Abstract
Wingless-type mouse mammary tumor virus integration site (WNT) signaling molecules are locally secreted glycoproteins that play a role in a number of physiological and pathological developmental processes. Components of the WNT signaling pathway have been demonstrated to impact reproductive functions including embryonic development of the sex organs, and regulation of follicle maturation controlling steroidogenesis in the postnatal ovary. Emerging evidence underscores the complexity of WNT signaling molecules in regulation of dynamic changes that occur in the ovary during the reproductive cycle. While disruption in the WNT signaling cascade has been recognized to have deleterious consequences to normal sexual development, more recent studies are beginning to highlight the importance of these molecules in adult ovarian function related to follicle development, corpus luteum formation, steroid production and fertility. Hormonal regulation of WNT genes and expression of members of the WNT signaling network, including WNT ligands, frizzled receptors and downstream signaling components that are expressed in the postnatal ovary at distinct stages of the estrous cycle, suggest a crucial role in normal ovarian function. Similarly, FSH stimulation of T cell factor-dependent gene expression requires input from β-catenin, a lynchpin molecule in canonical WNT signaling, further indicating β-catenin participation in regulation of follicle maturation. This review will focus on the multiple functions of WNT signaling in folliculogenesis in the adult ovary.
Introduction
The adult ovary is a dynamic organ undergoing constant changes throughout the estrous cycle as follicles progress from immature preantral follicles to more developed preovulatory follicles and eventually formation of the corpus luteum following ovulation. The multifaceted process of folliculogenesis relies on synchronized input of hormones exchanged between the hypothalamus, pituitary, and the gonads. While the initial stages of follicle development occur largely in the absence of gonadotropin input, transition from preantral to a preovulatory follicle occurs as a result of increased follicle-stimulating hormone (FSH) and luteinizing hormone (LH) responsiveness (Richards 1980) along with involvement of numerous other local hormones and growth factors (Findlay 1993, Monget & Bondy 2000).
The actions of the gonadotropins are also dependent on other signaling pathways and a diverse set of intraovarian factors expressed in cell specific manner at defined stages of follicular growth (Richards et al. 2002a). One more recently identified regulator of ovarian function is the wingless-type mouse mammary tumor integration site family (WNT) of signaling molecules. WNTs are highly conserved signaling molecules that act through β-catenin dependent and β-catenin independent pathways to regulate important processes of cellular growth and differentiation including cell proliferation, cell fate specifications, embryonic induction and the generation of cell polarity (Cadigan & Nusse 1997, Miller et al. 1999, Komiya & Habas 2008). Misregulation of WNT signal transduction can lead to a variety of pathologies including development of carcinomas of the breast, colon, skin, and ovary (Polakis 2000, Giles et al. 2003, Logan & Nusse 2004, Boerboom et al. 2005). The foundational study establishing a requirement of WNT signaling molecules in the female ovary was performed by Vainio et al. (1999). This group utilized mice null for Wnt4 to demonstrate a role for this molecule in early ovarian development and suppression of the male reproductive tract. Wnt4 null females have sex-reversed ovaries that express genes associated with testicular development, along with a reduced number of oocytes at birth. Evaluation of Wnt4 in the postnatal ovary using this mouse model was not possible as the homozygous mutation results in death shortly after birth due to renal failure. Subsequent work aimed at elucidating the importance of WNT signaling in the postnatal ovary has identified multiple Wnt/WNT family member transcripts expressed at specific stages of follicle development within the adult ovary of mice, rats, humans and cattle (Hsieh et al. 2002, Ricken et al. 2002, Wang et al. 2009, Gupta et al. 2014). In addition, functional studies in the adult ovary have shown a fundamental requirement of WNT signaling for normal ovarian function and fertility. Though our understanding of contributions of WNT signaling to the regulation of folliculogenesis has grown tremendously in recent years, much still remains unknown about the broader physiological involvement of WNT signaling in the adult ovary. This review will focus on the role of WNT ligands, downstream signaling molecules, and their interaction with various hormones in the maturation of the ovarian follicle.
WNT Signaling
The WNT signaling pathway is a conserved pathway among many species that controls numerous developmental processes as well as disease states. WNTs can initiate three separate signal transduction cascades through interaction of the ligand with their cognate frizzled (FZ) receptor. Most mammalian genomes are comprised of 19 structurally related Wnt genes (Logan & Nusse 2004) which encode secreted glycoproteins that interact with a large extracellular cysteine-rich domain on FZ seven-transmembrane receptors (Bhanot et al. 1996, Dann et al. 2001). In general, WNT proteins range in length from 350–400 amino acids, and are approximately 40 kDa in size (Cadigan & Nusse 1997, Clevers & Nusse 2012). WNTs contain 20% to 85% identity among species and are defined by their nearly identical primary sequence that contains 23–24 specifically spaced cysteine residues (Cadigan & Nusse 1997, Miller 2002). While WNTs have been classified as morphogens capable of specifying cell fate in a concentration dependent manner, in most contexts they are short-range molecules acting predominately on cells that are close to each other (Christian 2000, Sato et al. 2011, Strand & Micchelli 2011). The paracrine or autocrine quality of WNTs is likely reflective of the low (~200 ng/mL) expression levels of these proteins (Willert et al. 2003).
The activity of WNT signaling is dependent on cellular context and the particular combination in which the more than 15 receptor and co-receptors are expressed (reviewed in Niehrs (2012)). The ten FZ proteins are membrane bound receptors belonging to the G-protein coupled receptor family (Slusarski et al. 1997, Liu et al. 2001, Foord et al. 2005, Bjarnadottir et al. 2006) and are thought to bind to WNT proteins promiscuously. Frizzled proteins contain a conserved 120-amino acid cysteine-rich domain (CRD) which mediates the binding of WNT ligands (MacDonald & He 2012) with nanomolar affinity (Kd of 1–10 nM) (Hsieh et al. 1999, Rulifson et al. 2000, Wu & Nusse 2002). Differences in affinity of specific WNTs with different FZ may determine which signaling branch is activated (He et al. 1997). Transduction of a WNT signal involves an interaction between WNT and FZ as well as cooperation with single pass coreceptors, low-density lipoprotein receptor-related protein 5 or 6 (LRP 5/6) or receptor tyrosine kinase-like orphan receptor 1 or 2 (ROR 1/2), to direct β-catenin dependent or β-catenin independent pathways, respectively. The main WNT signaling pathways include the canonical WNT/β-catenin (β-catenin dependent) and non-canonical (β-catenin independent) planar cell polarity, and WNT/Ca2+ pathways. Upon binding of WNT to the FZ/co-receptor complex the signal is relayed to the downstream cytoplasmic phosphoprotein dishevelled (DVL) which is pivotal in all three pathways (Boutros & Mlodzik 1999, Sheldahl et al. 2003).
