Abstract
Bone is a regenerative tissue, capable of healing itself after fractures. However, some circumstances such as critical size defects, malformations, and tumor destruction may exceed the skeleton’s capacity for self-repair. In addition, bone mass and strength decline with age, leading to an increase in fragility fractures. Therefore the ability to generate large numbers of patient-specific osteoblasts would have enormous clinical implications for the treatment of skeletal defects and diseases. This review will highlight recent advances in the derivation of pluripotent stem cells, and in their directed differentiation towards bone-forming osteoblasts.
Keywords: Bone, Skeleton Regeneration, Stem Cells
Introduction
The ancient Greek myth of Prometheus, forced to endure repeated destruction of his liver by an eagle as punishment for giving fire to humans, has underscored our enduring fascinating with organ regeneration. Recent advances in stem cell biology hold promise that tissue regeneration may soon transition from mythology into reality. Stem cells are unique in their ability to both self-renew and give rise to differentiated tissues, and thus represent an appealing cellular population for regenerative medicine. In this review I will focus on pluripotent stem cells and their potential applications in skeletal biology and regenerative medicine in murine and human models.
The direct use of stem cells in diseases affecting the skeleton, particularly in combination with tissue engineering, can be readily applied to critical defects in bone, for example as a result of trauma or tumor-mediated destruction. In contrast, osteoporosis is one of the most common degenerative diseases of aging, resulting in fragility fractures in 50% of women and 25% of men over the age of 50, but characterized by diffuse bone loss. Comprised of over 206 discrete and uniquely shaped elements, the skeleton presents unique challenges to efforts aimed at the regenerative process. However, stem cells may prove useful in disease modeling and drug screening to identify pathways and compounds that effective increase bone mass or strength.
Within the bone marrow, mesenchymal stem cells can give rise to osteoblasts, adipocytes, and chondrocytes among other tissues of mesodermal origin (1-4). When needed, as in the case of fracture healing osteoblast progenitors can rapidly expand and form new bone. While adult bone marrow MSCs may be an appealing source of patient-derived osteoblasts, in reality their use for clinical applications is limited by low frequencies within the bone marrow, the need for invasive acquisition by bone marrow biopsy, limited self-renewal potential, and decreasing numbers and differentiation capacity with age (5). In recent decades the ability to derive and differentiate pluripotent stem cells has generated great excitement as a potential limitless source of cells and tissues for regenerative medicine. Here I will review the generation of murine and human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells, and their potential applications in skeletal diseases.
Embryonic stem cells
Embryonic stem cells are derived from the inner cell mass of the blastocyst, and are self-renewing and pluripotent. Mouse ES cells were first described in 1981 (6, 7), and combined with homologous recombination technology have made possible the generation of genetically engineered mice that have revolutionized disease modeling in vertebrate organisms. Human ES cells were first isolated in 1998 (8). The ability of ES cells to contribute to any tissue makes them ideal for regenerative purposes, and intensive efforts have focused on differentiating ES cells into various tissues. However, human ES cells in particular have the severe limitation that their derivation requires the destruction of human embryos, and it is not possible to generate patient-specific lines. This potentially limits their utility in cell transplant, although some have proposed that by generating banks of HLA-typed ES lines it would be possible to match most recipients (9).
A key component of any regenerative strategy involving stem cells is directed differentiation into the tissue of choice. As a model system for understanding lineage commitment and differentiation, embryonic stem cells have proven invaluable. Insights from the embryology and developmental biology have been used to direct the induction of endoderm, mesoderm, and ectoderm in differentiating ES cells (reviewed in (10)), with reports of directed differentiation into a variety of tissues including neurons (11-13), cardiomyocytes (14, 15), and pancreatic progenitors (16).
Directed differentiation of ES cells into osteoblasts
The earliest reports of directed differentiation of murine (5, 17) and human (18) ES cells into osteoblasts relied on embryoid body formation, in which ES cells are aggregated into clusters, usually in hanging droplets, to induce tri-germ layer differentiation. After several days in culture embryoid bodies are disassociated and plated in osteogenic medium, typically consisting of ascorbic acid (AA) and an organic phosphate source such as β-glycerophosphate (βGP) (Table 1). Additional factors such as dexamethasone, retinoic acid (RA), bone morphogenetic proteins (BMPs) and vitamin D3 (VD3) have variously been reported to enhance osteogenic differentiation of ES cells (19-23). Early studies relied predominantly on in vitro assays of osteogenic differentiation, including mineral deposition, alkaline phosphatase activity, and expression of markers of osteoblast differentiation (5, 17, 18, 20, 22, 23). However, these assays can be positive even when only a small percentage of cells have differentiated into the osteoblast lineage, and EB differentiation typically results in marked heterogeneity containing cells of multiple lineages. Therefore measuring the efficiency of osteogenic differentiation is challenging. Use of flow cytometry for cell surface markers of osteoblast lineage cells is one approach, however such markers must often used in combination, and vary among research groups (3). Another strategy is the use of fluorescent reporters targeted to the osteoblast lineage. Xin et al. used zinc finger nuclease technology to direct the osteoblast-specific fluorescent reporter Col2.3GFP to a “safe harbor” site in human ES cells (24). This now enables the use of flow cytometry to quantitate GFP+ cells that have differentiated into the osteoblast lineage.
