Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the most fatal cancers world-wide, partly because methods are lacking to detect disease at an early, operable stage. Noninvasive PDAC precursors called intraductal papillary mucinous neoplasms (IPMNs) exist, and strategies are needed to aid in their proper diagnosis and management. Data support the importance of mi(cro)RNAs in the progression of IPMNs to malignancy, and we hypothesized that miRNAs may be shed from IPMN tissues and detected in blood. Our primary goals were to measure the abundance of miRNAs in archived pre-operative plasma from individuals with pathologically-confirmed IPMNs and healthy controls and discover plasma miRNAs that distinguish between IPMN patients and controls and between ‘malignant’ and ‘benign’ IPMNs. Using novel nCounter technology™ to evaluate 800 miRNAs, we showed that a 30-miRNA signature distinguished 42 IPMN cases from 24 controls (area underneath the curve (AUC)= 74.4 (95% CI:62.3-86.5, p=0.002). The signature contained novel miRNAs and miRNAs previously implicated in pancreatic carcinogenesis that had 2-4 fold higher expression in cases than controls. We also generated a 5-miRNA signature that discriminated between 21 malignant (high-grade dysplasia and invasive carcinoma) and 21 benign (low- and moderate-grade dysplasia) IPMNs (AUC=73.2 (95% CI: 57.6-73.2, p=0.005)), and showed that paired plasma and tissue samples from patients with IPMNs can have distinct miRNA expression profiles. This study suggests feasibility of using new cost-effective technology to develop a miRNA-based blood test to aid in pre-operative identification of malignant IPMNs that warrant resection while sparing individuals with benign IPMNs the morbidity associated with overtreatment.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer deaths in the United States, with a five-year survival rate of only 6% (1). Approximately 85% of cases present with metastases, which can be partly explained by a lack of accurate methods to detect disease at an early, operable stage (1).The detection and treatment of noninvasive precursor lesions may offer the greatest hope in reducing morbidity and mortality. Three noninvasive PDAC precursor lesions (‘precancers’) exist: pancreatic intraepithelial neoplasia (PanIN), mucinous cystic neoplasms (MCNs), and intraductal papillary mucinous neoplasms (IPMNs) (2, 3). PanINs are microscopic lesions, while MCNs and IPMNs are macroscopic cysts accounting for over half of the ~150,000 asymptomatic pancreatic cysts detected incidentally in the general population each year by imaging(4). Once detected, endoscopic ultrasound (EUS)-guided fine needle aspirations are often performed to assess the degree of dysplasia, but imaging features and biomarkers obtained from such invasive procedures do not reliably predict disease severity pre-operatively(3). Noninvasive approaches are needed to aid in IPMN management and prevent progression to malignancy.
miRNAs are biomarkers that regulate one-third of all protein-coding genes and promote carcinogenesis by regulating tumor suppressors and oncogenes or serving these functions themselves (5). miRNAs are excellent candidate biomarkers of early disease because of their tissue-specific expression patterns(5), their remarkable stability in tissue (6) and biofluids (7) due to their small size and protection from endogenous RNase activity, and their ability to regulate hundreds of genes and biological pathways (5). Recent studies by our group (8) and others (9-11) have evaluated genome-wide miRNA expression in IPMN tissue, and provide data to suggest that key miRNAs may reliably differentiate low-risk/benign IPMNs (ie. low- and moderate-grade) that can be monitored from high-risk/malignant IPMNs (ie. high-grade and invasive) that warrant resection. These tissue-based findings combined with discoveries that miRNAs can be readily and reliably detected in circulation (7, 12) raise the possibility that a noninvasive test that measures blood levels of miRNAs may be able to differentiate between individuals with IPMNs and non-diseased controls and between malignant and benign IPMNs. Of only two published studies (13, 14) that have focused on evaluating blood-based genome-wide miRNA expression in early-stage PDAC patients versus controls, one (13) included pre-operative serum from 20 patients with IPMNs.
The primary objective of the current study was to measure the abundance of 800 miRNAs in archived plasma obtained pre-operatively from individuals with IPMNs and disease-free controls using a novel digital amplification-free quantification method called nCounter technology™ (Nanostring, Inc, Seattle, Washington)(15). We then sought to discover circulating miRNAs that may a) differentiate between patients with IPMNs and non-diseased controls, b) distinguish malignant from benign IPMNs, and/or c) reflect the paired tumor miRNA expression profile. To our knowledge, this is the first study to conduct genome-wide profiling of circulating miRNAs exclusively among patients with cancer precursors using digital technology.
Materials and Methods
Study population and biospecimens
A prospectively maintained clinical database was retrospectively reviewed to identify individuals who underwent a pancreatic resection for an IPMN between 2006 and 2011 at Moffitt Cancer Center and Research Institute (Moffitt) and had provided written consent for blood to be donated pre-operatively for research through protocols approved by the Institutional Review Board (IRB) of the University of South Florida, including Total Cancer Care (16). IRB approval was granted for the research described herein (IRB#Pro4971). The diagnosis and degree of dysplasia was histologically confirmed using World Health Organization (WHO) guidelines (17). The final diagnosis represented the most severe grade of dysplasia observed in the neoplastic epithelium. None of the cases received pre-operative chemotherapy or radiation. Age- and gender- matched healthy controls with no current or prior history of pancreatic disease or symptoms who presented to Moffitt’s Cancer Screening and Prevention Center during the same time period and donated blood through a related IRB-approved protocol using the same procedures were also eligible for inclusion.
Blood was collected from consented participants via phlebotomy in a 7-mL EDTA tube and processed for plasma within two hours using standard procedures(18). The tube was inverted 3 times and spun at 3600 rpm for 8 minutes and then aliquoted into 0.5 mL bar-coded cryovials and banked at −80°C. Demographic, clinical, and epidemiologic data was collected from an electronic questionnaire, the medical record, Moffitt’s cancer registry, and other source systems.