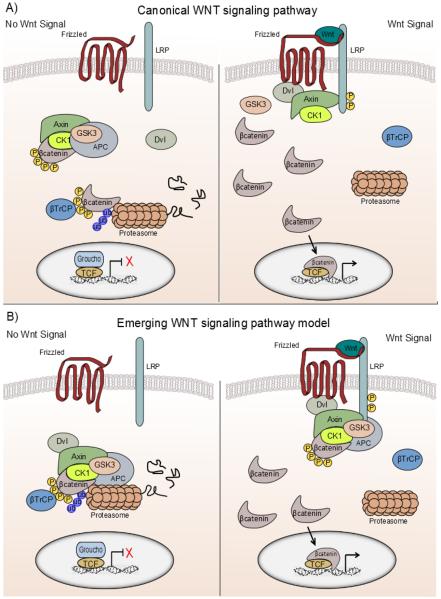
The most extensively dissected and therefore the best understood WNT pathway is the canonical WNT signaling cascade that signals through the transcriptional co-factor, β-catenin to regulate gene expression. In addition to the WNT/FZ complex, the canonical WNT/β-catenin pathway also requires the presence of a single-span transmembrane molecule identified in vertebrates as LRP5/6 (Pinson et al. 2000) to relay a signal. The prevailing view regarding the mechanism regulating cytoplasmic β-catenin has been that in the absence of WNT ligand, constitutively active casein kinase-1 (CK1) and glycogen synthase kinase-3 β(GSK3β) phosphorylate β-catenin, captured by the degradation complex, at four specific serine and threonine residues (Ser33, Ser37, Thr41, Ser45) in the N-terminal region (Liu et al. 2002) targeting β-catenin for ubiquitination and degradation by the proteasome (Figure 1A) (Aberle et al. 1997). Interaction of WNT and FZ/LRP receptors promotes hyperphosphorylation of DVL, and inhibits the β-catenin degradation complex made up of adenomatous polyposis coli (APC), GSK3β and the scaffold protein AXIN1, effectively blocking phosphorylation and degradation of cytoplasmic β-catenin. Activation of the receptor complex promotes recruitment of AXIN1 protein to the phosphorylated tail of LRP (Tamai et al. 2004). The 200 amino acid LRP5/6 cytoplasmic domain contains five PPPSPxS motifs that are conserved from invertebrates to humans (MacDonald & He 2012). WNT-mediated phosphorylation of LRP5/6 at the PPPSPxS motif occurs via GSK3β and CK1 to provide a docking site for AXIN1 (Davidson et al. 2005, Zeng et al. 2005). Association of AXIN1 with LRP was thought to facilitate disassociation of the degradation complex, resulting in stabilized β-catenin. Emerging data evaluating endogenous destruction complex components changes this view slightly (Li et al. 2012). In the absence of a WNT signal, the cytoplasmic degradation complex binds and phosphorylates β-catenin. Within the complex, β-TrCP subsequently ubiquitinates phosphorylated β-catenin thereby removing it from the complex by proteosomal degradation. In this model, β-catenin phosphorylation, ubiquitination, and degradation by the proteasome are all occurring within the AXIN1 degradation complex without physical disassociation of the complex (Li et al. 2012). This alternate model also demonstrates that in the presence of a WNT ligand, the complex remains largely intact showing only the disassociation of β-TrCP as AXIN1 binds to phosphorylated LRP. The degradation complex continues to bind and phosphorylate β-catenin but ubiquitination and degradation do no occur without the presence of β-TrCP. Phosphorylated β-catenin within the complex saturates and inactivates the degradation complex allowing newly synthesized, nonphosphorylated β-catenin to accumulate (Li et al. 2012) (Figure 1B). Interestingly, others have also suggested that only newly synthesized β-catenin is able to transduce a signal (Staal et al. 2002). Cytoplasmic β-catenin then translocates to the nucleus to activate transcription by displacing transcriptional repressors such as Groucho (Cavallo et al. 1998, Cinnamon & Paroush 2008) and associating with the T-cell factor (TCF)/lymphoid enhancer binding factor (LEF) family of transcription factors to alter target gene transcription (Molenaar et al. 1996, Riese et al. 1997, Behrens et al. 1998). Though the presence of many WNT signaling pathway components have been identified in the adult ovary of rodents and more recently in bovine, many questions remain regarding their mechanistic role in ovarian follicle development.
Figure 1. A new model for regulation of β-catenin in canonical WNT signaling pathway is emerging.
This overview provides comparisons and contrasts between the current model and the emerging model. A) The prevailing dogma for canonical WNT signaling denotes that in absence of a WNT signal, β-catenin is phosphorylated at N-terminal sites by the multi-protein degradation complex. Phosphorylated β-catenin is targeted for ubiquitination and subsequent degradation by the proteasome. WNT binding to the frizzled/LRP co-receptor complex promotes association of AXIN1 to the phosphorylated tail of LRP resulting in disassociation of the degradation complex and stabilization of β-catenin. Unphosphorylated β-catenin accumulates in the cytoplasm and translocates to the nucleus where it can restore transcriptional activity of TCF/LEF genes normally bound by repressor complexes containing Groucho-related proteins. B) An emerging view of canonical WNT signaling relies on an intact degradation complex to regulate β-catenin. In the absence of a WNT signal, the degradation complex binds β-catenin and subsequent phosphorylation, ubiquitination, and proteosomal degradation occur within the AXIN1/GSK3β/APC complex. In the presence of a WNT signal, activation of the frizzled /LRP co-receptors promotes association of the intact AXIN1 degradation complex with the phosphorylated tail of LRP and the disassociation of β-TrCP. The degradation complex still binds and phosphorylates β-catenin, but ubiquitination by β-TrCP fails to occur. Phosphorylated β-catenin saturates the complex, effectively inactivating the complex and allowing newly synthesized β-catenin to initiate gene transcription. Figure modified from Clevers and Nusse (2012), for details see Li et al. (2012).
The Role of WNT in Follicle Development
The presence and activity of WNT signaling components in the ovary is not unexpected given the variety of physiological processes known to be regulated by the WNT family of proteins. Members of the WNT family are divided into two functional groups with the canonical WNTs (Wnt-1, -2, -3A and -8) classified by their ability to induce secondary dorsal-ventral axis in Xenopus embryos and to transform mammary epithelial cell lines (Wong et al. 1994, Shimizu et al. 1997). Canonical WNT signaling is governed by the interaction of β-catenin with other molecules to regulate cellular decisions related to proliferation, differentiation, and morphogenesis (Willert & Jones 2006, Komiya & Habas 2008, Angers & Moon 2009). A series of studies have identified the expression and regulation of WNT ligands and downstream WNT signaling components in the developing follicle and corpus luteum of rats, mice, humans, and cattle (Hsieh et al. 2002, Ricken et al. 2002, Harwood et al. 2008, Wang et al. 2009, Castanon et al. 2012, Gupta et al. 2014) (Table 1). However, characterization of specific WNT molecules during folliculogenesis has been focused primarily on Wnt2/WNT2 and Wnt4/WNT4 in mice, rats and humans, although recent studies have unveiled contributions of frizzled receptor agonist, WNT3A in follicular development and steroid production of mice and rats (Li et al. 2014, Stapp et al. 2014).
Table 1.
