Abstract
Background
Randomised controlled trials have demonstrated significant reductions in colorectal cancer (CRC) incidence and mortality associated with polypectomy. However, little is known about whether polypectomy is effective at reducing CRC risk in routine clinical practice. The aim of this investigation was to quantify CRC risk following polypectomy in a large prospective population-based cohort study.
Methods
Patients with incident colorectal polyps between 2000 and 2005 in Northern Ireland (NI) were identified via electronic pathology reports received to the NI Cancer Registry (NICR). Patients were matched to the NICR to detect CRC and deaths up to 31st December 2010. CRC standardised incidence ratios (SIRs) were calculated and Cox proportional hazards modelling applied to determine CRC risk.
Results
During 44,724 person-years of follow-up, 193 CRC cases were diagnosed amongst 6,972 adenoma patients, representing an annual progression rate of 0.43%. CRC risk was significantly elevated in patients who had an adenoma removed (SIR 2.85; 95% CI: 2.61 to 3.25) compared with the general population. Male sex, older age, rectal site and villous architecture were associated with an increased CRC risk in adenoma patients. Further analysis suggested that not having a full colonoscopy performed at, or following, incident polypectomy contributed to the excess CRC risk.
Conclusions
CRC risk was elevated in individuals following polypectomy for adenoma, outside of screening programmes.
Impact
This finding emphasises the need for full colonoscopy and adenoma clearance, and appropriate surveillance, after endoscopic diagnosis of adenoma.
Keywords: colorectal cancer, colorectal polyps, adenomas, polypectomy, epidemiology
Introduction
In the UK, colorectal cancer (CRC) is the fourth most common incident cancer and second most common cause of cancer-related death.(1) The UK has inferior CRC survival rates to comparable countries, commonly attributed to later clinical presentation.(2, 3) CRC typically originates from precancerous colorectal polyps, providing an opportunity for prevention if detected early and successfully excised.
The vast majority of colorectal polyps are either adenomas or hyperplastic polyps.(4) Adenomas are dysplastic polyps which can progress via the adenoma-carcinoma sequence to invasive cancer. Hyperplastic polyps are classified within the serrated group of colorectal polyps, and are traditionally considered benign. A related subtype of serrated polyps, namely sessile serrated polyps (also known as sessile serrated adenomas), may progress to carcinoma via the serrated carcinogenesis pathway,(5, 6) but these are relatively rare.(7) Individuals who have only hyperplastic polyps removed at endoscopy are not typically entered into surveillance.(8) Individuals with adenomas detected will usually be entered into a surveillance regime at an interval of 1, 3 or 5 years primarily depending on the number and size of adenomas detected or genetic predisposition to CRC.(8, 9)
The ultimate aim of clearing the bowel of adenomas, and entering patients into surveillance, is to reduce CRC risk. Building upon the historic findings of Winawer and colleagues,(10) several recent randomised controlled trials (11–14) and population-based case-control studies (15, 16) have demonstrated the success of polypectomy in reducing CRC risk. The US Prostate, Lung, Colorectal and Ovarian cancer screening trial has reported a significant 21% reduced risk of CRC amongst the intervention arm who underwent flexible sigmoidoscopy at baseline plus 3 or 5 years later, compared with usual care.(11) A ‘one-off’ flexible sigmoidoscopy was also effective at reducing CRC risk by 23% in a UK trial,(12) while similar reductions were observed in Italian, but not Norwegian, trials.(13, 14)
However, little is known about whether polypectomy is effective at reducing CRC risk in routine clinical practice. The aforementioned trials (11–14) and case-control studies (15, 16) may incur selection bias due to modest response rates limiting the likelihood of participants being representative of the general population. Few studies have investigated CRC risk following polypectomy in the general population. A French study observed a 2.2-fold greater incidence of CRC amongst advanced adenoma patients compared with the general population,(17) whilst a large Dutch study concluded that excess CRC risk in adenoma patients was limited to the first few years of follow-up.(18) In addition, given the enhanced opportunity for polyp detection within bowel cancer screening programmes,(19, 20) the appropriate clinical management of patients following polypectomy is even more pertinent.
The aim of this investigation was to quantify CRC risk following polypectomy of adenomas in a large population-based study.
