Abstract
Dissemination of tumor cells is an essential step in metastasis. Direct contact between a macrophage, Mena over-expressing tumor cell and endothelial cell [Tumor MicroEnvironment of Metastasis (TMEM)], correlates with metastasis in breast cancer patients. Here we show, using intravital high-resolution two-photon microscopy, that transient vascular permeability and tumor cell intravasation occur simultaneously and exclusively at TMEM. The hyperpermeable nature of tumor vasculature is described as spatially and temporally heterogeneous. Using real-time imaging we observed that vascular permeability is transient, restricted to TMEM, and required for tumor cell dissemination. VEGFA signaling from Tie2Hi TMEM macrophages causes local loss of vascular junctions, transient vascular permeability and tumor cell intravasation, demonstrating a role for TMEM within the primary mammary tumor. These data provide insight into the mechanism of tumor cell intravasation and vascular permeability in breast cancer, explaining the value of TMEM density as a predictor of distant metastatic recurrence in patients.
Keywords: metastasis, breast cancer, intravital multiphoton microscopy, tumor-associated macrophage, TMEM, Mena, transendothelial migration
INTRODUCTION
For almost two decades tumor vasculature has been described as abnormal with increased vascular permeability (1, 2). Vascular endothelial growth factor A (VEGFA) is known to promote vascular permeability, and inhibition of VEGFA results in the normalization of tumor vasculature and a decrease in permeability (3, 4). Due to the significant effects of VEGFA on tumor angiogenesis and vascular permeability inhibitors of VEGF signaling have become an important research focus in the development of anti-tumor therapies.
Tumor-associated macrophages (TAMs) have been implicated in tumor progression, angiogenesis and metastasis (5, 6). A subpopulation of perivascular TAMs that have features of pro-tumorigenic macrophages, promoting tumor angiogensis and metastasis, has been identified as Tie2-expressing macrophages (TEMs) (7). Perivascular macrophages are also an essential component of the microanatomical sites termed “Tumor MicroEnvironment of Metastasis” (TMEM) that consist of a TAM in direct contact with a Mammalian enabled (Mena) over-expressing tumor cell and endothelial cell (8). TMEM have been associated with tumor cell intravasation (9, 10) and TMEM density predicts distant metastatic recurrence in breast cancer patients independently of other clinical prognostic indicators (8, 11). However, the mechanistic link between perivascular macrophages and tumor cell intravasation remained unclear. Further, hyperpermeability in tumor vasculature is not uniform, but rather is spatially and temporally heterogeneous (12). In a VEGFA overexpression model inducing vascular permeability, the presence of macrophages at vascular branch points was observed at hotspots of vascular permeability (4). Although hyperpermeability of tumor vasculature is widely accepted, a mechanistic understanding of the heterogeneity of vascular permeability, the contribution of TAMs, and the link with tumor cell intravasation has not been described.
Here we show, using intravital high-resolution two-photon microscopy, that transient vascular permeability events are restricted to TMEM sites of Tie2Hi/VEGFAHi perivascular macrophages. Local loss of vascular junctions at TMEM results in transient vascular permeability and tumor cell intravasation in the spontaneous autochthonous mouse mammary cancer model where the mouse mammary tumor virus long terminal repeat drives the polyoma middle T antigen (MMTV-PyMT), the human patient-derived xenograft model, TN1, and human metastatic breast cancer.
RESULTS
TMEM-associated tumor cells and macrophages are stationary in TMEM structures
To examine the functional role of TMEM in tumor cell dissemination we used the spontaneous autochthonous mouse mammary cancer model where the mouse mammary tumor virus long terminal repeat drives the polyoma middle T antigen (MMTV-PyMT), in which tumors exhibit histology similar to human luminal breast cancer, and progress to metastasis (13). Immunohistochemistry (IHC) revealed that TMEM structures in mouse tumors have the same microanatomical structure as identified in humans (fig. S1A) (11). TMEM density and circulating tumor cells increase with tumor progression with elevated TMEM scores in late carcinoma (LC) as compared to early carcinoma (EC) as seen by IHC (fig. S1A–C) though total perivascular macrophage (including macrophages not associated with tumor cells) density is not significantly different (13). High-resolution imaging demonstrates that in TMEM structures tumor cells and macrophages extend protrusions but are relatively non-migratory and stay in direct contact over time (fig. S1D).
