Abstract
The human opsonin ficolin-2 (L-ficolin) is an innate pattern-recognizing molecule that binds to acetylated moieties. Upon binding, ficolin-2 activates complement through the lectin pathway, opsonizing the target to promote phagocytic clearance. Ficolin-2 has been found to interact with a growing number of pathogenic bacteria, fungi, and viruses. Ficolin-2 also has proposed roles in host homeostasis, including the clearance of apoptotic cells. Consequently, there is an increased interest in studying ficolin-2, and access to purified ficolin-2 is necessary for these studies. Ficolin-2 purified from serum, plasma, or cell culture supernatants has been a useful tool in the characterization of ficolin-2 function; however, available protocols are laborious and inefficient, requiring additional processing of starting materials (e.g., polyethylene glycol precipitation or dialysis) and multiple steps of purification. Here, we investigated a simple solution to the problem: use of a simple, disposable bioreactor requiring only standard tissue culture equipment. Using this system, we generated cell culture supernatants containing high concentrations of recombinant ficolin-2, which permitted rapid purification of high-purity recombinant ficolin-2 without processing the supernatants. Purified recombinant ficolin-2 retained its binding capacity and supported complement activation in vitro. Bioreactor cultivation will likely be generally useful in the production of other recombinant proteins in the study of the complement system.
Keywords: complement, lectin pathway, L-ficolin, innate immunity, pattern recognition molecule, recombinant protein
1. INTRODUCTION
Since its discovery in the late 1990s, ficolin-2 (also known as L-ficolin) has become a molecule of great interest. Ficolin-2 functions as an innate immune opsonin and participates in the lectin pathway of complement activation, similarly to mannose binding lectin (MBL) (Faro et al., 2008). As part of innate immunity, ficolin-2 has been described to interact with acetylated and other moieties on an ever-expanding list of pathogenic bacterial and viral targets (Fujieda et al., 2012; Kilpatrick and Chalmers, 2012; Pan et al., 2012; Luo et al., 2013; Brady et al., 2014a; Hamed et al., 2014; Vassal-Stermann et al., 2014), but it has also been shown to bind to apoptotic cells (Kuraya et al., 2005; Jensen et al., 2007) and mitochondria (Brinkmann et al., 2013), suggesting important roles in host homeostasis.
Unlike its biological function, the structure of ficolin-2 is well-understood. Ficolin-2 is comprised of an N-terminal cysteine rich domain, a long, collagen-like domain, and a C-terminal fibrinogen-like domain, which is the ligand-binding domain (Matsushita et al., 1996). Ficolin-2 trimerizes through the collagen-like domain, and the trimers form higher-order oligomers through disulfide bonds in the N-terminal domain, with the most commonly observed species existing as a 12-mer (a tetramer of trimers) arranged in a bouquet-like structure (Ohashi and Erickson, 2004). Ficolin-2 is produced in the liver and is found primarily in the serum (Matsushita et al., 1996; Ohashi and Erickson, 2004), where it associates with MBL/ficolin-associated serine proteases (MASPs) (Matsushita et al., 2000). Upon ficolin-2 binding to a target, the MASPs become activated and cleave C4 and C2 to form the classical C3 convertase C4b2a and initiate complement activation.
Studies of ficolin-2 function require purified ficolin-2, and several protocols are described to purify ficolin-2 from human serum or plasma using agarose or sepharose beads conjugated to GlcNAc or CysNAc (Krarup et al., 2004; Faro et al., 2008; Matsushita et al., 2014). However, the reported protocols are apparently inefficient, with multiple steps of purification and low reported yields. Recombinant protein expression offers flexibility, and a common approach is the translational fusion of the protein to an affinity tag such as a polyhistidine tag. However, the secreted nature of ficolin-2 requires that the tag be placed at the C-terminal end of the protein. Indeed, commercially available recombinant ficolin-2 from R&D Systems (catalog no. 2428-FC) has a polyhistidine tag at the C-terminus. Since binding of ficolin-2 occurs through the C-terminus, tagging may alter or interfere with ficolin-2 target recognition; indeed, a C-terminally his-tagged ficolin-2 produced in our laboratory failed to bind serotype 11A bacteria (unpublished observation), a natural ficolin-2 target (Brady et al., 2014a). Thus, recombinant ficolin-2 in its natural form is desirable.
