Abstract
Background
A potential protective role for estrogen in colon carcinogenesis has been suggested based on exogenous hormone use, but it is unclear from previous studies whether endogenous estrogens are related to colorectal cancer (CRC) risk. These few prior studies focused on parent estrogens; none evaluated effects of estrogen metabolism in postmenopausal women.
Methods
We followed 15,595 women (ages 55–80) enrolled in B~FIT (Breast and Bone Follow-up to the Fracture Intervention Trial (FIT)) who donated blood between 1992 and 1993 for cancer through December 2004. A panel of 15 estrogen metabolites (EM), including estradiol and estrone, were measured in serum from 187 CRC cases and a subcohort of 501 women not using exogenous hormones at blood draw. We examined EM individually, grouped by pathway (hydroxylation at the C-2, C-4, or C-16 position), and by ratios of the groupings using Cox proportional hazards regression models.
Results
No significant associations were seen for estrone (HRQ4 v Q1=1.15, 95% CI=0.69–1.93, ptrend=0.54), estradiol (HRQ4 v Q1= 0.98, 95% CI=0.58–1.64, ptrend>0.99) or total EM (the sum of all EM; HRQ4 v Q1=1.35. 95% CI=0.81–2.24, ptrend=0.33). Most metabolites in the 2-, 4- or 16-pathway were unrelated to risk, although a borderline trend in risk was associated with high levels of 17-epiestriol.
Conclusion
Circulating estrogens and their metabolites were generally unrelated to CRC risk in postmenopausal women.
Impact
Additional studies are needed to understand how exogenous estrogen may prevent CRC
Keywords: Estrogen metabolism, postmenopausal women, CRC risk
Introduction
Exogenous hormones are linked to CRC risk reduction (1,2), yet risks associated with circulating estrogens are elevated (3,4), or null (5). In normal colonic tissue, ERα enhances cell growth and is expressed at low levels, while the anti-proliferative ERβ is abundant (6). In neoplastic colonic tissue this is reversed. Estradiol binds to both receptors with comparably high affinity, but at least one estrogen metabolite (EM), 17-epiestriol, shows a binding preference for ERβ (7). We speculated that selective binding of EM to ERα or ERβ may influence colon carcinogenesis. Thus, we investigated the association between a panel of EM and CRC in B~FIT.
Materials and Methods
FIT, a randomized trial designed to test alendronate, screened 22,695 postmenopausal women aged 55 to 80 from 1992 to 1993, who provided a bone mineral density scan (BMD), blood and information on demographic, lifestyle and reproductive factors through a self-administered questionnaire. B~FIT comprises 15,595 of the FIT screenees followed for incident cancer through December 2004. An additional questionnaire covering subsequent cancer diagnoses was sent to these participants (8).
Self-reported cancers were confirmed by medical records and/or cancer registries. Additional cases were identified from cancer registries. Vital status was determined by the National Death Index. All participants provided written informed consent and Institutional Review Board approval was obtained from all clinical sites and the National Cancer Institute.
This analysis was part of a case-cohort study within B~FIT examining EM in relation to CRC, breast, endometrial and ovarian cancer (8). We studied 187 CRC cases (10 in the subcohort) and 501 subcohort women. 15 EM were measured in serum: estrone, estradiol, 2-hydroxyestrone, 2-methoxyestrone, 2-hydroxyestradiol, 2-methoxyestradiol, 2-hydroxyestrone-3-methyl ether 4-hydroxyestrone, 4-methoxyestrone, 4-methoxyestradiol 16α-hydroxyestrone, estriol, 17-epiestriol, 16-ketoestradiol, and 16-epiestriol.
EM were examined individually, by pathway (C-2, C-4 or C-16 hydroxylation, with EM in each pathway summed), and by ratios of pathways. Cox proportional hazards regression models with robust variance adjustment were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). For cases not in the subcohort, follow-up began 6 months prior to CRC diagnosis and ended at diagnosis. For the subcohort, follow-up began at baseline and ended at CRC diagnosis for cases (n=10), and death or end of follow-up for non-cases (n=491). Covariates examined included BMI, parity, smoking, postmenopausal hormone use, and ages at menarche, birth of first child and menopause. The final model adjusted for clinic site, trial participation status, age at blood collection and total BMD. No adjustment was made for multiple comparisons.