Table 1.
Osteogenic differentiation of embryonic stem cells.
| Embryoid body (EB) vs monolayer (ML) |
Factors tested |
Additional conditions (eg bioreactor) |
Assays (mineral deposition, gene expression, implant) |
Reference |
|---|---|---|---|---|
| Mouse | ||||
| EB | AA, βGP, dex |
|
(5) | |
| EB | AA, βGP, RA, BMP2 |
|
(70) | |
| EB | AA, βGP, VD3 |
|
(22) | |
| EB | AA, βGP, dex |
|
(20) | |
| EB | AA, βGP, VD3, RA, BMP2 |
|
(23) | |
| ESCs seeded on scaffold to form cartilage matrix |
|
(25) | ||
| EB | AA, βGP, dex | Decellularized osteogenic extracellular matrix |
|
(26) |
| ML | AA, βGP, dex | CultiSpher S microcarrier |
|
(29) |
| EB | AA, βGP, dex |
|
(88) | |
| EB | AA, βGP, VD3 | 3D type I collagen gel |
|
(30) |
| EB | AA, βGP, dex, PPARγ inhibitor, RA |
|
(89) | |
| EB | AA, βGP, VD3 | Cyclic loading |
|
(32) |
| EB | AA, βGP, dex, BMP4 |
|
(21) | |
| Human | ||||
| EB | AA, βGP, dex |
|
(18) | |
| EB | AA, βGP, dex |
|
(19) | |
| ML | Various | HA/TCP particles or carpet |
|
(31) |
| EB | Lentiviral expression of Runx2 and BMP-2- enriched hydrogel |
|
(90) | |
| ML | AA, βGP, dex | Decellularized bone scaffolds and perfused bioreactor culture |
|
(27) |
| ML | AA, βGP, dex | Mechanical strain on BioFlex plates |
|
(33) |
| ML | AA, βGP | Decellularized osteogenic matrix on PLGA scaffold |
|
(28) |
| ML | Zinc finger nuclease targeting of Col2.3GFP and hydroxyapatite /collagen matrix scaffold |
|
(24) | |
Abbreviations: AA – ascorbic acid; βGP – β-glycerophosphate; RA – retinoic acid; VD3 – 1,25OH-vitamin D3; PDLLA – poly-D, L-lactide; ALP – alkaline phosphatase; BMSC – bone marrow stromal cell; PLGA – poly(lactic-co-glycolic acid); HA/TCP – hydroxyapatite/tricalcium phosphate
To further increase the efficiency of osteogenic differentiation of ES cells, several groups have examined the use of 3-dimensional scaffolds in culture. Jukes et al. differentiated ES cells into a cartilage matrix to recapitulate endochondral ossification, and when these matrices were transplanted into critical-size cranial defects in rats, they underwent cartilage hypertrophy, calcification, and ultimately replacement by bone (25). Other scaffolds that have been tested include decellularized osteoblast-derived extracellular matrix alone (26, 27) or on a PLGA scaffold (28), microcarriers (29), type I collagen gel (30), and hydroxyapatite/tricalcium phosphate (HA/TCP) (24, 31). Furthermore, investigators have tried to simulate mechanical loading in culture with approaches such as cyclic loading in a compression chamber (32) or culture on BioFlex plates (33). In the future a combination of these approaches will likely further enhance our ability to differentiate ES cells into osteoblasts.
As a word of caution, as mentioned above osteogenic differentiation of ES cells is frequently assessed by in vitro assays such as mineral deposition and expression of osteoblast markers. However, in vitro assays do not reliably predict bone formation in vivo (31), therefore osteogenic capacity is ideally evaluated in vivo. The most common in vivo assays of bone formation are subcutaneous implantation (usually combined with a carrier) in immunocompromised mice (19, 27, 29, 31, 34), or healing of a critical-size defect in the calvaria (24, 25) or burr-hole fracture in mice (29, 30).