Expression of WNT ligand and FZ receptor in adult mammalian ovaries
| Gene | Species, Reference | |
|---|---|---|
| Wnt1/WNT1 | Whole ovary on days 0 – 21 postpartum Luteinized granulosa cells from healthy and endometrial afflicted ovaries |
mouse, (Harwood et al. 2008) human, (Sanchez et al. 2014) |
| Wnt2/WNT2 | Granulosa cells of all growing follicles collected from eCG/hCG stimulated ovaries Granulosa cells of all stages of follicles Cultured granulosa cells treated with FSH Whole ovary following PMSG/hCG stimulation Cumulus cells obtained from oocytes collected for in vitro fertilization |
rat, (Ricken et al. 2002) mouse, (Wang et al.2010) bovine, (Castanon et al. 2012) mouse, (Hsieh et al. 2002) human, (Wang et al. 2009) |
| Wnt2b/WNT2B | Whole ovary on days 0 – 21 postpartum Ovarian surface epithelium from gonadotropin stimulated ovaries Granulosa cells from dominant follicles Theca interna from large and small antral follicles |
mouse, (Harwood et al. 2008) rat, (Ricken et al. 2002) bovine, (Abedini et al. 2015) bovine, (Hatzirodos et al. 2014) |
| Wnt3/WNT3 | Whole ovary immediately postpartum and on days 8–12 postpartum Luteinized granulosa cells from healthy and endometrial afflicted ovaries |
mouse, (Harwood et al. 2008) human, (Sanchez et al. 2014) |
| Wnt3a | Whole ovary on days 6 to 21 postpartum Whole ovary following PMSG/hCG stimulation |
mouse, (Harwood et al. 2008) mouse, (Hsieh et al. 2002) |
| Wnt4/WNT4 | Granulosa cells throughout follicular development as well as luteal cells Granulosa and luteal cells from hormone stimulated ovaries Luteinized granulosa cells from healthy and endometrial afflicted ovaries Luteal cells Granulosa cells from primary, secondary and antral follicles and theca cells from antral follicles Cumulus cell oocyte complex |
mouse (Hsieh et al. 2002, Harwood et al. 2008) rat, (Hsieh et al. 2002) human, (Sanchez et al. 2014) porcine, (Kiewisz et al. 2011) human, (Jaaskelainen et al. 2010) mouse, (Hernandez-Gonzalez et al. 2006) |
| Wnt5a/WNT5A | Whole ovary on days 0–21 postpartum Luteinized granulosa cells from healthy and endometrial afflicted ovaries Granulosa cells from dominant follicles Luteal cells Theca cells from normal and PCOS ovaries Whole ovary following PMSG/hCG stimulation |
mouse, (Harwood et al. 2008) human, (Sanchez et al. 2014) bovine, (Abedini et al. 2015) porcine, (Kiewisz et al. 2011) human, (Wood et al. 2003) mouse, (Hsieh et al. 2002) |
| Wnt5b/WNT5B | Whole ovary on days 6 to 21 postpartum Granulosa cells from dominant follicles |
mouse, (Harwood et al. 2008) bovine, (Abedini et al. 2015) |
| Wnt6 | Whole ovary on days 0 – 21 postpartum | mouse, (Harwood et al. 2008) |
| Wnt7a/WNT7A | Whole ovary on days 0 – 21 postpartum Luteal cells Whole ovary following PMSG/hCG stimulation |
mouse, (Harwood et al. 2008) porcine, (Kiewisz et al. 2011) mouse,(Hsieh et al. 2002) |
| Wnt7b | Whole ovary on days 6–12 postpartum | mouse,(Harwood et al. 2008) |
| Wnt8 | Whole ovary following PMSG/hCG stimulation | mouse, (Hsieh et al. 2002) |
| WNT8B | Granulosa cells from dominant follicles | bovine, (Abedini et al. 2015) |
|
Wnt9b
Wnt10a Wnt10b |
Whole ovary on days 0 – 21 postpartum | mouse, (Harwood et al. 2008) |
| Wnt11/WNT11 | Whole ovary on days 0 – 21 postpartum Granulosa cells from dominant follicles Whole ovaries following PMSG/hCG stimulation |
mouse, (Harwood et al. 2008) bovine, (Abedini et al. 2015) mouse, (Hsieh et al. 2002) |
| Wnt16/WNT16 | Whole ovary on days 0 – 21 postpartum Granulosa cells from dominant follicles |
mouse, (Harwood et al. 2008) bovine, (Abedini et al. 2015) |
| Fzd1 | Whole ovary on days 0 – 21 postpartum Cumulus cell oocyte complex Granulosa cells of pre-ovulatory follicles from ovaries following PMSG/hCG |
mouse, (Harwood et al. 2008) mouse, (Hernandez-Gonzalez et al. 2006) mouse, (Hsieh et al. 2002) |
| Fzd2 | Whole ovary on days 0 – 21 postpartum Cumulus cell oocyte complex Whole ovary following PMSG/hCG stimulation |
mouse, (Harwood et al. 2008) mouse, (Hernandez-Gonzalez et al. 2006) |
| Fzd3 | Whole ovary following PMSG/hCG stimulation | mouse, (Hsieh et al. 2002) |
| Fzd4 | Whole ovary on days 0 – 21 postpartum PMSG/hCG stimulated, pregnant and postpartum ovaries as well as CL |
mouse, (Harwood et al. 2008) mouse, rat, (Hsieh et al. 2002) |
| Fzd5 | Whole ovary on days 0 – 21 postpartum | mouse, (Harwood et al. 2008) |
| Fzd6/FZD6 | Whole ovary on days 0 – 21 postpartum Granulosa cells from follicles at the emergence, predeviation, onset of deviation, and early dominance stage Whole ovary followinq PMSG/hCG stimulation |
mouse, (Harwood et al. 2008) bovine, (Gupta et al. 2014) mouse, (Hsieh et al. 2002) |
|
Fzd7
Fzd8 Fzd10 |
Whole ovary on days 0 – 21 postpartum | mouse, (Harwood et al. 2008) |
| Fzd9 | Whole ovary on days 0 – 21 postpartum | mouse, (Harwood et al. 2008) |
Wnt2 expression is detected in granulosa cells of immature rat ovaries at all stages of follicle development (Ricken et al. 2002) with the greatest WNT2 immunoreactivity in mouse cumulus and mural granulosa cells and in large, healthy preantral and antral follicles (Wang et al. 2010). Supporting a role of WNT2 during these distinct stages of follicle growth is the demonstrated increased expression of WNT2 mRNA in response to FSH-treatment in cultured bovine granulosa cells (Castanon et al. 2012) and WNT2 in human cumulus cells collected after gonadotropin stimulation (Wang et al. 2009). Likewise, RNAi-mediated knockdown of Wnt2 inhibits granulosa cell proliferation as indicated by reduced 5-ethynyl-2'-deoxyuridine (EdU) incorporation into DNA and marked decrease in proliferating cell nuclear antigen (PCNA) accumulation (Wang et al. 2010). Overexpression of WNT2 via transduction of granulosa cells with a WNT2 encoding retrovirus conversely increased the proportion of EdU-positive cells and abundance of PCNA, events that are expected to promote cell proliferation (Wang et al. 2010). Additionally, WNT2/Wnt2 overexpression increases cytoplasmic and nuclear accumulation of β-catenin in mouse granulosa cells (Wang et al. 2010) and in a rat granulosa cell line (DC3) that displays characteristics of early stage follicle development (Finnson et al. 2012). The mechanism by which WNT2 controls β-catenin is seemingly by regulating cytoplasmic accumulation of GSK3β as WNT2 knockdown granulosa cells have increased cytoplasmic GSK3β that results in reduced β-catenin. Moreover, si-RNA knockdown of β-catenin reduced granulosa cell expression of PCNA and prevents WNT2 overexpression to enhance DNA synthesis of mouse granulosa cells (Wang et al. 2010). These data indicate that regulation of granulosa cell proliferation relies on intact WNT2/β-catenin signaling.
