria were: use of soy isoflavones one year before the study, use of soy nuts or milk during study, dieting during the study, no desire to complete the study, presenting adverse effects of the supplement, and pregnancy during the study.
Study Design
The study protocol was approved by the Ethics Committee of the National Nutrition and Food Technology Research Institute of Shahid Beheshti University of Medical Science (NNFTRI-92-459). All the patients signed an informed consent to be aware of all the qualifications for the study, advantages and disadvantages of this intervention. At the start point, all the patients were randomly assigned to take either soy isoflavones or the placebo (containing corn starch) twice per day for 6 weeks. The placebos were produced manually were identical to the real supplements in appearance, taste and size.
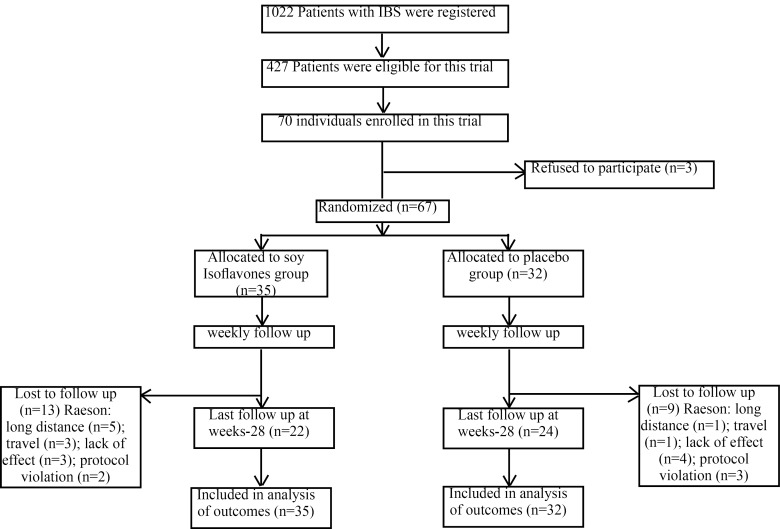
The baseline data of the patients were collected using valid questionnaires. Patients’ adherence was assessed by calling them up every week and counting the remained capsules at the end of the intervention. In accordance with a random sampling approach, of the 1022 subjects registered in IBS database at gastroenterology clinics of Shariati Hospital and Masoud Clinic, Tehran, Iran, 427 were selected for this clinical trial based on the eligibility criteria (Figure 1). The subjects who agreed to participate were randomly assigned to take either soy isoflavones or placebo. The computer generated codes were adopted by a statistician to randomize all the participants and they were handed out to the interviewer.
Fig. 1 .
The flow chart of study participants
All the participants and research team were unaware of the assignment until the endpoint of intervention. In the first session, baseline data were collected and half of the capsules were handed out to the participants. At week 3, the remaining capsules were delivered to all of them. Each capsule of soy isoflavones (21st century Co., USA) consisted 20 mg of isoflavones (10 mg of diadzein, 8.5 mg genistein, 1.5 mg glycetin). Both groups were advised to maintain a normal diet and not change their daily dietary intakes.
Clinical, psychological, and dietary intake assessments
The weight, height, and waist circumference of all participants were measured. The body mass index (BMI) was calculated by dividing weight (kg) by height (m2).
After random assignment, all the participants were asked to answer the questions of a validated IBS quality of life (IBS- QOL) questionnaire. It was shown that the IBS- QOL questionnaire is the most widely validated QOL instruments in patients with IBS. It has enough psychometric qualifications to assess QOL in patients with Persian language.11 The IBS-QOL consists of 34 items that has 5-choice response scale (0 to 4) and it encompasses all aspects of Health Related Quality of Life (HRQOL) measures including dysphoria (8 items), interference with activity (7 items), body image (4 items), food avoidance (3 items), health related worry (3 items), sexual worry (2 items), social reactions (4 items), and relationships (3 items).11
Moreover, IBS-SSS questionnaire was used to assess the clinical outcomes. This instrument consists of 5 items that determine the severity of abdominal pain, frequency of pain, severity of flatulence, satisfaction regarding to bowel movements, and the impact of IBS on quality of life by Visual Analogue Scale (VAS). The total score of IBS-SSS is from 0 to 500 and the higher scores imply more severity of disease symptoms. The differences of scores in this instrument are positively associated with scores of QOL and anxiety and depression.10 A 100-mm VAS was used to assess the pain, bloating, flatulence, and stool emergency and calculate the total score of the disease.12,13
The patients received prospective serial assessment of nutritional intake with three days of written food records. All enrolled subjects received instructions to record their daily dietary intake for three days including a weekend day at the first and the end of the study. Dietary intakes were analyzed by Nutritionist 4 (First DataBank, San Bruno, CA), incorporating use of food scales, and models to enhance portion size accuracy. National composition food tables were used as a reference.14
Follow up
Each participant was assessed every week by phone call. Any adverse effects was recorded. Also, capsule counts at the end of intervention was used to determine adherence to intervention and the VAS questionnaire on severity score 0 to 100 was filled in for the long term effect of supplementation even after cessation of the intervention. The last follow-up was two weeks after the end of intervention in June 2014.