Induced pluripotent stem cells
In 2006, Shinya Yamanaka reported the astonishing finding that introduction of only 4 factors – Oct3/4, Sox2, c-Myc, and Klf4 – was sufficient to convert murine fibroblasts into pluripotent stem cells (35), termed induced pluripotent stem (iPS) cells. The following year following the derivation of iPS cells from human somatic cells was reported using OCT4, SOX2, NANOG and LIN28 (36). These cells resemble ES cells in morphology and growth kinetics, and as confirmation of pluripotency form teratomas in vivo and contribute to chimeric embryos when injected into blastocysts. The ability to derive such induced pluripotent stem (iPS) cells from easily accessible somatic cells has transformed stem cell biology – it is now possible to generate unlimited numbers of patient-matched stem cells for disease modeling, drug screening, and regenerative therapies – and for this discovery Dr. Yamanaka was awarded the 2012 Nobel Prize in Physiology or Medicine.
Since then there have been significant technical advances focused on increasing the safety of pluripotency factor delivery methods (reviewed in (37, 38)). Particularly when considering applications involving cells as regenerative therapy in humans, the importance of inducing pluripotency without permanent integration of genetic elements, as might occur with viral transduction, is paramount (39). To that end, recent promising studies have focused on delivery via episomal plasmids (40) or as recombinant proteins (41). In early studies the efficiency of pluripotency induction was very low, typically less than 1%; while reprogramming is likely initiated in a much higher percentage of somatic cells expressing the pluripotency factor, most fail to achieve a pluripotent state (38). A variety of small molecules targeting epigenetic modifiers, MAPK, Wnt and TGF-β signaling pathways have been reported to enhance efficiency of pluripotency induction (42-46).
From the first reports of iPS cells, there has been great interest in how similar they truly are to ES cells, and in a related vein, whether iPS cells derived from different somatic cell sources retain an epigenetic “memory” of their cells of origin. Initial reports focused on the similarities between iPS and ES cells; perhaps not surprisingly, these were then followed by reports that iPS and ES cells have differential gene expression (47) and DNA methylation (48, 49). Several groups have noted persistent donor cell gene expression in iPS cells (50, 51) and epigenetic memories of donor cells (34, 52, 53). However, these studies were generally performed with small numbers of iPS and ES cell lines. More recently researchers have noted that when large numbers of ES and iPS cell lines are compared, it becomes more difficult to distinguish differences between pluripotent cell lines (54-56) due to high inherent variability (reviewed in Yamanaka 2012).
Unfettered by the ethical and logistical constraints that overshadow the isolation of embryonic stem cells, induced pluripotent stem (iPS) cells can be derived from a wide array of tissues. From the first reports of patient-derived iPS cells (57, 58), iPS technology has enabled the generation of disease-specific stem cell lines that can be used for studying disease pathogenesis as well as screening for novel therapeutic targets. Combined with gene editing techniques, there is hope that iPS cells can also be used for regenerative and curative therapies (reviewed in (59)). Already in mice researchers have reported the treatment of sickle cell anemia and Fanconi anemia using iPS cells (60, 61).
Highlighting the potential of iPS cell modeling to study skeletal diseases, Matsumoto et al. generated iPS cells from skin fibroblasts of patients with fibrodysplasia ossificans progressiva (FOP). When induced to undergo endochondral development, FOP-iPS cells demonstrated increased mineral deposition and enhanced chondrogenesis in vitro. (62). iPS cells have also been used to investigate the molecular mechanisms underlying osteosarcoma development in patients with Li-Fraumeni familial cancer syndrome, and identified dysregulation of the imprinted gene H19 during osteogenesis as a key factor (63). Other potential skeletal diseases amenable to iPS cell modeling include fibrous dysplasia/McCune Albright syndrome and osteogenesis imperfecta. For monogenic diseases, gene editing approaches may one day provide a source of healthy replacement cells. For any potential cell-based therapy involving iPS cells, several safety issues remain to be resolved including risks of teratoma or other neoplasm formation, immunogenicity of transplanted cells, and heterotopic differentiation (reviewed in (64)).