Vascular permeability and tumor cell intravasation occur concurrently at TMEM
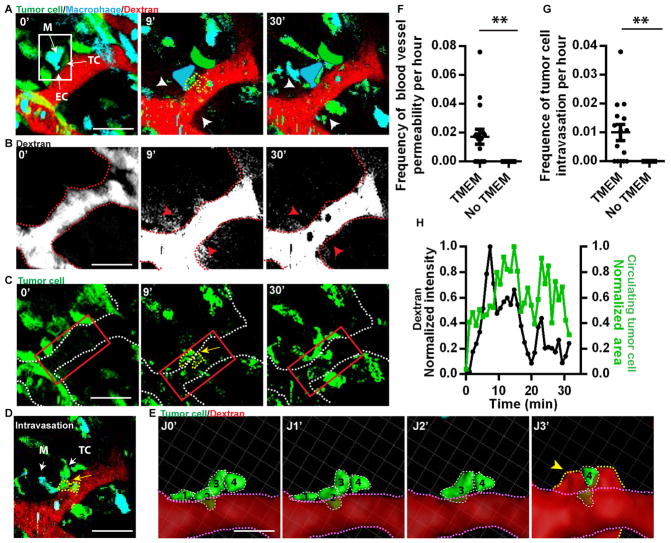
To directly observe TMEM function in vivo we used extended time-lapse IVM with high spatial and temporal resolution. To visualize blood flow, vessels were labeled with a high molecular weight compound (155 kDa dextran or quantum dots) (1, 14) (Fig. 1, 2, 3 and fig. S2). In PyMT LC, migratory tumor cells and macrophages stream towards TMEM at sites with vascular permeability whereupon tumor cells undergo transendothelial migration at TMEM (Fig. 1A–E, fig. S2A–E). In LC, transient, local blood vessel permeability was observed at TMEM sites by the extravasation of quantum dots (fig. S2A and B) or 155 kDa dextran-tetramethylrhodamine (TMR) (Fig. 2A, B, 3C fig. S2C–E, and Movie S1). Further, tumor cell intravasation occurs at TMEM sites concurrently with transient permeability (Fig. 2A–H and S2C–E). Transient vascular permeability at TMEM is spatially and temporally heterogeneous (fig. S2F), with events of permeability and tumor cell intravasation at TMEM occurring predominantly at vascular branch points (fig. S2G). Transendothelial crossing of tumor cells is visualized by the hourglass shape of tumor cells as they are partially in the vessel lumen and partially in the tissue (Fig. 1C, 2A, C–E and fig. S2E). During transendothelial migration of tumor cells, the TMEM tumor cell and macrophage neither migrate nor intravasate, indicating that tumor cells entering the blood vessel at TMEM are supplied by the migratory stream of cells (Fig 1A, B and D). The stationary phenotype of these cells is consistent with previous results showing macrophage contact -initiated invadopodium formation uniquely in the TMEM tumor cell (9) and that perivascular invadopodium-containing tumor cells are relatively non-motile in vivo (15).
Fig. 1. Motile tumor cells intravasate at TMEM.
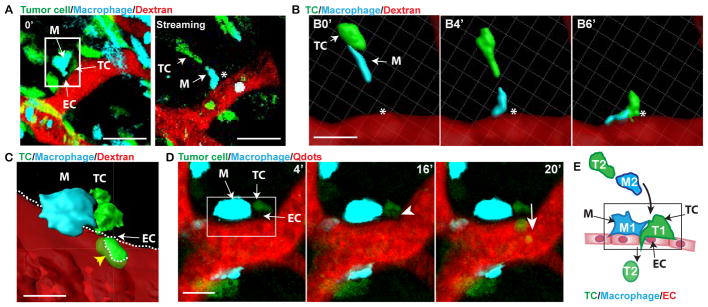
(A) Time 0′ in the left panel indicating TMEM (white box) from time-lapse IVM. Macrophages (M, cyan), Tumor cells (TC, green) and blood vessels (155 kDa Dextran-TMR (red)). Right panel is a single time point from time lapse of tumor cell and macrophage streaming towards non-migratory TMEM (asterisk, TMEM position from left panel). Streams and TMEM are in different focal planes. Scale bar, 50 μm. (B) 3D reconstruction of time-lapse IVM from (A) of TC and macrophage streaming towards TMEM (asterisk). Scale bar, 20 μm. (C) 3D reconstruction of TC intravasation (yellow arrowhead) at TMEM (luminal surface of the endothelium dashed white line). (D) IVM time-lapse of tumor cell intravasation at TMEM (white box in 4′ panel containing stationary TMEM-Macrophage (M), -Tumor cell (TC) and -endothelial cell boundary (EC)(arrows)). A non-TMEM TC arrives at TMEM (arrowhead in panel 16′) and undergoes transendothelial migration (arrow in panel 20′) while TMEM-macrophage and -TC remain immobile. Scale bar = 10 μm. (E) Schematic summary diagram of panels A–D where TC (green, T2) and macrophage (blue, M2) stream towards non-migratory TMEM (black box, T1 and M1), where the TC (T2) undergoes transendothelial migration.
Fig. 2. Transient, local blood vessel permeability events accompany intravasation, at TMEM.
(A) IVM time-lapse of 155 kDa dextran-TMR extravasation and tumor cell intravasation. TMEM (white box). Blood vessel permeability sites (white arrows) and intravasating TC (yellow dashed line, 9′). Clearance of dextran and decrease of CTC at 30′. Scale bar, 50 μm. At 9′ and 30′ TMEM tumor cells and macrophages are added in false color to increase visibility after bleaching. (B) Isolated 155 kDa dextran-TMR channel from (A). Red arrows mark dextran extravasation (white). Dashed red line indicates the luminal side of the endothelium. (C) Isolated tumor cell channel from (A). Yellow arrowhead marks site of intravasating TC (yellow dashed line) at TMEM. White dashed line marks the luminal surface of the endothelium. Red box indicates the region adjacent to TMEM with elevated CTC. (D) Single time point of tumor cell intravasation (yellow dashed line) by time-lapse IVM. Scale bar, 50 μm. (E) 3D reconstruction of time-lapse IVM from (D) of tumor cell intravasation at TMEM. Transmigrating tumor cells (individually numbered, dashed white lines) are isolated from other cell types for clarity with time in minutes from start (J0′) to end of transmigration (J3′). The luminal endothelial surface is outlined in a pink dashed line. Extravascular dextran (red) at TMEM indicated with a yellow arrowhead and outlined in a yellow dashed line. (F) Frequency of blood vessel permeability events in the presence of TMEM or away from TMEM in 100 μm windows (See fig. S4) (n = 16, **, P = 0.0034). (G) Frequency of tumor cell intravasation events in the presence of TMEM or away from TMEM in 100 μm windows (n = 16, **, P = 0.0012). (H) Quantification of extravascular dextran intensity and CTC area at TMEM over time from (A). (●) Extravascular dextran, (■) CTC.