RNA isolation from plasma and quality control
One 0.5 mL cryovial of plasma was retrieved and thawed from each study participant. To control for variance in the starting material and the efficiency of RNA extraction, RNA spike-in miRNAs (synthetic control templates) were used. Total RNA isolation was performed on 500 uL of plasma using the Plasma/Serum Circulating and Exosomal RNA Purification Mini Kit (Slurry Format) from Norgen Biotek (Ontario, Canada). After adding lysis buffer, 1000 attomoles of the synthetic RNA oligonucleotides (spike-in oligos) osa-miR-414, cel-mi-248, and ath-miR-159a (Operon, Inc, Huntsville, AL) were added.No-template control samples were run to monitor the baseline for spike-ins. RNA was eluted in 100 μL water, concentrated to 20 μL using Amicon Ultra 0.5 mL centrifugal filters (Sigma-Alrich, St. Louis, MO), and 3 μL was used for each assay.
Total RNA concentration and integrity were assessed using the NanoDrop spectrophotometer (NanoDrop Technologies, Waltham, MA) and an Agilent Bioanalyzer (Agilent, Santa Clara, CA). Since hemolysis (rupturing of erythrocytes) can be a source of variation in studies of circulating miRNAs, we assessed the possibility of hemolysis based on recommended guidelines(19-21): a) prior to RNA extraction, samples were visually inspected for a pink/red hue, b) oxygen hemoglobin absorbance of isolated RNA was measured at λ=414 nm, with values exceeding 0.2 indicative of hemolysis, and c) signal levels were evaluated post-hoc for cellular miRNAs likely to be elevated in the presence of hemolysis (miR-451, miR-16).
High-throughput measurement of miRNA abundance
The nCounter™ Human v2 miRNA Expression Assay Codeset (Nanostring Technologies, Seattle, WA, USA) was used to quantify the abundance of 800 human miRNAs and built-in controls: positive controls (spiked RNA at various concentrations to assess overall assay performance, n=6) and negative controls (alien probes for background calculation, n=8).This platform involves direct amplification-free digital measurement of miRNA abundance using color-coded probe pairs (15). Using 3 μL of the extracted plasma RNA as input, preparation involved multiplexed ligation of DNA sequences to mature miRNAs through sequence-specific bridges. Excess tags and bridges were removed via an enzymatic step, and the tagged miRNA probes were hybridized to a color-coded reporter probe pair for data collection. Reporter probes were counted for each miRNA using the nCounter Digital Analyzer at 555 fields of view.
Data Processing and Quality Control
For each sample, background-corrected measures of miRNA expression were estimated by subtracting the negative control average plus two standard deviation (SD) cut-point from the raw miRNA counts.miRNAs with less than 20% of samples above the negative control cut-point (ie. low-expression probes) were removed from downstream analysis. Human messenger RNA (mRNA) housekeeping genes included in the codeset (ACTB, B2M, GAPDH, RPL19 and RPLP0) were used to evaluate possible sample contamination. Data for each sample was normalized using the geometric mean of the 3 spike-in oligos. Biological normalization was then performed using the four most stable/invariant circulating miRNAs as endogenous controls. Normalized data was log2-transformed prior to signature selection.
Statistical Analysis
Descriptive statistics were calculated using frequencies and percents for categorical variables and means and standard deviations (SD) for continuous variables. Data analysis was performed to (a) identify a plasma miRNA signature that differentiates between IPMN cases and non-diseased controls, (b) identify a plasma miRNA signature that distinguishes malignant (pathologically-confirmed as high-grade or invasive) from benign IPMNs (pathologically-confirmed as low-or moderate-grade), (c) discover pathways controlled by the most deregulated miRNAs, and (d) explore paired tissue and circulating miRNA expression (Supplementary Fig. S1).
Identification of plasma miRNA signatures
For (a) and (b), linear models for microarray data (LIMMA) (22) was used to identify miRNAs that differentiate between IPMN cases and controls and between malignant and benign IPMNs, respectively. Since miRNAs can be over- or under-expressed, we used principal component analysis (PCA) to combine the most deregulated miRNAs and generate an overall ‘IPMN-risk score’ based on the first principal component (PC1). PC1 accounts for the largest variability in the data and represents the overall combined effect of the IPMN-risk miRNA signatures. Specifically, IPMN-risk score, defined by PC1 as Σwixi, is a weighted average expression among the IPMN-risk miRNAs, where xi represents miRNA i expression level, wi is the corresponding weight (PC1’s loading coefficient for miRNA i) with Σwi2 = 1, and the wi values maximize the variance of Σwixi. This approach has been used to derive gene signatures previously (23-26).Receiver operating characteristic (ROC) curves were generated to measure the predictive power of the IPMN-risk signatures in discriminating between groups. Estimates of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. For the analysis of malignant versus benign cases, we also conducted multivariable logistic regression analysis to assess whether the identified miRNA signature was associated with malignant IPMN status independent of known prognostics factors (ie. main-duct involvement, lesion size, serum CA-19-9 level) (27).
Pathway enrichment analysis
To explore the potential roles of identified circulating miRNAs in pancreatic carcinogenesis, pathway-based bioinformatics analysis (c) was conducted. Using Tarbase 6.0 (28) we obtained a list of genes experimentally-validated to target the identified miRNAs. The target genes were then analyzed to identify enriched KEGG pathways (29) predicted to be regulated by the circulating miRNAs.
Exploring paired tissue and circulating miRNA expression
For 12 IPMN cases (4 low-grade, 8 high-grade) that had matching tumor tissue that underwent genome-wide miRNA profiling previously (8), spearman correlation analysis was used to evaluate the relationship between the mean abundance of each miRNA in pre-operative plasma compared to paired tissue.
