Abstract
In this study, the effects of combining ursolic acid (UA) + resveratrol (Res), for possible combined inhibitory effects on skin tumor promotion were evaluated. UA, Res and the combination of UA + Res were applied topically prior to TPA treatment on mouse skin to examine their effect on TPA-induced signaling pathways, epidermal hyperproliferation, skin inflammation, inflammatory gene expression and skin tumor promotion. The combination of UA + Res produced a greater inhibition of 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced epidermal hyperproliferation. The combination of UA + Res inhibited TPA-induced signaling pathways, including EGFR, STAT3, Src, Akt, Cox-2, Fas, NF-κB, p38 MAPK, c-Jun, and JNK1/2 while increasing levels of tumor suppressors such as p21 and PDCD4 to a greater extent compared to the groups treated with the individual compounds. UA + Res also induced a dramatic increase of p-AMPK-αThr172. Combined treatment with UA + Res resulted in a greater inhibition of expression of proinflammatory cytokines including IL-1α, IL-1β, and IL-22. Furthermore, NF-κB, Egr-1, and AP-1 DNA binding activities after TPA treatment were dramatically decreased by the combination of UA + Res. Treatment with UA + Res during skin tumor promotion with TPA produced greater inhibition of tumor multiplicity and tumor size than with either agent alone. Collectively, the greater ability of the combination of UA + Res to inhibit skin tumor promotion was due to the greater inhibitory effects on growth factor and inflammatory signaling, skin inflammation and epidermal hyperproliferation induced by TPA treatment.
Keywords: Ursolic acid, resveratrol, combination, chemoprevention, skin tumor promotion
Introduction
Ursolic acid (UA) is a natural pentacyclic triterpenoid carboxylic acid found in many plants including P. fructescen (Japanese basil), rosemary, apples, elder flowers and many others. UA has been shown to have apoptotic, anti-inflammatory and anti-tumorigenic effects in various cancer models including prostate, ovary, stomach, intestine, and skin (1–3). Further studies have revealed that UA has broad-spectrum anti-carcinogenic effects including prevention of DNA damage, inhibition of EGFR/MAPK signaling, inhibition of angiogenesis, activation of apoptotic pathways, and inhibition of Akt/mTOR, NF-κB, Cox-2, and STAT3 signaling pathways (1, 4). Although several studies have reported that UA inhibited carcinogen and 12-O-tetracanoylphorbol-13-acetate (TPA)-induced inflammation, hyperplasia and tumor promotion in mouse skin (2, 3, 5), its inhibitory mechanism on skin tumor promotion is not fully understood. Recently, several studies reported that UA has an anti-obesity effect and mimics some of the effects of calorie restriction (CR) by modulating Akt/mTOR signaling pathways (6–8). UA has also been shown to activate the LKB1/AMPK pathway for inhibition of adipogenesis (9).
Resveratrol (Res) is a phytoalexin and is present in grapes, berries, peanuts and red wine. Res has been shown to have cardiovascular benefit and anti-diabetic effects in both mice and humans. In addition, Res was shown to inhibit skin tumor promotion and also inhibit the growth of many cancer cell lines, including breast, prostate, colon and liver (5, 10–13). Mechanisms associated with the anti-tumor promoting effects of Res include inhibition NF-κB, AP-1, and Cox-2 (10, 13, 14). Several reports have suggested that Res also mimics some of the effects of CR on life span in worms and other model organisms, especially by inhibiting inflammation and mTOR signaling (15, 16). Res also mimics effects of CR by increasing SirT1 and AMPK activation (17). Boily et al. have suggested that the anti-promoting effect of Res on mouse skin is at least partially mediated by SirT1 (18).
Emerging evidence suggests that combinations of phytochemicals may be an effective strategy to achieve a greater chemopreventive effect than with single agents (19–21). Several studies have shown that combinations of natural compounds can produce potential synergistic inhibitory effects in various cancers (e.g., Res + grape seed extract and ellagic acid + grape seed extract) (12, 20–24). Recently, Junco et al. reported that Res potentiates the growth inhibitory effect of UA in mouse skin papilloma and carcinoma cell lines (25). Thus, combining agents may provide the most rational and effective approach to cancer chemoprevention. In addition, using combinations of phytochemicals may produce overall effects that more similarly mimic CR.
In the present study, topical treatment with a combination of UA + Res produced a greater inhibitory effect on skin tumor promotion by TPA than with either agent alone. Further mechanistic studies revealed that this combination produced a greater inhibition of multiple growth factor and inflammation signaling pathways as well as greater upregulation of tumor suppressor genes such as p21 and PDCD4. Interestingly, the combination of UA + Res induced a dramatic increase of p-AMPK-αThr172 and its downstream target p-Ulk1Ser555. Collectively, the current data suggest that combined treatment of UA + Res is a more effective inhibitor of skin tumor promotion than either UA or Res given alone. The mechanism for this greater inhibition appears to be multi-faceted with similarities to changes observed with CR.
Materials and Methods
Animals and diets
For all experiments except the labeling retaining cell assay (LRC assay), Female Hsd : ICR (CD-1)mice 6–7 weeks of age were used and purchased from Harlan Laboratories Inc. (Houston, TX). For LRC assays, 10-day old mice were obtained by breeding FVB/N female and male mice (purchased from the National Cancer Institute). Mice were group housed in a 12 hr dark/12 hr light cycle at 24 °C for all experiments. For the short-term experiments, mice were fed a regular chow diet. For tumor experiments, mice received either an overweight control diet (D12450B, 10 Kcal% fat; Research Diets Inc.) or an obesity-inducing diet (D12492, 60 Kcal% fat; Research Diets Inc). All animal experiments were conducted in accordance with both Institutional as well as NIH guidelines under an approved IACUC protocol.
Two-stage skin carcinogenesis assays
Female ICR mice (n=30/group) 7–8 weeks of age were shaved on the dorsal skin and then 48 hrs later initiated with a single topical application of 25 nmol of 7, 12-dimethylbenz[a]anthracene (DMBA; Sigma-Aldrich) in 0.2 ml acetone or acetone vehicle. Two weeks after initiation, mice were randomized to receive one of the two experimental diets (overweight control and obesity-inducing diet) for 6 weeks before starting treatment with the tumor promoter, TPA. During tumor promotion, mice received 2 µmol of UA (Sabinsa Corporation) and 2 µmol of Res (Orchid Chemicals & Pharmaceuticals Ltd.) 15 min prior to each TPA application. For the combination, Res was given 30 min and UA was given 15 min prior to each 6.8 nmol dose of TPA (LC Laboratories) to allow time for absorption of each compound prior to TPA application. All other aspects of the tumor experiments were as previously described (26–28).