However, purification of ficolin-2 can be laborious, and purification of recombinant ficolin-2 presents further challenges due to low concentrations of ficolin-2 in the supernatants, requiring handling and concentration of large volumes of supernatant prior to purification (Lacroix et al., 2009). To avoid these challenges, we have investigated use of bioreactors, which can permit high-concentration yields of secreted proteins. We show that a simple and inexpensive disposable bioreactor produces culture supernatants with high concentrations of ficolin-2 and that ficolin-2 can be directly purified from these supernatants without processing using GlcNAc-agarose, yielding high-purity ficolin-2.
2. MATERIALS AND METHODS
2.1 Culture conditions
Generation of the human ficolin-2-expressing cell line huf2E by transfection of Chinese Hamster Ovary K1 (CHO) cells with FCN2 cDNA (Genbank accession no. BC069825) in pcDNA3.1(−) was described previously (Brady et al., 2014a; Brady et al., 2014b). All cell culture was performed in a humidified 37°C incubator with 5% CO2. For conventional cell culture, a confluent 150 cm2 tissue culture flask of huf2E was rinsed with 5 ml Hanks’ Balanced Saline Solution without magnesium or calcium (HBSS−/−) and digested with 5 ml trypsin (0.05%)-EDTA solution (Gibco 15400) in HBSS−/− for 5 minutes at 37°C. After digestion, cells were thoroughly resuspended from the flask by pipetting, and 250 μl (1/20 flask, 1.6 × 106 cells) were inoculated into 100 ml fresh Dulbecco's Modified Eagle's Medium/Nutrient Mixture F12 (DMEF, Thermo SH30023) supplemented to 10% heat-inactivated fetal bovine serum (Thermo SH30080) (DMEF-FBS). Geneticin (Life Technologies 10131027) was added to a final concentration of 750 μg/ml. Supernatants were harvested at 14 days, and cells were passaged at harvest as described above.
For bioreactor culture, 1.6 × 106 huf2E cells (prepared as described above) were inoculated into 15 ml DMEF-FBS in the cell chamber of a CELLine Bioreactor Flask (Wheaton Science Products WCL1000-3); 150 ml DMEF was added to the nutrient chamber. Both chambers were supplemented to 750 μg/ml Geneticin. After 14 days of incubation, the nutrient medium was discarded. Cell chamber material (typically 20-25 ml) was removed and centrifuged at 314 × g to recover cells. Cells were resuspended in 2 ml DMEF-FBS, and 1/20 cells (~5.2 × 106) were inoculated into 15 ml fresh DMEF-FBS in the cell chamber of the same bioreactor. Fresh nutrient medium and Geneticin were added as described above. It should be noted that the CELLine bioreactor is available with an adherent surface; as CHO cells are compatible with either unit, we used the suspension model and have not tested our protocols with the adherent cell model.
2.2 SDS-PAGE and immunoblotting
Ficolin-2 purity was determined through SDS-10%PAGE of a 15 μl aliquot of the indicated fractions and silver staining using Pierce Color Silver Stain Kit (Thermo Scientific 24597). Ficolin-2-containing fractions were detected through either immunoblot of SDS-10%PAGE of a 5 μl aliquot of the indicated fractions or dot blot of 100 μl serial dilutions of the indicated fractions on a 96-well dot blotting apparatus (Bio-Rad 170-6545). All immunoblots were performed on 0.45 μm nitrocellulose membranes and blocked in 5% powdered skim milk in tris-buffered saline supplemented with 0.05% tween-20. Immunodetection of ficolin-2 was achieved using a biotinylated anti-ficolin-2 antibody (R&D Systems BAF2428) and streptavidinconjugated alkaline phosphatase (Life Technologies 43-4322), both at a dilution of 1:1000, and development using 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium chloride in 1 M tris, pH 8.8.
2.3 Buffers
The dialysis buffer (referred to hereafter as “wash buffer”) reported by Lacroix, et al, (145 mM NaCl, 5 mM CaCl2, 20 mM Tris, pH 7.4) was the base for all other buffers (Lacroix et al., 2009), but because some solutes were acidic, solutions for purification were made from a 10X wash buffer solution and not adjusted for pH until the final solution was assembled. For elution from GlcNAc column, 500 mM N-acetyl-L-cysteine (CysNAc) was dissolved in 10X wash buffer and water and adjusted to pH 7.4 with 50% w/w NaOH before being brought to 1X (“elution buffer”).