Materials and Methods
Subject classification
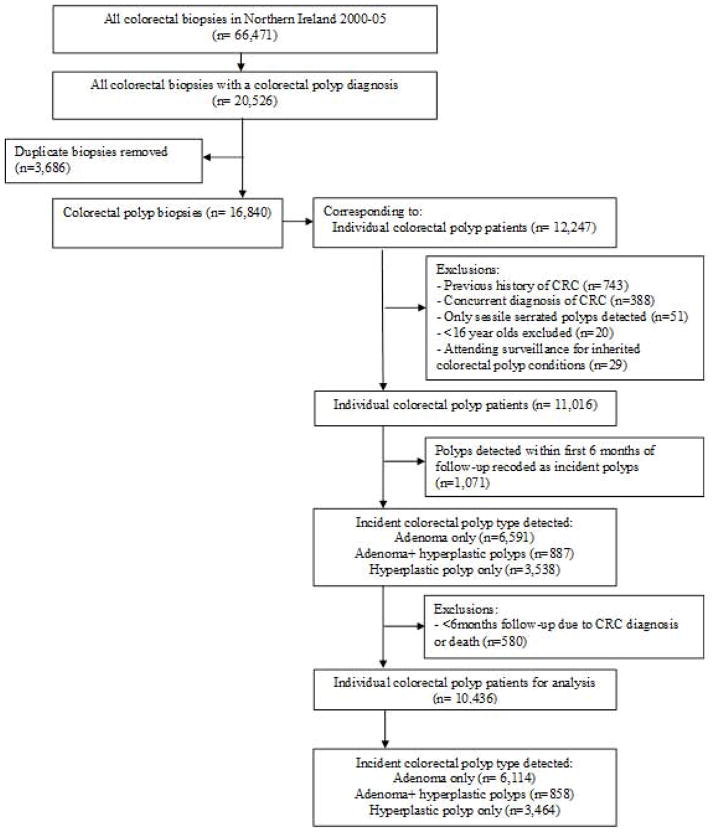
The Northern Ireland colorectal polyp register (NICPR) was derived from electronic pathology reports relating to all colorectal biopsies within Northern Ireland between 2000 and 2005. The NICPR has ethical approval from ORECNI: 10/NIR02/53. This timeframe precedes bowel cancer screening in this region, and therefore reflects clinical investigation of a symptomatic population. As outlined in Figure 1, relevant SNOMED morphology codes for adenomas and hyperplastic polyps were extracted. Few polyps were diagnosed or coded as sessile serrated polyps during this time period, and therefore these were excluded from analysis. Individuals aged <16 years were also removed. We applied two strategies for identifying and excluding patients who may be genetically predisposed to CRC due to polyposis syndromes. Firstly, patients were removed from the NICPR if they attended a genetic follow-up service at the Belfast Health and Social Care Trust (the sole referral centre in Northern Ireland). Secondly, we conducted a medical note review as part of a nested case-control study (described in detail below) to identify participants with a personal or family history of colorectal polyps or polyposis syndromes.
Figure 1.
Development of the Northern Ireland colorectal polyp register 2000–05.
CRC: Colorectal cancer.
Patients were categorised into an ‘adenoma register’ if their incident polyp was recorded as an adenomatous polyp(s) with or without a concurrent hyperplastic polyp(s) diagnosis. Patients who only received a hyperplastic polyp diagnosis were grouped into a ‘hyperplastic polyp register’. SNOMED coding was used to identify tubular, tubulovillous or villous adenoma subtypes. Information on polyp size was not readily available, and so patients with ‘advanced risk’ adenomas were considered to be those with multiple adenomas of any histological type or at least one adenoma including a villous component i.e. villous or tubulovillous. In order to minimise misclassification of polyps detected but not removed at first investigation, all polyps detected within six months of the baseline polyp were reclassified as ‘incident’ polyps. Subsequent polyps were those diagnosed at least six months after incident polyps, and up to 31st December 2005.
Outcome classification
The Northern Ireland Cancer Registry collects information on all cancer diagnoses since 1993. All individuals diagnosed with CRC (ICD-10 codes C18-C20) up to 31st December 2010 were matched on the basis of name, date of birth and/or address to the NICPR. Individuals with a previous or concurrent CRC diagnosis were excluded from the NICPR. CRC cases diagnosed at least six months after their incident polyp were considered to be incident CRC, and were considered for further case note review as part of a nested case-control study, as detailed below. The NICPR was also matched to death records supplied by the Northern Ireland Registrar General’s Office to the Northern Ireland Cancer Registry to detect patients who died by 31st December 2010.
Nested case control study
Patients who developed CRC at least six months post-incident polyp diagnosis were included in a nested case-control study. Controls were matched 1:1 to CRC cases by age (±1 year), sex, year of incident polyp diagnosis and were alive at the time of their matched cases’ CRC diagnosis. Tumour verification officers reviewed hospital case notes for information on all CRC and polyp diagnoses, polyp characteristics (including size, number and dysplasia grade), personal and family history of chronic bowel diseases and all lower gastrointestinal investigations conducted (including endoscopies) from one year prior to incident polyp diagnosis up to end of follow-up. A small number of individuals who were found to have a personal history of polyposis syndromes (or a family history of colorectal polyps as a proxy for an undiagnosed polyposis syndrome) were excluded from further nested case-control study analysis due to the likelihood that they are genetically predisposed to CRC. We did not exclude individuals reporting a family history of CRC, since only a small proportion (5–10%)(21) of these cases are likely to be due to genetic conditions and a known family history may simply reflect shared lifestyle or other non-hereditary CRC risk factors between family members. Cases for whom we were able to retrieve notes to enable inclusion within the case-control study did not differ from those not included with respect to age, sex, polyp location, morphology or advanced status, and are therefore representative of the entire CRC case set.
Statistical analysis
Patients were followed up from the date of their incident polyp diagnosis (excluding the first six months of follow-up) until their date of CRC diagnosis, date of death or 31st December 2010. Descriptive characteristics were compared using independent t-tests and chi-squared tests for continuous and categorical variables, respectively. Individuals with less than six months follow-up were excluded from analysis. CRC incidence was calculated per 100-person years of follow-up, comparing observed incidence with that of expected incidence in the Northern Ireland population between 2000 and 2010. Cox proportional hazards models were applied to investigate the association between CRC risk and demographics or incident polyp characteristics. Stratified analysis was conducted by these variables to explore effect modification. Assumptions for Cox proportional hazards models were checked by visual inspection of Kaplan-Meier plots. Standardised CRC incidence ratios (SIRs) and corresponding 95% confidence intervals (CI) were analysed per 100,000 population, and separately for males and females. Sensitivity analysis was performed after excluding those with less than one year of follow-up. For the nested case-control study, CRC risk was assessed between comparative groups by applying conditional logistic regression analysis to generate odds ratios (OR) and 95% CI. Statistical analysis was conducted using Intercooled Stata version 11.0 (StataCorp, College Station, TX, USA).