Directed differentiation of iPS cells into osteoblasts
Both murine (65, 66) and human (67) iPS cells have been differentiated into osteoblasts, following protocols similar to those used to direct ES cells into the osteoblast lineage (Table 2) (reviewed in (37)). Some groups have relied on differentiation into mesenchymal stem cell (MSC) intermediates followed by osteogenic differentiation (65, 67). In addition to AA, βGP and dexamethasone, other factors that have been studied for their abilities to improve the differentiation of iPS cells into osteoblasts include RA (65, 66, 68-70), transforming growth factor β (TGF-β) (70-73), fibroblast growth factors (FGFs) (70, 71, 73) and BMPs (70, 73). Adenoviral expression of the osteoblast transcription factor Runx2 can also increase osteoblast differentiation from iPS cells (66). More recently, Kanke et al. developed a stepwise approach based on serum-free monolayer culture without EB formation, using small molecule inducers to direct iPS cells first into mesodermal intermediates followed by skeletal progenitors and then osteoblasts with high yield (74). As with ES cells, in vitro assays of osteogenic differentiation do not predict successful formation of bone in vivo (70), therefore iPS-derived osteoblasts should also be tested by in vivo assays of bone formation such as subcutaneous implantation in syngeneic (68) or immunocompromised (70, 75, 76) mice. In another model, iPS-derived MSCs have been used to rescue a murine model of hind-limb ischemia (67).
Table 2.
Osteogenic differentiation of induced pluripotent stem cells.
| Embryoid body (EB) vs monolayer (ML) |
Factors tested |
Additional conditions (eg bioreactor) |
Assays (mineral deposition, gene expression, implant) |
Reference |
|---|---|---|---|---|
| Mouse | ||||
| EB | RA, AA, βGP, dex |
Adenoviral expression of Runx2 |
|
(66) |
| EB | RA, TGF-b1, AA, βGP, dex |
|
(65) | |
| EB | RA, AA, βGP, dex |
Gelfoam sponges |
|
(68) |
| EB | RA, AA, βGP, dex |
|
(69) | |
| Human | ||||
| iPSC-derived MSCs |
|
(67) | ||
| ML | AA, βGP, dex | Decellularized bone scaffolds and perfused bioreactor culture |
|
(75) |
| AA, βGP, dex | PCL scaffold |
|
(76) | |
| EB | AA, βGP, dex FGF-2, TGF- β1, BMP-2/7 |
|
(73) | |
| ML | Various | HA/TCP particles or carpet |
|
(70) |
| EB | AA, βGP, dex, VD3 |
Calcium phosphate cement scaffold |
|
(91) |
| EB | AA, βGP, dex FGF-2, IGF-1, TGF-β |
|
(71) | |
TNAP – tissue-nonspecific alkaline phosphatase; PCL – poly(caprolactone); MSC – mesenchymal stem cell
Direct reprogramming
While iPS cells hold great promise given the ability to derive patient-matched cells as a source for regenerative therapy, the low efficiency of iPS cell derivation from specific patients, potential risk of teratoma formation, and protracted time required for directed differentiation into tissues of choice are still practical limitations. The discovery of MyoD demonstrated that a single master transcription factor had the ability to directly convert fibroblasts into myoblasts (77). Since then there has been great interest in identifying master transcription factors for other lineages; now with the understanding that induction of pluripotency requires multiple transcription factors, investigators have begun to identify combinatorial factors that can directly reprogram one differentiated cell type into another without the need for a pluripotent intermediate (38). The cell of origin typically shares a common developmental origin with the target cell (reviewed in (78)). In 2008 the conversion of exocrine to endocrine pancreas cells was accomplished with 3 factors (79). This has been followed by protocols for reprogramming fibroblasts into neuronal cells (80), hepatocytes (81), cardiac myocytes (82), and hematopoietic progenitors (83). More recent studies have demonstrated direct reprogramming in vivo in mice to generate pancreatic beta cells (79), cardiomyocytes (84, 85), and neurons (86, 87). To date, there have not been any studies of directed reprogramming into osteoblasts. However, it is reasonable to expect that a combination of transcription factors, likely including Runx2, required for osteoblast differentiation, can be similarly identified to reprogram cells into osteoblasts.
Conclusions
Recent advances in the study of pluripotent stem cells may soon enable the generation of unlimited numbers of osteoblasts. Induced pluripotency and direct reprogramming further provide the opportunity to derive disease-specific and patient-matched cell lines that have tremendous potential for regenerative approaches to skeletal disease, in addition to understanding disease pathogenesis and screening for novel therapeutic compounds. Further improvements in differentiation efficiency and tissue engineering will likely accelerate this translational process. Several issues remain to be clarified, including the ideal cell types for reprogramming, as well as safety from teratoma formation.