All statistical analyses were performed using SAS version 9.4 and R version 3.11.To visualize miRNA expression patterns, we generated heatmaps and performed unsupervised, hierarchical clustering.
Results
Study Population
Pre-operative plasma samples were evaluated for 44 IPMN cases and 25 non-diseased controls that were frequency-matched to cases on age-group and gender. Three samples (2 from cases and 1 from a control) were excluded prior to normalization and statistical analysis due to presumed cellular contamination characterized by ribosomal RNA bands or high mRNA counts, leaving samples from 66 participants (42 cases, 24 controls) for analysis.Study population characteristics are shown in Table 1. Cases and controls were well-matched on age (mean age: 69.0 vs 69.1 years). Most subjects were white, non-Hispanic. The distribution of low-, moderate-, high-grade, and invasive IPMN cases was 9.5%, 40.5%, 31%, and 19%, respectively.
Table 1.
Characteristics of the Study Population (N=66)
| Variable | IPMN cases (n=42) |
Healthy controls (n=24) |
|---|---|---|
| Age at diagnosis/interview, mean (SD)(yrs) | 69.0 (10.7) | 69.1 (9.6) |
| Gender, male: female, n (%) | 19:23 (45:55) | 12:12 (50:50) |
| Race, n (%) | ||
| White, Non-Hispanic | 37 (88) | 24 (100) |
| Other | 5 (12) | 0 (0) |
| Family history of pancreatic cancer, n (%) | ||
| Yes | 4 (17) | 1 (4) |
| No | 15 (83) | 23 (96) |
| Ever Smoker, n (%) | ||
| Yes | 21 (50) | 11 (46) |
| No | 21 (50) | 3 (13) |
| Unknown | 0 (0) | 10 (42) |
| IPMN Grade, n (%) | ||
| Low | 4 (9.5 ) | - |
| Moderate | 17 (40.5) | - |
| High | 13 (31) | - |
| Invasive | 8 (19) | - |
Data represent counts (percentages) unless otherwise indicated. Counts may not add up to the total due to missing values, and percentages may not equal 100 due to rounding.
Exploratory analysis of circulating miRNAs in IPMNs versus non-diseased controls
A total of 558 of the 800 miRNAs evaluated (69.8%) had >80% of signals below background and were excluded, leaving 242 miRNAs for normalization and statistical analysis. This proportion of detectable circulating miRNAs is comparable or better than that of other studies (14, 30-32). No difference in the frequency or the amount of hemolysis was observed in the case versus control samples.
After technical normalization with spike-in oligos and biological normalization with the most invariant miRNAs in the dataset (miR-378e, miR-579, miR-30e-5p, and miR-570-3p), 30 miRNAs differentiated cases from controls using an FDR <0.15 (Table 2). The candidate miRNAs had 2.09-4.32 fold higher expression in cases compared to controls (Table 2). The circulating miRNA that was most significantly associated with IPMN case status was miR-145-5p (P=8.6 × 10−5), with expression levels 3.78-fold higher in cases than controls and an AUC of 0.79 (95% CI: 69.3-90.3) in classifying between groups (Table 2; Supplementary Fig. S2A and S2B).
Table 2.
The top 30 miRNAs deregulated in plasma of IPMN cases versus non-diseased controls
| miRNA ID | Mean Normalized Counts Cases (n=42) |
Mean Normalized Counts Controls (n=24) |
Fold- Change |
P | False Discovery Rate |
AUC (95% CI) |
|---|---|---|---|---|---|---|
| hsa-miR-145-5p | 3.26 | 1.34 | 3.78 | 8.61E-05 | 0.0208 | 79.3 (68.3, 90.3) |
| hsa-miR-4454 | 10.58 | 9.46 | 2.18 | 0.0018 | 0.1043 | 72.4 (59.6, 85.2) |