Results
Over a six year time period, n=6,972 individuals had a least one adenoma removed at their index colonoscopic investigation (Figure 1). There was a slight male predominance for adenoma patients, and the mean age at adenoma removal was 62.3 years. The majority of adenomas were detected and removed from the colon, and 22% of individuals had multiple adenomas removed at their first investigation in this time period (Table 1).
Table 1.
Characteristics of individuals diagnosed with incident colorectal adenomas in Northern Ireland 2000–05.
| Variable | Adenoma a register n= 6,972 (%)b |
|---|---|
| Sex | |
| Female | 3,157 (45.3) |
| Male | 3,815 (54.7) |
| Age at diagnosis (years, mean ± SD) | 62.7 ± 13.1 |
| Age groups at diagnosis (years) | |
| 16–<50 | 1,237 (17.7) |
| 50–<60 | 1,609 (23.1) |
| 60–<70 | 1,916 (27.5) |
| 70–<80 | 1,613 (23.1) |
| ≥80 | 597 (8.6) |
| Year of diagnosis | |
| 2000 | 1,004 (14.4) |
| 2001 | 990 (14.2) |
| 2002 | 1,016 (14.6) |
| 2003 | 1,280 (18.4) |
| 2004 | 1,278 (18.3) |
| 2005 | 1,404 (20.1) |
| Topography of index polyp(s) | |
| Colon only | 4,815 (69.1) |
| Rectum only | 1,687 (24.2) |
| Colon & rectum | 470 (6.7) |
| Number of incident polyps a | |
| 1 | 5,414 (77.7) |
| ≥2 | 1,558 (22.4) |
| Adenoma histology c | |
| Tubular only | 1,771 (25.4) |
| Villous/tubulovillous | 3,368 (48.3) |
| Unspecified | 1,833 (26.3) |
| Advanced adenomas d | 3,819 (54.8) |
| Subsequent adenoma e | 870 (12.5) |
| Subsequent hyperplastic polyp e | 447 (6.4) |
Incorporates n=858 individuals diagnosed with concurrent hyperplastic polyps as outlined in Figure 1. These are excluded from the ≥2 polyp number category unless multiple adenomas were diagnosed.
Percentages may not total 100 due to rounding.
Individuals with ≥1 villous or tubulovillous adenoma were classified as villous/tubulovillous, even if they also had a tubular adenoma at incident diagnosis.
Individuals with multiple adenomas or ≥1 villous/tubulovillous adenomas were classified as advanced. The polyp register did not contain detailed information on polyp size.
Subsequent defined as ever diagnosis ≥6 months after incident polyps and up to date of colorectal cancer diagnosis or end of 31st December 2005.
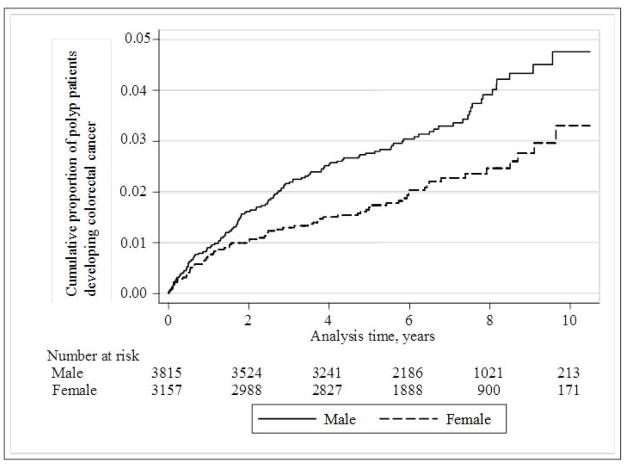
During a total of 44,724 person-years of follow-up, 193 patients were diagnosed with incident CRC. The rate of progression was relatively stable over time, and was consistently higher in males than females (Figure 2). Over this period, 0.43% of adenoma patients developed CRC each year, as shown in Table 2. CRC risk was significantly elevated in males and in individuals aged 60 years or older with adenomas. Rectal adenoma patients had an increased CRC risk (HR 1.70; 95% CI: 1.27–2.28) compared with those who had only colonic adenomas excised. No CRC developed in patients who had adenomas removed from both the colon and rectum. Those with tubulovillous or villous adenomas were more likely to develop CRC compared with those with tubular adenomas (HR 1.51; 95% CI: 1.02–2.23).
Figure 2.
Kaplan-Meier plot of the cumulative proportion of incident colorectal adenoma patients developing colorectal cancer, stratified by sex.
Table 2.
Colorectal cancer risk at least six months after incident colorectal adenoma diagnosis.