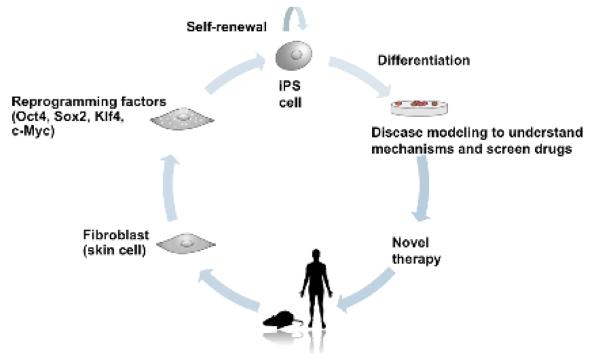
Figure 1. Derivation and applications of induced pluripotent stem cells.
iPS cells can be derived from patient-derived somatic cells by the introduction of the reprogramming factors Oct4, Sox2, Klf4 and c-Myc. Self-renewing iPS cells can then be directed to differentiate into cell lineages of choice, and can be used for either disease modeling or drug screening, or as cell-based regenerative therapies. Figure courtesy of James Oh.
Acknowledgments
Conflict of Interest
Dr. Joy Y. Wu has received research support from the National Institute of Health and was funded by NIH grant OD008466.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
All studies by Joy Y. Wu involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19(3):180–92. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5):267–74. [PubMed] [Google Scholar]
- 3.Panaroni C, Tzeng YS, Saeed H, Wu JY. Mesenchymal progenitors and the osteoblast lineage in bone marrow hematopoietic niches. Curr Osteoporos Rep. 2014;12(1):22–32. doi: 10.1007/s11914-014-0190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Buttery LD, Bourne S, Xynos JD, Wood H, Hughes FJ, Hughes SP, Episkopou V, Polak JM. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 2001;7(1):89–99. doi: 10.1089/107632700300003323. [DOI] [PubMed] [Google Scholar]
- 6.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 7.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78(12):7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 9.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137(1):13–7. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–80. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172(2):383–97. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- 12.Schuldiner M, Eiges R, Eden A, Yanuka O, Itskovitz-Eldor J, Goldstein RS, Benvenisty N. Induced neuronal differentiation of human embryonic stem cells. Brain Res. 2001;913(2):201–5. doi: 10.1016/s0006-8993(01)02776-7. [DOI] [PubMed] [Google Scholar]
- 13.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19(12):1129–33. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 14.Kehat I, Gepstein A, Spira A, Itskovitz-Eldor J, Gepstein L. High-resolution electrophysiological assessment of human embryonic stem cell-derived cardiomyocytes: a novel in vitro model for the study of conduction. Circ Res. 2002;91(8):659–61. doi: 10.1161/01.res.0000039084.30342.9b. [DOI] [PubMed] [Google Scholar]
- 15.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91(6):501–8. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 16.Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50(8):1691–7. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- 17.Phillips BW, Belmonte N, Vernochet C, Ailhaud G, Dani C. Compactin enhances osteogenesis in murine embryonic stem cells. Biochem Biophys Res Commun. 2001;284(2):478–84. doi: 10.1006/bbrc.2001.4987. [DOI] [PubMed] [Google Scholar]
- 18.Sottile V, Thomson A, McWhir J. In vitro osteogenic differentiation of human ES cells. Cloning Stem Cells. 2003;5(2):149–55. doi: 10.1089/153623003322234759. [DOI] [PubMed] [Google Scholar]
- 19.Bielby RC, Boccaccini AR, Polak JM, Buttery LD. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 2004;10(9-10):1518–25. doi: 10.1089/ten.2004.10.1518. [DOI] [PubMed] [Google Scholar]
- 20.Bourne S, Polak JM, Hughes SP, Buttery LD. Osteogenic differentiation of mouse embryonic stem cells: differential gene expression analysis by cDNA microarray and purification of osteoblasts by cadherin-11 magnetically activated cell sorting. Tissue Eng. 2004;10(5-6):796–806. doi: 10.1089/1076327041348293. [DOI] [PubMed] [Google Scholar]
- 21.Camargos BM, Tavares RL, Del Puerto HL, Andrade LO, Camargos AF, Reis FM. BMP-4 increases activin A gene expression during osteogenic differentiation of mouse embryonic stem cells. Growth Factors. 2015;33(2):133–8. doi: 10.3109/08977194.2014.984805. [DOI] [PubMed] [Google Scholar]
- 22.zur Nieden NI, Kempka G, Ahr HJ. In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation. 2003;71(1):18–27. doi: 10.1046/j.1432-0436.2003.700602.x. [DOI] [PubMed] [Google Scholar]
- 23.zur Nieden NI, Price FD, Davis LA, Everitt RE, Rancourt DE. Gene profiling on mixed embryonic stem cell populations reveals a biphasic role for beta-catenin in osteogenic differentiation. Mol Endocrinol. 2007;21(3):674–85. doi: 10.1210/me.2005-0438. [DOI] [PubMed] [Google Scholar]
- 24.Xin X, Jiang X, Wang L, Stover ML, Zhan S, Huang J, Goldberg AJ, Liu Y, Kuhn L, Reichenberger EJ, et al. A Site-Specific Integrated Col2.3GFP Reporter Identifies Osteoblasts Within Mineralized Tissue Formed In Vivo by Human Embryonic Stem Cells. Stem Cells Transl Med. 2014;3(10):1125–37. doi: 10.5966/sctm.2013-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jukes JM, Both SK, Leusink A, Sterk LM, van Blitterswijk CA, de Boer J. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105(19):6840–5. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans ND, Gentleman E, Chen X, Roberts CJ, Polak JM, Stevens MM. Extracellular matrix-mediated osteogenic differentiation of murine embryonic stem cells. Biomaterials. 2010;31(12):3244–52. doi: 10.1016/j.biomaterials.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 27 •.Marolt D, Campos IM, Bhumiratana S, Koren A, Petridis P, Zhang G, Spitalnik PF, Grayson WL, Vunjak-Novakovic G. Engineering bone tissue from human embryonic stem cells. Proc Natl Acad Sci U S A. 2012;109(22):8705–9. doi: 10.1073/pnas.1201830109. This article reported the use of tissue engineering techniques to promote bone formation from ES cells.