| hsa-let-7f-5p | 8.27 | 6.38 | 3.69 | 0.0028 | 0.1043 | 73.8 (62.0, 85.6) |
| hsa-miR-146a-5p | 8.55 | 6.55 | 4.00 | 0.0032 | 0.1043 | 72.4 (60.2, 84.6) |
| hsa-let-7d-5p | 7.33 | 5.42 | 3.75 | 0.0043 | 0.1043 | 69.1 (55.7, 82.4) |
| hsa-let-7a-5p | 11.43 | 9.83 | 3.05 | 0.0044 | 0.1043 | 68.6 (55.2, 81.9) |
| hsa-miR-142-3p | 12.71 | 11.29 | 2.68 | 0.0060 | 0.1043 | 67.2 (53.3, 81.0) |
| hsa-miR-423-5p | 8.62 | 7.04 | 2.99 | 0.0061 | 0.1043 | 69.4 (56.2, 82.7) |
| hsa-miR-22-3p | 10.06 | 8.93 | 2.18 | 0.0072 | 0.1043 | 68.8 (55.8, 81.7) |
| hsa-miR-107 | 7.64 | 5.84 | 3.48 | 0.0073 | 0.1043 | 72.3 (59.3, 85.4) |
| hsa-miR-29c-3p | 6.97 | 5.11 | 3.63 | 0.0076 | 0.1043 | 67.4 (53.6, 81.1) |
| hsa-miR-148a-3p | 8.23 | 6.50 | 3.31 | 0.0078 | 0.1043 | 70.8 (57.8, 83.9) |
| hsa-miR-340-5p | 5.28 | 3.18 | 4.32 | 0.0079 | 0.1043 | 70.8 (57.5, 84.2) |
| hsa-miR-181a-5p | 5.36 | 3.34 | 4.06 | 0.0079 | 0.1043 | 69.6 (56.6, 82.7) |
| hsa-miR-335-5p | 3.44 | 1.92 | 2.87 | 0.0083 | 0.1043 | 69.4 (56.3, 82.6) |
| hsa-let-7i-5p | 8.79 | 7.31 | 2.80 | 0.0092 | 0.1043 | 71.0 (58.3, 83.7) |
| hsa-miR-337-5p | 3.19 | 1.71 | 2.79 | 0.0092 | 0.1043 | 71.4 (58.2, 84.7) |
| hsa-miR-1260b | 3.28 | 1.63 | 3.15 | 0.0093 | 0.1043 | 67.9 (54.6, 81.1) |
| hsa-miR-593-3p | 2.46 | 1.40 | 2.09 | 0.0097 | 0.1043 | 68.7 (55.2, 82.1) |
| hsa-miR-27a-3p | 4.29 | 2.61 | 3.20 | 0.0097 | 0.1043 | 68.8 (55.3, 82.2) |
| hsa-let-7g-5p | 11.95 | 10.70 | 2.38 | 0.0099 | 0.1043 | 68.5 (55.4, 81.5) |
| hsa-miR-191-5p | 10.56 | 9.29 | 2.40 | 0.0100 | 0.1043 | 68.2 (54.9, 81.4) |
| hsa-miR-24-3p | 8.15 | 6.21 | 3.83 | 0.0100 | 0.1043 | 69.4 (56.3, 82.4) |
| hsa-miR-20a- 5p+hsa- miR-20b-5p |
10.36 | 9.15 | 2.30 | 0.0103 | 0.1043 | 68.1 (54.9, 81.2) |
| hsa-miR-26a-5p | 10.23 | 8.91 | 2.50 | 0.0114 | 0.1100 | 72.4 (60.2, 84.6) |
| hsa-miR-23a-3p | 11.16 | 10.05 | 2.16 | 0.0130 | 0.1206 | 66.7 (53.2, 80.2) |
| hsa-miR-199a- 3p+hsa-miR-199b- 3p |
10.53 | 8.95 | 2.99 | 0.0150 | 0.1344 |
69.5 (56.5, 82.6) |
| hsa-miR-126-3p | 13.23 | 11.90 | 2.51 | 0.0168 | 0.1406 | 65.8 (52.0, 79.5) |
| hsa-miR-98 | 4.12 | 2.51 | 3.05 | 0.0174 | 0.1406 | 67.4 (54.0, 80.8) |
| hsa-miR-15b-5p | 11.24 | 9.83 | 2.65 | 0.0174 | 0.1406 | 65.8 (52.3, 79.3) |
Abbreviations: AUC=Area underneath the curve; CI=confidence interval
Comparisons conducted with t-tests
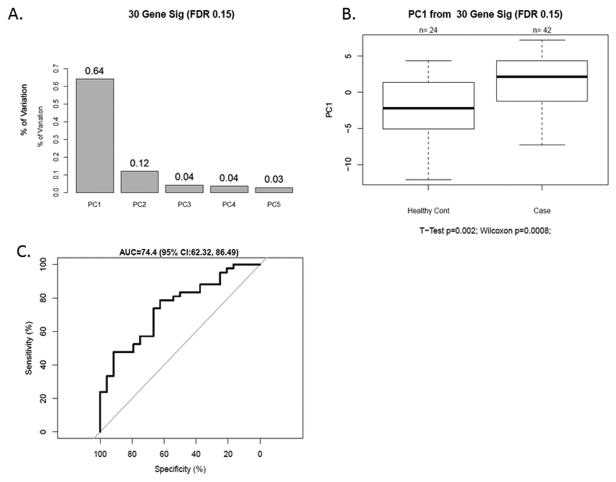
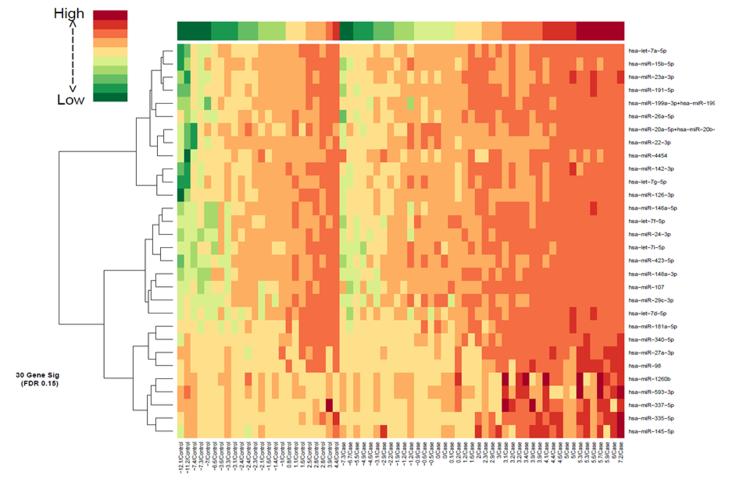
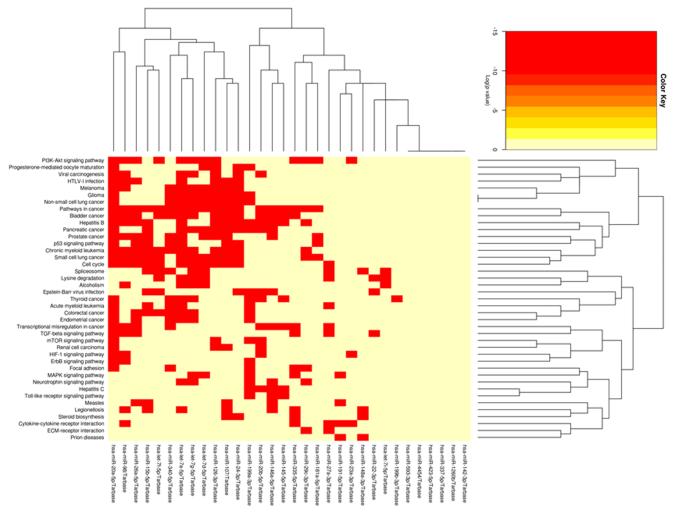
The 30 miRNA IPMN-risk signature, represented by PC1, explained 64% of the variability in the data, suggesting it represents the signature well (Fig. 1A). The overall expression of PC1 was higher in cases than controls (p=0.002, Fig. 1B), was significantly associated with IPMN status (odds ratio (OR) 95% CI: =1.23 (1.07-1.41), p=0.003), and had an AUC value of 74.4 (95% CI: 62.3-86.5) (Fig. 1C). The sensitivity, specificity, PPV, and NPV for IPMN detection were 78.6%, 62.5%, 78.6% and 62.5%, respectively. A heatmap of gene expression for the 30 miRNA signature in our cohort is displayed in Fig. 2. Pathway enrichment analysis revealed numerous pathways predicted to be regulated by the 30 miRNA IPMN-risk signature (Fig. 3), with ‘Pathways in Cancer,’ ‘p53 signaling,’ and ‘PI3K-AKT signaling’ comprising the top-ranked pathways with p=1.3×10−26, p=1.2×10−24, and p=4.0×10−17, respectively.