| Group | No. cases | Person-years follow-up | No. colorectal cancers | Incidence, % per year (95% CI) | Adjusted a hazard ratio (95% CI) |
|---|---|---|---|---|---|
|
Adenoma register
| |||||
| All | 6,972 | 44,724 | 193 | 0.43 (0.37–0.50) | N/A |
| Sex | |||||
| Female | 3,157 | 20,656 | 68 | 0.33 (0.26–0.42) | 1.00 |
| Male | 3,815 | 24,068 | 125 | 0.52 (0.43–0.62) | 1.69 (1.26–2.27) |
| Age groups (years) | |||||
| <50 | 1,237 | 8,817 | 18 | 0.20 (0.12–0.32) | 1.00 |
| 50–<60 | 1,609 | 11,186 | 29 | 0.26 (0.17–0.37) | 1.27 (0.71–2.29) |
| 60–<70 | 1,916 | 12,529 | 51 | 0.41 (0.30–0.53) | 1.99 (1.16–3.41) |
| 70–<80 | 1,613 | 9,414 | 60 | 0.64 (0.49–0.82) | 3.10 (1.83–5.26) |
| ≥80 | 597 | 2,778 | 35 | 1.26 (0.88–1.75) | 6.16 (3.48–10.91) |
| Topography of index polyp(s) | |||||
| Colon | 4,815 | 30,686 | 120 | 0.39 (0.32–0.47) | 1.00 |
| Rectum | 1,687 | 11,042 | 73 | 0.66 (0.52–0.83) | 1.70 (1.27–2.28) |
| Colon & rectum | 470 | 2,997 | 0 | 0.00 (0.00–0.00) | / |
| No. incident adenomas | |||||
| 1 | 5,414 | 34,864 | 161 | 0.46 (0.39–0.54) | 1.00 |
| ≥2 | 1,558 | 9,861 | 32 | 0.32 (0.22–0.46) | 0.67 (0.45–0.97) |
| Morphology b | |||||
| Tubular only | 1,771 | 11,524 | 36 | 0.31 (0.22–0.43) | 1.00 |
| Villous/tubulovillous | 3,368 | 21,508 | 97 | 0.45 (0.37–0.55) | 1.51 (1.02–2.23) |
| Unspecified | 1,833 | 11,692 | 60 | 0.51 (0.39–0.66) | 1.68 (1.11–2.54) |
| Advanced adenomas c | 3,819 | 24,373 | 105 | 0.43 (0.35–0.52) | 1.13 (0.83–1.54) |
| Concurrent hyperplastic polyp(s) | |||||
| Adenoma only | 6,114 | 39,051 | 182 | 0.47 (0.40–0.54) | 1.00 |
| Adenoma+hyperplastic | 858 | 5,674 | 11 | 0.19 (0.10–0.35) | 0.45 (0.24–0.82) |
| Subsequent adenoma d | 870 | 6,572 | 20 | 0.30 (0.19–0.47) | 0.72 (0.45–1.16) |
| Subsequent hyperplastic polyp d | 447 | 3,507 | 4 | 0.11 (0.03–0.29) | 0.31 (0.11–0.85) |
|
| |||||
| Hyperplastic register | |||||
|
| |||||
| All | 3,439 | 21,807 | 38 | 0.17 (0.12–0.24) | N/A |
Adjusted for age at incident polyp diagnosis (<50, 50–<60, 60–<70, 70–<80, ≥80 years), sex, year of incident polyp diagnosis (2000–2005), number of incident polyps diagnosed (1, ≥2) and subsequent adenoma diagnosed (yes/no).
Individuals with ≥1 villous or tubulovillous adenoma were classified as villous/tubulovillous, even if they also had a tubular adenoma at incident diagnosis.
Individuals with multiple adenomas or ≥1 villous/tubulovillous adenomas were classified as advanced. The polyp register did not contain detailed information on polyp size.
Subsequent defined as ever diagnosis ≥6 months after incident polyps and up to date of colorectal cancer diagnosis or end of 31st December 2005.
Several factors were associated with reduced risk of CRC amongst adenoma cases, including being diagnosed with concurrent hyperplastic polyps (HR 0.45; 95% CI: 0.24–0.82), or subsequent polyps (Table 2). Individuals diagnosed with multiple incident adenomas also had a decreased risk of CRC (HR 0.67; 95% CI: 0.45–0.97). Sensitivity analysis removing the first year of follow-up revealed largely similar results (Supplementary Table 1). As shown in Table 2, the risk of progression to CRC was lower in patients diagnosed with only hyperplastic polyps (0.17% per year), compared with adenoma patients. CRC risk in hyperplastic polyp patients did not differ significantly by sex, polyp location or multiplicity, however individuals aged 60–<80 years were at significantly increased risk of CRC compared with younger patients (data not shown).
As outlined in Table 3, individuals diagnosed with adenomas were almost three times more likely to develop CRC than the general population (SIR 2.85; 95% CI: 2.61–3.25). This heightened risk was observed in both males and females, for those with advanced adenomas, and after excluding cases in the first year of follow-up. Unexpectedly, hyperplastic polyp patients also had an increased CRC risk compared with the general population (SIR 1.79; 95% CI: 1.45–2.33), which did not alter in sensitivity analysis excluding incident hyperplastic polyp patients who had a subsequent adenoma (SIR 1.80; 95% CI: 1.46–2.36). Overall, an excess of 125 and 18 CRC cases developed amongst adenoma and hyperplastic polyp patients, respectively.
Table 3.
Standardised incidence ratios of colorectal cancer in individuals diagnosed with colorectal adenomas.
| Group | No. cases | No. CRC expected | No. CRC observed | SIR (95% CI) |
|---|---|---|---|---|
| Adenomas a | ||||
| All | 6,972 | 68 | 193 | 2.85 (2.61–3.25) |
| Female | 3,157 | 25 | 68 | 2.69 (2.30–3.33) |
| Male | 3,815 | 45 | 125 | 2.76 (2.47–3.25) |
| Advanced adenomas b | 3,819 | 38 | 105 | 1.75 (2.43–3.28) |
| All, excluding CRC in 1st year of follow-up | 6,834 | 68 | 157 | 2.32 (2.10–2.69) |
|
| ||||
| Hyperplastic polyps | ||||
| All | 3,464 | 23 | 41 | 1.79 (1.34–2.14) |
| Female | 1,668 | 9 | 17 | 1.89 (1.34–2.78) |
| Male | 1,796 | 14 | 24 | 1.67 (1.26–2.34) |
| No subsequent adenoma | 3,382 | 22 | 40 | 1.80 (1.46–2.36) |
CRC: Colorectal cancer. SIR: Standardised Incidence Ratios.