Short-term treatment protocol
For a number of experiments, mice were treated using a short-term treatment protocol involving 4 applications of TPA. For this protocol, groups of mice (7–8 weeks of age) were shaved on the dorsal skin and then two days later treated twice weekly for two weeks with 0.2 ml acetone vehicle, UA (2 µmol) or Res (2 µmol) 15 min prior to each 6.8 nmol of TPA treatment. For the combination, mice received UA (2 µmol) and Res (2 µmol) 15 min and 30 min prior to TPA treatment, respectively. Mice were then sacrificed at various times thereafter for collection of epidermal tissue.
Label retaining cell (LRC) assay
For these experiments, 10-day old FVB/N mice were injected with BrdU [50 µg/g body weight (B.W.)] i.p every 12 hrs over 2 days. Seventy days later, mice were shaved on the dorsal skin and then treated with the short-term protocol. Mice were sacrificed 48 hrs after the last treatment and dorsal skin samples were prepared and analyzed as previously described (28).
Histological analyses
For analysis of epidermal thickness and labeling index (LI) as well as the number of dermal inflammatory cells, mice were shaved on the dorsal skin and then treated with the short-term protocol. All procedures for these analyses were as previously described (26–28).
Preparation of epidermal protein lysates, cytosolic fractions, nuclear fractions and RNA
Groups of mice were treated with the short-term protocol and then sacrificed 6 hrs after the last TPA treatment. After sacrifice, epidermal protein lysates were collected as previously described (26). For electrophoretic mobility shift assays (EMSAs), the epidermal cytosolic and nuclear fractions were isolated using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific Inc). The protein lysates and nuclear/cytosolic fractions were used immediately or stored at −80 °C until used. Epidermal RNA samples were isolated as previously described (29, 30) and subjected to quantitative real-time PCR (qRT-PCR) analysis.
Western blot analysis
Western blot analyses were performed as previously described (26). Antibodies used are listed in supplemental Table 1.
EMSA
EMSA was performed using a DNA-protein binding detection kit according to the manufacturer’s protocol (Thermo Scientific Inc.). See supplemental Table 2 for the sequences of the NF-κB, Egr-1 and AP-1 oligos used.
qRT-PCR analysis
qRT-PCR analyses were performed as previously described (29, 30). cDNA (150 ng) was mixed with 2× TaqMan gene expression master mix (AB Applied Biosciences), 20× primer sets (IL-1α, IL-1β, IL-22, ribosomal 18 S), and nuclease-free water in a total volume of 10 µl. For qPCR of Cox-2 mRNA, 2× iTaq™ universal SYBR® green supermix (Bio-rad), 1 µM primers (Cox-2, and GAPDH), and nuclease-free water were added to cDNA (150 ng). The mixtures were then subjected to qRT-PCR using ViiA™ 7 real time instrument and analysis software.
Statistical analysis
For comparisons of quantitative protein expression, gene expression, epidermal thickness, labeling index, the number of infiltrated inflammatory cells, transcriptional activities, and tumor multiplicity and tumor size, the Mann-Whitney U test was used. A one-tailed Fisher’s exact test and Mantel-Cox test was used for comparisons of tumor incidence and tumor latency, respectively. Significance in all cases was set at p ≤ 0.05.
Results
Effect of UA + Res on skin tumor promotion by TPA
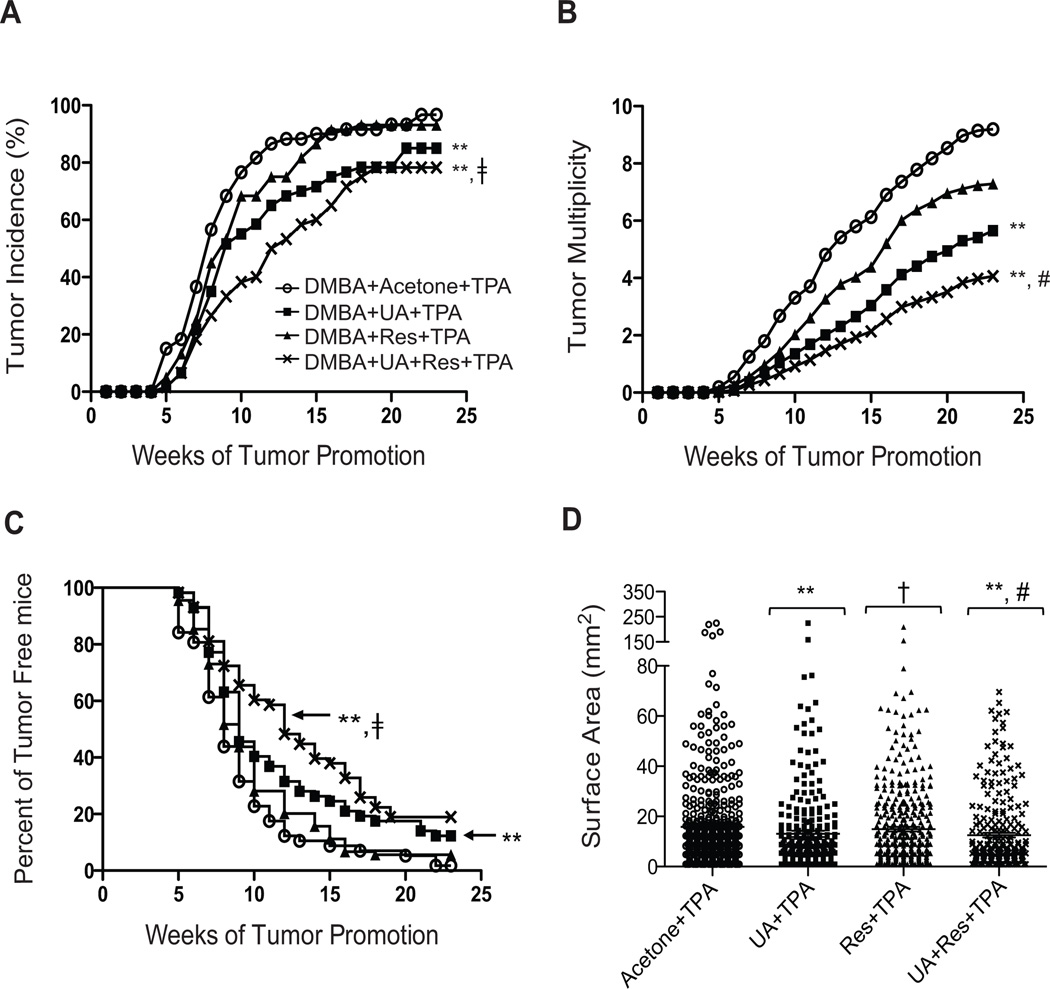
The ability of a combination of UA + Res to inhibit skin tumor promotion by TPA was evaluated in ICR mice maintained on either an overweight control diet or a diet-induced obesity diet (DIO diet). After completion of the tumor experiment, the tumor responses in both diet groups were similar for all groups (see supplemental Figs. 1A and 1B). Therefore, the data for the corresponding treatment groups on each diet were combined as presented in Fig. 1. As shown in Fig. 1, pretreatment with UA or Res alone inhibited tumor multiplicity by 38.6% and 20.8%, respectively. The reduction in tumor multiplicity with UA was statistically significant (p<0.05; Mann-Whitney U test) compared to the group treated with TPA alone. Pretreatment with the combination of UA + Res resulted in 56% reduction in tumor multiplicity that was significantly lower when compared to both the Res + TPA and UA + TPA groups (p<0.05; Mann-Whitney U test). Furthermore, the incidence of papillomas in the mice treated with UA + Res was significantly lower than that observed in the TPA and Res + TPA treated groups (p<0.05; Fisher’s exact test) but not the UA + TPA group. An effect on tumor latency was also observed as shown in Fig. 1C. In this regard, the percent of tumor-free mice treated with the combination of UA + Res was significantly higher than that of the TPA only and Res + TPA groups over the 23 week observation period (p<0.05; Mantel-Cox test) but not the UA + TPA treated group.