2.4 Purification of ficolin-2
All steps were performed at room temperature (~20°C) unless otherwise noted. All column applications were by gravity flow. Ten milliliters of ficolin-2-containing bioreactor supernatants were applied to a 1 ml bed of GlcNAc-agarose (Sigma-Aldrich A2278) (previously equilibrated with 20 ml wash buffer) in a 10 ml polypropylene chromatography column (Bio-Rad 731-1550). The column was washed six times with 10 ml wash buffer prior to five 1-ml elutions with elution buffer. Ficolin-2 containing fractions were determined by dot blotting, pooled, and dialyzed against ≥ 500 volumes wash buffer at 4°C overnight.
Dialyzed ficolin-2 was supplemented with glycerol to 10% and concentrated using 3 kDa molecular weight cutoff microcentrifuge concentrator columns (Millipore UFC500396) to achieve a concentration of ~ 1 mg/ml.
2.5 Quantitation of ficolin-2
Ficolin-2 from supernatants was quantitated absolutely using a human ficolin-2 ELISA kit (Hycult Biotech HK336) or relatively by dot blot. Concentration of recovered ficolin-2 was determined by measuring absorbance at 280 nm in a 1 cm quartz cuvette (using either wash buffer or wash buffer made with 10% glycerol as a blank) assuming an extinction coefficient of 1.767 (mg/ml)−1 cm−1 calculated using the Protein Calculator (http://protcalc.sourceforge.net/) based on the molecular weight and the method of Gill and von Hippel (Gill and von Hippel, 1989).
2.6 Ficolin-2 functional assays
Ficolin-2 was assayed for binding and complement activation on serotype 11A Streptococcus pneumoniae (strain JC03 (Calix et al., 2014)) using flow-cytometric assays as previously described (Brady et al., 2014a; Brady et al., 2014b; Brady et al., 2014c). C1q-depleted serum was purchased from Quidel (A509); normal human serum (NHS) was obtained from a consented, healthy adult donor in glass serum collection tubes under an IRB-approved protocol. NHS was previously determined to have 4.95 μg/ml ficolin-2 (Brady et al., 2014c); commercial C1q-depleted serum is depleted of ficolin-2 (Brady et al., 2014b). Sera were used at 5% final concentration where indicated. Briefly, JC03 was opsonized with ficolin-2 (or in buffer control) at 4 °C for 1 h, washed by centrifugation and resuspension, incubated in the presence of serum (or buffer control) for 1 h at 37°C, and probed for ficolin-2 deposition and complement deposition with specific antibodies before analysis by flow cytometry. C3 and C4 deposition were detected by Pierce Complement C3 Antibody (Thermo Scientific LF-MA0132, used at 1:1000), detected by a phycoerythrin-conjugated goat-anti-mouse secondary antibody (Southern Biotech 1030-09, used at 1:2500), and a fluorescein isothiocyanate-conjugated anti-C4b/C4c antibody (Thermo Scientific PA1-28407, used at 1:200). Due to incompatible fluorophores, ficolin-2 binding was detected in parallel in identically prepared samples using the Pierce LFicolin (clone 19) monoclonal antibody (Thermo Scientific ABS005-19-02, used at 1:1000) and a phycoerythrin-conjugated goat-anti-mouse IgG secondary antibody (Southern Biotech 1030-09).
3. RESULTS
3.1 Bioreactor cultivation produces high concentrations of ficolin-2
The low abundance of ficolin-2 in standard CHO cell expression cultures results in the need for extensive processing of supernatants prior to purification (Lacroix et al., 2009). In an effort to further streamline ficolin-2 purification, we grew our ficolin-2-producing cell line, huf2E (Brady et al., 2014a; Brady et al., 2014b), in a CELLine bioreactor using the same passaging/expression schedule as the conventional cultures. Ficolin-2 concentrations from three harvested bioreactor supernatants were compared to three harvested standard culture supernatants by ELISA. Bioreactor supernatants contained 16-39-fold more concentrated ficolin-2 (Figure 1).