Figure 1. A 30-miRNA signature discriminates IPMN Cases (N=42) from Healthy Controls (N=24).
A) Percentage of variation explained in the first 5 principal components using the 30 miRNA signature. B) Association of the 30-miRNA signature with case-control status. Box plots were used to display the distribution of the IPMN-risk malignancy score within each group. Two-sample t-tests were used to determine associations between the continuous PC1 score and case-control status. C) Receiver operating characteristic (ROC) curve analysis using miRNA expression to discriminate IPMN cases from healthy controls. The 30-miRNA signature PC1 yielded an area underneath the curve (AUC) value of 74.4 (95% CI: 62.3-86.5) in differentiating between groups.
Figure 2.
Heatmap of the 30-miRNA signature in IPMN cases and non-diseased controls.
Figure 3.
Heatmap of the KEGG pathways enriched for genes targeted by the 30 differentially expressed miRNAs.
Exploratory analysis of circulating miRNAs in malignant versus benign IPMNs
We evaluated the ability of the 30-miRNA signature (PC1) to discriminate between the malignant and benign IPMNs and observed it did not perform well (AUC=60.8 (95% CI: 42.5-79.1)). However, a focused analysis of the 42 IPMN cases showed that five miRNAs (miR-200a-3p, miR-1185-5p, miR-33a-5p, miR-574-3p, and miR-663b) discriminated between malignant and benign IPMNs (p<0.05) (Supplementary Table S1). The 5 miRNA IPMN-risk signature characterized by PC1 explained 50% of the variability (Supplementary Figure S3A).The overall expression of PC1 was lower in malignant compared to benign IPMNs (p=0.005, Supplementary Figure S3B), was significantly associated with malignant status (OR (95% CI): =0.36 (0.16, 0.83), p=0.017), and had an AUC value of 73.2 (95% CI: 57.6-88.9) in discriminating between groups (Supplementary Figure S3C). Estimates of sensitivity, specificity, PPV, and NPV of PC1 in detecting malignant IPMNs were 80.9%, 52.5%, 63.0%, and 73.3%.‘Cell cycle’ (p=0.0008) and ‘Wnt signaling’ (P=0.005) were among top-ranked pathways predicted to be regulated by the 5 miRNA IPMN-risk signature.
Clinical factors associated (p<0.05) with an increased risk of malignancy included cyst size greater >3 cm (OR (95% CI)=6.4 (1.4-28.5, p=0.015), main duct involvement (OR (95% CI)=18.4 (2.8-122.4, p=0.002), and lower serum albumin levels (OR (95% CI)=0.08 (0.01-0.62).Cyst size was negatively correlated with the 5 miRNA PC1 score (spearman correlation=−0.53, p=0.001), while serum albumin levels were positive correlated with the 5-miRNA signature (spearman correlation=0.47, p=0.002). Mean serum CA 19-9 levels were higher in the malignant compared to benign group, but the difference was not statistically significant (p=0.130). The PC1 score for the 5-miRNA signature was not independently associated with malignant status after adjustment for main duct involvement (p=0.008).
IPMN tumors tissue and paired plasma have distinct miRNA expression profiles
We evaluated matched plasma and tissue specimens from 12 IPMN cases.Among 160 miRNA probes evaluated in both specimen types, expression levels of only 3 (1.9%) were significantly positively correlated (r>0.60, p<0.05), and included miR-484, miR-330, and miR-574-3p (Supplementary Table S2).Of these three miRNA probes, one (miR-574-3p) was represented in the 5-miRNA signature.For the miRNAs that were ranked as the most highly expressed in plasma of IPMN cases versus controls (miR-146a-5p, miR-340-5p, and miR-181a-5p), correlations with tissue miRNA expression were small (r<0.60) (Supplementary Table S2). These data suggest plasma and tissue samples from patients with IPMNs can have distinct miRNA profiles.
Discussion
This is the first report to interrogate plasma miRNA expression levels exclusively in individuals newly-diagnosed with IPMNs and healthy controls.We used nCounter technology™ formiRNA quantitation as an alternative to microarray and PCR-based methods to more accurately detect and quantify low miRNA levels present in blood and to eliminate the need for pre-amplification which may compromise measurement reliability(33). We also implemented an extensive quality control and data analysis pipeline to account for pre-analytical and technical factors that can affect circulating miRNA levels and result in biases that do not reflect underlying biology.This study demonstrates the feasibility of evaluating plasma miRNAs using nCounter technology™, and our results suggest that miRNAs in plasma warrant further evaluation as noninvasive biomarkers for the detection of PDAC precursors.