Incorporates n=858 individuals diagnosed with concurrent hyperplastic polyps as outlined in Figure 1.
Individuals with multiple adenomas or ≥1 villous/tubulovillous adenomas were classified as advanced. The polyp register did not contain detailed information on polyp size.
A post-hoc nested case-control study was conducted amongst CRC cases arising in adenoma and hyperplastic polyp cases (n=193, 82.5%) and matched controls whose medical notes were available for review, to determine further factors that may explain the excess CRC risk. A small number of CRC cases were identified to have a personal history of polyposis syndromes (n=3) or family history of colorectal polyps (n=7), or both (n=1) and were excluded from further analysis since they are likely to be genetically predisposed to a greater CRC risk, leaving n=148 case-control pairs in the remaining adenoma analysis and n=34 pairs in the hyperplastic polyp analysis. As shown in Table 4, a small proportion of excess CRC risk could be attributed to concurrent inflammatory bowel disease (OR 2.25; 95%CI: 0.98–5.17). Patients with large or right-sided incident polyps or adenomas with high-grade dysplasia had non-significantly increased risks of developing CRC.
Table 4.
Colorectal cancer risk factors from a case note review of patients with colorectal adenoma who developed colorectal cancer and matched controls.
| Adenoma patients | |||
|---|---|---|---|
|
| |||
| Factor | Controls n=148 (%) |
CRC cases n=148 (%) |
Odds ratio a (95% CI) |
| Previous adenoma before 2000 | |||
| No/unknown | 132 (89.2) | 136 (91.9) | 1.00 |
| Yes | 16 (10.8) | 12 (8.1) | 0.69 (0.30–1.62) |
| Personal history of IBD | |||
| No/unknown | 137 (92.6) | 127 (85.8) | 1.00 |
| Yes | 11 (7.4) | 21 (14.2) | 2.25 (0.98–5.17) |
| Family history of CRC | |||
| No/unknown | 127 (85.8) | 130 (87.8) | 1.00 |
| Yes | 21 (14.2) | 18 (12.2) | 0.83 (0.42–1.65) |
| Presence of HGD | |||
| No/unknown | 135 (91.2) | 121 (81.8) | 1.00 |
| Yes | 13 (8.8) | 27 (18.2) | 3.33 (1.34–8.30) |
| No. incident polyps | |||
| 1 | 94 (63.5) | 105 (71.0) | 1.00 |
| ≥2 | 54 (36.5) | 43 (29.0) | 0.70 (0.43–1.16) |
| Right-sided polyp b | |||
| No/unknown | 134 (85.4) | 124 (83.8) | 1.00 |
| Yes, ≥1 right-sided | 23 (14.7) | 24 (16.2) | 1.22 (0.66–2.28) |
| Polyp size | |||
| Small/unknown | 112 (75.7) | 103 (69.6) | 1.00 |
| Large (≥1cm) | 36 (24.3) | 45 (30.4) | 1.35 (0.81–2.24) |
| Full colonoscopy c | |||
| No/unknown | 37 (25.0) | 50 (33.8) | 1.00 |
| Yes,1 | 49 (33.1) | 45 (30.4) | 0.66 (0.36–1.21) |
| Yes, ≥2 | 62 (41.9) | 53 (35.8) | 0.59 (0.32–1.09) |
| Any other investigations d | |||
| No/unknown | 23 (15.5) | 18 (12.2) | 1.20 (0.58–2.47) |
| Yes,1 e | 62 (41.9) | 38 (25.7) | 1.00 |
| Yes, ≥2 | 63 (42.6) | 92 (62.2) | 2.18 (1.32–3.60) |
| Did not attend for planned follow-up procedure | |||
| No/unknown | 137 (92.6) | 132 (89.2) | 1.00 |
| Yes | 10 (6.8) | 13 (8.8) | 1.45 (0.62–3.41) |
| Yes, missed>1 procedure | 1 (0.7) | 3 (2.0) | 3.64 (0.36–37.01) |
CRC: Colorectal cancer; HGD: High grade dysplasia; IBD: Inflammatory bowel disease.
Adjusted for matching criteria (age, sex, incident polyp type (adenoma/hyperplastic) and year of diagnosis at incident polyp.
Includes polyps detected in caecum, ascending colon, hepatic flexure and transverse colon
Number of full colonoscopies performed within 1 year prior to incident polyp diagnosis and before censor date (CRC diagnosis or matched follow-up time for controls).
Any other investigative procedures (excluding full colonoscopy) including incomplete or unspecified colonoscopy, proctoscopy, rigid or flexible sigmoidoscopy or barium enema performed within 1 year prior to incident polyp diagnosis.
Chosen as reference category to reflect that many individuals attended for a sigmoidoscopy or other non-full colonoscopy investigation at their first investigation, which was then followed up by a full colonoscopy.
Conversely, those with multiple adenomas, a CRC family history or an adenoma removed prior to the initiation of our study period (and therefore potentially undergoing surveillance) did not have an increased CRC risk. Adenoma patients who had a full colonoscopy during the follow-up period had a non-significant reduced risk of CRC, particularly if they had multiple (two or more) full colonoscopies performed (OR 0.59; 95% CI: 0.32–1.09). If patients had attended for investigative procedures other than full colonoscopy (for example sigmoidoscopy or barium enema), they had a significant increased risk of CRC (OR 2.18; 95% CI: 1.32–3.60). In addition, individuals who did not attend for a planned procedure were at an increased risk of CRC, which was exaggerated if multiple appointments were missed (Table 4).