Primary and secondary outcomes
The primary outcome measure was a statistically significant dedecrease in IBS-SSS. The secondary outcome measure was a statistically significant improvement in IBS-QOL scores.
Statistical Analyses
Quantitative variables are reported as mean (SD) and qualitative variables are presented through frequencies (percentages). The independent t test was used to compare the mean of quantitative outcomes between the two groups. We used analysis of covariance (ANOCVA) to compare the mean of postoperative continuous outcomes between the two groups by adjustment on preoperative results.
We compared the distribution of qualitative variables between the two groups using chi-square test or Fisher exact test. Statistical analysis was performed using STATA software (version 11, StataCorp, College Station, TX, USA). P values less than 0.05 were considered as statistically significant. We analyzed the data according to intention to treat principle.
RESULTS
Characteristics of the patients
All data on participants’ enrollment and detainment are shown in figure 1. The baseline characteristics of the two groups were similar according to demographic data such as age, smoking, menopause status, and clinical data. Nonetheless, the mean of weight, and BMI were significantly different between the two groups (Table 1). Moreover, the mean of dietary food items were not significantly different between the two groups before and after the intervention (Table 2).
Table 1 . Demographic characteristics of the study participants .
| Baseline Characteristic | Soy (n=22) | Placebo (n=23) | P value |
| Age, Mean (SD) (y) | 45.54 (9.89) | 40.04 (13.39) | 0.126 |
| Weight, Mean (SD) (kg) | 73.95 (9.73) | 66.28 (12.39) | 0.026* |
| BMI ,Mean (SD) (kg/m2) | 28.83 (4.64) | 25.27 (4.07) | 0.009* |
| Smoking, (%) | 2 (9.1%) | 2 (8.7%) | 1.00 |
| Menopause, (%) | 10 (45.54%) | 7 (30.4%) | 0.299 |
| Soy consumption (%) | 0 (0.0%) | 0 (0.0%) | 1.00 |
| SSS baseline, Mean (SD) | 23.64 (8.17) | 24.78 (11.82) | 0.708 |
| QOl baseline, Mean (SD) | 64.41 (27.78) | 46.70 (31.37) | 0.052 |
| Total score, Mean (SD) | 21.14 (14.63) | 20.00 (13.40) | 0.787 |
Note: Significances are based on independent t test for quantitative variables and Pearson’s chi-square test / Fisher exact test for qualitative factors. *: Statistically significant. BMI: Body mass index, SSS: Severity scoring system, QOL: Quality of life.
Table 2 . Dietary intake of selected nutrients in the study participants at baseline .
| Intake | Soy (n=22)Mean (SD) | Placebo (n=23)Mean (SD) | p value |
| Calorie | 1528.97 (433.04) | 1538.25 (504.98) | 0.948 |
| Protein | 69.04 (25.29) | 62.17 (32.72) | 0.437 |
| Carbohydrate | 209.85 (64.40) | 202.16 (70.63) | 0.713 |
| Total fat | 49.21 (19.90) | 57.34 (29.22) | 0.284 |
| Cholesterol | 192.15 (120.80) | 227.81 (266.28) | 0.559 |
| Vitamin C | 103.57 (113.65) | 132.57 (155.80) | 0.484 |
| Calcium | 553.89 (247.79) | 634.78 (281.25) | 0.313 |
| Vitamin D | 1.00 (1.15) | 1.27 (2.64) | 0.663 |
| Zinc | 6.89 (3.88) | 7.45 (4.32) | 0.649 |
| Magnesium | 200.74 (133.44) | 220.03 (100.30) | 0.585 |
| Phosphor | 889.59 (409.13) | 965.47 (495.98) | 0.580 |
| Fiber | 19.08 (17.86) | 23.38 (18.58) | 0.433 |
| Ferrous | 10.67 (3.29) | 9.90 (4.29) | 0.506 |
| Vitamin B2 | 5.10 (18.86) | 1.22 (0.59) | 0.309 |
| Fluoride | 1118.69 (961.16) | 1251.68 (784.77) | 0.613 |
Note: Significances are based on independent t test.