Figure 1.
Effect of UA + Res on skin tumor promotion in ICR mice. Panel A, Incidence of tumors (percentage of mice with papillomas). The percentage of mice with papillomas in the group treated with UA + Res +TPA was significantly lower than TPA (**) or Res + TPA (‡) treated groups (p<0.05; Fisher’s exact test). Panel B, tumor multiplicity (average number of papillomas per mouse). Both the UA and the UA + Res pretreated groups had significantly reduced tumor multiplicity compared to the TPA-treated group (**, p< 0.05). The tumor multiplicity in the UA + Res + TPA-treated (#, p<0.05; Mann-Whitney U test) was also significantly lower than both UA + TPA and Res + TPA-treated group. Panel C. tumor latency (tumor free survival). Significant differences were observed between the UA + TPA and UA + Res + TPA compared to the TPA only group (**, p<0.05; Mantel-Cox test). Percent of tumor free mice in the combination group (‡, p<0.05; Mantel-Cox test) was greater than the Res + TPA group. Panel D, tumor size. The surface area of papillomas was measured at the 23rd week. **, p<0.05 when compared to TPA group; †, p<0.05 when compared to UA + TPA group; and #, p<0.05 when compared to both the Res + TPA and UA + TPA groups. The Mann-Whitney U test was used for all statistical comparisons of tumor size.
As shown in Fig. 1D, the combination of UA + Res significantly reduced the size of papillomas compared to the TPA only group as well as both the UA + TPA and Res + TPA groups (p<0.05; Mann-Whitney U test). Thus, the combination was more effective at reducing both the number and size of papillomas when compared to either agent given alone.
Body weight gain for the 23 week experiment for each group is shown in Supplemental Fig. 1C and 1D. As expected, there were significant differences in body weight between untreated mice on the overweight control and DIO diets (41.57 g ± 2.49 and 56.57 g ± 2.39, respectively; p< 0.05; Mann-Whitney U test). No significant differences were observed in body weight between the treated groups in mice on either the control diet or DIO diet. Overall, these data suggest that the combined treatment of UA + Res, at the doses used, had a greater inhibitory effect on skin tumor promotion compared to the groups pretreated with either of the compounds alone and with no apparent toxicity.
Effect of UA + Res Treatment on TPA-induced epidermal hyperproliferation and LRCs
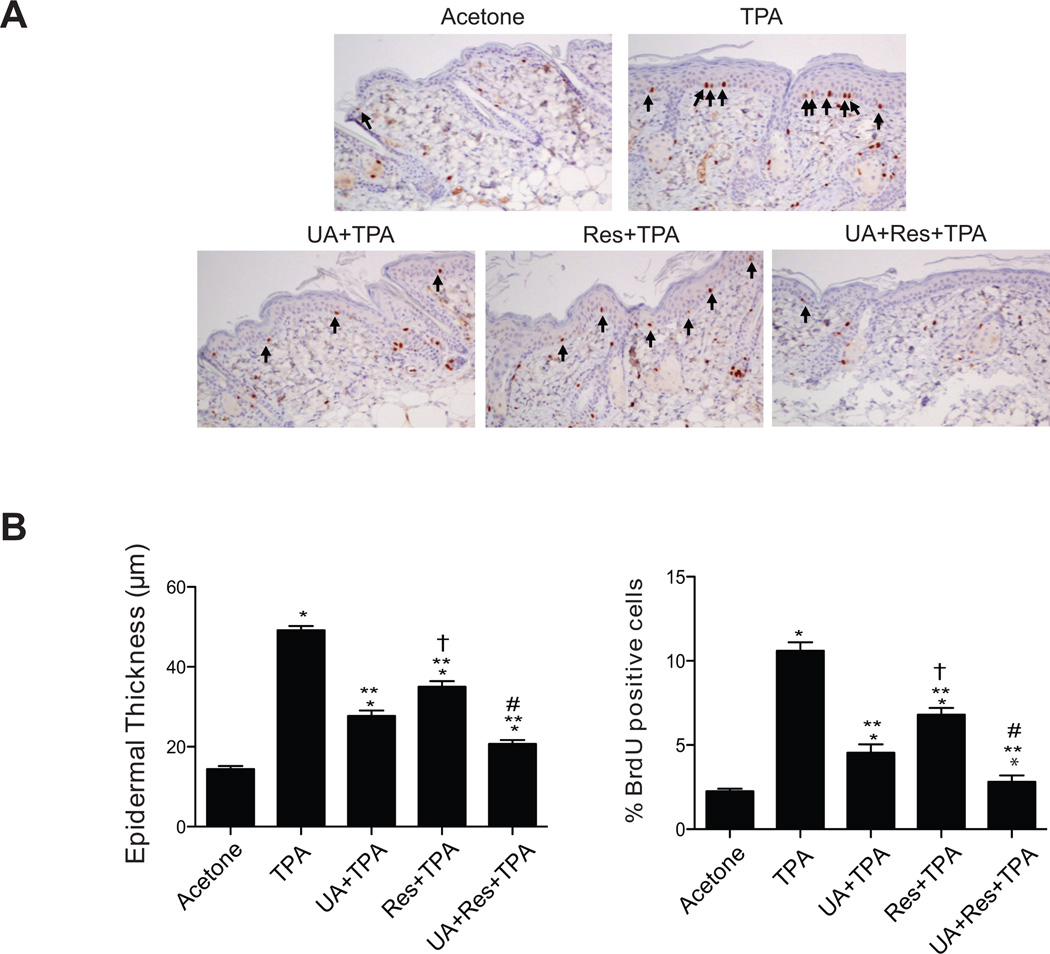
As shown in Figs. 2A and 2B, UA or Res alone significantly reduced BrdU incorporation and epidermal thickness following TPA treatment (p<0.05; Mann-Whitney U test). However, treatment with the combination of UA + Res produced a greater inhibition of BrdU incorporation and epidermal thickness induced by TPA compared to that observed with either of the compounds given alone with TPA (p<0.05; Mann-Whitney U test).
Figure 2.
