Abstract
Hypertension is a widespread condition that affects millions of people around the world and has a great impact in public health. The classic Renin Angiotensin System is a complex system comprised of multiple peptides and pathways that have been the motive of great drug development over the years to control hypertension. However, there are still patients whose hypertension is very difficult to control with the current drugs and strategies, thus motivating further research in this field. In the last two decades, important discoveries have expanded our knowledge of this system and new pathways are emerging that are helping us understand the complex interaction taking place not only in the periphery but also in the central nervous system where the renin angiotensin system is also very active. A new arm, called the ACE2/Ang-(1-7)/Mas receptor axis was shown to exert anti-hypertensive properties and serve as a counterbalance to the classic ACE/Angiotensin-II/AT1 receptor axis; in this way, modulating or even counteracting the negative effects of Angiotensin-II in blood pressure regulation and water retention. Modulation of this new axis through ACE2 activation, ADAM17 regulation or AT1 receptor internalization are some of the novel avenues and challenges that have the potential to become target for new drug research and development for the treatment of hypertension.
Keywords: ADAM17, oxidative stress, DOCA-salt hypertension, antioxidant, ACE2, Angiotensin II
Hypertension is classically defined in adults as systolic blood pressure (BP) higher than 140 mmHg and/or diastolic BP higher than 90 mmHg. Pre-hypertension is defined as systolic BP >120 mmHg and diastolic BP > 80 mmHg, but not reaching hypertension levels according to the Joint National Committee (JNC) 8 for hypertension guidelines [James et al., 2014]. Thresholds for pediatric patients vary according to age.
Hypertension is a multifactorial disease, the etiology of which is not completely understood [Yemane et al., 2010]. It has reached epidemic proportions worldwide and contributes significantly to the burden of heart disease, stroke, kidney failure, disability and premature death [Neupane et al., 2014]. It is estimated that about 17 million deaths occur worldwide because of cardiovascular diseases every year, of which, complications of hypertension alone account for 9.4 million deaths [2011]. A third of US adults are reported to be pre-hypertensive [Yoon et al., 2015]. Prevention is an important step in the pre-hypertensive group to reduce the number of people affected by high BP and consequently the number of deaths due to cardiovascular complications arising from hypertension. Emphasis is placed on those with pre-hypertension in order to prevent the development of high BP by adopting a healthier lifestyle like increasing exercise activity, reducing salt consumption and reducing smoking, among others. However in spite of the effort and self-care, there is still a great population of patients who will eventually require pharmacological treatment.
Current strategies to manage hypertension
According to the recent Joint National Committee (JNC) 8 meeting, the current recommendations for treatment of hypertension are mainly based on the use, alone or in combination, of 4 classes of medications which include: diuretics, calcium channel blockers, angiotensin receptor blockers (ARBs) and angiotensin converting enzyme inhibitors (ACEI). All of these medications have proved to be adequate to decrease BP enough to be maintained below the threshold levels for the age population [James et al., 2014]. There is however a >30% of patients whose hypertension cannot be controlled [Yoon et al., 2015] with the current recommendations and require novel interventions or additional drugs to their treatment regimen with variable rate of success [Persell, 2011]. Therefore, the question of finding new treatment strategies is still a major concern in pharmacological research.