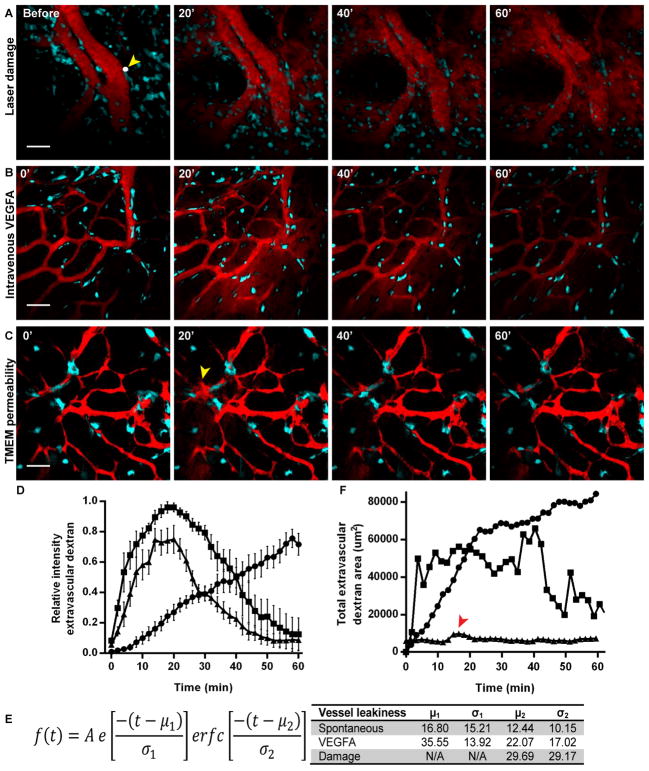
Fig. 3. TMEM-mediated vascular permeability is transient and localized.
(A) Time lapse imaging demonstrates that laser-induced damage to the endothelium creates a hole allowing for extravasation of 155 kDa dextran-TMR. The location of the hole is marked by a white dot (2 μm) and a yellow arrowhead. 155 kDa dextran-TMR extravasates and increases over time up to 60′ filling the field of view and not clearing from the tissue (n = 4). Scale bar, 50 μm. (B) 155 kDa dextran-TMR is injected by tail vein i.v. catheter followed by 8 μg of VEGFA165 at 0′. VEGFA165 induces blood vessel permeability in all of the blood vessels in the field of view. Peak extravascular dextran is observed at 20′ followed by clearance by 60′ after resealing of vascular junctions (n = 4). Scale bar, 50 μm. (C) Spontaneous vascular permeability at TMEM is both transient and local. Local peak extravasation of 155 kDa dextran-TMR occurs after 20′ (yellow arrowhead) and clears within 60′ (n = 11). Scale bar, 50 μm. (D) Quantification of average relative intensity of extravascular 155 kDa dextran-TMR after (●) laser damage (n = 4), (■) intravenous VEGFA165 n = 4 and (▲) spontaneous permeability (n =11). (E) Table of parameters from curve fitting to an Exponentially Modified Gaussian function using data from (D). (F) Quantification of total extravascular 155 kDa dextran-TMR area after laser-induced damage, i.v. injection of VEGFA165 or spontaneous permeability at TMEM from individual animals represented in (A), (B) and (C). Peak of 155 kDa dextran-TMR area in spontaneous permeability at TMEM indicated with a red arrowhead. (●) laser damage, (■) intravenous VEGFA165 and (▲) spontaneous vascular permeability at TMEM.
The peak of extravascular dextran intensity and the appearance of circulating tumor cells coincide temporally and spatially (Fig. 2A–E, H, fig. S2, and Movie S1) demonstrating a direct link between localized blood vessel permeability and tumor cell intravasation at TMEM. The coincidence of spontaneous, transient vascular permeability with tumor cell intravasation at TMEM also has been observed in a patient-derived xenograft model of triple-negative breast cancer, TN1 (fig. S3).
To confirm that TMEM is associated with transient vascular permeability and tumor cell intravasation a 100 μm window, the approximate width of a TMEM site, was consecutively slid along all blood vessels (window measurement) to quantify the frequency of tumor cell intravasation and vascular permeability events in the presence or absence of TMEM (fig. S4). Vascular permeability and tumor cell intravasation occur exclusively within the 100 μm window when it contains a TMEM, but never when the 100 μm window does not contain a TMEM in PyMT (Fig. 2F and G). Similar results were observed in the human TN1 model (fig. S3C and D) highlighting the importance of TMEM in transient vascular permeability and tumor cell intravasation.
Vascular permeability at TMEM is a highly localized and transient event
Tumor vasculature has been previously described as abnormal with increased vascular permeability, which has been attributed to larger vascular intercellular openings (1, 12, 16). However, vascular permeability is not spatially or temporally uniform, with hotspots at vascular branch points (4, 12). Here we demonstrate that vascular permeability is transient, occurs exclusively at TMEM sites, and is temporally heterogeneous, explaining the previously unresolved heterogeneity in vascular permeability (Fig. 2F and fig. S2E). Events of spontaneous, local vascular permeability and tumor cell intravasation at TMEM occur predominantly at vascular branch points, consistent with previous reports of vascular permeability (fig. S2G). If tumor blood vessels were uniformly leaky high-molecular weight vascular probes would extravasate immediately and continuously after injection. While the high-molecular weight probe, 155 kDa dextran-TMR, remains in the vasculature in the absence of transient TMEM-associated permeability events for the duration of the time-lapse imaging, a low molecular weight dextran, 10 kDa dextran-FITC, below the molecular cutoff size of the endothelium (1, 14) leaks from blood vessels and clears from the vascular space (fig. S5).