Effect of UA + Res on TPA-induced epidermal hyperproliferation in ICR mice. Female ICR mice at 7–8 weeks of age maintained on standard chow diet were treated topically with the short-term protocol. Dorsal skin was collected at 48 hrs after the last treatment for histological evaluation. Sections were stained with H&E and for BrdU incorporation. Panel A, representative sections of BrdU stained skin. Arrows indicate BrdU-positive cells. Magnification, × 20 Panel B, quantitative analysis of the effects of UA, Res or UA + Res on TPA-induced epidermal thickness and labeling index (% BrdU positive cells). The values in panel B represent the means ± SEM. *, p<0.05 when compared to the acetone treated group; **, p<0.05 when compared to the TPA treated group; †, p<0.05 when compared to the UA + TPA group; #, p<0.05 when compared to UA + TPA and Res + TPA group. All statistical analyses were performed using the Mann-Whitney U test.
As shown in supplemental Fig. 2, LRCs in acetone-treated mice were confined to the hair follicle bulge-region as expected based on previous studies (28, 30). However, after a two-week treatment regimen with TPA, the LRCs can be seen moving up and out of the hair follicle into the interfollicular epidermis (supplemental Fig. 2A). Pretreatment with UA or Res partially inhibited the effect of TPA on proliferation and migration of LRCs. However, treatment with the combination of UA + Res prior to application of TPA produced a greater inhibitory effect on the proliferation and migration of these cells compared to the UA or Res only treated groups (supplemental Fig. 2B; p<0.05; Mann-Whitney U test).
Effect of UA + Res on TPA-induced epidermal signaling pathways
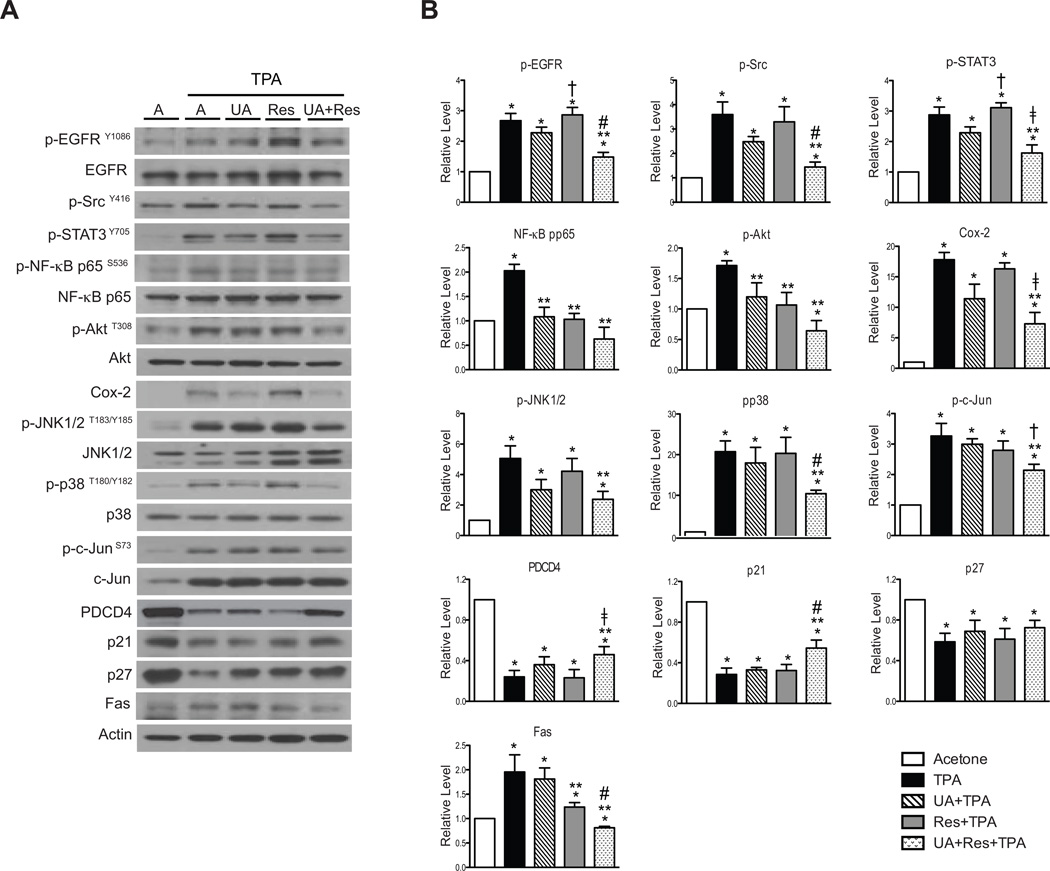
As shown in the Fig. 3A and 3B, UA + Res significantly inhibited TPA-activated p-STAT3Tyr705, p-AktThr308, p-NF-κB p65Ser536, p-JNK1/2Thr183/Tyr185, p-c-JunSer73, and p-p38 MAPKThr180/Tyr182, whereas UA or Res alone either produced no significant effects or a moderate inhibition of phosphorylation of these proteins relative to the combination. Cox-2 induction by TPA was not significantly decreased by pretreatment with either UA or Res alone, however, the combination of UA + Res produced a statistically significant inhibition of Cox-2 induction by TPA. The levels of several tumor suppressors were also evaluated (see again Fig. 3A and 3B). The combination of UA + Res significantly reversed the effect of TPA on PDCD4 and p21 levels while pretreatment with either compound alone had no effect. In contrast, none of the treatments reversed the effects of TPA treatment on p27 levels.
Figure 3.
Effect of UA + Res on TPA-induced signaling pathways in epidermis of female ICR mice. Western blot analyses were performed using pooled epidermal protein lysates from mice (n=4–5/group) receiving treatment with the short-term protocol. Panel A, representative Western blot analyses of multiple signaling pathways. Panel B, quantitative evaluation of Western blot data. Values represent means ± SEM from at least 3 independent experiments. *, p<0.05 when compared to the acetone treated group; **, p<0.05 when compared to the TPA treated group; †, p<0.05 when compared to the UA + TPA treated group; ‡, p<0.05 when compared to the Res + TPA treated group; and #, p<0.05 when compared to the UA + TPA and Res + TPA treated groups. The Mann-Whitney U test was used for all statistical comparisons.
The phosphorylation of both EGFRTyr1086 and SrcTyr416 was also significantly inhibited by the combination of UA + Res at the doses and time points examined whereas neither UA nor Res alone significantly inhibited phosphorylation of these proteins. On the other hand, the increased level of Fas induced by TPA was decreased by treatment with Res alone and the combination of UA + Res but not with UA. Again, the combination was the most effective at inhibiting the increase in Fas seen following treatment with TPA.