The Renin Angiotensin System
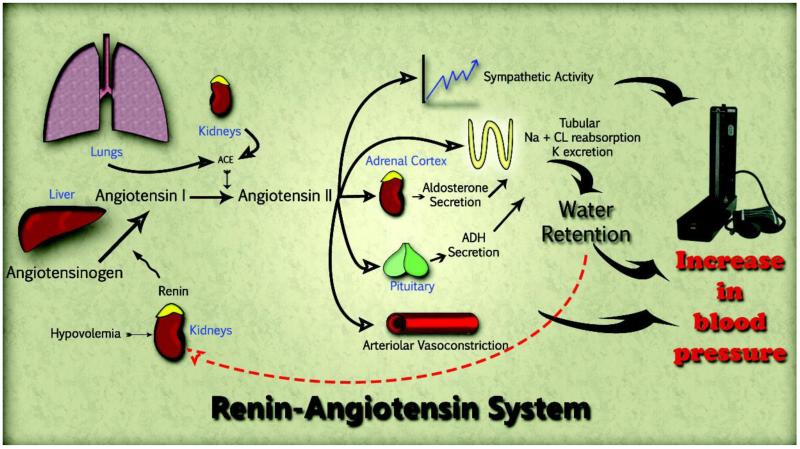
The classic renin angiotensin system (RAS) is a complex system composed of numerous peptides, enzymes and receptors that are involved in BP regulation and fluid homeostasis. It starts with angiotensinogen (AGT) generated in the liver. AGT is converted to angiotensin (Ang)-I by renin that is released from juxtaglomerular cells of the kidney under salt deprivation and fluid imbalance. Ang-I acts as a substrate for angiotensin converting enzyme (ACE) which cleaves it to form Ang-II (Fig 1). Ang-II signaling via G protein-coupled AT1 receptors (AT1R) promotes vasoconstriction, increases sympathetic tone, vasopressin release and aldosterone secretion. All of these effects ultimately increase the effective fluid volume and elevate BP. To a lesser extent Ang-II also signals through a second G protein-coupled receptor, namely AT2 (AT2R), promoting vasodilatory effects opposite to AT1R through the release of NO.
Figure 1.
Although the RAS is classically thought to act as an endocrine system with Ang-II circulating throughout the bloodstream, components have been shown to exist locally in several tissues including the heart, lung, adrenal gland, kidney, blood vessels and brain, to name only a few [Lavoie et al., 2003, Paul et al., 2006].
The brain RAS produces Ang peptides locally in several regions involved in the central regulation of BP, such as the paraventricular nucleus of the hypothalamus (PVN), subfornical organ (SFO), rostral ventrolateral medulla (RVLM), area postrema, and nucleus tractus solitarius (NTS) [Davisson, 2003, Gironacci et al., 2014]. Increased circulating Ang-II acts by controlling sodium and water intake by acting on structures of the antero-ventral region of the third ventricle (AV3V), stimulating the secretion of vasopressin from the PVN and supraoptic nuclei of the hypothalamus, and controlling autonomic function by increasing sympathetic nerve activity in several nuclei including the PVN and RVLM [Ferguson et al., 1997].
Over-activity of the brain RAS has been implicated in neurogenic hypertension and is associated with excessive levels of Ang-II [Grobe et al., 2010]. In addition, to the circumventricular organs (CVO), which are brain regions lacking a blood-brain barrier and allowing small, circulating peptides like Ang-II to cross [Johnson et al., 1993], evidence suggest that the blood brain barrier becomes “leaky” during the development of hypertension [Ueno et al., 2004] thus allowing more Ang-II to enter the central nervous system .
Therefore, not only locally-generated Ang-II interacting with RAS components within the blood-brain barrier (PVN and RVLM), but also circulating Ang-II can enter the brain and interact with Ang receptors, notably AT1R, to produce sympathetic activation in addition to peripheral effects and further exacerbate the over-activity of the brain RAS in neurogenic hypertension [Lazartigues et al., 2007, Xia et al., 2009].