Primary outcome
Eighty-two percent of the participants accomplished the 6 weeks intervention and the clinical outcomes were obtained from the participants at weeks 0 and 6 of the study. Moreover, the total score was measured at week 10 as follow-up measure. The loss to follow-up was the same at week 6 and week 10. The data of participants were entered in analysis of primary outcome measure as reduction of IBS-SSS, which was not significantly different between the two groups after adjustment for the confounding baseline covariates (Table 3).
Table 3 . The mean difference of the effect of soy isoflavones versus placebo on SSS, IBS-QOL and total score between the two groups .
| Characteristic | Soy (n=22) | Placebo (n=23) | Crude p-value |
Adjusted
p-value |
R 2 | ||
| Crude | Adjusted | Crude | Adjusted | ||||
| SSS, Mean (SE) | 12.77(1.74) | 13.36(2.09) | 19.74(2.52) | 19.18(2.04) | 0.029 | 0.068 | 0.371 |
| IBS-QOL, Mean(SE) | 41.68 (6.07) | 33.34(4.63) | 44.17(6.98) | 52.15(4.52) | 0.789 | 0.009 | 0.607 |
| Total score, Mean(SE) | 69.76 (5.39) | 68.97(3.88) | 26.30(3.30) | 27.03(3.68) | <0.001 | <0.001 | 0.734 |
Notes: Crude Significances are based on independent t test and adjusted significances are based on ANCOVA with factors age, BMI, IQB (IBS- QOL baseline) and before baseline value of each factor as covariates. SSS: Severity scoring system. IBS- QOL: Inflammatory bowel syndrome-Quality of life
Secondary outcomes
The mean IBS- QOL scores were significantly increased even after adjusting for age, BMI and baseline IBS-QOL score (p=0.009). Furthermore, the total score of IBS as a VAS measure was significantly reduced considering confounders (age, BMI, IQB (IBS- QOL baseline) at the end of the study (p<0.001). In patients who supplemented with soy isoflavones, the improvement of each item of IBS-QOL questionnaire was significantly more than the placebo group (p<0.05, Table 4).
Table 4 . The comparison of items of IBS- quality of life between means (SD) of the two groups in univariate analysis of variance* .
| Items of quality of life | Soy isoflavones group (n=22) | Control group (n=23) | Adjusted R 2 | Sig. |
| Dysphoria | 7.24(1.20) | 11.24 (1.17) | 0.56 | <0.001 |
| Interference | 8.01 (1.00) | 10.64 (0.98) | 0.52 | <0.001 |
| Body image | 3.95 (0.54) | 6.09 (0.53) | 0.69 | <0.001 |
| Health worry | 3.56 (0.68) | 5.42 (0.67) | 0.15 | 0.030 |
| Food avoidance | 3.85 (0.58) | 4.67 (0.56) | 0.41 | <0.001 |
| Social reaction | 3.46 (0.56) | 5.3 (0.54) | 0.66 | <0.001 |
| Sexual items | 1.59 (0.30) | 2.70 (0.30) | 0.66 | <0.001 |
| Relationship | 3.27 (0.50) | 5.09 (0.49) | 0.33 | <0.001 |
*The covariates in the model are age, baseline BMI, and item of IBS-QOL before intervention.
DISCUSSION
To our knowledge, this study was the first randomized, double blind, placebo controlled, clinical trial evaluating the effects of soy isoflavones supplementation on patients with IBS. The findings of our study indicate that administration of soy isoflavones supplementation may improve the QOL in patients with IBS, although it could not affect the IBS-SSS significantly. Our results confirm the results of the only recent study evaluating the effects of soy isoflavones on IBS in an experimental model of IBS. Moussa and colleagues15 have found that soy isoflavones mimic estradiol function and can abolish IBS- like symptoms through binding to estrogen receptors in enterocytes.