Results
Descriptive characteristics and HR for lifestyle/reproductive risk factors are presented (Table 1). Compared to the subcohort, cases were older at study entry, followed for fewer years, and had lower BMD at baseline. Current smokers experienced a 40% increased risk, and women with a late age at menopause had >2-fold excess risk, but HR for other factors were not statistically significant.
Table 1.
Select Characteristics and Hazard Ratios* for Lifestyle and Reproductive/Hormonal Risk Factors; CRC Case-Cohort Study, B~FIT
| Characteristics | CRC Cases | Subcohort | P value | |||
|---|---|---|---|---|---|---|
| Caucasian (N%) | 182 (97) | 475 (95) | ||||
| Years follow-up (mean (SD)) | 5.4 (3.1) | 10.2 (2.2) | <0.001 | |||
| Age at blood draw (mean, (SD)) | 69.8 (5.6) | 67.3 (6.2) | <0.001 | |||
| Years postmenopausal at blood draw (mean, (SD)) | 22.5 (8.2) | 20.7 (8.8) | 0.015 | |||
| Bone mineral density (femoral neck g/cm, mean | 0.74 (0.1) | 0.77 (0.1) | 0.030 | |||
| Lifestyle Risk Factors | CRC Cases | Subcohort | P trend | |||
| BMI at blood draw (kg/m2) | <25 | 78 | 209 | 1.00 | referent | |
| 25–29 | 72 | 157 | 1.18 | (0.78, 1.77) | ||
| 30–34 | 21 | 83 | 0.68 | (0.39, 1.21) | ||
| 35+ | 14 | 47 | 0.81 | (0.40, 1.65) | 0.232 | |
| Regular exercise program | Yes | 72 | 228 | 0.98 | (0.81, 1.17) | 0.975 |
| Cigarette use | Never | 111 | 259 | 1.00 | referent | |
| Former | 53 | 185 | 1.03 | (0.86, 1.25) | ||
| Current | 20 | 53 | 1.38 | (1.06, 1.81) | 0.849 | |
| Recent Alcohol consumption | None (past 30 | 95 | 210 | 1.00 | referent | |
| <3 days/week | 50 | 138 | 0.97 | (0.88, 1.22) | ||
| 3–6 days/week | 27 | 116 | 1.18 | (0.95, 1.46) | ||
| Daily | 15 | 37 | 1.13 | (0.83, 1.53) | 0.127 | |
| Hormonal and Reproductive Risk Factors | ||||||
| Age at menarche | <11 | 27 | 77 | 1.00 | referent | |
| 12, 13 | 93 | 280 | 0.94 | (0.55, 1.61) | ||
| 14+ | 58 | 125 | 1.36 | (0.77, 2.42) | 0.615 | |