Targeting the RAS
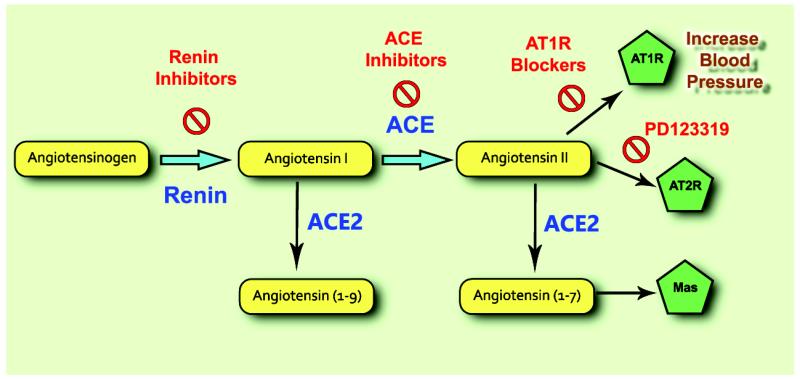
Strategies to treat hypertension and heart failure have focused on blockade (Fig 2) of Ang-II formation (i.e. ACEI and more recently renin inhibitors) and blockade of the ATIR (i.e. Angiotensin receptor blockers). Blockade of the RAS with these drugs is beneficial but not absolute and results in arterial and venous vasodilation, natriuresis and diuresis, decrease in sympathetic nervous activity, and inhibition of cardiac and vascular hypertrophy. These effects lead to a reduction in BP and less vascular remodeling in the pathology of hypertension [Brunner et al., 1979]. Accordingly, RAS inhibitors are also effective anti-hypertensive treatments in patients with low plasma renin levels, a phenomenon seen in neurogenic hypertension [Sigmund, 2010] and correlate with 25% of those diagnosed with low renin hypertension [2011]. Renin inhibitors, such as Aliskiren, target the conversion of AGT to Ang-I and thus reduce the substrate available for metabolism into Ang-II. Another peptide in the RAS, Ang-(1-12), can be cleaved by ACE and converted into Ang-I, followed by a second cleavage by ACE into Ang-II, thus bypassing the effects of renin [Westwood et al., 2012] and suggesting that renin inhibitors may not completely block the formation of Ang-II. Nagata et al. reported that administration of Ang-(1-12) in the periphery increases BP. This pressor response was attenuated by the ATIR blocker candesartan and the ACEI captopril [Nagata et al., 2006] suggesting that Ang-II stimulation of AT1R remains the main pressor mechanism.
Figure 2.
ACEI like captopril, lisinopril and enalapril, block the cleavage of Ang-I to Ang-II and lead to an overall decrease in circulating Ang-II levels. However, chymase has been shown to catalyze the formation of Ang-II from Ang-I [Park et al., 2013] independently of ACE. This was particularly evident in the face of diabetic and hypertensive nephropathy which allows Ang-II to bypass the effects of ACEI to some extent. ACEI also inhibit the catabolism of bradykinin [Tom et al., 2001], a molecule that produces vasodilatory effects via its B2 receptor and mediates inflammation through its B1 receptor. This dual action of ACEI provides great therapeutic benefits in treating cardiovascular diseases.
A final set of inhibitors targeting the RAS, blocks the AT1R, through which Ang-II promotes the majority of its signaling effects [Allen et al., 2000]. These drugs are commonly referred to as sartans or ARB. While only minor effects of Ang-II are attributed to the AT2R in normal conditions, the role of this receptor appears to be more prominent in pathological conditions (e.g. hypertension), as evidenced by recent data [Xu et al., 2011, Bruce et al., 2014, Sampson et al., 2015]. Blocking AT1R increases circulating Ang-II levels, leading to increased signaling through AT2R (and ACE2). Like ACEI, ARB also provide beneficial effects in the treatment of hypertension by decreasing Ang-II signaling via the ATIR.
The ACE2/ANG-(1-7)/Mas Axis
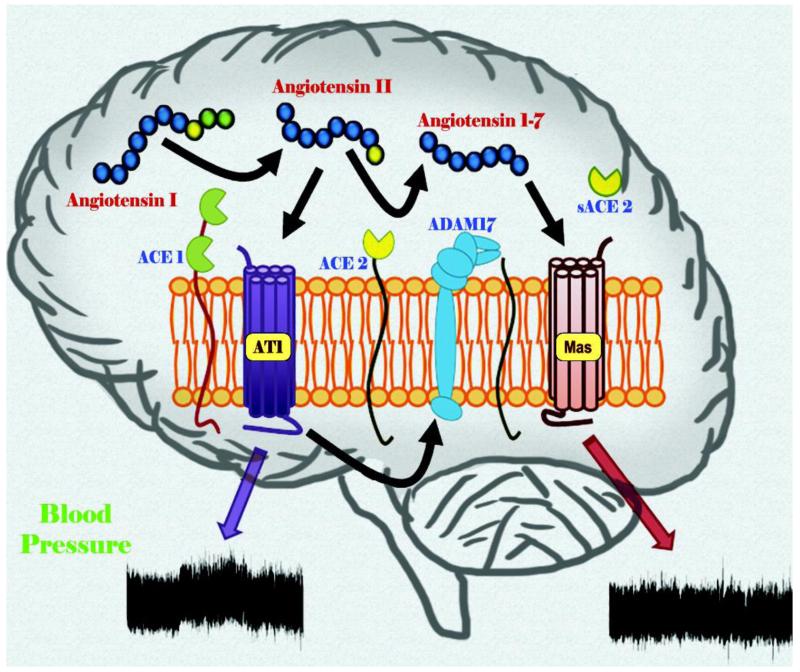
In the past decade, a new axis of the RAS, Angiotensin Converting Enzyme Type 2 (ACE2)/Ang-(1-7)/Mas receptor, has been identified (Fig. 3) and shown to exhibit compensatory mechanisms in the face of an overactive RAS [Xu et al., 2011]. The components of this new axis have been identified in regions of the brain involved in the central regulation of BP, as well as the periphery, and are thought to play a role in neurogenic hypertension [Lazartigues et al., 2007]. ACE2 is a homologue of ACE that cleaves Ang-II into the vasodilator peptide Ang-(1-7), which acts on the Mas receptor to produce effects opposite to Ang-II, such as vasodilatation, anti-proliferation and anti-hypertrophy [Ferrario et al., 2005]. ACE2 (and AT2R) may also be providing some of the beneficial effects seen with ARB, which increase Ang-II levels, by allowing for ACE2 to cleave more Ang-II substrate into Ang-(1-7). ACE2 can also cleave Ang-I into Ang-(1-9), which could then be converted into Ang-(1-7) by ACE or neprilysin [Vickers et al., 2002]. However, the affinity for this reaction is much less than for Ang-II and it appears that this path would only be activated when Ang-I levels are elevated, for example in the presence of ACEI due to blocking the conversion of Ang-I into Ang-II, leaving Ang-I available for cleavage by ACE2 (Fig 3).