Figure 1.
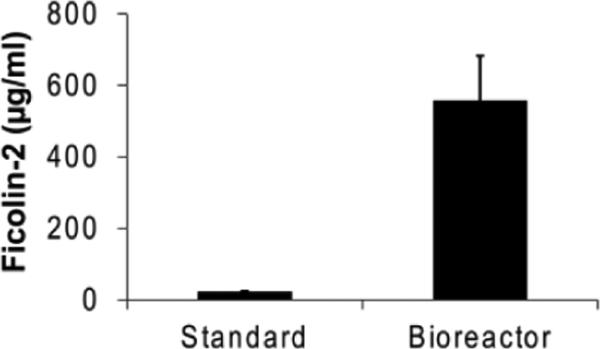
Recombinant ficolin-2 concentrations in huf2E supernatants. Three independent supernatants from the recombinant ficolin-2-producing cell line huf2E grown under standard culture conditions (“Standard”) or in a CELLine bioreactor (“Bioreactor”) were assayed for their ficolin-2 content by ELISA as described in Materials and Methods. Data are mean + SEM.
3.2 Ficolin-2 can be purified directly from bioreactor supernatants
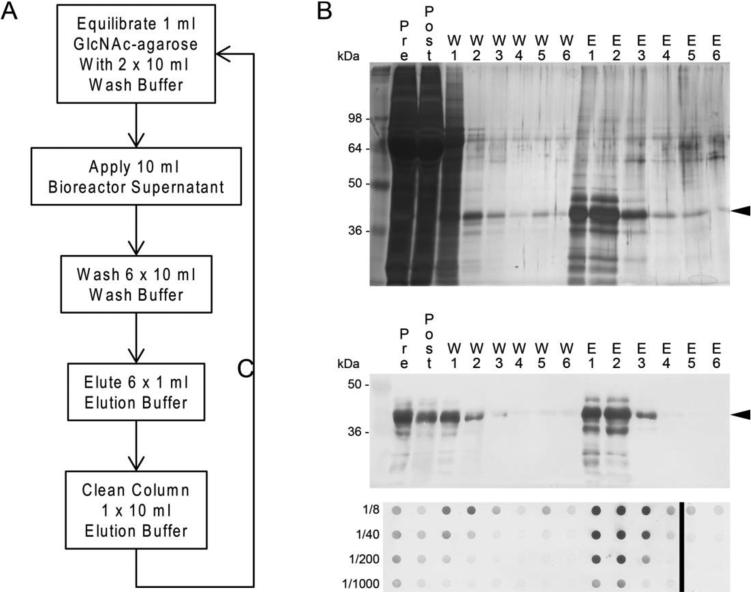
We hypothesized that in such great abundance, ficolin-2 could be purified directly from bioreactor supernatants without dialysis or concentration. We applied 10 ml (approximately half of a typical supernatant) of bioreactor supernatant to a 1 ml bed of GlcNAc-agarose, washed extensively, and eluted with elution buffer containing 500 mM CysNAc (a flowchart for purification is presented in Figure 2A). As shown in Figure 2, panels B and C, ficolin-2 eluted in high concentrations across the first three elution fractions (1 ml each) before steeply decreasing in the fourth fraction. Much material was retained in the flow-through (Figure 2C, “Post”), suggesting that repeat purifications may provide additional recovery. The eluted ficolin-2 was of high purity, as nearly all species detected in eluates correspond to species detected with a ficolin-2-specific antibody (Figure 2). Interestingly, a band of higher molecular weight than the major species was routinely detected in silver stain and immunoblot; however, it was not consistently observed by coomassie blue staining, suggesting that it is a very minor fraction of the protein (data not shown).
Figure 2.
Ficolin-2 purification from bioreactor supernatants using GlcNAc-agarose. A, schematic for ficolin-2 purification. B, silver stain of 15 μl aliquots of fractions from purification. Arrows indicate the major ficolin-2 bamd C, upper, immunoblot of 5 μl aliquots of purification samples; lower, dot immunoblot of 100 μl of purification samples at the indicated dilution. The vertical black line marks where another portion of the same dot blot is displayed beside the other portion. Fractions: Pre, starting material; Post, flow-through from GlcNAc column; W1-6, washes 1-6; E1-6, elutions 1-6.