These effects could be explained by the effects of soy isoflavones on gut barrier function and intestinal microflora. It has been shown that soy isoflavones, diadzein and genistein, can improve gut barrier function through binding to the ER β in enterocytes.16 It seems that soy isoflavones can improve epithelial tight junctions and protect against the onset of symptoms of IBS by imitating ovarian estradiol secreted during follicular period of menstrual cycle.17
Moreover, soy isoflavones can play a role as protective agents for gut environment due to their antioxidant properties, which have been shown previously.18,19 A recent study has shown that soy proteins and isoflavones can reduce IL-6 as an inflammatory cytokine20, which might be involved in pathogenesis of IBS.21 Furthermore, the pathogen bacteria overgrowth may be reduced in response to soy isoflavones supplementation.22
This study has some limitations; firstly the study population was not sufficient to analyze the subtypes of IBS. Moreover, the period of the supplementation was short to judge for the long term role of soy isoflavones on clinical and psychological parameters. Additionally, the lack of stool samples hinders the explanation for these results in aspect of microflora.
This study has several strengths; using valid questionnaires for assessment of IBS-SSS, and QOL is the most important advantage of this study. The randomized, placebo controlled, double blind design is another advantage of this study.
In conclusion, our results support the concept that soy isoflavones can improve the quality of life in patients with IBS. Further research with longer duration are needed to determine the effects of soy isoflavones on IBS symptoms and addressing its mechanism of action.
ACKNOWLEDGEMENT
This study was supported by a grant from the National Nutrition and Food Technology Research Institute of the Shahid Beheshti University of Medical Sciences and Digestive Disease Research Center of Tehran University of Medical Science. We are indebted to Shariati Hospital and Masoud Clinic for providing us with the equipments used in the study, as well as Mrs. Markarian, the laboratory specialist who performed all lab procedures, and Ms Leyla Perkins for English writing edition. We appreciate all participants for their patience and kindly collaboration.
Author Contributions: Dr. Hekmatdoost had full access to all of the data in the study and takes the responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Jalili, Hekmatdoost, Vahedi, Malekzadeh.
Data acquisition: Vahedi, Malekzadeh, Poustchi.
Analysis and interpretation of data: Janani, Jalili.
Drafting of the manuscript: Jalili, Hekmatdoost.
Critical revision of the manuscript for important intellectual content: Hekmatdoost, Vahedi, Malekzadeh, Poustchi, Jalili.
Statistical analysis: Janani, Jalili.
Obtained funding: Hekmatdoost, Vahedi, Malekzadeh, Poustchi.
Administrative, technical, or material support: Hekmatdoost, Vahedi, Poustchi, Malekzadeh, Jalili.
Study supervision: Hekmatdoost, Vahedi, Malekzadeh, Poustchi.
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this work.
Please cite this paper as:
Jalili M, Vahedi H, Janani L, Poustchi H, Malekzadeh R, Hekmatdoost A. Soy Isoflavones Supplementation for Patients with Irritable Bowel Syndrome: A Randomized Double Blind Clinical Trial. Middle East J Dig Dis 2015;7:170-6.