Non-significant increased CRC risks were observed for individuals with multiple and/or right-sided hyperplastic polyps, and those who did not attend for a planned follow-up investigation (data not shown).
Discussion
Overall, annual CRC risk amongst individuals following polypectomy of adenomas in the non-screening setting is 0.43%. Despite undergoing polypectomy, CRC risk is significantly elevated for incident adenoma patients compared with the general population.
The increased risk of CRC following adenoma removal observed in our study is in line with findings from a French study.(17) In their analysis of 5,779 adenoma patients diagnosed between 1990 and 1999, 87 CRC were diagnosed, resulting in an SIR of 2.23 (95%CI 1.67–2.92) for advanced adenomas.(17) As a result of further case note review, a small proportion of CRC cases in the current study were found to be associated with genetic predisposition (6.9% overall, and 8.5% in advanced adenoma cases). Applying these proportions to our overall SIR estimates would result in SIR of 2.65 and 2.53 for all and advanced adenomas, respectively, and are more comparable with the methods in the French study.(17) The largest study to date, published from a Dutch population, only observed an elevated SIR (2.8; 95% CI 2.5–3.1) in the second and third year of follow-up, which was then attenuated in later years.(18) This contrasts with our study findings, which illustrate a consistently increased CRC risk over a prolonged time period. Earlier population-based studies of smaller sample size, tended to observe either no association or a reduced risk of CRC following adenoma removal.(15, 16, 22) Randomised controlled trials of flexible sigmoidoscopy have also mostly demonstrated a reduction in CRC incidence.(11, 12, 14) Our findings indicate that the benefits seen for polyp removal in the tightly-controlled environment of trials and screened populations may not be replicated in the general population who are more likely to be symptomatic when presenting for clinical investigation.
As expected, being male or aged 60 years or older was associated with an increased risk of CRC following adenoma removal.(23, 24) Individuals whose incident adenomas contained a villous component also had increased CRC risk, which is consistent with previous reports.(23) Other factors that would be expected to increase CRC risk, including a personal history of inflammatory bowel disease, presence of high-grade dysplasia in the adenomas and large adenoma size,(9, 25) were directly associated with CRC risk in the detailed case note review aspect of our study. All of these findings are reassuring for the robustness of our data, but collectively, these factors only account for a relatively small proportion of the excess CRC cases found in this population.
Several factors indirectly suggested that incomplete clearance of adenomas from the bowel may have led to the higher future CRC risk, which is consistent with previous study findings.(26–29) Firstly, having only rectal adenomas diagnosed was associated with an increased risk of CRC, implying the colon may not have been examined. No CRC occurred amongst individuals who had adenomas diagnosed in both the colon and rectum. Similarly, having multiple adenomas removed was inversely associated with CRC risk. Finally, having concurrent adenoma and hyperplastic polyp removal at incident polypectomy (which suggests more thorough primary clearance of the bowel) or subsequent hyperplastic polyp detection (which indicates follow-up endoscopy) was associated with a reduced risk of CRC. This was also corroborated by results from the case note review, in which individuals undergoing a full colonoscopy had decreased risks of CRC, whereas patients who had other investigative procedures such as incomplete colonoscopy, flexible sigmoidoscopy or barium enema had an increased risk of CRC. These findings have implications for current surveillance guidelines following polypectomy.(9) Our results suggest that a significant proportion of patients diagnosed with colorectal adenoma do not undergo full colonoscopy and adenoma clearance, and this incurs an increased risk of CRC. The reasons for this are unclear but merit further consideration. While patient choice is foremost in decision making, understanding potential barriers for colonoscopy compliance is an important area for future research. Having multiple full colonoscopies following incident adenoma removal was inversely associated with CRC risk, which argues for the benefit of continued surveillance.(30) The increased yield of adenomas and CRC when performing full colonoscopy compared with other procedures is well established.(31–33)
We also observed an unexpected increased CRC risk amongst individuals having only hyperplastic polyps removed. The potential for hyperplastic polyps to be a marker of adenomas has been previously debated.(34, 35) Our results do not support this finding, since an increased CRC risk remained when individuals with subsequent adenomas were removed from analysis. However, we were unable to account for adenomas detected after 2005 and undetected concurrent or subsequent adenomas may still explain the excess CRC risk seen. Sessile serrated polyps represent another subset of serrated polyps, much rarer than hyperplastic polyps, and have only been well described in recent years.(36) Sessile serrated polyps are more common in the proximal colon, are often multiple, are larger than typical hyperplastic polyps and are now accepted as the precursors to serrated pathway CRCs.(37, 38) Sessile serrated polyps were not frequently reported by pathologists during this study period and it is likely many such polyps were misdiagnosed as hyperplastic polyps.(36, 39) This is supported by our findings that multiple and right-sided ‘hyperplastic polyps’ carried an increased risk of CRC progression.
Our study has several strengths, including its large size, length of follow-up and relevance to current practice, given the timeframe of adenoma patients studied. The population-based nature of the study is important to emphasise, demonstrating CRC risk following polypectomy in a ‘real world’ setting. This is the first insight into this association in a UK population, amongst whom healthcare is free at the point of access. Northern Ireland has a relatively stable population with limited migration,(40) therefore the completeness of passive follow-up is excellent. Furthermore, our results were not diluted with bowel cancer screening-detected adenomas/cancers since a screening programme only commenced in mid-2010. This study does have some limitations that should be considered. In the NICPR we did not have information on polyps diagnosed prior to 2000 or after 2005, or information on polyp size or detailed polyp topography. However, we overcame these limitations by conducting a detailed note review in a nested case-control study. The number of polyps in patients with more than one polyp was also underestimated due to the potential for SNOMED codes to have been entered only once by the reporting pathologist, if a patient had multiple polyps diagnosed of the same histological type at one episode. Comparison with the case note review data suggests that approximately 10% of patients with multiple adenomas were misclassified as singular in the overall register.