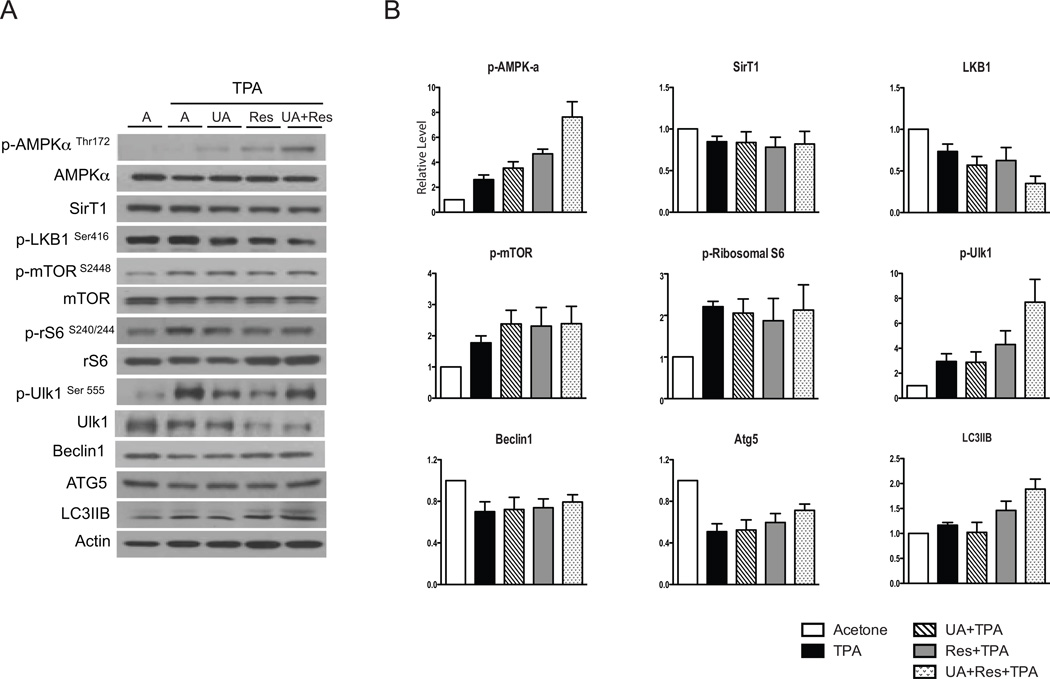
As shown in Fig. 4A and 4B, treatment with TPA alone produced a ~2.5-fold increase in p-AMPK-αThr172 compared to the acetone treated control group. Both UA and Res when given with TPA further increased p-AMPK-αThr172, while the combination of UA + Res together with TPA produced an even greater activation of AMPK-α that was significantly greater than with either UA or Res given alone (p<0.05). The level of SirT1 was not changed by treatment with TPA or pretreatment with any of the compounds given together with TPA, including the combination of UA + Res (again see Fig. 4A and 4B). TPA treatment reduced the level of p-LKB1Ser428 compared to the acetone group, however, neither UA nor Res had any further effect. In contrast, the level of p-LKB1Ser428 was further decreased when the combination of UA + Res was given before TPA treatment. TPA treatment led to activation of mTORC1 signaling as previously reported (26, 28, 31), however, the levels of p-mTORC1Ser2448 and its downstream target, p-S6-ribosomal proteinSer240/244, were not affected by treatment with UA, Res or the combination of UA + Res. Notably, the level of p-Ulk1Ser555 was significantly increased when the combination of UA + Res was given together with TPA. The combination of UA + Res when given together with TPA significantly increased the level of LC3IIB compared to the acetone, TPA and UA + TPA groups while the levels of ATG5 and Beclin1 that were reduced by TPA treatment were not significantly altered further by any of the treatments.
Figure 4.
Effect of UA + Res on TPA-induced AMPK and mTORC1 signaling pathways in epidermis of female ICR mice. Western blot analyses were performed using pooled epidermal protein lysates from mice (n=4–5/group) that received multiple treatments with short-term protocol. Panel A, representative Western blot analyses. Panel B, quantitative evaluation of the effect of UA, Res or UA + Res on TPA-induced AMPK-α signaling pathway. Values represent means ± SEM from at least 3 independent experiments. *, p<0.05 when compared to acetone group; **, p<0.05 when compared to TPA group; †, p<0.05 when compared to UA + TPA group; ‡, p<0.05 when compared to the Res + TPA treated group; and #, p<0.05 when compared to UA + TPA and Res + TPA group. The Mann-Whitney U test was used for statistical comparisons.
Effect of UA + Res on TPA-induced inflammation and inflammatory gene expression
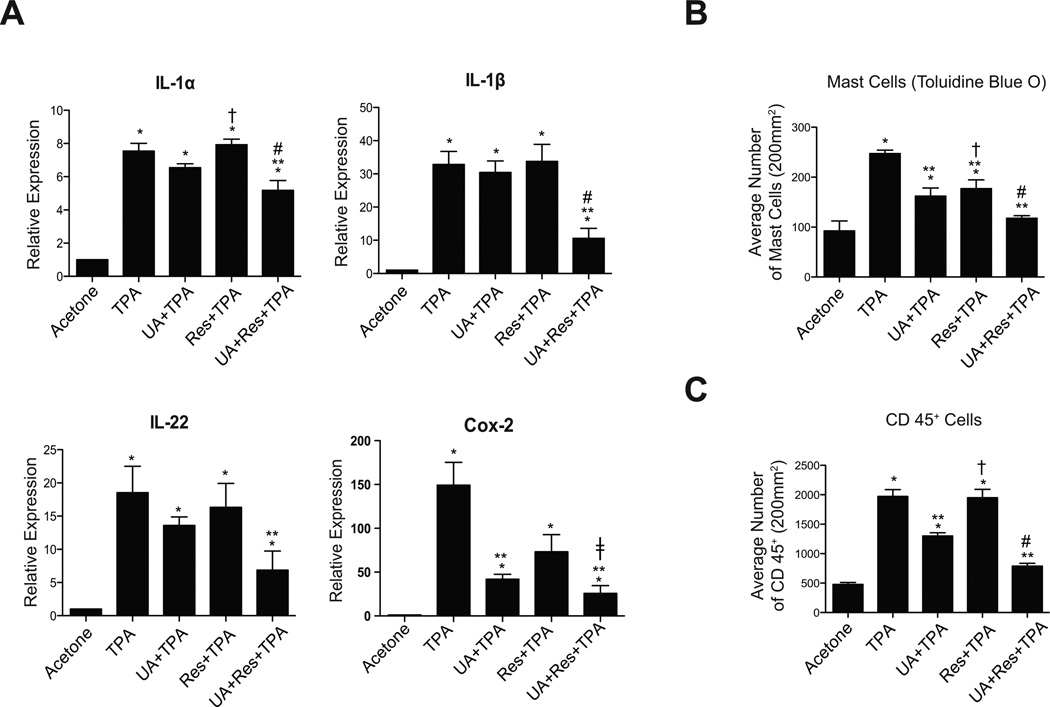
As shown in Fig. 5A, the levels of IL-1α, IL-1β, IL-22 and Cox-2 mRNA were increased following treatment with TPA (given twice weekly for two weeks) and significantly decreased in the UA + Res pretreated group (p<0.05; Mann-Whitney U test). With the exception of UA pretreatment on Cox-2 mRNA, neither UA nor Res pretreatment significantly reduced the mRNA levels of these inflammatory genes. As shown in Supplemental Fig. 3 and Figs. 5B–C, pretreatment with UA and Res decreased the number of mast cells in the dermis seen following TPA treatment, however, an additional decrease in the number of dermal mast cells was observed after treatment with UA + Res + TPA (p<0.05; Mann-Whitney U test). UA alone and UA + Res also produced a significant decrease in the number of CD45+ cells in dermis (p<0.05). Again, the combination of UA + Res produced the greatest reduction in the numbers of both mast cells and CD45+ cells.