Figure 3.
Several studies have highlighted ACE2 as a pivotal player in counterbalancing the vasoconstrictive actions of Ang-II in the development of cardiovascular diseases. The development of Ang-II-induced hypertension was blunted by overexpression of ACE2 in the central nervous system [Feng et al., 2010]. In similar studies, rodents were also protected from impaired baroreflex and autonomic dysfunction induced by Ang-II when ACE2 was overexpressed in the brain [Yamazato et al., 2007, Xia et al., 2009]. Other studies have reported that ACE2 prevents cardiac hypertrophy in Ang-II infused animals [Crackower et al., 2002, Diez-Freire et al., 2006] [Feng et al., 2012]. Finally, high BP is decreased by ACE2 overexpression from lentivirus introduced to the RVLM of spontaneously hypertensive rats [Yamazato et al., 2007]. In these studies, ACE2 not only reduces the levels of Ang-II but also increases the levels of Ang-(1-7), promoting beneficial effects in various cardiovascular diseases. Therefore, therapies to enhance ACE2 could become a new approach for the treatment of cardiovascular diseases.
Potential new targets and new challenges
Although not part of the compensatory RAS per se, the previously identified (Pro)-renin receptor (PRR) [Nguyen et al., 2008] has recently be shown to play a critical role in the central regulation of BP. Not only the PRR was shown to be expressed in key brain nuclei involved in the regulation of autonomic function but it is also up-regulated with hypertension [Li et al., 2012, Zubcevic et al., 2013, Li et al., 2014]. Interestingly, one of these groups recently reported the generation of a new PRR antagonist, termed PRO20, capable of dose-dependently inhibiting PRR-induced hypertension in mice [Li et al., 2015]. Prevention of increased Ang-II levels within the central nervous system was shown to contribute to the anti-hypertensive effects of PRO20 which could potentially be the first of a new class of anti-hypertensive agents.
At the other end of the RAS cascade, Ala1-Ang-(1-7), aka Alamandine, was identified as a new metabolite of Ang-(1-7) through an unidentified enzyme [Lautner et al., 2013]. Alamandine can also be formed, via ACE2, from Ang-A, an analogue of Ang-II identified in human plasma [Jankowski et al., 2007, Etelvino et al., 2014]. So far, reports show that Alamandine has similar properties to Ang-(1-7), capable of promoting nitric oxide signaling, reducing Ang-A-mediated vasoconstriction and with anti-hypertensive properties. Alamandine was reported to bind the MrgD receptor which can also be activated, in vitro, by β-alanine, GABA, BABA and Ang-(1-7) [Solinski et al., 2014]. However recent studies have suggested that heterodimerization of receptors, including the AT2R, could mediate the effects of Alamandine and Ang-(1-7) [Villela et al., 2015]. More work is needed to determine the specificity of Alamandine for these receptors in vivo. Of particular interest, a new endopeptidase was recently identified in the brain and kidney, capable of hydrolyzing Ang-(1-7) and Alamandine [Wilson et al., 2015]. Once identified, this new endopeptidase might be of importance as its inhibition would theoretically prolong Ang-(1-7) and Alamandine half-life and therefore their anti-hypertensive properties.