| CRC Cases | Subcohort | P trend | ||||
| Age first live birth | Nulliparous | 24 | 47 | 1.00 | referent | |
| <20 | 23 | 50 | 0.87 | (0.41, 1.85) | ||
| 20–24 | 71 | 216 | 0.66 | (0.36, 1.21) | ||
| 25–29 | 52 | 128 | 0.82 | (0.44, 1.52) | ||
| 30+ | 18 | 60 | 0.50 | (0.24, 1.07) | 0.136 | |
| Parity | Nulliparous | 24 | 47 | 1.00 | referent | |
| 1 | 15 | 60 | 0.35 | (0.16, 0.77) | ||
| 2 | 49 | 143 | 0.67 | (0.35, 1.26) | ||
| 3 | 47 | 115 | 0.86 | (0.46, 1.63) | ||
| 4+ | 52 | 136 | 0.75 | (0.40, 1.39) | 0.564 | |
| Ever breastfeed | Yes | 103 | 274 | 1.04 | (0.73, 1.47) | 0.984 |
| Age at menopause | <40 | 27 | 82 | 1.00 | referent | |
| 40–44 | 27 | 81 | 1.23 | (0.64, 2.35) | ||
| 45–49 | 62 | 176 | 1.16 | (0.65, 2.08) | ||
| 50–54 | 60 | 145 | 1.48 | (0.84, 2.60) | ||
| 55+ | 11 | 17 | 2.51 | (1.08, 5.85) | 0.064 | |
| Postmenopausal estrogen use | Never | 129 | 334 | 1.00 | referent | |
| <1 year | 12 | 49 | 0.74 | (0.37, 1.47) | ||
| 1–4 years | 30 | 64 | 1.25 | (0.74, 2.10) | ||
| 5–9 years | 4 | 27 | 0.35 | (0.12, 1.04) | ||
| 10+ years | 10 | 23 | 0.94 | (0.41, 2.15) | 0.608 |
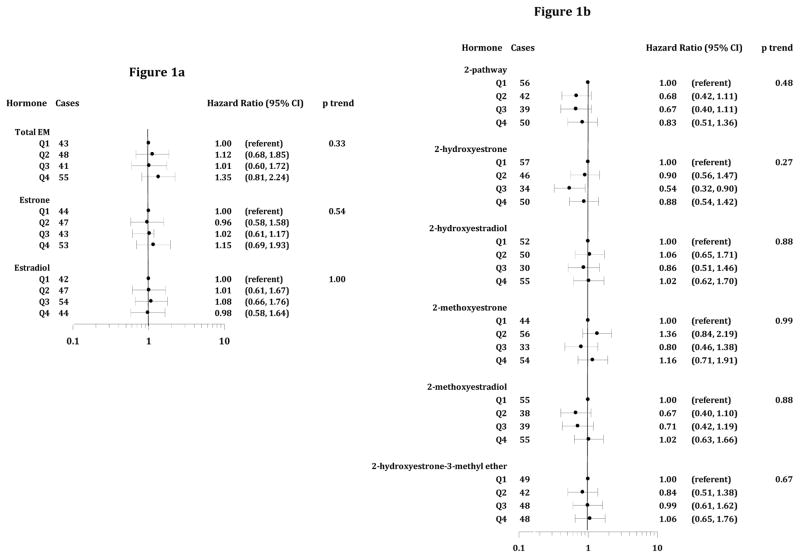
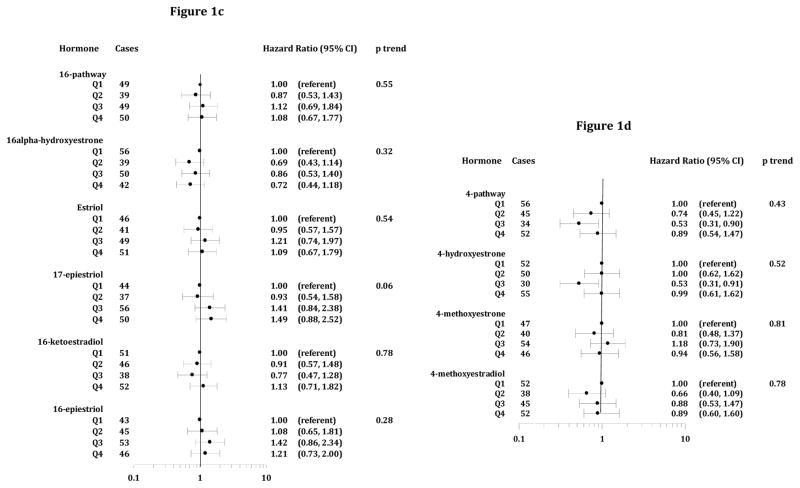
Women with higher total EM were not at elevated risk compared to those in the lowest quartile (Figure 1a). HR for estrone and estradiol were not significant, and there was no evidence of dose response. HR and trends were not significant for 2-, or 4-pathway metabolites, except for reduced risks for moderately high 2-hydroxyestrone (HRQ3 v Q1=0.54, 95% CI=0.32–0.90) and 4-hydroxyestrone (HRQ3 v Q1=0.53, 95% CI=0.31–0.91). For the 16-pathway, a trend of increasing risk with higher 17-epiestriol was suggested, but results for other metabolites were null. HR for quartiles of pathway ratios (2 vs 4-pathway, 2 vs 16-pathway and 4 vs 16-pathway) showed no association (results not shown).
Figure 1.