We show that a 30-miRNA gene signature can partially discriminate IPMN cases from healthy controls ((AUC)= 74.4 (95% CI:62.3-86.5, p=0.002)).Several of the highlighted miRNAs (let-7a-5p, let-7d-5p, miR-1260b, miR-142-3p, and miR-146a-5p, miR-23a-3p) are unaffected by hemolysis (19, 21), minimizing red blood cell contamination as a potential source of confounding. The identified miRNAs had 2-4-fold higher expression in cases compared to controls, and included miRNAs previously shown to be up-regulated (miR-107, miR-145, miR-146a, miR-15b, miR-181a, miR-24) or down-regulated (miR-142, miR-148a) in PDAC versus normal tissues (34). Noteworthy, several candidate miRNAs (miR-145-5p and miR-335) may be involved in inhibiting cancer stem cell properties of PDACs by targeting the transcription factor, OCT4 (35, 36). Candidate miRNAs such as miR-1260b and miR-4454 are novel and also of interest because validated targets include key drivers in pancreatic carcinogenesis, SMAD4 (37) and NF-κβ (38), respectively.The biological plausibility of findings was enhanced by pathway-based analysis which predicted that the identified miRNAs may affect critical pathways involved in PDAC initiation and progression.
Although previous studies (33, 39, 40) have evaluated miRNA expression in blood of PDAC patients and healthy controls, the implications for early detection were limited because most cases had locally advanced or metastatic disease.Of two studies(13, 14) that focused on evaluating blood-based genome-wide miRNA expression in early-stage PDAC patients, one (13) included pre-operative serum from patients with IPMNs (N=20; 4 low-grade;16 moderate-/high-grade).Li et al (13) measured 735 miRNAs by qRT-PCR using Taqman MicroRNA Arrays, and found that miR-1290 had the best diagnostic performance for subjects with PDAC (n=41) relative to healthy controls (n=19), and that serum miR-1290 levels were higher among the 20 IPMN cases compared to controls (AUC=0.76 (95% CI: 0.61-0.91). Although miR-1290 was evaluated in the nCounter miRNA codeset, this miRNA was not analyzed because 93% of the 69 samples had values below background.Two miRNAs from our 30-miRNA gene signature, miR-24 and miR-146a, were among the miRNAs that distinguished sera of patients with PDAC from healthy controls with AUCs>0.70 in the study by Li et al (13), but comparisons of these levels in IPMNs versus healthy controls were not reported. None of the circulating miRNAs identified in our study overlapped with three miRNAs (miR-642b, miR-885,5-p, miR-22) highlighted in a small study of plasma miRNA expression in early PDAC patients versus controls (14).
Analysis revealed a 5-miRNA signature (comprising miR-200a-3p, miR-1185-5p, miR-33a-5p, miR-574-3p, and miR-663b) that partially discriminates between malignant and benign IPMNs (AUC= 73.2 (95% CI: 57.6-88.9)). These miRNAs had 2-3 fold lower expression in malignant compared to benign cases.Consistent with our findings, miR-200a down-regulation has been implicated in epithelial-to-mesenchymal transition and early metastasis (34).On the other hand, miR-200a was shown to be hypomethylated and overexpressed in PDAC (compared to normal) tissue and in sera of PDAC patients versus controls (41). miR-574-3p can act as a tumor suppressor and regulate cell signaling pathways (42, 43), suggesting low levels of miR-574-3p may regulate oncogenes that promote malignant IPMN status. It is noteworthy that the candidate miRNAs identified here and in our previous tissue-based study of IPMNs (8) have lower expression in malignant compared to benign IPMNs, supporting a tumor suppressor rather than an oncogenic role. Also consistent with existing data (3), cyst size > 3 cm and main duct involvement were associated with malignant IPMN status in our cohort. We also showed that lower serum albumin levels are correlated with malignant IPMN status, a finding in line with evidence that lower serum albumin levels are associated with poorer survival in PDAC patients (44). Larger studies that account for clinical and radiologic factors are needed to confirm and expand upon these findings.
In the subset of IPMN cases for which paired pre-operative plasma and tissue miRNA data was available, plasma miRNA levels were not strongly correlated with paired tissue miRNA expression. This observation suggests it will be necessary to further explore the origin of circulating miRNAs in IPMN patients. Discrepancies between plasma and tumor miRNA profiles have been reported (30, 45), challenging the hypothesis that the origin of circulating miRNAs is from tumors as a result of cell death and lysis. Alternative explanations for the origin of circulating miRNAs include: blood or normal cell contamination, heterogeneity of the primary tumor, dietary sources, and locoregional inflammation reflecting a response of the host microenvironment to disease (20, 46-48).
Despite the strengths and novelty of this study, there are limitations that should be considered. First, the relatively small sample size limits the ability to draw firm conclusions.However, archived plasma from 42 cases and 24 controls provided at least 85% power to detect a miRNA expression difference of 2-fold and above, assuming a set of 800 miRNAs, a standard deviation of 1, and a 15% FDR. Technical validation of the Nanostring assay and external validation of findings in a large, multi-center prospective investigation of serial plasma miRNA measurements (pre- and post-surgery or during surveillance) is indicated for individuals newly-diagnosed with various types of pancreatic cysts (including IPMNs, MCNs, and benign, non-mucinous cysts) and early-stage PDAC and those at high genetic risk for developing PDAC. To increase diagnostic accuracy and improve outcomes, it may also be necessary to integrate novel classes of molecular markers, imaging features, and clinical characteristics.Future investigations of the most promising miRNAs in animal models may also be instrumental in providing biological clues for developing early detection and prevention strategies to impede progression to pancreatic malignancy.
In summary, these data support further development of a plasma miRNA assay for IPMN management. Large-scale studies with rigorous designs and incorporation of epidemiologic and clinical data are needed to further explore the potential for circulating miRNAs to be utilized clinically as novel biomarkers for IPMNs.