3.3 Purified ficolin-2 is functional
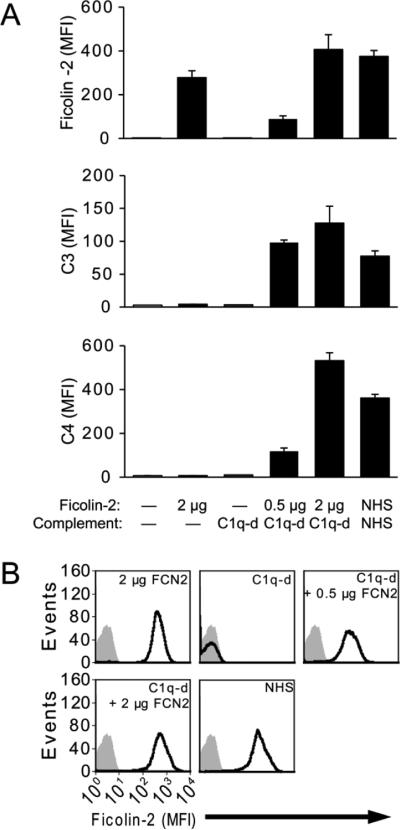
It is essential that purified ficolin-2 is functional. We previously demonstrated that ficolin-2 binds to and can activate complement upon serotype 11A pneumococcus (Brady et al., 2014a). Therefore, we tested our purified, recombinant ficolin-2 for its ability to bind to serotype 11A pneumococci and support complement deposition on their surface in C1q-depleted serum, which is otherwise depleted of ficolin-2 (Brady et al., 2014b). Purified, recombinant ficolin-2 bound to serotype 11A bacteria in a dose-dependent manner (Figure 3) and was able to support C3 and C4 deposition in the presence of C1q-depleted serum (Figure 3A).
Figure 3.
Purified recombinant ficolin-2 is functional. A, detection of ficolin-2, C3, and C4 on serotype 11A bacteria incubated with or without purified recombinant ficolin-2 and/or 5% serum as a complement source, as indicated, for 1 h . All events with forward scatter and side scatter ≥ 10 were analyzed. Data shown are geometric mean fluorescent intensities + SD of three independently-prepared replicates. Due to incompatible fluorophores with C3 detection, ficolin-2 binding was analyzed in identically-prepared wells on the same plate at the same time. B, representative histograms of ficolin-2 binding from panel A. Gray shading indicates no ficolin-2, no serum control. C1q-d, C1q-depleted serum; NHS, normal human serum; FCN2, ficolin-2.
4. DISCUSSION
Existing protocols for the purification of ficolin-2 are inefficient, especially for recombinant ficolin-2, and require many steps of processing and purification. Using CELLine bioreactors, we were able to generate CHO cell supernatants with ficolin-2 concentrations approaching 1 mg/ml – about 20-40 fold more than the conventional cell culture of the same cell line, which provided about 1.5 mg/ml of ficolin-2 per month after purification, with little optimization. Repeated purifications of the starting material are likely to increase recovery, as supernatants still retained ample ficolin-2 after the first purification (Figure 2). The high concentration of ficolin-2 facilitates the purification of high-purity recombinant ficolin-2 without the need for laborious processing. The recombinant ficolin-2 retained its functional capabilities, activating complement on serotype 11A pneumococcus, a natural ficolin-2 target (Brady et al., 2014a). Purified ficolin-2 could also be biotinylated, retaining both its binding and complement-activating abilities (data not shown).
Disposable bioreactors offer a convenient way to increase the relative concentration of recombinant protein to concentrations of other components of the medium that may interfere with purification, especially in an untagged system. For example, the only existing protocol for purification of recombinant ficolin-2 requires concentrating supernatants 20-fold (Lacroix et al., 2009); however, concentrations of FBS corresponding to 4-fold concentrated supernatant (i.e., 40% FBS) are sufficient to partially inhibit ficolin-2 binding (data not shown). Concentrating supernatants also increases the concentration of albumin, which is present in mg/ml quantities and is the most persistent contaminant during ficolin-2 purification. Bioreactor cultivation relieves the need for concentrating supernatants and the complications it introduces.