- 28.Rutledge K, Cheng Q, Pryzhkova M, Harris GM, Jabbarzadeh E. Enhanced differentiation of human embryonic stem cells on extracellular matrix-containing osteomimetic scaffolds for bone tissue engineering. Tissue Eng Part C Methods. 2014;20(11):865–74. doi: 10.1089/ten.tec.2013.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alfred R, Taiani JT, Krawetz RJ, Yamashita A, Rancourt DE, Kallos MS. Large-scale production of murine embryonic stem cell-derived osteoblasts and chondrocytes on microcarriers in serum-free media. Biomaterials. 2011;32(26):6006–16. doi: 10.1016/j.biomaterials.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Taiani JT, Krawetz RJ, Yamashita A, Pauchard Y, Buie HR, Ponjevic D, Boyd SK, Rancourt DE, Matyas JR. Embryonic stem cells incorporate into newly formed bone and do not form tumors in an immunocompetent mouse fracture model. Cell Transplant. 2013;22(8):1453–62. doi: 10.3727/096368912X658755. [DOI] [PubMed] [Google Scholar]
- 31.Kuznetsov SA, Cherman N, Robey PG. In vivo bone formation by progeny of human embryonic stem cells. Stem Cells Dev. 2011;20(2):269–87. doi: 10.1089/scd.2009.0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehnes DD, Price FD, Shrive NG, Hart DA, Rancourt DE, Zur Nieden NI. Embryonic stem cell-derived osteocytes are capable of responding to mechanical oscillatory hydrostatic pressure. J Biomech. 2015 doi: 10.1016/j.jbiomech.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Li X, Meikle MC, Islam I, Cao T. Short periods of cyclic mechanical strain enhance triple-supplement directed osteogenesis and bone nodule formation by human embryonic stem cells in vitro. Tissue Eng Part A. 2013;19(19-20):2130–7. doi: 10.1089/ten.tea.2012.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, Huo H, Loh YH, Aryee MJ, Lensch MW, et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. 2011;29(12):1117–9. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 37.Illich DJ, Demir N, Stojkovic M, Scheer M, Rothamel D, Neugebauer J, Hescheler J, Zoller JE. Concise review: induced pluripotent stem cells and lineage reprogramming: prospects for bone regeneration. Stem Cells. 2011;29(4):555–63. doi: 10.1002/stem.611. [DOI] [PubMed] [Google Scholar]
- 38• •.Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10(6):678–84. doi: 10.1016/j.stem.2012.05.005. This review, authored by the discoverer of iPS cells, summarizes the history and potential applications of iPSC technology.