In conclusion, this large population-based study demonstrates that CRC risk remains significantly higher than that of the general population following adenoma removal, outside of screening programmes. Our findings suggest the increased CRC risk may be partially due to incomplete examination and/or adherence to follow-up colonoscopies. There is a need for full colonoscopy and adenoma clearance after a diagnosis of colorectal adenoma to reduce risk of subsequent CRC. Further research is required to identify optimal surveillance protocols, and adherence to these, following adenoma removal.
Supplementary Material
Acknowledgments
Funding support: This work was supported by funding from Cancer Focus Northern Ireland (formerly the Ulster Cancer Foundation) awarded to Marie M. Cantwell, Anna T. Gavin and Liam J. Murray. The Northern Ireland Cancer Registry (for which Anna T. Gavin is Director) is funded by the Public Health Agency for Northern Ireland.
We would like to acknowledge the contribution of IT and data management staff in the Northern Ireland Cancer Registry, namely Dr Richard Middleton, Mr Giulio Napolitano and Mr Colin Fox, for their help in collecting and extracting data that contributed to the Northern Ireland Colorectal Polyp Register. Special gratitude is extended to Mrs Donna Floyd and Mrs Michelle McGaughey for their assistance in piloting and designing the data extraction form used in the case note review for the nested case-control study, and to Mrs Donna Floyd, Mrs Rosemary Ward and Mrs Kate Donnelly (all Northern Ireland Cancer Registry) for conducting the case note review. We would also like to acknowledge the input of Dr Khaled Kasim in initiating cleaning and analysis of the Northern Ireland colorectal polyp register database.
Footnotes
Disclosures: The authors do not have any potential conflicts of interest to declare.
References
- 1.Cancer Research UK. [Accessed August 28, 2013];Bowel Cancer Statistics. 2013 Available at: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bowel/
- 2.Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): An analysis of population-based cancer registry data. Lancet. 2011;377:127–38. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris EJ, Sandin F, Lambert PC, Bray F, Klint A, Linklater K, et al. A population-based comparison of the survival of patients with colorectal cancer in England, Norway and Sweden between 1996 and 2004. Gut. 2011;60:1087–93. doi: 10.1136/gut.2010.229575. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton SR, Bosman FT, Boffetta P, Ilyas M, Morreau H, Nakamura SI, et al. Carcinoma of the colon and rectum. In: Bosman FT, Carniero F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4. Lyon: IARC; 2010. [Google Scholar]
- 5.Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, et al. Serrated lesions of the colorectum: Review and recommendations from an expert panel. Am J Gastroenterol. 2012;107(9):1315, 29. doi: 10.1038/ajg.2012.161. quiz 1314, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Carr NJ, Mahajan H, Tan KL, Hawkins NJ, Ward RL. Serrated and non-serrated polyps of the colorectum: Their prevalence in an unselected case series and correlation of BRAF mutation analysis with the diagnosis of sessile serrated adenoma. J Clin Pathol. 2009;62:516–8. doi: 10.1136/jcp.2008.061960. [DOI] [PubMed] [Google Scholar]
- 8.Atkin WS, Saunders BP British Society for Gastroenterology, Association of Coloproctology for Great Britain and Ireland. Surveillance guidelines after removal of colorectal adenomatous polyps. Gut. 2002;51 (Suppl 5):V6–9. doi: 10.1136/gut.51.suppl_5.v6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–89. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 10.Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. the national polyp study workgroup. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 11.Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–57. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: A multicentre randomised controlled trial. Lancet. 2010;375(9726):1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 13.Hoff G, Grotmol T, Skovlund E, Bretthauer M Norwegian Colorectal Cancer Prevention Study Group. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: Randomised controlled trial. BMJ. 2009;338:b1846. doi: 10.1136/bmj.b1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, et al. Once-only sigmoidoscopy in colorectal cancer screening: Follow-up findings of the Italian randomized controlled trial--SCORE. J Natl Cancer Inst. 2011;103:1310–22. doi: 10.1093/jnci/djr284. [DOI] [PubMed] [Google Scholar]
- 15.Brenner H, Chang-Claude J, Rickert A, Seiler CM, Hoffmeister M. Risk of colorectal cancer after detection and removal of adenomas at colonoscopy: Population-based case-control study. J Clin Oncol. 2012;30:2969–76. doi: 10.1200/JCO.2011.41.3377. [DOI] [PubMed] [Google Scholar]
- 16.Brenner H, Chang-Claude J, Jansen L, Seiler CM, Hoffmeister M. Role of colonoscopy and polyp characteristics in colorectal cancer after colonoscopic polyp detection: A population-based case-control study. Ann Intern Med. 2012;157:225–32. doi: 10.7326/0003-4819-157-4-201208210-00002. [DOI] [PubMed] [Google Scholar]
- 17.Cottet V, Jooste V, Fournel I, Bouvier AM, Faivre J, Bonithon-Kopp C. Long-term risk of colorectal cancer after adenoma removal: A population-based cohort study. Gut. 2012;61:1180–6. doi: 10.1136/gutjnl-2011-300295. [DOI] [PubMed] [Google Scholar]