Besides the promising targets described above, researchers are also facing new challenges with the previously identified target: ACE2. Our laboratory recently identified 2 new post-translational mechanisms that may impair the ongoing efforts to elevate ACE2 activity and reinforce the enzyme’s compensatory activity.
ADAM17 (A Disintegrin and Metalloproteinase 17) is a type I transmembrane protein that belongs to a superfamily of Zn-dependent metalloproteases. ADAM17 plays a key role in the regulation of the proteolytic release from cellular membranes of some cytokines, chemokines, growth factors and their receptors [Dreymueller et al., 2012]. Increased ADAM17-mediated shedding has been described in a variety of diseases such as ischemia, heart failure, arthritis, atherosclerosis, diabetes, cancer, neurological and immune diseases. Recently, we demonstrated enhanced ADAM17 expression through a RAS over-activity-mediated mechanism leading to ACE2 down-regulation from the plasma membrane through shedding of its catalytic site, in the brain of DOCA-salt hypertensive mice [Xia et al., 2013]. At the same time, treatment of neurons with Ang-II was shown to up-regulate the expression of ADAM17. Furthermore, knockdown of ADAM17 in the central nervous system using chronic infusion of small interference RNA blunted the development of hypertension in these mice. This may suggest a deleterious effect of ADAM17 activity on ACE2 compensatory effects and potentially a new target for the treatment of neurogenic hypertension.
Another mechanism of ACE2 down-regulation has been proposed by our group, by suggesting that the hypertensive effects of ANG-II are in part mediated by internalization of membrane bound ACE2 and subsequent lysosomal degradation of this carboxypeptidase through an AT1R-dependent mechanism [Deshotels et al., 2014]. Interestingly, a similar mechanism of AT1R-mediated internalization was very recently highlighted for another enzyme, Cox2 [Sood et al., 2014]. This feed-forward mechanism, supporting the reduction of ACE2 would limit the formation and therefore availability of ANG-(1-7), as well as Alamandine, and enhance the hypertensive actions of ANG-II.
These studies emphasize the need for a better understanding of the mechanisms limiting ACE2 compensatory effects before the introduction of new therapies to enhance ACE2 activity.
Conclusion
Certainly, a great advance has been made in understanding the role of the renin-angiotensin system and ACE2 in the brain. The potential therapeutic implications of ACE2 and downstream peptides like Ang-(1-7) and Alamandine, make for ideal target candidates for further research in hypertension, heart failure, and other cardiovascular diseases. Increasing brain ACE2 by stimulating endogenous ACE2 activity and/or expression, or administration of exogenous ACE2 may provide beneficial effects in some pathologic conditions, mostly by reducing sympathetic activity, oxidative stress and inflammation which are the main mechanisms activated by the ACE/Ang-II/AT1R axis. Although the discovery of ACE2 and Alamandine have been recent advancements in our understanding of the compensatory RAS, further clarification of its role and therapeutic potential in the central nervous system in health and disease is needed.
Acknowledgements
This work was supported by the National Institutes of Health [HL093178, GM103514]; and The American Heart Association [12EIA8030004].