Bioreactors are a useful solution to the limitations of standard cell culture in the production of recombinant proteins. Large-scale bioreactors are available for industry or industry-oriented laboratories, but two bioreactor systems are suitable for research laboratories. One system is the hollow, fiber-based bioreactor (Fibercell), which circulates medium through a cell chamber in which the cells adhere to a fibrous matrix, and this has been used to produce recombinant proteins (e.g., (Arthos et al., 2002)). However, the system requires other supporting equipment (e.g., pumps), an expense that may deter investigators. Another is the CELLine Bioreactor, which does not require supporting equipment apart from the usual requirements for cell culture. Although its use was reported in 2001 in the production of a recombinant fusion peptide (Docagne et al., 2001), it has not been reported since for use in recombinant protein production and has instead been primarily used for monoclonal antibody production (e.g., (Bruce et al., 2002)). We show that this simple CELLine bioreactor can be useful in producing recombinant proteins for research laboratories with only standard cell culture equipment.
Even under the improved conditions bioreactor cultivation provides, ficolin-2 purification has offered myriad pitfalls. In the development of this protocol, we made additional technical discoveries that may be valuable to others. GlcNAc has been reported to elute ficolin-2 from GlcNAc- or CysNAc-agarose or -sepharose (e.g., refs. (Krarup et al., 2004; Faro et al., 2008; Lacroix et al., 2009; Matsushita et al., 2014)). In our system, GlcNAc in concentrations up to 450 mM resulted in diffuse elution, with low concentrations of ficolin-2 across many fractions rather than most eluted material being confined to a few fractions (as in Figure 2); thus, CysNAc is the more efficient eluent in our protocol. Given its efficiency, it may be worthwhile to consider CysNAc in established protocols purifying ficolin-2 from serum or plasma. A previous report of ficolin-2 purification from CHO cell supernatants described concentration of ficolin-2 to 0.1-0.4 mg/ml using spin concentrators (Lacroix et al., 2009). We found that ficolin-2 would precipitate at concentrations presumably above this range in spin concentrators; by supplementing to 10% glycerol prior to concentration, however, we were able to achieve concentrations ~ 1 mg/ml. Even in the presence of 10-20% glycerol, spin concentration reduced the apparent recovery of ficolin-2 (data not shown); we therefore advise that dialysis should be used for buffer exchange and the use of spin concentrators (preferably in the microcentrifuge format, as apparent loss was greater in the 15-ml format) be limited to concentrating the ficolin-2 solution. In addition, we confirmed the reported requirement for 10% FBS in the medium for proper ficolin-2 oligomerization (Lacroix et al., 2009), as reduction to even 5% FBS in the medium resulted in the production of low-order ficolin-2 oligomers as observed using native PAGE (data not shown). Increasing FBS to 20% showed no added benefit.
The study of ficolin-2 has expanded rapidly since ficolin-2 was identified, and purified recombinant ficolin-2 will be a useful tool in further study of this molecule. Bioreactor cultivation eliminates the need for processing large volumes of serum or culture supernatant to purify ficolin-2 through many steps with apparently poor efficiency. Many serum/plasma components, including MBL (Matsushita et al., 2000), bind to GlcNAc-agarose. The use of CysNAc-agarose can provide greater selectivity (Krarup et al., 2004), but the resin is not commercially available, and its generation requires toxic chemicals (Krarup et al., 2004). Use of recombinant protein rather than serum or plasma sidesteps the need for this selectivity.
Pathogenesis research is revealing the involvement of complement pathways in many processes. The complement system has many participating proteins, including several activating molecules and numerous regulators. Recombinant proteins are a powerful tool in dissecting molecular interactions, but efficient protocols for their purification are needed to employ them. Our approach will help to advance studies in this growing field by allowing time, incubator space, and other laboratory resources to be directed towards more fruitful ends and with appropriate modifications may be widely applicable to the in vitro production of other related proteins.
Highlights.