Supplementary Material
Acknowledgements
The authors wish to thank the individuals who contributed specimens and data for this research, and thank Vonetta Williams, Kavita Ghia, Michelle Fournier, Marek Wloch, and Tania Mesa for their technical assistance.
Grant Support:
This study was supported in part by Institutional Research Grant number 93-032-16 from the American Cancer Society (J. Permuth-Wey) and by the National Cancer Institute (NCI)/United States Public Health Service (R01 CA-129227, M. Malafa). The research was also made possible through the Total Cancer Care™ Protocol at the H. Lee Moffitt Cancer Center & Research Institute and by the Collaborative Data Services, Tissue, Molecular Genomics, Biostatistics, and Cancer Informatics Core Facilities at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292).
Footnotes
Conflicts of Interest:
A provisional patent application has been filed.
References
- 1.American Cancer Society . Cancer Facts and Figures 2014. American Cancer Society; Atlanta: 2014. [Google Scholar]
- 2.Berman JJ, Albores-Saavedra J, Bostwick D, Delellis R, Eble J, Hamilton SR, et al. Precancer: a conceptual working definition -- results of a Consensus Conference. Cancer detection and prevention. 2006;30:387–94. doi: 10.1016/j.cdp.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Hruban RH, Maitra A, Kern SE, Goggins M. Precursors to pancreatic cancer. Gastroenterol Clin North Am. 2007;36:831–49. doi: 10.1016/j.gtc.2007.08.012. vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sachs T, Pratt WB, Callery MP, Vollmer CM., Jr. The incidental asymptomatic pancreatic lesion: nuisance or threat? J Gastrointest Surg. 2009;13:405–15. doi: 10.1007/s11605-008-0788-0. [DOI] [PubMed] [Google Scholar]
- 5.Medina PP, Slack FJ. microRNAs and cancer: an overview. Cell cycle (Georgetown, Tex) 2008;7:2485–92. doi: 10.4161/cc.7.16.6453. [DOI] [PubMed] [Google Scholar]
- 6.Xi Y, Nakajima G, Gavin E, Morris CG, Kudo K, Hayashi K, et al. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. Rna. 2007;13:1668–74. doi: 10.1261/rna.642907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Permuth-Wey J, Chen YA, Fisher K, McCarthy S, Qu X, Lloyd MC, et al. A Genome-Wide Investigation of MicroRNA Expression Identifies Biologically-Meaningful MicroRNAs That Distinguish between High-Risk and Low-Risk Intraductal Papillary Mucinous Neoplasms of the Pancreas. PLoS One. 2015;10:e0116869. doi: 10.1371/journal.pone.0116869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthaei H, Schulick RD, Hruban RH, Maitra A. Cystic precursors to invasive pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2011;8:141–50. doi: 10.1038/nrgastro.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubezky N, Loewenstein S, Ben-Haim M, Brazowski E, Marmor S, Pasmanik-Chor M, et al. MicroRNA expression signatures in intraductal papillary mucinous neoplasm of the pancreas. Surgery. 2013;153:663–72. doi: 10.1016/j.surg.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Caponi S, Funel N, Frampton AE, Mosca F, Santarpia L, Van der Velde AG, et al. The good, the bad and the ugly: a tale of miR-101, miR-21 and miR-155 in pancreatic intraductal papillary mucinous neoplasms. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24:734–41. doi: 10.1093/annonc/mds513. [DOI] [PubMed] [Google Scholar]
- 12.Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8:467–77. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li A, Yu J, Kim H, Wolfgang CL, Canto MI, Hruban RH, et al. MicroRNA Array Analysis Finds Elevated Serum miR-1290 Accurately Distinguishes Patients with Low-Stage Pancreatic Cancer from Healthy and Disease Controls. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganepola GA, Rutledge JR, Suman P, Yiengpruksawan A, Chang DH. Novel blood-based microRNA biomarker panel for early diagnosis of pancreatic cancer. World journal of gastrointestinal oncology. 2014;6:22–33. doi: 10.4251/wjgo.v6.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–25. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 16.Fenstermacher DA, Wenham RM, Rollison DE, Dalton WS. Implementing personalized medicine in a cancer center. Cancer J. 2011;17:528–36. doi: 10.1097/PPO.0b013e318238216e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adsay NVFN, Furukawa T, Hruban RH, Klimstra DS, Kloppel G, et al. Intraductal Papillary Mucinous Neoplasm of the Pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumors of the digestive system. WHO Press; Lyon: 2010. pp. 304–313. [Google Scholar]
- 18.Blondal T, Jensby Nielsen S, Baker A, Andreasen D, Mouritzen P, Wrang Teilum M, et al. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods. 2013;59:164–9. doi: 10.1016/j.ymeth.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Blondal T, Jensby Nielsen S, Baker A, Andreasen D, Mouritzen P, Wrang Teilum M, et al. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods. 2013;59:S1–6. doi: 10.1016/j.ymeth.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Pritchard CC, Kroh E, Wood B, Arroyo JD, Dougherty KJ, Miyaji MM, et al. Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev Res (Phila) 2012;5:492–7. doi: 10.1158/1940-6207.CAPR-11-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirschner MB, Edelman JJ, Kao SC, Vallely MP, van Zandwijk N, Reid G. The Impact of Hemolysis on Cell-Free microRNA Biomarkers. Front Genet. 2013;4:94. doi: 10.3389/fgene.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- 23.Chen DT, Hsu YL, Fulp WJ, Coppola D, Haura EB, Yeatman TJ, et al. Prognostic and predictive value of a malignancy-risk gene signature in early-stage non-small cell lung cancer. Journal of the National Cancer Institute. 