Additional recent data also indicates that in mouse granulosa cells WNT2 can regulate gap junction signaling pathways important for ovarian folliculogenesis (Wang et al. 2013). In WNT2 si-RNA treated mouse granulosa cells, connexin 43, a gap junction protein required for follicular development beyond the early preantral stages, and gap junctional intercellular communication between cells was reduced (Wang et al. 2013). While WNT2 appears to be important for follicle maturation and granulosa cell proliferation, female mice null for Wnt2 are reported to be fertile (Monkley et al. 1996), suggesting compensatory activity of other molecules, possibly other WNTs. Though defects in placental vascularization are observed in Wnt2-null females, no data specifically related to ovarian function have been reported (Monkley et al. 1996). Together these data suggest that Wnt2 expression is regulated by FSH and contributes to preantral to antral maturation of the follicle through granulosa cell proliferation mediated by β-catenin.
Wnt4 expression is found in rat and murine granulosa cells throughout follicle development (Hsieh et al. 2002) and in mouse cumulus oocyte complexes (Hernandez-Gonzalez et al. 2006). Conversely, WNT4 is not detected in human cumulus granulosa cells obtained from oocytes prior to in vitro fertilization (Wang et al. 2009). In adult rodent granulosa cells Wnt4 is elevated in response to hCG stimulation and remains elevated in the corpora lutea (Hsieh et al. 2002). Likewise, estrus synchronization of gilts utilizing PGF2α/PMSG/hCG increased expression of WNT4 in luteal tissue compared to control females (Kiewisz et al. 2011). Targeted deletion of Wnt4 in mouse granulosa cells resulted in subfertile females with smaller ovaries and fewer healthy antral follicles at 42-d of age compared with control mice (Boyer et al. 2010). These results suggest that WNT4 originating from the granulosa cells is necessary for follicle maturation. Adenoviral overexpression of WNT4 in cultured granulosa cells from eCG-treated mice results in increased expression of ovarian β-catenin target genes, Cyp11a1, Cyp19a1 and StAR (Boyer et al. 2010). Furthermore, WNT4 was shown to regulate expression of steroidogenic genes in vivo as granulosa cells isolated from Wnt4-null mice treated for 48 hr with eCG, followed by an ovulatory dose of hCG had lower expression of Cyp11a1, Cyp19a1 and StAR, compared to controls (Boyer et al. 2010). Similarly, eCG-treated Wnt4-null mice had lower serum progesterone at 0, 12 and 24 hr after hCG, compared to controls. Further evidence of WNT4 signaling via β-catenin is found in the fetal mouse ovary where constitutively active β-catenin is able to prevent germ cell loss in Wnt4 KO ovaries (Liu et al. 2010). Data suggests that β-catenin can mediate the events of WNT4 that are important in regulation of antral follicle maturation and steroidogenesis.
Similar to WNT ligands, FZ receptors have been shown to be expressed at specific stages during ovarian follicular maturation, ovulation, and luteinization (Table 1). A number of FZ receptors have been detected in granulosa cells; however, little is known about the physiological relevance of FZ in adult folliculogenesis. In the mouse ovary, Fz1 expression is selectively and transiently induced in large ovulatory follicles by an ovulatory dose of hCG (Hsieh et al. 2002). Evaluation of Fz1 expression in progesterone receptor (PR) knockout mice, which fail to ovulate when hormonally stimulated, show an altered expression of Fz1 compared with PR heterozygotes. In this model, the initial increase of Fz1 expression is comparable in ovaries of PR knockout and PR heterozygotes, however, by 12 h after LH-stimulation (a time point just prior to ovulation) expression of Fz1 was reduced in PR knockout ovaries compared to PR heterozygotes (Hsieh et al. 2002). While these data indicate that LH-mediated induction of Fz1 appears to depend on PR, Fz1 deficient mice are fertile (Yu et al. 2010) with only marginal differences in litter size reported (Lapointe et al. 2012). Therefore, Fz1 does not appear to be necessary in processes related to rupture. In contrast to Fz1, Fz4 displays distinct expression in the adult rodent corpus luteum of gonadotropin-treated and pregnant mice and is required for fertility. Mice lacking Fz4 receptor demonstrate follicle development that is responsive to hormone stimulation, and results in the expected genes expression profiles involved in early follicle development (Hsieh et al. 2005). Furthermore, adult female Fz4-null mice exhibit normal ovulation and ability to produce fertilized oocytes but are sterile as a consequence of failure of embryo implantation. This inability to establish a successful implantation is due to the impaired formation of the corpora lutea and the associated reduction of luteal-specific gene expression and progesterone production (Hsieh et al. 2005).
Of note, Lrp4, a member of the low-density lipoprotein receptor family implicated in a number of diverse biological functions has been detected in follicular cells of the adult mouse ovary (Yamaguchi et al. 2006). While the ligand or LRP4 remains unknown, it is closely related to the WNT co-receptors LRP5/6 (Zong et al. 2012). Expression of Lrp4 specific to the migratory primordial germ cells and adult gonad but not in embryo or germ cell derived stem cells suggest Lrp4 may be a marker distinguishing germ cells from embryo-derived pluripotent stem cells (Yamaguchi et al. 2006).
Gonadotropin Regulation of WNT Gene Expression
There is also evidence that select Wnt family gene expression is hormonally regulated in rodent ovaries. For example, Wnt4 expression is elevated in rat granulosa cells following hCG stimulation, and high expression of Wnt4 is detected in terminally differentiated luteal cells (Hsieh et al. 2002). Additionally, genetically modified mice that hypersecrete LH (Tg(Cga-LHB/CGB)94Jhn/J) also develop granulosa cell tumors which display alterations in members of the WNT signaling pathway (Owens et al. 2002). Specifically, Wnt4 and secreted frizzled related protein-4 (SFRP-4), a proposed inhibitor of the WNT pathway, are dramatically decreased in granulosa cell tumors, while a WNT receptor, Fz10 was increased in these same granulosa cell tumors. However, it was the work of Parakh et al. (2006) which provided the first direct indication that β-catenin was required for FSH/cAMP-induction of Cyp19a1 expression in a human granulosa tumor cell line (KGN), and in primary cultures of rat granulosa cells. This increased expression of Cyp19a1 in response to FSH was determined in KGN cells to be mediated by functional interactions of β-catenin with steroidogenic factor-1 (NR5A1). In subsequent studies, conditional deletion of β-catenin in primary cultures of mouse granulosa cells similarly resulted in a compromised ability of FSH to stimulate Cyp19a1 expression as well as consequent estradiol production, reinforcing a role for β-catenin in steroid production from the ovary (Hernandez Gifford et al. 2009). A requirement for β-catenin in FSH regulation of steroid production has more recently been identified in granulosa cells of large bovine antral follicles, as high estrogen producing follicles demonstrate an increase in β-catenin protein accumulation compared to follicles with low intrafollicular estradiol concentrations (Castanon et al. 2012). Consistent with β-catenin's role in regulation of steroidogenesis is the demonstrated ability of FSH to directly increase β-catenin protein accumulation (Castanon et al. 2012, Stapp et al. 2014) and β-catenin/TCF dependent transcriptional activity in granulosa cells (Fan et al. 2010, Stapp et al. 2014). In addition, Law et al. (2013) showed that FSH via PKA stimulates phosphorylation of β-catenin on Ser552 and Ser675 leading to its activation. FSH stimulation of transcriptionally active β-catenin promotes NR5A1 and TCF-regulated gene expression, including Lhcgr (Law et al. 2013). Together these data confirm that activation of β-catenin facilitates FSH-mediated actions in ovarian follicular cells.