Further, transient permeability events are distinct from mechanical damage to the endothelium. After creating a 2 μm hole in the endothelium with a laser, 155 kDa dextran-TMR extravasates continuously, filling the field of view (Fig. 3A). By contrast, VEGFA-mediated permeability is transient (12). Intravenous injection of VEGFA165, the soluble isoform of VEGFA with properties of native VEGF (17), results in vascular permeability with peak intensity of extravascular dextran at 20 min (Fig 3B). Spontaneous vascular permeability at TMEM follows similar kinetics to VEGFA165-mediated permeability with peak intensity of extravascular dextran at 20 min but is restricted to individual TMEM sites (Fig. 3C). The curves obtained for average intensity of extravascular 155 kDa dextran-TMR after laser damage, VEGFA165 and spontaneous permeability were fit to an exponentially modified Gaussian function (Fig 3D and E). While the curve for laser damage does not have a clearance term as dextran continues to extravasate for the entire time-lapse, both the VEGFA165 and spontaneous curves have similar extravasation and clearance rates. A significant difference between VEGFA165 and spontaneous TMEM-mediated permeability is that permeability at TMEM is highly local, while VEGFA165 results in dextran extravasation from all blood vessels within a FOV. Thus the area of extravascular 155 kDa dextran-TMR from local TMEM-mediated permeability is markedly less than permeability from VEGFA165 or laser-induced damage (Fig 3F) further emphasizing the local nature of TMEM-mediated vascular permeability.
TMEM-associated macrophages are essential for vascular permeability and tumor cell intravasation
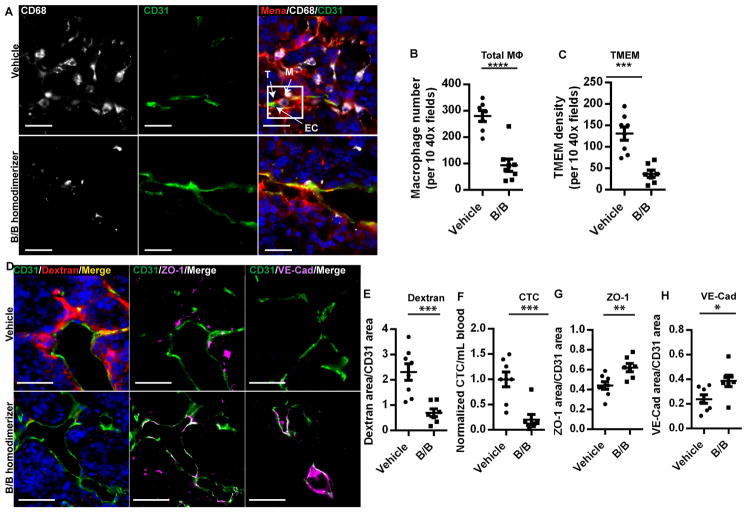
To determine if TMEM macrophages regulate vascular permeability and tumor cell intravasation, macrophages were depleted in the mammary tumor using the previously characterized mouse model, MAFIA (macrophage fas-induced apoptosis) (18, 19) with orthotopic MMTV-PyMT tumor implants. Depletion of macrophages is systemic, including the mammary tumor, thus resulting in a depletion of TAM and TMEM by 67% and 72% respectively (Figure 4A–C). When macrophages are depleted, extravascular dextran decreases, as does the number of circulating tumor cells (Fig. 4D–F). These data demonstrate that macrophages are essential for vascular permeability and tumor cell intravasation at TMEM.
Fig. 4. Macrophage depletion reduces vascular permeability and tumor cell intravasation.
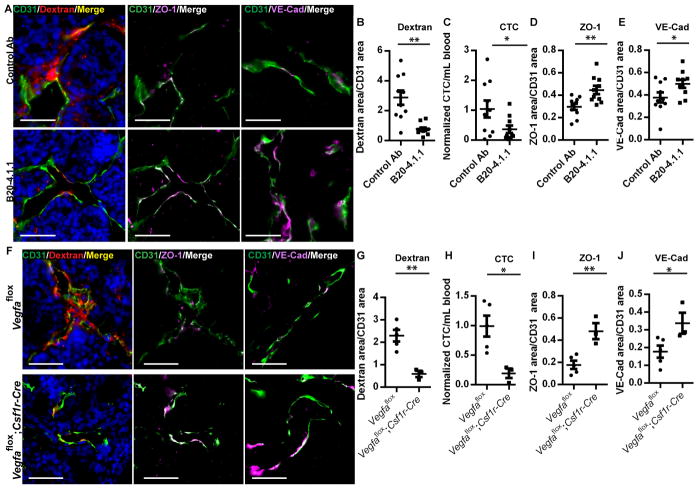
(A) Immunofluorescence imaging of tumor sections stained for TMEM. Vasculature (CD31, green), tumor cells (Mena, red) and macrophages (CD68, gray) and DAPI (blue). TMEM are outlined in a white box. Scale bar, 20 μm. (B) Quantification of total CD68+ macrophages in tumor tissue (****, P < 0.0001), (C) in TMEM (***, P = 0.0003). (D) Immunfluorescence imaging of tumor sections stained for vasculature (CD31, green), 155 kDa dextran-TMR (red) and DAPI (blue), ZO-1 (magenta) or VE-Cadherin (magenta) as indicated demonstrating changes in vascular permeability by extravascular dextran and vascular junction staining. Scale bar, 50 μm. (E) Quantification of extravascular 155 kDa dextran-TMR from (D) (vehicle n = 7, B/B homodimerizer n = 8; ***, P = 0.0009) (F) circulating tumor cells (***, P = 0.0007) (G) Vascular ZO-1 from (D) (**, P = 0.006) and (H) Vascular VE-Cadherin from (D) (* P = 0.02).