Figures 1a–d. Hazard Ratios and 95% Confidence Intervals, Estrogen Metabolites and CRC (Parent Estrogens, 2-Pathway, 16-Pathway and 4-Pathway EM, figures 1a–d, respectively). Time scale for HR estimates is age at entry (baseline) through age at diagnosis or censoring (exit). For cases not in the subcohort, follow-up began 6 months prior to their CRC diagnosis. HR are adjusted for clinic, age at blood draw, bone mineral density, and trial participation.
Discussion
This first prospective study of EM and CRC risk found no evidence for associations with these hormones. There was a suggestion of a slight effect for 17-epiestriol, but this may have occurred by chance given the number of comparisons. Our lack of association is consistent with results from one study (5) but not others (3, 4) showing modest elevated risks for estradiol or estrone. Reasons for these disparate findings are not clear. Women in our study were older (mean age 67 vs 60 to 64 in prior studies), but all involved postmenopausal women not using menopausal hormones at blood draw. The studies were of comparable size; however, unlike others, BMI was not associated with CRC in our study. Additionally, immunoassays for estrogens in prior studies may not detect the low levels in postmenopausal women.
Estrogen is not central to the etiology of CRC, but the change from predominantly ERβ expression in the healthy colon to ERα in neoplastic tissue suggests it may afford protection in the healthy colon, but promote tumorigenesis once neoplastic changes occur. However, results from observational studies of endogenous and exogenous estrogens are not consistent (1, 2).
In summary, while our apparent lack of association between endogenous estrogens is at odds with findings of reduced risk associated with exogenous hormone use, additional well-powered studies are needed to improve our understanding of the exogenous hormone and CRC association.
Acknowledgments
Funding: The original FIT study was supported by Merck Research Laboratories. B~FIT was supported by the National Cancer Institute [contract # N02-CP-D.C. Bauer, J. A. Tice].
The authors wish to thank Stephanie Litwack-Harrison, MPH for study management; Eric Boyd, and Vicky Chia, PhD for assistance in data management; and the B~FIT participants for their contributions to this study.
Footnotes
Declaration of conflict of interest: The authors declare no conflicts of interest regarding this publication.
References
- 1.Lin KJ, Cheung WY, Lai JY, Giovannucci EL. The effect of estrogen vs. combined estrogen-progestogen therapy on the risk of colorectal cancer. Int J Cancer. 2012;130:419–30. doi: 10.1002/ijc.26026. [DOI] [PubMed] [Google Scholar]
- 2.Tsilidis KK, Allen NE, Key TJ, Bakken K, Lund E, Berrino F, et al. Oral contraceptives, reproductive history and risk of colorectal cancer in the European prospective investigation into cancer and nutrition. Br J Cancer. 2010;103:1755–9. doi: 10.1038/sj.bjc.6605965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clendenen TV, Koenig KL, Shore RE, Levitz M, Arslan AA, Zeleniuch-Jacquotte A. Postmenopausal levels of endogenous sex hormones and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:275–81. doi: 10.1158/1055-9965.EPI-08-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, et al. Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res. 2008;68:329–37. doi: 10.1158/0008-5472.CAN-07-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin JH, Zhang SM, Rexrode KM, Manson JE, Chan AT, Wu K, et al. Association between sex hormones and colorectal cancer risk in men and women. Clinical Gastroenterology and Hepatology. 2013;11:419–24. doi: 10.1016/j.cgh.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao C, Dahlman-Wright K, Gustafsson JA. Estrogen receptor β: an overview and update. Nucl Recept Signal. 2008;6:e003. doi: 10.1621/nrs.06003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu BT, Han GZ, Shim JY, Wen Y, Jiang XR. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor α and β subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology. 2006;147:4132–50. doi: 10.1210/en.2006-0113. [DOI] [PubMed] [Google Scholar]
- 8.Dallal CM, Tice JA, Buist DS, Bauer DC, Lacey JV, Jr, Cauley JA, et al. Estrogen metabolism and breast cancer risk among postmenopausal women: a case-cohort study with B~FIT. Carcinogenesis. 2014;35:346–55. doi: 10.1093/carcin/bgt367. [DOI] [PMC free article] [PubMed] [Google Scholar]