- 18.Loeve F, van Ballegooijen M, Boer R, Kuipers EJ, Habbema JD. Colorectal cancer risk in adenoma patients: A nation-wide study. Int J Cancer. 2004;111:147–51. doi: 10.1002/ijc.20241. [DOI] [PubMed] [Google Scholar]
- 19.Mansouri D, McMillan DC, Roxburgh CS, Moug SJ, Crighton EM, Horgan PG. Flexible sigmoidoscopy following a positive faecal occult blood test within a bowel screening programme may reduce the detection of neoplasia. Colorectal Dis. 2013;15:1375–81. doi: 10.1111/codi.12377. [DOI] [PubMed] [Google Scholar]
- 20.Lee TJ, Nickerson C, Goddard AF, Rees CJ, McNally RJ, Rutter MD. Outcome of 12 month surveillance colonoscopy in high risk patients in the NHS bowel cancer screening programme. Colorectal Dis. 2013;15:e435–42. doi: 10.1111/codi.12278. [DOI] [PubMed] [Google Scholar]
- 21.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–32. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 22.Loeve F, van Ballegooijen M, Snel P, Habbema JD. Colorectal cancer risk after colonoscopic polypectomy: A population-based study and literature search. Eur J Cancer. 2005;41:416–22. doi: 10.1016/j.ejca.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Martinez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832–41. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levi F, Randimbison L, La Vecchia C. Trends in the subsite distribution of colorectal carcinomas and polyps: An update. Cancer. 1998;83:2040–2. doi: 10.1002/(sici)1097-0142(19981101)83:9<2040::aid-cncr22>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 25.Eaden JA, Mayberry JF British Society for Gastroenterology, Association of Coloproctology for Great Britain and Ireland. Guidelines for screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut. 2002;51 (Suppl 5):V10–2. doi: 10.1136/gut.51.suppl_5.v10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn SB, Han DS, Bae JH, Byun TJ, Kim JP, Eun CS. The miss rate for colorectal adenoma determined by quality-adjusted, back-to-back colonoscopies. Gut Liver. 2012;6:64–70. doi: 10.5009/gnl.2012.6.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bensen S, Mott LA, Dain B, Rothstein R, Baron J. The colonoscopic miss rate and true one-year recurrence of colorectal neoplastic polyps. polyp prevention study group. Am J Gastroenterol. 1999;94:194–9. doi: 10.1111/j.1572-0241.1999.00796.x. [DOI] [PubMed] [Google Scholar]
- 28.Bressler B, Paszat LF, Vinden C, Li C, He J, Rabeneck L. Colonoscopic miss rates for right-sided colon cancer: A population-based analysis. Gastroenterology. 2004;127:452–6. doi: 10.1053/j.gastro.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 29.Hixson LJ, Fennerty MB, Sampliner RE, Garewal HS. Prospective blinded trial of the colonoscopic miss-rate of large colorectal polyps. Gastrointest Endosc. 1991;37:125–7. doi: 10.1016/s0016-5107(91)70668-8. [DOI] [PubMed] [Google Scholar]
- 30.Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–8. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 31.Graser A, Stieber P, Nagel D, Schäfer C, Horst D, Becker CR, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58:241–8. doi: 10.1136/gut.2008.156448. [DOI] [PubMed] [Google Scholar]
- 32.Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. Colorectal cancers not detected by screening flexible sigmoidoscopy in the prostate, lung, colorectal, and ovarian cancer screening trial. Gastrointest Endosc. 2012;75:612–20. doi: 10.1016/j.gie.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 33.Castells A, Bessa X, Quintero E, Bujanda L, Cubiella J, Salas D, et al. Risk of advanced proximal neoplasms according to distal colorectal findings: Comparison of sigmoidoscopy-based strategies. J Natl Cancer Inst. 2013;105:878–86. doi: 10.1093/jnci/djt117. [DOI] [PubMed] [Google Scholar]
- 34.Sciallero S, Costantini M, Bertinelli E, Castiglione G, Onofri P, Aste H, et al. Distal hyperplastic polyps do not predict proximal adenomas: results from a multicentric study of colorectal adenomas. Gastrointest Endosc. 1997;46:124–30. doi: 10.1016/s0016-5107(97)70059-2. [DOI] [PubMed] [Google Scholar]
- 35.Viel JF, Studer JM, Ottignon Y, Hirsch JP Franche-Comté Polyp Surveillance Study Group. Predictors of colorectal polyp recurrence after the first polypectomy in private practice settings: a cohort study. PloS one. 2012;7:e50990. doi: 10.1371/journal.pone.0050990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bettington M, Walker N, Rosty C, Brown I, Clouston A, Wockner L, et al. Critical appraisal of the diagnosis of the sessile serrated adenoma. Am J Surg Pathol. 2014;38:158–66. doi: 10.1097/PAS.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 37.Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367–386. doi: 10.1111/his.12055. [DOI] [PubMed] [Google Scholar]
- 38.McDonald SA, Preston SL, Lovell MJ, Wright NA, Jankowski JA. Mechanisms of disease: from stem cells to colorectal cancer. Nat Clin Pract Gastroenterol Hepatol. 2006;3:267–74. doi: 10.1038/ncpgasthep0473. [DOI] [PubMed] [Google Scholar]
- 39.Gill P, Wang LM, Bailey A, East JE, Leedham S, Chetty R. Reporting trends of right-sided hyperplastic and sessile serrated polyps in a large teaching hospital over a 4-year period (2009–2012) J Clin Pathol. 2013;66:655–8. doi: 10.1136/jclinpath-2013-201608. [DOI] [PubMed] [Google Scholar]
- 40.Northern Ireland Statistics and Research Agency. [Accessed February 25, 2014];Migration Statistics. 2012 Available at: http://www.nisra.gov.uk/demography/default.asp18.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.