Ficolin-2 is a human serum molecule of increasing immunological interest
Protocols for purification of recombinant ficolin-2 are laborious and inefficient
Disposable bioreactor culture allows single-step purification of ficolin-2
Recovered ficolin-2 is of high-purity and retains complement-activating ability
Disposable bioreactors should simplify purification of many recombinant proteins
ACKNOWLEDGMENTS
This work was supported by NIH grants T32 HL105346 (KAG) and R56 AI031473 (MHN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arthos J, Cicala C, Steenbeke TD, Chun TW, Dela Cruz C, Hanback DB, Khazanie P, Nam D, Schuck P, Selig SM, Van Ryk D, Chaikin MA, Fauci AS. Biochemical and biological characterization of a dodecameric CD4-Ig fusion protein: implications for therapeutic and vaccine strategies. J Biol Chem. 2002;277:11456–64. doi: 10.1074/jbc.M111191200. doi: 10.1074/jbc.M111191200. [DOI] [PubMed] [Google Scholar]
- Brady AM, Calix JJ, Yu J, Geno KA, Cutter GR, Nahm MH. Low invasiveness of pneumococcal serotype 11A is linked to ficolin-2 recognition of O-acetylated capsule epitopes and lectin complement pathway activation. J Infect Dis. 2014a;210:1155–65. doi: 10.1093/infdis/jiu195. doi: 10.1093/infdis/jiu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM, Geno KA, Dalecki AG, Cheng X, Nahm MH. Commercially available complement component-depleted sera are unexpectedly codepleted of ficolin-2. Clin Vaccine Immunol. 2014b;21:1323–9. doi: 10.1128/CVI.00370-14. doi: 10.1128/CVI.00370-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM, Spencer BL, Falsey AR, Nahm MH. Blood collection tubes influence serum ficolin-1 and ficolin-2 levels. Clin Vaccine Immunol. 2014c;21:51–5. doi: 10.1128/CVI.00607-13. doi: 10.1128/CVI.00607-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann CR, Jensen L, Dagnaes-Hansen F, Holm IE, Endo Y, Fujita T, Thiel S, Jensenius JC, Degn SE. Mitochondria and the lectin pathway of complement. J Biol Chem. 2013;288:8016–27. doi: 10.1074/jbc.M112.430249. doi: 10.1074/jbc.M112.430249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce MP, Boyd V, Duch C, White JR. Dialysis-based bioreactor systems for the production of monoclonal antibodies--alternatives to ascites production in mice. J Immunol Methods. 2002;264:59–68. doi: 10.1016/s0022-1759(02)00081-9. doi: 10.1016/S0022-1759(02)00081-9. [DOI] [PubMed] [Google Scholar]
- Calix JJ, Brady AM, Du VY, Saad JS, Nahm MH. Spectrum of pneumococcal serotype 11A variants results from incomplete loss of capsule O-acetylation. J Clin Microbiol. 2014;52:758–65. doi: 10.1128/JCM.02695-13. doi: 10.1128/JCM.02695-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docagne F, Colloc'h N, Bougueret V, Page M, Paput J, Tripier M, Dutartre P, MacKenzie ET, Buisson A, Komesli S, Vivien D. A soluble transforming growth factor-beta (TGF-beta ) type I receptor mimics TGF-beta responses. J Biol Chem. 2001;276:46243–50. doi: 10.1074/jbc.M010915200. doi: 10.1074/jbc.M010915200. [DOI] [PubMed] [Google Scholar]
- Faro J, Chen Y, Jhaveri P, Oza P, Spear GT, Lint TF, Gewurz H. L-ficolin binding and lectin pathway activation by acetylated low-density lipoprotein. Clin Exp Immunol. 2008;151:275–83. doi: 10.1111/j.1365-2249.2007.03538.x. doi: 10.1111/j.1365-2249.2007.03538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujieda M, Aoyagi Y, Matsubara K, Takeuchi Y, Fujimaki W, Matsushita M, Bohnsack JF, Takahashi S. L-ficolin and capsular polysaccharide-specific IgG in cord serum contribute synergistically to opsonophagocytic killing of serotype III and V group B streptococci. Infect Immun. 2012;80:2053–60. doi: 10.1128/IAI.06232-11. doi: 10.1128/IAI.06232-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989;182:319–26. doi: 10.1016/0003-2697(89)90602-7. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- Hamed MR, Brown RJ, Zothner C, Urbanowicz RA, Mason CP, Krarup A, McClure CP, Irving WL, Ball JK, Harris M, Hickling TP, Tarr AW. Recombinant human L-ficolin directly neutralizes hepatitis C virus entry. J Innate Immun. 2014;6:676–84. doi: 10.1159/000362209. doi: 10.1159/000362209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ML, Honore C, Hummelshoj T, Hansen BE, Madsen HO, Garred P. Ficolin-2 recognizes DNA and participates in the clearance of dying host cells. Mol Immunol. 2007;44:856–65. doi: 10.1016/j.molimm.2006.04.002. doi: 10.1016/j.molimm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DC, Chalmers JD. Human L-ficolin (ficolin-2) and its clinical significance. J Biomed Biotechnol. 2012;2012:1–10. doi: 10.1155/2012/138797. doi: 10.1155/2012/138797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krarup A, Thiel S, Hansen A, Fujita T, Jensenius JC. L-ficolin is a pattern recognition molecule specific for acetyl groups. J Biol Chem. 2004;279:47513–9. doi: 10.1074/jbc.M407161200. doi: 10.1074/jbc.M407161200. [DOI] [PubMed] [Google Scholar]
- Kuraya M, Ming Z, Liu X, Matsushita M, Fujita T. Specific binding of L-ficolin and H-ficolin to apoptotic cells leads to complement activation. Immunobiology. 2005;209:689–97. doi: 10.1016/j.imbio.2004.11.001. doi: 10.1016/j.imbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Lacroix M, Dumestre-Perard C, Schoehn G, Houen G, Cesbron JY, Arlaud GJ, Thielens NM. Residue Lys57 in the collagen-like region of human L-ficolin and its counterpart Lys47 in H-ficolin play a key role in the interaction with the mannan-binding lectin-associated serine proteases and the collectin receptor calreticulin. J Immunol. 2009;182:456–65. doi: 10.4049/jimmunol.182.1.456. doi: 10.4049/jimmunol.182.1.456. [DOI] [PubMed] [Google Scholar]
- Luo F, Sun X, Wang Y, Wang Q, Wu Y, Pan Q, Fang C, Zhang XL. Ficolin-2 defends against virulent Mycobacteria tuberculosis infection in vivo, and its insufficiency is associated with infection in humans. PLoS One. 2013;8:e73859. doi: 10.1371/journal.pone.0073859. doi: 10.1371/journal.pone.0073859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Endo Y, Fujita T. Cutting edge: complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J Immunol. 2000;164:2281–4. doi: 10.4049/jimmunol.164.5.2281. doi: 10.4049/jimmunol.164.5.2281. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Endo Y, Taira S, Sato Y, Fujita T, Ichikawa N, Nakata M, Mizuochi T. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J Biol Chem. 1996;271:2448–54. doi: 10.1074/jbc.271.5.2448. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Kilpatrick D, Shiraki H, Liu Y, Tateishi K, Tsujimura M, Endo Y, Fujita T. Purification, measurement of concentration, and functional complement assay of human ficolins. Methods in molecular biology. 2014;1100:141–59. doi: 10.1007/978-1-62703-724-2_12. doi: 10.1007/978-1-62703-724-2_12. [DOI] [PubMed] [Google Scholar]
- Ohashi T, Erickson HP. The disulfide bonding pattern in ficolin multimers. J Biol Chem. 2004;279:6534–9. doi: 10.1074/jbc.M310555200. doi: 10.1074/jbc.M310555200. [DOI] [PubMed] [Google Scholar]
- Pan Q, Chen H, Wang F, Jeza VT, Hou W, Zhao Y, Xiang T, Zhu Y, Endo Y, Fujita T, Zhang XL. L-ficolin binds to the glycoproteins hemagglutinin and neuraminidase and inhibits influenza A virus infection both in vitro and in vivo. J Innate Immun. 2012;4:312–24. doi: 10.1159/000335670. doi: 10.1159/000335670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassal-Stermann E, Lacroix M, Gout E, Laffly E, Pedersen CM, Martin L, Amoroso A, Schmidt RR, Zahringer U, Gaboriaud C, Di Guilmi AM, Thielens NM. Human L-ficolin recognizes phosphocholine moieties of pneumococcal teichoic acid. J Immunol. 2014;193:5699–708. doi: 10.4049/jimmunol.1400127. doi: 10.4049/jimmunol.1400127. [DOI] [PubMed] [Google Scholar]