Figure 5.
Effect of UA + Res on TPA-induced inflammatory gene expression and inflammatory cell infiltration. Epidermal RNA samples were prepared from groups of female ICR mice (n=4–5/group) treated using the short-term protocol. RNA samples were then subjected to qRT-PCR analysis as described in Materials and Methods. Panel A, qRT-PCR analysis of IL-1α, IL-1β, IL-22, and Cox-2. mRNA levels of IL-1α, IL-1β, and IL-22 were normalized to 18S and the mRNA level of Cox-2 was normalized to GAPDH. Panel B, quantitative evaluation of the effect of UA, Res and UA + Res on the number of mast cells in the dermis 48 hrs after the last TPA treatment. Positive cells were counted per 200 mm2. Panel C, quantitative analysis of the effect of UA, Res and UA + Res on the number of CD45 positive cells in the dermis 48 hrs after the last TPA treat. Positive cells were counted per 200 mm2. The graphs in all cases represent means ± SEM of at least 3 independent experiments. *, p<0.05 when compared to acetone group; **, p<0.05 when compared to TPA group; †, p<0.05 when compared to UA + TPA group; ‡, p<0.05 when compared to Res + TPA group; and #, p<0.05 when compared to UA + TPA and Res + TPA group. The Mann-Whitney U test was used for statistical comparisons.
Effect of UA + Res on NF-κB, Egr-1, and AP-1 DNA binding activities induced by TPA
Treatment with TPA significantly increased the amount of NF-κB, Egr-1 and AP-1 bound to their consensus DNA binding oligos (supplemental Figs. 4A–C and supplemental Figs. 5A–C). The TPA-induced increase in DNA binding activity of all three transcription factors was significantly reduced by pretreatment with the combination of UA + Res. Pretreatment with UA or Res alone significantly reduced binding of NF-κB and UA pretreatment significantly reduced binding of AP-1. Thus, the combination of UA + Res was highly effective at inhibiting the activation of all three of these transcription factors by TPA. Additionally, the nuclear translocation of NF-κB, Egr-1 and AP-1 induced by TPA was also significantly inhibited by UA + Res to a greater extent than pretreatment with either UA or Res (supplemental Figs. 4G–I and supplemental Figs. 5D–F).
Discussion
In the present study, topical application of UA + Res followed by TPA treatment inhibited skin tumor promotion to a greater extent when compared to the groups treated with either Res + TPA or UA + TPA alone. Further analyses revealed that the greater ability of the combination to inhibit skin tumor promotion correlated with a greater ability to inhibit epidermal proliferation induced by TPA. In addition, combined treatment with UA + Res produced greater inhibitory effects on TPA-induced epidermal signaling pathways including EGFR, STAT3, Fas, Src, Akt, Cox-2, NF-κB, p38 MAPK, and JNK1/2. Notably, treatment with the combination also increased the levels of the tumor suppressor proteins p21 and PDCD4 compared to the groups treated with the individual compounds. Both UA and Res treatment followed by TPA increased AMPK-α activation, however, the combination of UA + Res together with TPA produced an even greater activation of AMPK-α. Further studies revealed that the activation of NF-κB, Egr-1, and AP-1 (DNA binding activity and nuclear translocation) were significantly inhibited by the combination of UA + Res compared to the groups treated with either UA or Res alone. The combination also produced greater effect on TPA-induced inflammation and inflammatory gene expression. Overall, the current data indicate that combined treatment with UA + Res led to a greater inhibitory effect on skin tumor promotion than either compound alone via effects on multiple events and pathways critical to the process of skin tumor promotion.
As noted in the Introduction, UA was previously shown to have inhibitory effects on TPA-induced skin inflammation as well as skin carcinogenesis (2, 5). Res was also shown to be as an effective inhibitor on skin tumor promotion (5, 10, 13, 32) along with inhibitory effects on breast, colorectal, hepatic, pancreatic, and prostate cancers (11). Recently, several studies have shown potential combinatorial chemopreventive effects with these agents in preclinical models of cancer. For example, melatonin was shown to potentiate the inhibitory effect of UA on proliferation and apoptosis in colon cancer cells by modulating multiple signaling pathways including caspases, PARP, NF-κB and Cox-2 (33). Kowalczyk et al. (5) tested a combination of 2% calcium D-glucarate (CG) given in the diet, with either 2.5 µmol of Res or 1 µmol of UA applied topically in two-stage skin carcinogenesis model. In this study, UA applied alone and in combination with CG showed inhibitory effects on skin tumor incidence and multiplicity.
Combinations of Res with other phytochemicals have been shown to have a greater inhibitory effect in several tumor models. For example, combined dietary administration of Res, quercetin and catechin (combinations at 0.5, 5 or 25 mg/kg) reduced primary tumor growth of breast cancer xenografts in a nude mouse model (34). Res + black tea polyphenol inhibited mouse skin tumor growth by modulating MAPKs and p53 (32). In other studies, Res + curcumin produced a better chemopreventive effect by maintaining adequate zinc and regulating p21 and Cox-2 level during lung carcinogenesis (35). Genistein + Res also reduced the most severe grade of prostate cancer in the SV-40 tag rat (23).
As shown in the current study, the combination of UA + Res was a more effective inhibitor of skin tumor promotion by TPA in ICR mice than either agent alone at the dose used and this was true for both tumor multiplicity and tumor size (see again Fig. 1). Although we did not design the current studies to analyze the development of squamous cell carcinomas (SCCs), papillomas are considered premalignant tumors and previous studies have shown that reductions in numbers of papillomas leads to reductions in SCCs (27, 36, 37). As noted above, previous studies have shown that UA broadly inhibited a number of signaling pathways including EGFR, MAPK, Akt/mTOR, NF-κB, Cox-2 and STAT3 in a variety of cell types, including mouse epidermis in vivo (1, 4). Furthermore, mechanisms associated with the anti-tumor promoting effects of Res include modulation of NF-κB, Cox-2, mTORC1, and SirT1 (10, 13, 17, 18). In our current study, we evaluated a number of oncogenic signaling molecules including EGFR, Src, STAT3, Fas, NF-κB, Akt, p38, JNK1/2, c-Jun, and mTOR as well as the tumor suppressors p27, p21, PDCD4, and AMPK. As shown in Figs. 3 and 4 we found that the combination of UA + Res was more effective at altering these pathways during tumor promotion than either UA or Res given alone. In particular, the combination was significantly more effective at inhibiting TPA-induced activation (phosphorylation) of EGFR, Src and p38 MAPK and at altering the levels of Fas (decrease) and p-AMPK-αThr172 and p21 (increase) compared to either UA or Res given alone. In addition, the combination of UA + Res produced the greatest inhibition of NF-κB, Egr-1 and AP-1 DNA binding activities and nuclear translocation (supplemental Figs. 4 and 5). All three of these transcription factors are known to be upregulated during skin tumor promotion and skin carcinogenesis (38, 39). Thus, the combination of UA + Res produced a more global and robust inhibition of epidermal signaling pathways compared to either UA or Res given alone.