References
- (Cdc), C.F.D.C.A.P Vital Signs: Prevalence, Treatment, and Control of Hypertension--United States, 1999-2002 and 2005-2008. MMWR Morb Mortal Wkly Rep. 2011;60:103–108. [PubMed] [Google Scholar]
- Allen AM, Zhuo J, Mendelsohn FAO. Localization and Function of Angiotensin At1 Receptors. Am J Hypertens. 2000;13:31S–38S. doi: 10.1016/s0895-7061(99)00249-6. [DOI] [PubMed] [Google Scholar]
- Bruce E, Shenoy V, Rathinasabapathy A, Espejo A, Horowitz A, Oswalt A, et al. Selective Activation of At2 Receptor Attenuates Progression of Pulmonary Hypertension and Inhibits Cardiopulmonary Fibrosis. Br J Pharmacol. 2014 doi: 10.1111/bph.13044. [Epub ahead of print]: doi: 10.1111/bph.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner HR, Gavras H, Waeber B, Kershaw GR, Turini GA, Vukovich RA, et al. Oral Angiotensin-Converting Enzyme Inhibitor in Long-Term Treatment of Hypertensive Patients. Ann Intern Med. 1979;90:19–23. doi: 10.7326/0003-4819-90-1-19. [DOI] [PubMed] [Google Scholar]
- Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, et al. Angiotensin-Converting Enzyme 2 Is an Essential Regulator of Heart Function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- Davisson RL. Physiological Genomic Analysis of the Brain Renin-Angiotensin System. Am J Physiol - Regul Integr Comp Physiol. 2003;285:R498–R511. doi: 10.1152/ajpregu.00190.2003. [DOI] [PubMed] [Google Scholar]
- Deshotels MR, Xia H, Sriramula S, Lazartigues E, Filipeanu CM. Angiotensin Ii Mediates Angiotensin Converting Enzyme Type 2 Internalization and Degradation through an Angiotensin Ii Type I Receptor-Dependent Mechanism. Hypertension. 2014;64:1368–1375. doi: 10.1161/HYPERTENSIONAHA.114.03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Freire C, Vazquez J, Correa De Adjounian MF, Ferrari MF, Yuan L, Silver X, et al. Ace2 Gene Transfer Attenuates Hypertension-Linked Pathophysiological Changes in the Shr. Physiol Genomics. 2006;27:12–19. doi: 10.1152/physiolgenomics.00312.2005. [DOI] [PubMed] [Google Scholar]
- Dreymueller D, Pruessmeyer J, Groth E, Ludwig A. The Role of Adam-Mediated Shedding in Vascular Biology. Eur J Cell Biol. 2012;91:472–485. doi: 10.1016/j.ejcb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Etelvino GM, Peluso AAB, Santos RAS. New Components of the Renin-Angiotensin System: Alamandine and the Mas-Related G Protein-Coupled Receptor D. Curr Hypertens Rep. 2014;16:1–6. doi: 10.1007/s11906-014-0433-0. [DOI] [PubMed] [Google Scholar]
- Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, et al. Brain-Selective Overexpression of Human Angiotensin-Converting Enzyme Type 2 Attenuates Neurogenic Hypertension. Circ Res. 2010;106:373–382. doi: 10.1161/CIRCRESAHA.109.208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AV, Bains JS. Actions of Angiotensin in the Subfornical Organ and Area Postrema: Implications for Long Term Control of Autonomic Output. 1997;24:96–101. doi: 10.1111/j.1440-1681.1997.tb01790.x. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Trask AJ, Jessup JA. Advances in Biochemical and Functional Roles of Angiotensin-Converting Enzyme 2 and Angiotensin-(1-7) in Regulation of Cardiovascular Function. Am J Physiol Heart Circ Physiol. 2005;289:H2281–2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gironacci MM, Cerniello FM, Longo Carbajosa NA, Goldstein J, Cerrato BD. Protective Axis of the Renin-Angiotensin System in the Brain. Clin Sci. 2014;127:295–306. doi: 10.1042/CS20130450. [DOI] [PubMed] [Google Scholar]
- Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, et al. The Brain Renin-Angiotensin System Controls Divergent Efferent Mechanisms to Regulate Fluid and Energy Balance. Cell Metab. 2010;12:431–442. doi: 10.1016/j.cmet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Oparil S, Carter B, Cushman W, Dennison-Himmelfarb C, Handler J, et al. Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report from the Panel Members Appointed to the Eighth Joint National Committee (Jnc 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- Jankowski V, Vanholder R, Van Der Giet M, Tolle M, Karadogan S, Gobom J, et al. Mass-Spectrometric Identification of a Novel Angiotensin Peptide in Human Plasma. Arterioscler Thromb Vasc Biol. 2007;27:297–302. doi: 10.1161/01.ATV.0000253889.09765.5f. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Gross PM. Sensory Circumventricular Organs and Brain Homeostatic Pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- Lautner RQ, Villela DC, Fraga-Silva RA, Silva N, Verano-Braga T, Costa-Fraga F, et al. Discovery and Characterization of Alamandine: A Novel Component of the Renin-Angiotensin System. Circ Res. 2013;112:1104–1111. doi: 10.1161/CIRCRESAHA.113.301077. [DOI] [PubMed] [Google Scholar]
- Lavoie JL, Sigmund CD. Minireview: Overview of the Renin-Angiotensin System--an Endocrine and Paracrine System. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- Lazartigues E, Feng Y, Lavoie JL. The Two Faces of the Tissue Renin-Angiotensin Systems: Implication in Cardiovascular Diseases. Curr Pharm Des. 2007;13:1231–1245. doi: 10.2174/138161207780618911. [DOI] [PubMed] [Google Scholar]
- Lazartigues E, Feng Y, Lavoie JL. The Two Faces of the Tissue Renin-Angiotensin Systems: Implication in Cardiovascular Diseases. Curr Pharm Des. 2007;13:1231–1245. doi: 10.2174/138161207780618911. [DOI] [PubMed] [Google Scholar]
- Li W, Peng H, Cao T, Sato R, Mcdaniels SJ, Kobori H, et al. Brain-Targeted (Pro)Renin Receptor Knockdown Attenuates Angiotensin Ii-Dependent Hypertension. Hypertension. 2012;59:1188–1194. doi: 10.1161/HYPERTENSIONAHA.111.190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Peng H, Mehaffey EP, Kimball CD, Grobe JL, Van Gool JMG, et al. Neuron-Specific (Pro)Renin Receptor Knockout Prevents the Development of Salt-Sensitive Hypertension. Hypertension. 2014;63:316–323. doi: 10.1161/HYPERTENSIONAHA.113.02041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Sullivan MN, Zhang S, Worker CJ, Xiong Z, Speth RC, et al. Intracerebroventricular Infusion of the (Pro)Renin Receptor Antagonist Pro20 Attenuates Deoxycorticosterone Acetate-Salt-Induced Hypertension. Hypertension. 2015;65:352–361. doi: 10.1161/HYPERTENSIONAHA.114.04458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and Identification of Proangiotensin-12, a Possible Component of the Renin-Angiotensin System. Biochem Biophys Res Commun. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- Neupane D, Mclachlan CS, Sharma R, Gyawali B, Khanal V, Mishra SR, et al. Prevalence of Hypertension in Member Countries of South Asian Association for Regional Cooperation (Saarc): Systematic Review and Meta-Analysis. Medicine (Baltimore) 2014;93:e74. doi: 10.1097/MD.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen G, Contrepas A. Physiology and Pharmacology of the (Pro)Renin Receptor. Current Opinion in Pharmacology. 2008;8:127–132. doi: 10.1016/j.coph.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Park S, Bivona BJ, Ford SM, Xu S, Kobori H, De Garavilla L, et al. Direct Evidence for Intrarenal Chymase-Dependent Angiotensin Ii Formation on the Diabetic Renal Microvasculature. Hypertension. 2013;61:465–471. doi: 10.1161/HYPERTENSIONAHA.111.202424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M, Poyan Mehr A, Kreutz R. Physiology of Local Renin-Angiotensin Systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- Persell SD. Prevalence of Resistant Hypertension in the United States, 2003-2008. Hypertension. 2011;57:1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- Sampson AK, Irvine JC, Shihata WA, Dragoljevic D, Lumsden N, Huet O, et al. Compound 21 Prevents Endothelial Inflammation and Leukocyte Adhesion in Vitro and in Vivo. Br J Pharmacol. 2015 doi: 10.1111/bph.13063. [Epub ahead of print]: doi: 10.1111/bph.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund CD. Divergent Mechanism Regulating Fluid Intake and Metabolism by the Brain Renin-Angiotensin System. Am J Physiol Regul Integr Comp Physiol. 2010;302:R313–R320. doi: 10.1152/ajpregu.00575.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinski HJ, Gudermann T, Breit A. Pharmacology and Signaling of Mas-Related G Protein-Coupled Receptors. Pharmacol Rev. 2014;66:570–597. doi: 10.1124/pr.113.008425. [DOI] [PubMed] [Google Scholar]