β-catenin's participation in regulation of steroidogenesis has also been linked to LH-mediated production of progesterone from bovine corpora lutea. In cultured bovine luteal cells, LH stimulation of cAMP/PKA results in phosphorylation and inhibition of GSK3β allowing stabilization of β-catenin (Roy et al. 2009). Increased levels of transcriptionally active β-catenin interact with the proximal promoter of the StAR gene and successively increase StAR mRNA expression and progesterone synthesis. However, it appears that β-catenin alone is insufficient to modulate steroid pathways and that contributions of the gonadotropins are integral for β-catenin to maximally impact steroidogenesis in ovarian cells. Overexpression of adenoviral Δ90 β-catenin, a β-catenin mutant lacking N-terminal GSK3β phosphorylation sites involved in its targeted degradation, resulted in only modest regulation of Cyp19a1 and Cyp11a2 mRNA in granulosa cells (Parakh et al. 2006) and had no effect on progesterone concentrations in media from cultured luteal cells (Roy et al. 2009).
Negative Feedback Loops Regulate WNT/β-catenin
Whereas previous studies utilizing overexpression systems indicate β-catenin participates in gonadotropin induction of steroidogenic enzyme expression and steroid output, a recent study from Stapp et al. (2014) revealed a previously unappreciated inhibition of steroidogenesis with concomitant stimulation of FSH and canonical WNT signaling pathways. Exposure of primary rat granulosa cells to recombinant WNT3A at a minimal effective dose of 50 ng/mL caused specific induction of canonical WNT signaling as determined by increased expression of the WNT target gene, Axin2 and stimulation of the β-catenin/TCF promoter reporter TOPflash (Stapp et al. 2014). Unexpectedly, WNT3A induction of β-catenin resulted in down-regulation of FSH-mediated expression of key steroidogenic enzymes (StAR, Cyp11a1, and Cyp19a1) and ovarian differentiation factors (Lhcgr and inhibin alpha). Co-incubation of FSH and WNT3A repressed FSH-induced steroidogenic enzyme expression that further translated to a reduction in estradiol and progesterone production (Stapp et al. 2014). In agreement with these findings, WNT pathway agonist/GSK3β inhibitor, LiCl and WNT3A significantly decreased estradiol concentration in cultured mouse follicles, while treatment with a WNT inhibitor increased culture media concentrations of estradiol (Li et al. 2014).
The noted up-regulation of Axin2, a negative regulator of WNT signaling, in response to co-stimulation of granulosa cells with WNT3A and FSH allowed for detection of a negative feedback mechanism whereby FSH regulates canonical WNTs in an effort to control TCF responsive genes. These data provide valuable insight into the physiological functions of β-catenin in the adult ovary. The notion of creating a negative feedback loop to ensure β-catenin remains controlled is consistent with the detection of WNT/β-catenin signaling antagonists WNT inhibitory factor 1 (Wif1), naked cuticle homolog 1 (Nkd1), dickkopf 4 (Dkk4) and Axin2 in ovaries of mice which constitutively express β-catenin (Boerboom et al. 2006). Similarly, overactivation of β-catenin has negative effects on LH-induced cumulus-oocyte complex expansion, ovulation, luteinization and progesterone production (Fan et al. 2010). Granulosa cells from mice expressing dominant stable β-catenin have muted expression of StAR, Cyp11a1, and Lhcgr following forskolin and PMA-treatment that is meant to mimic the effects of LH in vitro (Fan et al. 2010).
Modulators of β-catenin Suppression
Negative feedback mechanisms that limit the duration of a signaling event following initial stimulus are present in most signal transduction pathways. The data mentioned above provide evidence that FSH via β-catenin/TCF pathway upregulates FSH target genes involved in granulosa cell maturation and differentiation. WNT ligands appear to be another FSH target that may function in a feedback manner by up-regulating Axin2 mRNA expression. Axin1 is a known negative regulator of the canonical WNT signaling pathway; however, the significance of the Axin1 homologue Axin2 in granulosa cells remains to be characterized. AXIN2 is thought to act as a scaffold protein to facilitate phosphorylation of β-catenin by GSK3β resulting in its consequent degradation (Jho et al. 2002). Induction of Axin2, therefore, may exert an inhibitory effect on β-catenin to effectively shut down β-catenin/TCF gene transcription (Figure 2). Numerous FSH target genes in granulosa cells are TCF responsive, including but not limited to Cyp19a1, Inha, Foxo1 and Lhcgr (Law et al. 2013).
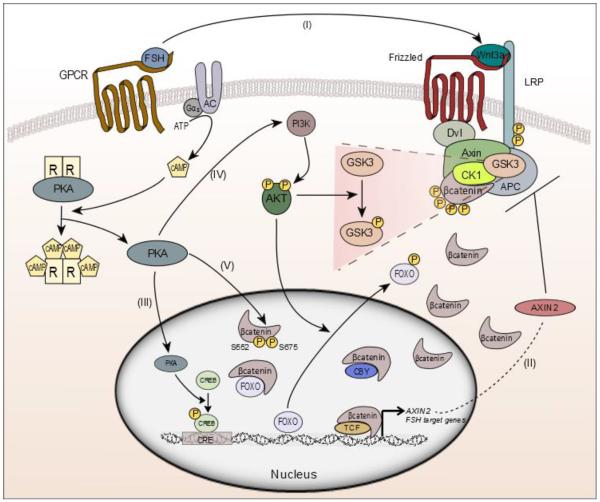
Figure 2. FSH regulation of WNT contributes to a negative feedback mechanism to regulate TCF responsive genes in the granulosa cells.
(I) FSH regulates induction of several WNT ligands, any of which may contribute to negative feedback regulation. Recent data provide evidence that FSH regulates transduction of a WNT signal which in turn upregulates Axin2 and FSH target genes via the β-catenin /TCF pathway. (II) Axin2 induction may subsequently exert an inhibitory effect on β-catenin to effectively shut down β-catenin/TCF gene transcription. Alternative negative modulators including FOXO1/3A and Chibby can prevent β-catenin transcriptional activity by binding it in the nucleus. (III) FSH binding to the G-protein coupled FSH receptor stimulates adenylyl cyclase and promotes cAMP dependent PKA activity. This active kinase phosphorylates CREB to regulate expression of PKA target genes in granulosa cells. (IV) PKA also enhances the activity of PI3K leading to AKT phosphorylation. AKT phosphorylates FOXO leading to its export from the nucleus and releasing its inhibition on transcriptional activity of genes regulating granulosa cell proliferation and steroid production. Additionally, FOXO may bind to β-catenin in the nucleus to repress its transcriptional activity. This negative regulation could work to ensure that β-catenin remains controlled and its target genes are not overexpressed. (V) In addition, PKA regulates stability and activity of β-catenin by phosphorylation on Ser552 and Ser675 in granulosa cells providing a new layer of complexity to the intracellular mechanisms regulating follicle development.