References
- 1.Staudacher HM, Lomer MC, Anderson JL, Barrett JS, Muir JG, Irving PM. et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142:1510–8. doi: 10.3945/jn.112.159285. [DOI] [PubMed] [Google Scholar]
- 2.Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P. et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770–98. doi: 10.1136/gut.2007.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 4.Osterberg E, Blomquist L, Krakau I, Weinryb RM, Asberg M, Hultcrantz R. A population study on irritable bowel syndrome and mental health. Scand J Gastroenterol. 2000;35:264–8. doi: 10.1080/003655200750024128. [DOI] [PubMed] [Google Scholar]
- 5.Thompson WG, Heaton KW, Smyth GT, Smyth C. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut. 2000;46:78–82. doi: 10.1136/gut.46.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houghton LA, Lea R, Jackson N, Whorwell PJ. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50:471–4. doi: 10.1136/gut.50.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T. et al. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001;24:351–6. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- 8.Wada-Hiraike O, Imamov O, Hiraike H, Hultenby K, Schwend T, Omoto Y. et al. Role of estrogen receptor beta in colonic epithelium. Proc Natl Acad Sci U S A. 2006;103:2959–64. doi: 10.1073/pnas.0511271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloedon LT, Jeffcoat AR, Lopaczynski W, Schell MJ, Black TM, Dix KJ. et al. Safety and pharmacokinetics of purified soy isoflavones: single-dose administration to postmenopausal women. Am J Clin Nutr. 2002;76:1126–37. doi: 10.1093/ajcn/76.5.1126. [DOI] [PubMed] [Google Scholar]
- 10.Drossman DA, Chang L, Bellamy N, Gallo-Torres HE, Lembo A, Mearin F. et al. Severity in irritable bowel syndrome: a Rome Foundation Working Team report. Am J Gastroenterol. 2011;106:1749–59; quiz 1760. doi: 10.1038/ajg.2011.201. [DOI] [PubMed] [Google Scholar]
- 11.Gholamrezaei A, Zolfaghari B, Farajzadegan Z, Nemati K, Daghaghzadeh H, Tavakkoli H. et al. Linguistic validation of the Irritable Bowel Syndrome-Quality of Life Questionnaire for Iranian patients. Acta Med Iran. 2011;49:390–5. [PubMed] [Google Scholar]
- 12.Cappello C, Tremolaterra F, Pascariello A, Ciacci C, Iovino P. A randomised clinical trial (RCT) of a symbiotic mixture in patients with irritable bowel syndrome (IBS): effects on symptoms, colonic transit and quality of life. Int J Colorectal Dis. 2013;28:349–58. doi: 10.1007/s00384-012-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heaton KW, Ghosh S, Braddon FE. How bad are the symptoms and bowel dysfunction of patients with the irritable bowel syndrome? A prospective, controlled study with emphasis on stool form. Gut. 1991;32:73–9. doi: 10.1136/gut.32.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghaffarpour M, Houshiar-Rad A, Kianfar H. The manual for household measures, cooking yields factors and edible portion of foods. Tehran, Iran: National Institute of Nutrition and Food Technology; 1999.
- 15.Moussa L, Bezirard V, Salvador-Cartier C, Bacquie V, Houdeau E, Theodorou V. A new soy germ fermented ingredient displays estrogenic and protease inhibitor activities able to prevent irritable bowel syndrome-like symptoms in stressed female rats. Clin Nutr. 2013;32:51–8. doi: 10.1016/j.clnu.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT. et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 17.Braniste V, Leveque M, Buisson-Brenac C, Bueno L, Fioramonti J, Houdeau E. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol. 2009;587:3317–28. doi: 10.1113/jphysiol.2009.169300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erba D, Casiraghi MC, Martinez-Conesa C, Goi G, Massaccesi L. Isoflavone supplementation reduces DNA oxidative damage and increases O-beta-N-acetyl-D-glucosaminidase activity in healthy women. Nutr Res. 2012;32:233–40. doi: 10.1016/j.nutres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez-Escrig A, Tenorio MD, Espinosa-Martos I, Ruperez P. Health-promoting effects of a dietary fiber concentrate from the soybean byproduct okara in rats. J Agric Food Chem. 2008;56:7495–501. doi: 10.1021/jf800792y. [DOI] [PubMed] [Google Scholar]
- 20.Mangano KM, Hutchins-Wiese HL, Kenny AM, Walsh SJ, Abourizk RH, Bruno RS. et al. Soy proteins and isoflavones reduce interleukin-6 but not serum lipids in older women: a randomized controlled trial. Nutr Res. 2013;33:1026–33. doi: 10.1016/j.nutres.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckley MM, O’Halloran KD, Rae MG, Dinan TG, O’Malley D. Modulation of enteric neurons by interleukin-6 and corticotropin-releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. J Physiol. 2014;592:5235–50. doi: 10.1113/jphysiol.2014.279968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menon R, Watson SE, Thomas LN, Allred CD, Dabney A, Azcarate-Peril MA. et al. Diet complexity and estrogen receptor beta status affect the composition of the murine intestinal microbiota. Appl Environ Microbiol. 2013;79:5763–73. doi: 10.1128/AEM.01182-13. [DOI] [PMC free article] [PubMed] [Google Scholar]