- Sood R, Minzel W, Rimon G, Tal S, Barki-Harrington L. Down-Regulation of Cyclooxygenase-2 by the Carboxyl Tail of the Angiotensin II Type 1 Receptor. J Biol Chem. 2014;289:31473–31479. doi: 10.1074/jbc.M114.587576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom B, De Vries R, Saxena PR, Danser AHJ. Bradykinin Potentiation by Angiotensin-(1-7) and Ace Inhibitors Correlates with Ace C- and N-Domain Blockade. Hypertension. 2001;38:95–99. doi: 10.1161/01.hyp.38.1.95. [DOI] [PubMed] [Google Scholar]
- Ueno M, Sakamoto H, Liao Y-J, Onodera M, Huang C-L, Miyanaka H, et al. Blood-Brain Barrier Disruption in the Hypothalamus of Young Adult Spontaneously Hypertensive Rats. Histochem Cell Biol. 2004;122:131–137. doi: 10.1007/s00418-004-0684-y. [DOI] [PubMed] [Google Scholar]
- Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, et al. Hydrolysis of Biological Peptides by Human Angiotensin-Converting Enzyme-Related Carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- Villela D, Leonhardt J, Patel N, Joseph J, Kirsch S, Hallberg A, et al. Angiotensin Type 2 Receptor (At2r) and Receptor Mas: A Complex Liaison. Clin Sci (Lond) 2015;23:130–134. doi: 10.1042/CS20130515. [DOI] [PubMed] [Google Scholar]
- Westwood BM, Chappell MC. Divergent Pathways for the Angiotensin-(1-12) Metabolism in the Rat Circulation and Kidney. Peptides. 2012;35:190–195. doi: 10.1016/j.peptides.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Cruz-Diaz N, Marshall A, Pirro N, Su Y, Gwathmey T, et al. An Angiotensin-(1-7) Endopeptidase in the Kidney Cortex, Proximal Tubules and Human Hk-2 Epithelial Cells That Is Distinct from Insulin Degrading Enzyme. Am J Physiol Renal Physiol. 2015 doi: 10.1152/ajprenal.00609.2014. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Angiotensin Ii Type 1 Receptor-Mediated Reduction of Angiotensin-Converting Enzyme 2 Activity in the Brain Impairs Baroreflex Function in Hypertensive Mice. Hypertension. 2009;53:210–216. doi: 10.1161/HYPERTENSIONAHA.108.123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Sriramula S, Chhabra K, Lazartigues E. Brain Ace2 Shedding Contributes to the Development of Neurogenic Hypertension. Circ Res. 2013;113:1087–1096. doi: 10.1161/CIRCRESAHA.113.301811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Sriramula S, Lazartigues E. Ace2/Ang-(1-7)/Mas Pathway in the Brain: The Axis of Good. Am J Physiology - Regul Integr Comp Physiol. 2011;300:R804–817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of Angiotensin-Converting Enzyme 2 in the Rostral Ventrolateral Medulla Causes Long-Term Decrease in Blood Pressure in the Spontaneously Hypertensive Rats. Hypertension. 2007;49:926–931. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- Yemane H, Busauskas M, Burris SK, Knuepfer MM. Neurohumoral Mechanisms in Deoxycorticosterone Acetate (Doca)-Salt Hypertension in Rats. Exp Physiol. 2010;95:51–55. doi: 10.1113/expphysiol.2008.046334. [DOI] [PubMed] [Google Scholar]
- Yoon SS, Gu Q, Nwankwo T, Wright JD, Hong Y, Burt V. Trends in Blood Pressure among Adults with Hypertension: United States, 2003 to 2012. Hypertension. 2015;65:54–61. doi: 10.1161/HYPERTENSIONAHA.114.04012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubcevic J, Jun JY, Lamont G, Murca TM, Shi P, Yuan W, et al. Nucleus of the Solitary Tract (Pro)Renin Receptor-Mediated Antihypertensive Effect Involves Nuclear Factor-Kappab-Cytokine Signaling in the Spontaneously Hypertensive Rat. Hypertension. 2013;61:622–627. doi: 10.1161/HYPERTENSIONAHA.111.199836. [DOI] [PubMed] [Google Scholar]