Additional alternative scenarios for limiting a WNT signal exist including β-catenin's interaction with a nuclear molecule that could prevent it from binding transcriptional targets. One such candidate is Chibby (CBY1), a conserved nuclear associated antagonist of the WNT pathway which associates with the C-terminal domain of β-catenin and blocks its interaction with TCF/LEF transcription factors (Takemaru et al. 2003). The expression of Cby1 has been detected in a variety of adult human tissues (Takemaru et al. 2003). In COS7 cells, CBY1 protein is largely nuclear and its localization is unaffected by expression of WNT-1, -5a or β-catenin (Takemaru et al. 2003). While characterization and gonadotropin control of CBY1 in the ovary remains to be demonstrated, a recent study Finnson et al. (2012) identified the expression of CBY1 in a SV-40 transformed rat granulosa cell line (DC3). Overexpression of Wnt2 in DC3 cells led to β-catenin accumulation in the nucleus but failed to stimulate β-catenin/TCF-dependent transcription likely as a consequence of CBY1 association and suppression of endogenous β-catenin (Finnson et al. 2012).
Another molecule which may modulate follicular development is the Forkhead box-O (FOXO) family of transcription factors that are recognized for their involvement in the regulation of apoptosis, proliferation, and cell cycle arrest (Burgering & Medema 2003). FOXOs are downstream targets of PI3K/AKT pathway, and direct phosphorylation by AKT inhibits transcriptional activation of FOXO by causing their exclusion from the nucleus into the cytoplasm and subsequent degradation. FOXO transcription factors are found in the rodent ovary and are regulated by gonadotropins. In granulosa cells, FSH enhances Foxo1 gene expression in granulosa cells of the preovulatory follicle, and is rapidly down-regulated following hCG induced ovulation (Richards et al. 2002b, Fan et al. 2010) a pattern consistent with FOXO1 repression of granulosa cell proliferation and steroidogenesis (Park et al. 2005, Liu et al. 2009). Likewise, FOXO1 represses Lhcgr expression in granulosa cells and is present on the promotor of vehicle treated cells, but is removed from the promoter after FSH stimulation (Law et al. 2013). A study by Hoogeboom et al. (2008) proposed β-catenin to be a link between the WNT signaling and FOXO pathways, given the ability of FOXO3A to inhibit TCF-transcription by binding to β-catenin. To elucidate the role of WNT/β-catenin in regulation of early follicle development a recent study employed an in vitro follicle culture system utilizing isolated secondary follicles that were cultured in the presence or absence of WNT pathway activators and inhibitors (Li et al. 2014). In this study, WNT pathway activators, LiCl and WNT3A were found to decrease phosphorylation of FOXO3A while the WNT inhibitor, IWR-1, increased FOXO3A phosphorylation. In addition, FOXO3A targets, Bim, Puma and p27 were increased by WNT3A and LiCl and decreased by WNT inhibition (Li et al. 2014). Furthermore, activation of WNT/β-catenin resulted in a large number of abnormal follicles while suppression of this pathway promoted follicle growth (Li et al. 2014). Consistent with negative feedback results of WNT inhibiting FSH signaling responses, these data suggest that β-catenin signaling may be necessary for keeping follicle growth in check by negatively controlling early follicle development and that several different mechanisms may participate in this regulation.
Future Considerations
A large body of data definitively recognizes WNT signaling as an essential factor for proper development of the female mammalian gonad (Vainio et al. 1999, Heikkila et al. 2001, Biason-Lauber & Konrad 2008, Maatouk et al. 2008); however, the contribution of WNT family signaling components to ovarian folliculogenesis in the adult remains to be fully elucidated. It is suspected that the divergent roles or even opposing effects of WNT signaling is likely attributed to the different stages of follicle development and hormonal milieu present during the development of the ovarian follicle. It is clear that pituitary gonadotropins regulate ovarian events during the estrous cycle through the convergence of multiple signaling pathways. One newly recognized pathway is the canonical WNT signaling pathway which regulates levels of the downstream transcriptional co-factor, β-catenin shown to impact gonadotropin-responsive target gene expression and steroid production. Identification of WNT signaling in gonadotropin-mediated events in the adult ovary highlights the role of this pathway in regulation of normal follicle maturation, ovulation, and corpus luteum formation and function, but many questions in this field remain to be determined.
Functional studies in granulosa cells have evaluated the influences of only a few WNTs, namely WNT2, WNT4 and more recently WNT3A. A need therefore remains to determine if other WNTs known to be present in the adult ovary are involved in ovarian function. Although the non-canonical WNTs have been less characterized than the canonical WNT/β-catenin pathway, it is possible that these WNTs contribute to folliculogenesis and ovarian steroidogenesis. This idea is emphasized by the apparent discordant data in the literature regarding the effect of co-stimulation of the extracellular WNT and FSH signaling pathways on steroidogenic enzyme expression in granulosa cells. This difference is conceivably due to the use of two different WNT ligands employed in each study. Indeed, WNT3A and WNT4 have differing biological activities and as such are classified into two separate functional groups that can trigger distinct developmental outcomes (Wong et al. 1994, Du et al. 1995). However, the lines between these prototypical classifications are becoming blurred as data now suggests that WNT signaling is not strictly regulated by the ligand itself but that receptor context dictates the signal output (Mikels & Nusse 2006). Furthermore, a single WNT protein has been shown to simultaneously activate different branches of the WNT signaling pathway in the same cell dependent on WNT concentration (Nalesso et al. 2011). Together, these findings underscore the significance of evaluating the specific receptors present during different stages of follicular development along with defining which WNTs may be binding. Since WNT proteins have been shown to activate different pathways with distinct and independent outcomes depending on the concentration of WNT (Nalesso et al. 2011), it will be interesting to evaluate dose-dependent treatment paradigms at different stages of follicle development such as in granulosa, granulosa-lutein and differentiated luteal cells. Investigating changes that occur in the FZ and co-receptor complexes in follicular cells co-incubated with gonadotropin and WNT ligands has not been evaluated but would also be of value.
Follicles are exposed to various WNTs during follicle maturation that target β-catenin to the nucleus via the canonical WNT/β-catenin pathway to regulate target gene expression. Recent studies also identify a unique PKA-dependent regulation of β-catenin in response to FSH stimulation (Law et al. 2013) that regulates granulosa cell gene expression. It is interesting to consider whether PKA activated β-catenin regulates a similar set of genes as β-catenin that is regulated by GSK3β. Additionally, it remains to be determined if PKA-activation of β-catenin by both LH and FSH occurs in an equivalent fashion. Evaluation of WNT promoters for steroid response elements or other important regulatory regions may provide insight on the factors that may play a role in their function. In conclusion, the WNT signaling pathway encompasses multiple layers of complexity and while our understanding of the role of WNTs in regulation of postnatal ovarian function and steroidogenesis continues to expand, there are many important questions that need to be answered in order to gain a complete understanding of the contribution of this large family of signaling molecules to folliculogenesis.
Acknowledgements
The deepest gratitude is expressed to Belinda Gomez, Bahaa Aloqaily and Rita Flores for providing assistance in generating the figures and table. I would also like to sincerely thank Mary Hunzicker-Dunn for critical evaluation of this review.
Funding This work was supported by the Oklahoma Agric. Exp. Sta., Stillwater (OKL02934). The original research reported in this review was supported in part by National Institutes of Health (NIH) grant R15065668 from the Eunice Kennedy Shriver National Institutes of Child Health & Human Development; and Oklahoma Center for the Advancement of Science and Technology (OCAST) grant HR10-030S to J.A.H.G.
Footnotes
Declaration of interest The authors declare there is no conflict of interests which could be perceived as prejudicing the impartiality of the review.