TPA-induced epidermal hyperproliferation is required for its tumor promoting activity (37, 39, 40). Previous studies have shown that topical treatment of both 1 µmol of UA and 2 µmol of Res reduced TPA-induced BrdU incorporation in SENCAR mouse skin (5). In the current study, we observed that the combination of UA + Res together with TPA at a dose of 2 µmol each inhibited epidermal hyperproliferation to a greater extent than with either compound alone at the same dose. The greater inhibition of TPA-induced epidermal hyperproliferation with the combination was likely due to the greater effects observed on the multiple signaling pathways noted above. Skin inflammation is also known to be an important component of the process of skin tumor promotion by TPA involving the production of pro-inflammatory cytokines and infiltration of inflammatory cells (39, 41, 42). Again, as seen with the analyses of epidermal proliferation, the combination produced greater inhibition of inflammation. In this regard, the combination of UA + Res inhibited to a greater extent the increased expression of IL-1α, IL-1β, IL-22 and Cox-2 seen following treatment with TPA. In addition, as shown in Fig. 5B, the number of mast cells was decreased by either UA or Res treatment followed by TPA, however, the combination gave rise to an even greater inhibitory effect. The number of lymphocytes, monocytes and leukocytes (CD45+ cells) in the dermis were also significantly inhibited by the combination of UA + Res compared to the individual compounds alone. The greater inhibition of inflammation by the combination of UA + Res was likely due to the greater inhibition of inflammatory signaling pathways and to a greater reduction in NF-κB DNA binding activity.
In conclusion, the current study shows for the first time the efficacy of a combination of UA + Res for inhibition of tumor promotion in mouse skin. This combination of UA + Res produced a greater inhibition of skin tumor promotion by TPA compared to UA or Res alone. In addition, the combination targeted multiple TPA-induced signaling pathways involved in both epidermal proliferation and inflammation and produced effects on a number of these pathways greater than either compound alone. For the current experiments we choose to apply the compounds via the topical route. An important goal for future studies will be to examine the efficacy of this and other combinations, when given in the diet. Combining phytochemicals such as UA and Res appears to produce a CR mimetic type of effect by targeting multiple signaling pathways and should be explored further for potential cancer chemopreventive efficacy.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by a Pilot Project grant from the Cancer Therapy and Research Center (CTRC), The University of Texas Health Sciences Center at San Antonio, The CTRC Cancer Center Support Grant (P30 CA054174) and NCI grant CA164159 (to J. DiGiovanni and T. J. Slaga).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Zang LL, Wu BN, Lin Y, Wang J, Fu L, Tang ZY. Research progress of ursolic acid's anti-tumor actions. Chin J Integr Med. 2014;20:72–79. doi: 10.1007/s11655-013-1541-4. [DOI] [PubMed] [Google Scholar]
- 2.Tokuda H, Ohigashi H, Koshimizu K, Ito Y. Inhibitory effects of ursolic and oleanolic acid on skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer letters. 1986;33:279–285. doi: 10.1016/0304-3835(86)90067-4. [DOI] [PubMed] [Google Scholar]
- 3.Banno N, Akihisa T, Tokuda H, Yasukawa K, Higashihara H, Ukiya M, et al. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Bioscience, biotechnology, and biochemistry. 2004;68:85–90. doi: 10.1271/bbb.68.85. [DOI] [PubMed] [Google Scholar]
- 4.Yadav VR, Prasad S, Sung B, Kannappan R, Aggarwal BB. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins. 2010;2:2428–2466. doi: 10.3390/toxins2102428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowalczyk MC, Junco JJ, Kowalczyk P, Tolstykh O, Hanausek M, Slaga TJ, et al. Effects of combined phytochemicals on skin tumorigenesis in SENCAR mice. International journal of oncology. 2013;43:911–918. doi: 10.3892/ijo.2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayaprakasam B, Olson LK, Schutzki RE, Tai MH, Nair MG. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas) Journal of agricultural and food chemistry. 2006;54:243–248. doi: 10.1021/jf0520342. [DOI] [PubMed] [Google Scholar]
- 7.Rao VS, de Melo CL, Queiroz MG, Lemos TL, Menezes DB, Melo TS, et al. Ursolic acid, a pentacyclic triterpene from Sambucus australis, prevents abdominal adiposity in mice fed a high-fat diet. Journal of medicinal food. 2011;14:1375–1382. doi: 10.1089/jmf.2010.0267. [DOI] [PubMed] [Google Scholar]
- 8.Kunkel SD, Elmore CJ, Bongers KS, Ebert SM, Fox DK, Dyle MC, et al. Ursolic acid increases skeletal muscle and brown fat and decreases diet-induced obesity, glucose intolerance and fatty liver disease. PloS one. 2012;7:e39332. doi: 10.1371/journal.pone.0039332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Y, Li Y, Zhao T, Wang Y, Sun C. Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through LKB1/AMPK pathway. PloS one. 2013;8:e70135. doi: 10.1371/journal.pone.0070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis. 2006;27:1465–1474. doi: 10.1093/carcin/bgi349. [DOI] [PubMed] [Google Scholar]
- 11.Carter LG, D'Orazio JA, Pearson KJ. Resveratrol and cancer: focus on in vivo evidence. Endocrine-related cancer. 2014;21:R209–R225. doi: 10.1530/ERC-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowalczyk MC, Kowalczyk P, Tolstykh O, Hanausek M, Walaszek Z, Slaga TJ. Synergistic effects of combined phytochemicals and skin cancer prevention in SENCAR mice. Cancer Prev Res (Phila) 2010;3:170–178. doi: 10.1158/1940-6207.CAPR-09-0196. [DOI] [PubMed] [Google Scholar]
- 13.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 14.Kundu JK, Chun KS, Kim SO, Surh YJ. Resveratrol inhibits phorbol ester-induced cyclooxygenase-2 expression in mouse skin: MAPKs and AP-1 as potential molecular targets. BioFactors. 2004;21:33–39. doi: 10.1002/biof.552210108. [DOI] [PubMed] [Google Scholar]
- 15.Blagosklonny MV. An anti-aging drug today: from senescence-promoting genes to anti-aging pill. Drug discovery today. 2007;12:218–224. doi: 10.1016/j.drudis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Das S, Das DK. Anti-inflammatory responses of resveratrol. Inflammation & allergy drug targets. 2007;6:168–173. doi: 10.2174/187152807781696464. [DOI] [PubMed] [Google Scholar]