2011;103:1859–70. doi: 10.1093/jnci/djr420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen DT, Nasir A, Culhane A, Venkataramu C, Fulp W, Rubio R, et al. Proliferative genes dominate malignancy-risk gene signature in histologically-normal breast tissue. Breast cancer research and treatment. 2010;119:335–46. doi: 10.1007/s10549-009-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchion DC, Cottrill HM, Xiong Y, Chen N, Bicaku E, Fulp WJ, et al. BAD phosphorylation determines ovarian cancer chemosensitivity and patient survival. Clin Cancer Res. 2011;17:6356–66. doi: 10.1158/1078-0432.CCR-11-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopewell EL, Zhao W, Fulp WJ, Bronk CC, Lopez AS, Massengill M, et al. Lung tumor NF-kappaB signaling promotes T cell-mediated immune surveillance. The Journal of clinical investigation. 2013 doi: 10.1172/JCI67250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Vergoulis T, Vlachos IS, Alexiou P, Georgakilas G, Maragkakis M, Reczko M, et al. TarBase 6.0: capturing the exponential growth of miRNA targets with experimental support. Nucleic acids research. 2012;40:D222–9. doi: 10.1093/nar/gkr1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. http://www.genome.jp/kegg/
- 30.Suryawanshi S, Vlad AM, Lin HM, Mantia-Smaldone G, Laskey R, Lee M, et al. Plasma microRNAs as novel biomarkers for endometriosis and endometriosis-associated ovarian cancer. Clin Cancer Res. 2013;19:1213–24. doi: 10.1158/1078-0432.CCR-12-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fourie NH, Peace RM, Abey SK, Sherwin LB, Rahim-Williams B, Smyser PA, et al. Elevated circulating miR-150 and miR-342-3p in patients with irritable bowel syndrome. Experimental and molecular pathology. 2014 doi: 10.1016/j.yexmp.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu J, Wang Z, Liao BY, Yu L, Gao X, Lu S, et al. Human miR-1228 as a stable endogenous control for the quantification of circulating microRNAs in cancer patients. Int J Cancer. 2014 doi: 10.1002/ijc.28757. [DOI] [PubMed] [Google Scholar]
- 33.Schultz NA, Dehlendorff C, Jensen BV, Bjerregaard JK, Nielsen KR, Bojesen SE, et al. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA. 2014;311:392–404. doi: 10.1001/jama.2013.284664. [DOI] [PubMed] [Google Scholar]
- 34.Tang S, Bonaroti J, Unlu S, Liang X, Tang D, Zeh HJ, et al. Sweating the small stuff: microRNAs and genetic changes define pancreatic cancer. Pancreas. 2013;42:740–59. doi: 10.1097/MPA.0b013e3182854ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapoor S. miR-145 and its influence on tumor growth in systemic malignancies. Eur J Cancer Prev. 2014;23:233. doi: 10.1097/CEJ.0b013e328364747f. [DOI] [PubMed] [Google Scholar]
- 36.Gao L, Yang Y, Xu H, Liu R, Li D, Hong H, et al. miR-335 functions as a tumor suppressor in pancreatic cancer by targeting OCT4. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:8309–18. doi: 10.1007/s13277-014-2092-9. [DOI] [PubMed] [Google Scholar]
- 37.Hirata H, Hinoda Y, Shahryari V, Deng G, Tanaka Y, Tabatabai ZL, et al. Genistein downregulates onco-miR-1260b and upregulates sFRP1 and Smad4 via demethylation and histone modification in prostate cancer cells. Br J Cancer. 2014;110:1645–54. doi: 10.1038/bjc.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou F, Wang W, Xing Y, Wang T, Xu X, Wang J. NF-kappaB target microRNAs and their target genes in TNFalpha-stimulated HeLa cells. Biochimica et biophysica acta. 2014;1839:344–54. doi: 10.1016/j.bbagrm.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Chen J, Chang P, LeBlanc A, Li D, Abbruzzesse JL, et al. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res (Phila) 2009;2:807–13. doi: 10.1158/1940-6207.CAPR-09-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu R, Chen X, Du Y, Yao W, Shen L, Wang C, et al. Serum microRNA expression profile as a biomarker in the diagnosis and prognosis of pancreatic cancer. Clin Chem. 2012;58:610–8. doi: 10.1373/clinchem.2011.172767. [DOI] [PubMed] [Google Scholar]
- 41.Li A, Omura N, Hong SM, Vincent A, Walter K, Griffith M, et al. Pancreatic cancers epigenetically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels. Cancer Res. 2010;70:5226–37. doi: 10.1158/0008-5472.CAN-09-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiyomaru T, Yamamura S, Fukuhara S, Hidaka H, Majid S, Saini S, et al. Genistein up-regulates tumor suppressor microRNA-574-3p in prostate cancer. PLoS One. 2013;8:e58929. doi: 10.1371/journal.pone.0058929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su Y, Ni Z, Wang G, Cui J, Wei C, Wang J, et al. Aberrant expression of microRNAs in gastric cancer and biological significance of miR-574-3p. International immunopharmacology. 2012;13:468–75. doi: 10.1016/j.intimp.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang DX, Dai YD, Yuan SX, Tao L. Prognostic factors in patients with pancreatic cancer. Exp Ther Med. 2012;3:423–32. doi: 10.3892/etm.2011.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 102:1174–9. doi: 10.1038/sj.bjc.6605608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chin LJ, Slack FJ. A truth serum for cancer--microRNAs have major potential as cancer biomarkers. Cell research. 2008;18:983–4. doi: 10.1038/cr.2008.290. [DOI] [PubMed] [Google Scholar]
- 47.Berger F, Reiser MF. Micro-RNAs as potential new molecular biomarkers in oncology: have they reached relevance for the clinical imaging sciences? Theranostics. 2013;3:943–52. doi: 10.7150/thno.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neelakandan K, Babu P, Nair S. Emerging roles for modulation of microRNA signatures in cancer chemoprevention. Curr Cancer Drug Targets. 2012;12:716–40. doi: 10.2174/156800912801784875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.