References
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO Journal. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Biason-Lauber A, Konrad D. WNT4 and sex development. Sex Dev. 2008;2:210–218. doi: 10.1159/000152037. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir TK, Gloriam DE, Hellstrand SH, Kristiansson H, Fredriksson R, Schioth HB. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics. 2006;88:263–273. doi: 10.1016/j.ygeno.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Boerboom D, Paquet M, Hsieh M, Liu J, Jamin SP, Behringer RR, Sirois J, Taketo MM, Richards JS. Misregulated Wnt/beta-catenin signaling leads to ovarian granulosa cell tumor development. Cancer Research. 2005;65:9206–9215. doi: 10.1158/0008-5472.CAN-05-1024. [DOI] [PubMed] [Google Scholar]
- Boerboom D, White LD, Dalle S, Courty J, Richards JS. Dominant-stable beta-catenin expression causes cell fate alterations and Wnt signaling antagonist expression in a murine granulosa cell tumor model. Cancer Research. 2006;66:1964–1973. doi: 10.1158/0008-5472.CAN-05-3493. [DOI] [PubMed] [Google Scholar]
- Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mechanisms of Development. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Boyer A, Lapointe E, Zheng XF, Cowan RG, Li HG, Quirk SM, DeMayo FJ, Richards JS, Boerboom D. WNT4 is required for normal ovarian follicle development and female fertility. FASEB Journal. 2010;24:3010–3025. doi: 10.1096/fj.09-145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgering BM, Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. Journal of Leukocyte Biology. 2003;73:689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling; a common theme in animal development. Genes and Development. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Castanon BI, Stapp AD, Gifford CA, Spicer LJ, Hallford DM, Hernandez Gifford JA. Follicle-stimulating hormone regulation of estradiol production: possible involvement of WNT2 and beta-catenin in bovine granulosa cells. Journal of Animal Science. 2012;90:3789–3797. doi: 10.2527/jas.2011-4696. [DOI] [PubMed] [Google Scholar]
- Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature. 1998;395:604–608. doi: 10.1038/26982. [DOI] [PubMed] [Google Scholar]
- Christian JL. BMP, Wnt and Hedgehog signals: how far can they go? Current Opinion in Cell Biology. 2000;12:244–249. doi: 10.1016/s0955-0674(99)00082-4. [DOI] [PubMed] [Google Scholar]
- Cinnamon E, Paroush Z. Context-dependent regulation of Groucho/TLE-mediated repression. Current Opinion in Genetics and Development. 2008;18:435–440. doi: 10.1016/j.gde.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86–90. doi: 10.1038/35083601. [DOI] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- Du SJ, Purcell SM, Christian JL, McGrew LL, Moon RT. Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Molecular and Cellular Biology. 1995;15:2625–2634. doi: 10.1128/mcb.15.5.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, O'Connor A, Shitanaka M, Shimada M, Liu Z, Richards JS. Beta-catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Molecular Endocrinology. 2010;24:1529–1542. doi: 10.1210/me.2010-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay JK. An update on the roles of inhibin, activin, and follistatin as local regulators of folliculogenesis. Biology of Reproduction. 1993;48:15–23. doi: 10.1095/biolreprod48.1.15. [DOI] [PubMed] [Google Scholar]
- Finnson KW, Kontogiannea M, Li X, Farookhi R. Characterization of Wnt2 Overexpression in a Rat Granulosa Cell Line (DC3): Effects on CTNNB1 Activation. Biology of Reproduction. 2012;87:12. doi: 10.1095/biolreprod.111.096396. [DOI] [PubMed] [Google Scholar]
- Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin JP, Davenport AP, Spedding M, Harmar AJ. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacological Reviews. 2005;57:279–288. doi: 10.1124/pr.57.2.5. [DOI] [PubMed] [Google Scholar]
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochimica et Biophysica Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Gupta PS, Folger JK, Rajput SK, Lv L, Yao J, Ireland JJ, Smith GW. Regulation and regulatory role of WNT signaling in potentiating FSH action during bovine dominant follicle selection. PLoS One. 2014;9:e100201. doi: 10.1371/journal.pone.0100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood BN, Cross SK, Radford EE, Haac BE, De Vries WN. Members of the WNT signaling pathways are widely expressed in mouse ovaries, oocytes, and cleavage stage embryos. Developmental Dynamics. 2008;237:1099–1111. doi: 10.1002/dvdy.21491. [DOI] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- Heikkila M, Peltoketo H, Vainio S. Wnts and the female reproductive system. Journal of Experimental Zoology. 2001;290:616–623. doi: 10.1002/jez.1112. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, Richards JS. Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Molecular Endocrinology. 2006;20:1300–1321. doi: 10.1210/me.2005-0420. [DOI] [PubMed] [Google Scholar]
- Hernandez Gifford JA, Hunzicker-Dunn ME, Nilson JH. Conditional deletion of beta-catenin mediated by Amhr2cre in mice causes female infertility. Biology of Reproduction. 2009;80:1282–1292. doi: 10.1095/biolreprod.108.072280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. Journal of Biological Chemistry. 2008;283:9224–9230. doi: 10.1074/jbc.M706638200. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Rattner A, Smallwood PM, Nathans J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:3546–3551. doi: 10.1073/pnas.96.7.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M, Boerboom D, Shimada M, Lo Y, Parlow AF, Luhmann UF, Berger W, Richards JS. Mice null for Frizzled4 (Fzd4−/−) are infertile and exhibit impaired corpora lutea formation and function. Biology of Reproduction. 2005;73:1135–1146. doi: 10.1095/biolreprod.105.042739. [DOI] [PubMed] [Google Scholar]
- Hsieh M, Johnson MA, Greenberg NM, Richards JS. Regulated expression of Wnts and Frizzleds at specific stages of follicular development in the rodent ovary. Endocrinology. 2002;143:898–908. doi: 10.1210/endo.143.3.8684. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Molecular and Cellular Biology. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiewisz J, Kaczmarek MM, Morawska E, Blitek A, Kapelanski W, Ziecik AJ. Estrus synchronization affects WNT signaling in the porcine reproductive tract and embryos. Theriogenology. 2011;76:1684–1694. doi: 10.1016/j.theriogenology.2011.06.034. [DOI] [PubMed] [Google Scholar]
- Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe E, Boyer A, Rico C, Paquet M, Franco HL, Gossen J, DeMayo FJ, Richards JS, Boerboom D. FZD1 regulates cumulus expansion genes and is required for normal female fertility in mice. Biology of Reproduction. 2012;87:104. doi: 10.1095/biolreprod.112.102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law NC, Weck J, Kyriss B, Nilson JH, Hunzicker-Dunn M. Lhcgr Expression in Granulosa Cells: Roles for PKA-Phosphorylated beta-Catenin, TCF3, and FOXO1. Molecular Endocrinology. 2013;27:1295–1310. doi: 10.1210/me.2013-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Ji SY, Yang JL, Li XX, Zhang J, Zhang Y, Hu ZY, Liu YX. Wnt/beta-catenin signaling regulates follicular development by modulating the expression of Foxo3a signaling components. Molecular and Cellular Endocrinology. 2014;382:915–925. doi: 10.1016/j.mce.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, Mohammed S, Heck AJ, Maurice MM, Mahmoudi T, Clevers H. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Liu CF, Parker K, Yao HH. WNT4/beta-catenin pathway maintains female germ cell survival by inhibiting activin betaB in the mouse fetal ovary. PLoS One. 2010;5:e10382. doi: 10.1371/journal.pone.0010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, DeCostanzo AJ, Liu X, Wang H, Hallagan S, Moon RT, Malbon CC. G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science. 2001;292:1718–1722. doi: 10.1126/science.1060100. [DOI] [PubMed] [Google Scholar]