- 17.Chung JH, Manganiello V, Dyck JR. Resveratrol as a calorie restriction mimetic: therapeutic implications. Trends in cell biology. 2012;22:546–554. doi: 10.1016/j.tcb.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boily G, He XH, Pearce B, Jardine K, McBurney MW. SirT1-null mice develop tumors at normal rates but are poorly protected by resveratrol. Oncogene. 2009;28:2882–2893. doi: 10.1038/onc.2009.147. [DOI] [PubMed] [Google Scholar]
- 19.Hwang JT, Lee YK, Shin JI, Park OJ. Anti-inflammatory and anticarcinogenic effect of genistein alone or in combination with capsaicin in TPA-treated rat mammary glands or mammary cancer cell line. Ann N Y Acad Sci. 2009;1171:415–420. doi: 10.1111/j.1749-6632.2009.04696.x. [DOI] [PubMed] [Google Scholar]
- 20.Xu G, Ren G, Xu X, Yuan H, Wang Z, Kang L, et al. Combination of curcumin and green tea catechins prevents dimethylhydrazine-induced colon carcinogenesis. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2010;48:390–395. doi: 10.1016/j.fct.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Khatiwada J, Verghese M, Davis S, Williams LL. Green tea, phytic acid, and inositol in combination reduced the incidence of azoxymethane-induced colon tumors in Fisher 344 male rats. Journal of medicinal food. 2011;14:1313–1320. doi: 10.1089/jmf.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amin AR, Wang D, Zhang H, Peng S, Shin HJ, Brandes JC, et al. Enhanced anti-tumor activity by the combination of the natural compounds (−)-epigallocatechin-3-gallate and luteolin: potential role of p53. The Journal of biological chemistry. 2010;285:34557–34565. doi: 10.1074/jbc.M110.141135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harper CE, Cook LM, Patel BB, Wang J, Eltoum IA, Arabshahi A, et al. Genistein and resveratrol, alone and in combination, suppress prostate cancer in SV-40 tag rats. The Prostate. 2009;69:1668–1682. doi: 10.1002/pros.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh TC, Wu JM. Targeting CWR22Rv1 prostate cancer cell proliferation and gene expression by combinations of the phytochemicals EGCG, genistein and quercetin. Anticancer research. 2009;29:4025–4032. [PMC free article] [PubMed] [Google Scholar]
- 25.Junco JJ, Mancha A, Malik G, Wei SJ, Kim DJ, Liang H, et al. Resveratrol and P-glycoprotein inhibitors enhance the anti-skin cancer effects of ursolic acid. Molecular cancer research : MCR. 2013;11:1521–1529. doi: 10.1158/1541-7786.MCR-13-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Checkley LA, Rho O, Moore T, Hursting S, DiGiovanni J. Rapamycin is a potent inhibitor of skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer prevention research. 2011;4:1011–1020. doi: 10.1158/1940-6207.CAPR-10-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Checkley LA, Rho O, Angel JM, Cho J, Blando J, Beltran L, et al. Metformin inhibits skin tumor promotion in overweight and obese mice. Cancer prevention research. 2014;7:54–64. doi: 10.1158/1940-6207.CAPR-13-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rho O, Kiguchi K, Jiang G, DiGiovanni J. Impact of mTORC1 inhibition on keratinocyte proliferation during skin tumor promotion in wild-type and BK5.AktWT mice. Molecular carcinogenesis. 2014;53:871–882. doi: 10.1002/mc.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozeman R, Abel EL, Macias E, Cheng T, Beltran L, Digiovanni J. A novel mechanism of skin tumor promotion involving interferon-gamma (IFNgamma)/signal transducer and activator of transcription-1 (Stat1) signaling. Molecular carcinogenesis. 2014 doi: 10.1002/mc.22132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao D, Macias E, Carbajal S, Kiguchi K, DiGiovanni J. Constitutive Stat3 activation alters behavior of hair follicle stem and progenitor cell populations. Molecular carcinogenesis. 2015;54:121–133. doi: 10.1002/mc.22080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu ZH, Shvartsman MB, Lee AY, Shao JM, Murray MM, Kladney RD, et al. Mammalian target of rapamycin activator RHEB is frequently overexpressed in human carcinomas and is critical and sufficient for skin epithelial carcinogenesis. Cancer research. 2010;70:3287–3298. doi: 10.1158/0008-5472.CAN-09-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George J, Singh M, Srivastava AK, Bhui K, Roy P, Chaturvedi PK, et al. Resveratrol and black tea polyphenol combination synergistically suppress mouse skin tumors growth by inhibition of activated MAPKs and p53. PloS one. 2011;6:e23395. doi: 10.1371/journal.pone.0023395. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Wang J, Guo W, Chen W, Yu W, Tian Y, Fu L, et al. Melatonin potentiates the antiproliferative and pro-apoptotic effects of ursolic acid in colon cancer cells by modulating multiple signaling pathways. Journal of pineal research. 2013;54:406–416. doi: 10.1111/jpi.12035. [DOI] [PubMed] [Google Scholar]
- 34.Schlachterman A, Valle F, Wall KM, Azios NG, Castillo L, Morell L, et al. Combined resveratrol, quercetin, and catechin treatment reduces breast tumor growth in a nude mouse model. Translational oncology. 2008;1:19–27. doi: 10.1593/tlo.07100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malhotra A, Nair P, Dhawan DK. Curcumin and resveratrol synergistically stimulate p21 and regulate cox-2 by maintaining adequate zinc levels during lung carcinogenesis. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation. 2011;20:411–416. doi: 10.1097/CEJ.0b013e3283481d71. [DOI] [PubMed] [Google Scholar]
- 36.Moore T, Beltran L, Carbajal S, Hursting SD, DiGiovanni J. Energy balance modulates mouse skin tumor promotion through altered IGF-1R and EGFR crosstalk. Cancer prevention research. 2012;5:1236–1246. doi: 10.1158/1940-6207.CAPR-12-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009;4:1350–1362. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riggs PK, Rho O, DiGiovanni J. Alteration of Egr-1 mRNA during multistage carcinogenesis in mouse skin. Molecular carcinogenesis. 2000;27:247–251. doi: 10.1002/(sici)1098-2744(200004)27:4<247::aid-mc1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Rundhaug JE, Fischer SM. Molecular mechanisms of mouse skin tumor promotion. Cancers. 2010;2:436–482. doi: 10.3390/cancers2020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacology & therapeutics. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 41.Mueller MM. Inflammation in epithelial skin tumours: old stories and new ideas. European journal of cancer. 2006;42:735–744. doi: 10.1016/j.ejca.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Fujiki H, Sueoka E, Suganuma M. Tumor promoters: from chemicals to inflammatory proteins. Journal of cancer research and clinical oncology. 2013;139:1603–1614. doi: 10.1007/s00432-013-1455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.