- 39.Schlaeger TM, Daheron L, Brickler TR, Entwisle S, Chan K, Cianci A, DeVine A, Ettenger A, Fitzgerald K, Godfrey M, et al. A comparison of non-integrating reprogramming methods. Nat Biotechnol. 2015;33(1):58–63. doi: 10.1038/nbt.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, et al. A more efficient method to generate integration-free human iPS cells. Nature methods. 2011;8(5):409–12. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 41.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–6. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, Muhlestein W, Melton DA. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26(11):1269–75. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 43.Ichida JK, Blanchard J, Lam K, Son EY, Chung JE, Egli D, Loh KM, Carter AC, Di Giorgio FP, Koszka K, et al. A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5(5):491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin T, Ambasudhan R, Yuan X, Li W, Hilcove S, Abujarour R, Lin X, Hahm HS, Hao E, Hayek A, et al. A chemical platform for improved induction of human iPSCs. Nature methods. 2009;6(11):805–8. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marson A, Foreman R, Chevalier B, Bilodeau S, Kahn M, Young RA, Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3(2):132–5. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi Y, Do JT, Desponts C, Hahm HS, Scholer HR, Ding S. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2(6):525–8. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5(1):111–23. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84(6):911–21. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 49.Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R, Herb B, Ladd-Acosta C, Rho J, Loewer S, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet. 2009;41(12):1350–3. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh Z, Wilson KD, Wu Y, Hu S, Quertermous T, Wu JC. Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PLoS One. 2010;5(2):e8975. doi: 10.1371/journal.pone.0008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marchetto MC, Yeo GW, Kainohana O, Marsala M, Gage FH, Muotri AR. Transcriptional signature and memory retention of human-induced pluripotent stem cells. PLoS One. 2009;4(9):e7076. doi: 10.1371/journal.pone.0007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lister R, Pelizzola M, Kida YS, Hawkins RD, Nery JR, Hon G, Antosiewicz-Bourget J, O’Malley R, Castanon R, Klugman S, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471(7336):68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, et al. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat Cell Biol. 2011;13(5):541–9. doi: 10.1038/ncb2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bock C, Kiskinis E, Verstappen G, Gu H, Boulting G, Smith ZD, Ziller M, Croft GF, Amoroso MW, Oakley DH, et al. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144(3):439–52. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guenther MG, Frampton GM, Soldner F, Hockemeyer D, Mitalipova M, Jaenisch R, Young RA. Chromatin structure and gene expression programs of human embryonic and induced pluripotent stem cells. Cell Stem Cell. 2010;7(2):249–57. doi: 10.1016/j.stem.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Newman AM, Cooper JB. Lab-specific gene expression signatures in pluripotent stem cells. Cell Stem Cell. 2010;7(2):258–62. doi: 10.1016/j.stem.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 57.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321(5893):1218–21. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 58.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877–86. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merkle FT, Eggan K. Modeling human disease with pluripotent stem cells: from genome association to function. Cell Stem Cell. 2013;12(6):656–68. doi: 10.1016/j.stem.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 60.Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318(5858):1920–3. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 61.Raya A, Rodriguez-Piza I, Guenechea G, Vassena R, Navarro S, Barrero MJ, Consiglio A, Castella M, Rio P, Sleep E, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460(7251):53–9. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsumoto Y, Hayashi Y, Schlieve CR, Ikeya M, Kim H, Nguyen TD, Sami S, Baba S, Barruet E, Nasu A, et al. Induced pluripotent stem cells from patients with human fibrodysplasia ossificans progressiva show increased mineralization and cartilage formation. Orphanet J Rare Dis. 2013;8(190) doi: 10.1186/1750-1172-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee DF, Su J, Kim HS, Chang B, Papatsenko D, Zhao R, Yuan Y, Gingold J, Xia W, Darr H, et al. Modeling familial cancer with induced pluripotent stem cells. Cell. 2015;161(2):240–54. doi: 10.1016/j.cell.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.Fox IJ, Daley GQ, Goldman SA, Huard J, Kamp TJ, Trucco M. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science. 2014;345(6199):1247391. doi: 10.1126/science.1247391. This article reviews the potential clinical applications of iPSC technology to regenerative medicine.
- 65.Li F, Bronson S, Niyibizi C. Derivation of murine induced pluripotent stem cells (iPS) and assessment of their differentiation toward osteogenic lineage. J Cell Biochem. 2010;109(4):643–52. doi: 10.1002/jcb.22440. [DOI] [PubMed] [Google Scholar]
- 66.Tashiro K, Inamura M, Kawabata K, Sakurai F, Yamanishi K, Hayakawa T, Mizuguchi H. Efficient adipocyte and osteoblast differentiation from mouse induced pluripotent stem cells by adenoviral transduction. Stem Cells. 2009;27(8):1802–11. doi: 10.1002/stem.108. [DOI] [PubMed] [Google Scholar]
- 67.Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121(9):1113–23. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 68.Bilousova G, Jun du H, King KB, De Langhe S, Chick WS, Torchia EC, Chow KS, Klemm DJ, Roop DR, Majka SM. Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vivo. Stem Cells. 2011;29(2):206–16. doi: 10.1002/stem.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Egusa H, Kayashima H, Miura J, Uraguchi S, Wang F, Okawa H, Sasaki J, Saeki M, Matsumoto T, Yatani H. Comparative analysis of mouse-induced pluripotent stem cells and mesenchymal stem cells during osteogenic differentiation in vitro. Stem Cells Dev. 2014;23(18):2156–69. doi: 10.1089/scd.2013.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Phillips MD, Kuznetsov SA, Cherman N, Park K, Chen KG, McClendon BN, Hamilton RS, McKay RD, Chenoweth JG, Mallon BS, et al. Directed differentiation of human induced pluripotent stem cells toward bone and cartilage: in vitro versus in vivo assays. Stem Cells Transl Med. 2014;3(7):867–78. doi: 10.5966/sctm.2013-0154. This article compared the osteogenic differentiation capacity of iPSCs from fibroblasts vs bone marrow stromal cells, assayed by both in vitro and in vivo methods.