- Liu Z, Rudd MD, Hernandez-Gonzalez I, Gonzalez-Robayna I, Fan HY, Zeleznik AJ, Richards JS. FSH and FOXO1 regulate genes in the sterol/steroid and lipid biosynthetic pathways in granulosa cells. Molecular Endocrinology. 2009;23:649–661. doi: 10.1210/me.2008-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annual Review of Cell and Developmental Biology. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Human Molecular Genetics. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/beta-catenin signaling. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biology. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR. The Wnts. Genome Biology. 2002;3:3001. 1–3001.15. doi: 10.1186/gb-2001-3-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene. 1999;18:7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Monget P, Bondy C. Importance of the IGF system in early folliculogenesis. Molecular and Cellular Endocrinology. 2000;163:89–93. doi: 10.1016/s0303-7207(99)00244-0. [DOI] [PubMed] [Google Scholar]
- Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122:3343–3353. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- Nalesso G, Sherwood J, Bertrand J, Pap T, Ramachandran M, De Bari C, Pitzalis C, Dell'accio F. WNT-3A modulates articular chondrocyte phenotype by activating both canonical and noncanonical pathways. Journal of Cell Biology. 2011;193:551–564. doi: 10.1083/jcb.201011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- Owens GE, Keri RA, Nilson JH. Ovulatory surges of human CG prevent hormone-induced granulosa cell tumor formation leading to the identification of tumor-associated changes in the transcriptome. Molecular Endocrinology. 2002;16:1230–1242. doi: 10.1210/mend.16.6.0850. [DOI] [PubMed] [Google Scholar]
- Parakh TN, Hernandez JA, Grammer JC, Weck J, Hunzicker-Dunn M, Zeleznik AJ, Nilson JH. Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires beta-catenin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12435–12440. doi: 10.1073/pnas.0603006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Maizels ET, Feiger ZJ, Alam H, Peters CA, Woodruff TK, Unterman TG, Lee EJ, Jameson JL, Hunzicker-Dunn M. Induction of cyclin D2 in rat granulosa cells requires FSH-dependent relief from FOXO1 repression coupled with positive signals from Smad. Journal of Biological Chemistry. 2005;280:9135–9148. doi: 10.1074/jbc.M409486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes and Development. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Richards JS. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiological Reviews. 1980;60:51–89. doi: 10.1152/physrev.1980.60.1.51. [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, Lo YK, Sharma SC. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Progress in Hormone Research. 2002a;57:195–220. doi: 10.1210/rp.57.1.195. [DOI] [PubMed] [Google Scholar]
- Richards JS, Sharma SC, Falender AE, Lo YH. Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Molecular Endocrinology. 2002b;16:580–599. doi: 10.1210/mend.16.3.0806. [DOI] [PubMed] [Google Scholar]
- Ricken A, Lochhead P, Kontogiannea M, Farookhi R. Wnt signaling in the ovary: identification and compartmentalized expression of wnt-2, wnt-2b, and frizzled-4 mRNAs. Endocrinology. 2002;143:2741–2749. doi: 10.1210/endo.143.7.8908. [DOI] [PubMed] [Google Scholar]
- Riese J, Yu X, Munnerlyn A, Eresh S, Hsu SC, Grosschedl R, Bienz M. LEF-1, a nuclear factor coordinating signaling inputs from wingless and decapentaplegic. Cell. 1997;88:777–787. doi: 10.1016/s0092-8674(00)81924-8. [DOI] [PubMed] [Google Scholar]
- Roy L, McDonald CA, Jiang C, Maroni D, Zeleznik AJ, Wyatt TA, Hou X, Davis JS. Convergence of 3',5'-cyclic adenosine 5'-monophosphate/protein kinase A and glycogen synthase kinase-3-beta/beta-catenin signaling in corpus luteum progesterone synthesis. Endocrinology. 2009;150:5036–5045. doi: 10.1210/en.2009-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson EJ, Wu CH, Nusse R. Pathway specificity by the bifunctional receptor frizzled is determined by affinity for wingless. Molecular Cell. 2000;6:117–126. [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kuhl M, Moon RT. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. Journal of Cell Biology. 2003;161:769–777. doi: 10.1083/jcb.200211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, Kitajewski J. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth and Differentiation. 1997;8:1349–1358. [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Noort Mv M, Strous GJ, Clevers HC. Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep. 2002;3:63–68. doi: 10.1093/embo-reports/kvf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapp AD, Gomez BI, Gifford CA, Hallford DM, Hernandez Gifford JA. Canonical WNT signaling inhibits follicle stimulating hormone mediated steroidogenesis in primary cultures of rat granulosa cells. PLoS One. 2014;9:e86432. doi: 10.1371/journal.pone.0086432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M, Micchelli CA. Quiescent gastric stem cells maintain the adult Drosophila stomach. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17696–17701. doi: 10.1073/pnas.1109794108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemaru K, Yamaguchi S, Lee YS, Zhang Y, Carthew RW, Moon RT. Chibby, a nuclear beta-catenin-associated antagonist of the Wnt/Wingless pathway. Nature. 2003;422:905–909. doi: 10.1038/nature01570. [DOI] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Molecular Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Wang HX, Gillio-Meina C, Chen S, Gong XQ, Li TY, Bai D, Kidder GM. The canonical WNT2 pathway and FSH interact to regulate gap junction assembly in mouse granulosa cells. Biology of Reproduction. 2013;89:39. doi: 10.1095/biolreprod.113.109801. [DOI] [PubMed] [Google Scholar]
- Wang HX, Li TY, Kidder GM. WNT2 regulates DNA synthesis in mouse granulosa cells through beta-catenin. Biology of Reproduction. 2010;82:865–875. doi: 10.1095/biolreprod.109.080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Tekpetey FR, Kidder GM. Identification of WNT/beta-CATENIN signaling pathway components in human cumulus cells. Molecular Human Reproduction. 2009;15:11–17. doi: 10.1093/molehr/gan070. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes and Development. 2006;20:1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- Wong GT, Gavin BJ, McMahon AP. Differential transformation of mammary epithelial cells by Wnt genes. Molecular and Cellular Biology. 1994;14:6278–6286. doi: 10.1128/mcb.14.9.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Nusse R. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. Journal of Biological Chemistry. 2002;277:41762–41769. doi: 10.1074/jbc.M207850200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi YL, Tanaka SS, Kasa M, Yasuda K, Tam PP, Matsui Y. Expression of low density lipoprotein receptor-related protein 4 (Lrp4) gene in the mouse germ cells. Gene Expression Patterns. 2006;6:607–612. doi: 10.1016/j.modgep.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Yu H, Smallwood PM, Wang Y, Vidaltamayo R, Reed R, Nathans J. Frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: general implications for tissue fusion processes. Development. 2010;137:3707–3717. doi: 10.1242/dev.052001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Y, Zhang B, Gu S, Lee K, Zhou J, Yao G, Figueiredo D, Perry K, Mei L, Jin R. Structural basis of agrin-LRP4-MuSK signaling. Genes and Development. 2012;26:247–258. doi: 10.1101/gad.180885.111. [DOI] [PMC free article] [PubMed] [Google Scholar]