Since blood vessel permeability observed by IVM is restricted to TMEM, we examined if vascular junction protein localization was altered in the absence of macrophages, reflecting a requirement for macrophage-dependent signaling events to induce vascular permeability. Staining for vascular junction proteins ZO-1 and VE-Cadherin increased in the tumor vasculature after depletion of macrophages in MAFIA mouse tumors (Fig. 4D, G and H) indicating that macrophages are involved in vascular junction disassembly during vascular permeability events at TMEM.
Tie2-expressing macrophages are localized in TMEM structures
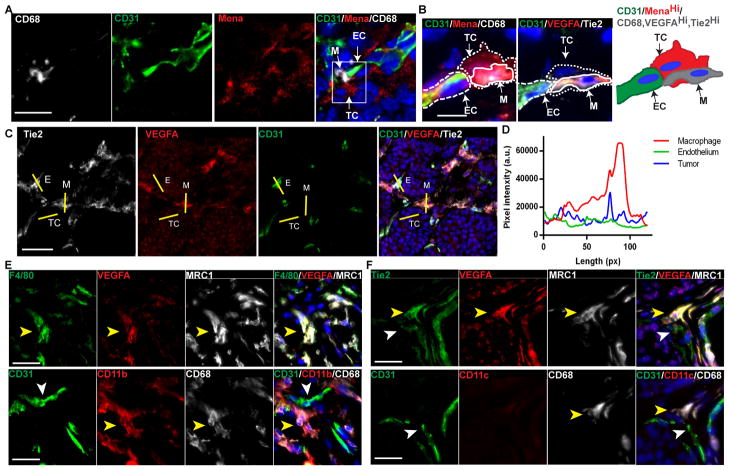
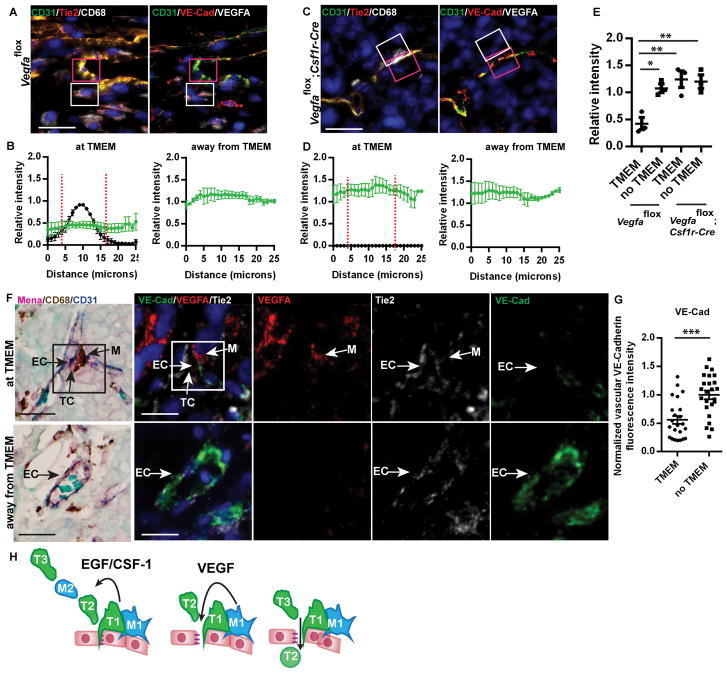
In PyMT mammary carcinoma, a subpopulation of TAMs has been identified as Tie2Hi perivascular macrophages (7, 20, 21). Tie2-expressing macrophages (TEMs) have been shown to upregulate the Tie2 tyrosine kinase receptor by 100 fold after recruitment to the tumor (22). TEMs have features of pro-tumorigenic macrophages and promote tumor angiogenesis (7). TEMs are further characterized as MRC1+/CD11b+/F4/80+/CD11c- and are associated with CD31+ tumor blood vessels (20). Thus, we sought to determine if Tie2-expressing macrophages are located in TMEM. Immunofluorescence of TMEM markers Mena (tumor cells), CD31 (endothelial cells) and CD68 (macrophage) (Fig. 5A) compared to Tie2, VEGFA and CD31 in sequential tissue sections demonstrates that Tie2Hi/VEGFAHi macrophages are enriched in TMEM structures (Fig. 5B and fig. S6A–E). VEGFA is elevated in Tie2Hi macrophages, as compared to the adjacent endothelial cells and surrounding tumor tissue (Fig. 5C and D). Further, 100% of Tie2Hi/VEGFAHi TMEM-associated macrophages express the TEM markers MRC1, CD11b and F4/80 while lacking CD11c (Fig. 5E, F and fig. S6).
Fig. 5. Tie2Hi/VEGFAHi TEMs are present in TMEM.