- 71.Kato H, Ochiai-Shino H, Onodera S, Saito A, Shibahara T, Azuma T. Promoting effect of 1,25(OH)2 vitamin D3 in osteogenic differentiation from induced pluripotent stem cells to osteocyte-like cells. Open Biol. 2015;5(2):140201. doi: 10.1098/rsob.140201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li CH, Amar S. Inhibition of SFRP1 reduces severity of periodontitis. J Dent Res. 2007;86(9):873–7. doi: 10.1177/154405910708600913. [DOI] [PubMed] [Google Scholar]
- 73.Ochiai-Shino H, Kato H, Sawada T, Onodera S, Saito A, Takato T, Shibahara T, Muramatsu T, Azuma T. A novel strategy for enrichment and isolation of osteoprogenitor cells from induced pluripotent stem cells based on surface marker combination. PLoS One. 2014;9(6):e99534. doi: 10.1371/journal.pone.0099534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74 ••.Kanke K, Masaki H, Saito T, Komiyama Y, Hojo H, Nakauchi H, Lichtler AC, Takato T, Chung UI, Ohba S. Stepwise differentiation of pluripotent stem cells into osteoblasts using four small molecules under serum-free and feeder-free conditions. Stem Cell Reports. 2014;2(6):751–60. doi: 10.1016/j.stemcr.2014.04.016. This article reported the use of small molecule compounds to recapitulate the developmental stages of osteoblast differentiation starting with pluripotent stem cells.
- 75.de Peppo GM, Marcos-Campos I, Kahler DJ, Alsalman D, Shang L, Vunjak-Novakovic G, Marolt D. Engineering bone tissue substitutes from human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2013;110(21):8680–5. doi: 10.1073/pnas.1301190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin GZ, Kim TH, Kim JH, Won JE, Yoo SY, Choi SJ, Hyun JK, Kim HW. Bone tissue engineering of induced pluripotent stem cells cultured with macrochanneled polymer scaffold. J Biomed Mater Res A. 2013;101(5):1283–91. doi: 10.1002/jbm.a.34425. [DOI] [PubMed] [Google Scholar]
- 77.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 78.Xu J, Du Y, Deng H. Direct lineage reprogramming: strategies, mechanisms, and applications. Cell Stem Cell. 2015;16(2):119–34. doi: 10.1016/j.stem.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 79.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455(7213):627–32. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–41. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475(7356):386–9. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 82.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375–86. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szabo E, Rampalli S, Risueno RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468(7323):521–6. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 84.Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110(11):1465–73. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485(7400):599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14(2):188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, Bjorklund A, Grealish S, Parmar M. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci U S A. 2013;110(17):7038–43. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Evans ND, Swain RJ, Gentleman E, Gentleman MM, Stevens MM. Gene-expression analysis reveals that embryonic stem cells cultured under osteogenic conditions produce mineral non-specifically compared to marrow stromal cells or osteoblasts. Eur Cell Mater. 2012;24(211-23) doi: 10.22203/ecm.v024a15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu Y, Al-Mansoori L, Opas M. Optimized osteogenic differentiation protocol from R1 mouse embryonic stem cells in vitro. Differentiation. 2015;89(1-2):1–10. doi: 10.1016/j.diff.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 90.Kim MJ, Park JS, Kim S, Moon SH, Yang HN, Park KH, Chung HM. Encapsulation of bone morphogenic protein-2 with Cbfa1-overexpressing osteogenic cells derived from human embryonic stem cells in hydrogel accelerates bone tissue regeneration. Stem Cells Dev. 2011;20(8):1349–58. doi: 10.1089/scd.2010.0311. [DOI] [PubMed] [Google Scholar]
- 91.Tang M, Chen W, Liu J, Weir MD, Cheng L, Xu HH. Human induced pluripotent stem cell-derived mesenchymal stem cell seeding on calcium phosphate scaffold for bone regeneration. Tissue Eng Part A. 2014;20(7-8):1295–305. doi: 10.1089/ten.tea.2013.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]