(A) Immunofluorescence imaging of TMEM. Macrophages (CD68, gray), blood vessels (CD31, green), tumor cells (Mena, red), and DAPI (blue). TMEM in white box (right panel). Scale bar, 15 μm. (B) Immunofluorescence imaging of VEGFAHi macrophages in TMEM in sequential sections. Scale bar, 10 μm. Tumor cell, spotted line; macrophages, solid line; and blood vessels, dashed line. Left panel: Macrophages (CD68, gray), tumor cells (Mena, red), blood vessels (CD31, green), and DAPI (blue). Sequential section (center panel): VEGFA (red), Tie2 (gray), blood vessels (CD31, green), and DAPI (blue). Schematic representation (right panel) of protein expression in TMEM; tumor cells with MenaHi (red), endothelial cells CD31 (green) and macrophages CD68, VEGFAHi and Tie2Hi (gray). M, macrophage; TC, tumor cell; and EC, endothelial cell. (C) Immunofluorescence images of Tie2, VEGFA and CD31. Yellow lines indicate regions of intensity profiling of VEGFA intensity for CD31 (EC), macrophage (M) and tumor tissue (TC). Scale bar, 25 μm. (D) Fluorescence intensity profile of VEGFA from (C) of macrophage, endothelial cell and tumor tissue. (E) Immunofluorescence imaging of sequential PyMT tumor sections for TEM markers. VEGFAHi TMEM macrophages express F4/80, MRC1, CD11b and CD68 as indicated by a yellow arrowhead in sequential sections. CD31+ endothelium is indicated by a white arrowhead. (F) VEGFAHi TMEM macrophages express Tie2, MRC1, and CD68 but not CD11c as indicated by a yellow arrowhead. CD31+/Tie2+ endothelium is indicated by a white arrowhead. Scale bar, 25 μm.
Inhibition of VEGFA signaling reduces vascular permeability and tumor cell intravasation
To investigate the importance of VEGFA in TMEM function we blocked VEGFA binding to VEGF receptors using a neutralizing antibody (B20-4.1.1), and found a decrease in extravascular dextran and circulating tumor cells (Fig. 6A–C). Binding of VEGFA to VEGR2 leads to junction disassembly (23). Vascular ZO-1 and VE-Cadherin staining increased during VEGFA inhibition suggesting an increase in integrity of endothelial adherens and tight junctions from reduced bioavailability of VEGFA, including VEGFA from TMEM (Fig. 6A, D and E).
Fig. 6. Inhibition of VEGFA or macrophage-specific ablation of Vegfa from Tie2Hi/VEGFAHi TMEM macrophages reduces vascular permeability and tumor cell intravasation.
(A) Immunfluorescence imaging of tumor sections after blocking VEGFA with anti-VEGFA blocking antibody (B20-4.1.1). Tumors are stained for vasculature (CD31, green), 155 kDa dextran-TMR (red) and DAPI (blue), ZO-1 (magenta) or VE-Cadherin (magenta) as indicated demonstrating changes in vascular permeability by extravascular dextran and vascular junction staining. Scale bar, 50 μm. (B) Quantification of extravascular 155 kDa dextran-TMR from (A) and (n = 10; **, P = 0.0015) (C) circulating tumor cells (*, P = 0.0497), (D) Vascular ZO-1 from (A) (**, P = 0.005) and (E) Vascular VE-Cadherin from (A) (*, P = 0.0463). (F) Immunfluorescence of tumor sections stained for vasculature (CD31, green), 155 kDa dextran-TMR (red) and DAPI (blue), ZO-1 (magenta) or VE-Cadherin (magenta) as indicated demonstrating changes in vascular permeability after ablation of Vegfa by extravascular dextran and vascular junction staining. Scale bar, 50 μm. (G) Quantification of extravascular 155 kDa dextran-TMR from (F) and (Vegfaflox n = 5, Vegfaflox;Csf1r-Cre n = 3; **; P = 0.0029) (H) circulating tumor cells (*, P = 0.0177) (I) Vascular ZO-1 from (F) (**, P = 0.0054) and (J) Vascular VE-Cadherin from (F) (*, P = 0.0457).
VEGFA signaling from Tie2Hi/VEGFAHi TMEM macrophages mediates vascular permeability and tumor cell intravasation
To determine if the subpopulation of Tie2Hi/VEGFAHi macrophages in TMEM are an essential source of VEGFA in the tumor microenvironment required for transient vascular permeability at TMEM and tumor cell intravasation, VEGFA was selectively ablated in monocytes and macrophages using the Vegfaflox/flox;Csf1r-Mer-iCre-Mer transgenic mouse depletion model of Vegfa that targets myeloid cells expressing Csf1r, including both Ly6CHi and Ly6CLo populations, including the TEM population (24). Macrophage-specific depletion of VEGFA reduced transient vascular permeability, and circulating tumor cells, while restoring vascular junctions (Fig. 6F–J). Immunofluorescence of sequential sections demonstrates that blood vessels adjacent to CD68+/Tie2Hi/VEGFAHi TMEM macrophage have significantly reduced vascular VE-Cadherin/CD31 relative intensity compared to regions of vasculature away from TMEM sites in Vegfaflox tumors (Fig. 7A and B). Further, when VEGFA has been ablated in Vegfaflox;Csf1r-Cre tumors VE-Cadherin/CD31 relative staining intensity is the same along the tumor vasculature as in regions away from TMEM (Fig. 7C and D). Therefore, vascular junction integrity, as measured by VE-Cadherin/CD31 relative staining intensity, is only significantly reduced in regions of vasculature adjacent to VEGFAHi TMEM macrophages in TMEM (Fig. 7E). Further, pericyte coverage of the vasculature is reduced in regions of VEGFAHi TEMs in TMEM as compared to regions away from VEGFAHi TMEM structures (fig. S6F – H). A decrease in pericyte coverage of vasculature has been correlated with increased metastasis and vascular permeability (25).
Fig. 7. Macrophage-specific ablation of Vegfa in PyMT implant tumors blocks blood vessel permeability and tumor cell intravasation at TMEM.
(A, C) Immunofluorescence of tumor sections stained for the presence of vascular junction proteins at TMEM macrophages. Tumor sections are stained for VE-cadherin (red), CD31 and VEGFA (gray). Sequential sections are stained for CD31 (green), Tie2 (red) and CD68 (gray). (A) Control tumors (Vegfaflox) or (C) after ablation of Vefga (Vegfaflox;Csf1r-Cre). CD68+ macrophage in TMEM outlined in white box, adjacent endothelium in TMEM in pink box. Yellow indicates merged signal of CD31 and Tie2 (left) or CD31 and VE-Cadherin (right). Decreased VE-Cadherin at TMEM (F, right) seen as decreased VE-Cadherin, resulting in green (CD31) at vascular junction. Scale bar, 15 μm. (B, D) Quantification of the relative intensity of VEGFA or vascular junction proteins (ratio of VE-cadherin to CD31 in blood vessels) in (A and C) at TMEM or away from TMEM in (B) control tumors (Vegfaflox) or (D) after ablation of Vegfa in Vegfaflox;Csf1r-Cre tumors along 25 μm lengths of blood vessel (n = 3). (●) Relative fluorescence intensity of VE-Cadherin/CD31, (■) Relative VEGFA intensity. Red dashed line indicates the presence of a CD68+ macrophage. (E) Quantification of average pixel intensity of VE-Cadherin/CD31immunofluorescence staining in 25 μm lengths of blood vessel at TMEM or away from TMEM in the presence of VEGFAHi macrophages (Vegfaflox , n = 3) or after macrophage-specific ablation of Vegfa (Vegfaflox;Csf1r-Cre, n = 3) from data in B and D. Post-ANOVA comparisons with significant difference indicated (*, **). (F) Human breast cancer tumor sections stained for the presence of vascular junction proteins at TMEM macrophages. Tumor sections are stained for TMEM; Mena (red), CD68 (brown) and CD31 (blue), (RBC in aqua) by IHC and for VE-cadherin (green), Tie2 (gray) and VEGFA (red) stained by immunofluorescence in sequential sections. TMEM outlined in black box in IHC and white box in immunofluorescence. Scale bar, 15 μm. (G) Quantification of normalized average pixel intensity of VE-Cadherin staining in vasculature at Tie2Hi/VEGFAHi macrophages of TMEM or away from TMEM in (n = 23 at TMEM, n = 24 away from TMEM in 5 individual patient samples, ***, P = 0.0001). (H) Cartoon summarizing TMEM macrophage-mediated induction of blood vessel permeability promotes tumor cell intravasation. TMEM assemble with close association between the non-migratory TMEM TC (green, T1) and Tie2Hi/VEGFAHi macrophage (blue, M1) on blood vessels. VEGFA destabilizes vascular junctions resulting in vascular permeability and TC (T2) intravasation.
To establish the relevance of Tie2Hi/VEGFAHi macrophages in TMEM structures in mediating vascular permeability and tumor cell dissemination in metastastic breast cancer, vascular junction staining was measured in human breast cancer patient samples. Staining of sequential sections demonstrates that blood vessels adjacent to Tie2Hi/VEGFAHi macrophages in TMEM have significantly reduced vascular VE-Cadherin fluorescence intensity compared to regions of vasculature away from TMEM (Fig. 7F, G and fig. S7).
Together these data establish that the Tie2Hi/VEGFAHi TMEM macrophages interact with endothelial cells through VEGFA signaling to mediate local, transient blood vessel permeability demonstrating the mechanism underlying the clinically-demonstrated association of TMEM density with metastatic recurrence of breast cancer.
DISCUSSION
Although the abnormality and permeability of tumor vasculature has been well characterized, the mechanism leading to spatial and temporal heterogeneity in permeability has not been resolved. The use of high-resolution multiphoton microscopy has allowed for the study of vascular permeability and tumor cell dissemination in mammary carcinoma at unprecedented spatial and temporal resolution. Our data show that in the PyMT authochthonous mouse mammary carcinoma and human patient-derived xenograft TN1 models, that vascular permeability is dynamic, localized, and restricted to TMEM. These data are consistent with previous findings that hyperpermeability of tumor vasculature is heterogeneous and often in the presence of perivascular macrophages (4), but further explains the observed heterogeneity and that tumor cell intravasation occurs at sites of vascular permeability.
The sites of dynamic tumor vascular permeability have been identified at sites of VEGFAHi perivascular macrophages at TMEM. The clinical significance of TMEM density in predicting metastatic risk has been recently expanded to a large cohort of patients, further emphasizing the importance of TMEM in breast cancer metastasis (11). These data demonstrate that Tie2Hi/VEGFAHi perivascular macrophages in TMEM share the characteristics of the pro-angiogenic and pro-metastatic Tie2-expressing macrophages (7). Thus, we have been able to expand our understanding of the function of this subset of TAMs in the tumor microenvironment in promoting metastasis.
Mechanistically, macrophage/tumor cell streams migrate to TMEM sites through the EGFR/CSF-1R paracrine loop (26). Elevated expression of VEGFA in the Tie2Hi TMEM macrophage results in transient permeability of tumor blood vessels proximal to TMEM that occurs by disassembling endothelial cell junctions. The simultaneous attraction of migratory tumor cells and transient blood vessel permeability results in a concurrent spike in tumor cell intravasation with vascular permeability at TMEM sites (Fig. 7H). These data, together with the clinical association of TMEM with distant metastatic tumor recurrence in human breast cancer patients explain why TMEM density can predict metastasis and argues for the development of therapeutic approaches targeted against both TMEM formation and function.
Materials and Methods
Mice
All studies involving mice were carried out in accordance with the National Institutes of Health regulation concerning the care and use of experimental animals and approved by the Albert Einstein College of Medicine Animal Care and Use Committee. PyMT (MMTV-PyMT) transgenic mice were bred in house. Mafia mice [C57BL/6-Tg(Csf1r-EGFP-NGFR/FKBP1A/TNFRSF6)2Bck/J] were obtained from The Jackson Laboratory and were implanted with tumor pieces (2 mm × 2mm) into the 4th mammary fat pad on the left side. For multiphoton microscopy transgenic mice were generated to label the myeloid lineage and mammary tumor cells by crossing MacBlue mice [Csf1r-GAL4VP16/UAS-enhanced cyan fluorescent protein (ECFP)](27) in C57BL/6 background with Tg(MMTV-iCre)-Tg(loxP-stop-loxP-PDendra2)jwp (28) mice of FVB background. The FVB macrophage-specific (Csf1r promoter), tamoxifen-inducible Cre expressing Tg(Csf1r-Mer-iCre)1jwp transgenic mouse was crossed with Vegfaflox/flox mice. Depletion of Vegfa in myeloid cells was induced by daily subcutaneous injection of 3 mg tamoxifen in corn oil per mouse for 2 d before injection of 155 kDa TMR-dextran (24). TN1 cells were isolated from a pleural effusion sample from a patient with triple negative breast cancer (ER-/PR-/Her2-) and transduced to express GFP (29). TN1-GFP patient-derived cells are only passaged in vivo in NOD.SCID mice (Jackson Laboratories) by orthotopic injection of 106 dissociated tumor cells mixed with 50% matrigel (BD Biosciences) into the 4th mammary fat pad on the left side of the mouse. No cell lines are used in this study.
Intravital imaging
Multiphoton intravital microscopy was performed as using a skin flap procedure as previously described (30) and a custom-built 2-laser multiphoton microscope (28). The animal was placed in a heated chamber maintained at physiological temperature during the course of imaging and monitored using MouseOx (Starr Life Sciences). 3 mg of 155 kDa tetramethylrhodamine-dextran (TMR-dextran) or 100 μL of 8 μM Qdots 705 (obtained from A. Smith, UIUC or Life Technologies Qdots ITK 705)(31–33) was administered via the tail vein catheter.
Inhibitory antibodies
Animals were administered 5 mg/kg B20-4.1.1 (Genentech) VEGFA neutralizing antibody or antibody isotype control by intravenous injection 6 h before termination of the experiment.
Macrophage depletion with B/B homodimerizer in Mafia mice
Animals were administered 10 mg/kg B/B homodimerizer (AP20187, Clontech) in diluted in 4 % ethanol, 10 % PEG-400 and 1.7 % Tween-20 or vehicle control by intraperitoneal injection daily for 5 days. 24 h after the last injection of B/B homodimerizer animals were administered 3 mg of 155 kDa dextran-tetramethylrhodamine for 1h and 100 uL of anti-CD31 antibody for 10 min.
Labeling of tumor vasculature and extravasation of 155 kDa dextran-TMR
One hour before the termination of the experiments with inhibitors or MAFIA mice 3 mg of 155 kDa TMR-dextran was administered by tail vein i.v. to label sites of vascular permeability. In tumor tissue several transient permeability events may occur at any given time due to the spatial and temporal heterogeneity of vascular permeability, thus quantitation of extravascular dextran over the course of 1 h will capture these dynamic events. Anti-mouse CD31-biotin was administered by tail vein i.v. for 10 minutes to label flowing blood vessels. Tumors were fixed in 4 % PFA and cryoprotected in 30 % sucrose in PBS before freezing in OCT. 5 μm sections of tumors were cut and immunofluorescence performed.
Circulating tumor cells for PyMT
Circulating tumor cells are isolated from anesthetized mice from blood drawn from the right ventricle of the heart. The use of blood burden experiments obtained by cardiac puncture are used for end point experiments to capture all of the CTCs in the animal blood at the experiment endpoint. Red blood cells are lysed using RBC lysis buffer (multi-species, eBioscience) before the cells are placed in culture with Dulcecco’s Modified Eagle Medium:Nutrient Mixture F-12 (DMEM/F-12) with 20 % FBS. Adherent tumor cells are counted at a time of no tumor cell growth, which is a count of CTC. Tumor cells have been identified by fluorescence microscopy as Dendra2- or CFP-expressing tumor cells as previously described (34, 35). Circulating tumor cells were also scored by quantifying tumor cells in blood vessels adjacent to TMEM as seen in Fig. 2 and fig. S4 in live animals. This method is described under Intravital microscopy image analysis in the Supplemental Material and Methods.
Immunofluorescence
Tumor sections were fixed and permeabilized with cold acetone, washed with PBS and blocked with block solution (1% BSA, 10 % FBS, and 0.0025 % fish skin gelatin in PBS-T). The following primary antibodies were used: rat anti-mouse CD68 (clone FA-11, Serotec), or AlexaFluor647-conjugated CD68 (eBioscience), mouse anti-Mena (from F. Gertler), rat anti-ZO-1 (clone R40.76, Millipore), goat anti-VE-Cadherin (clone C-19), rabbit anti-VEGFA (clone A-20, Santa Cruz Biotechnology), hamster anti-CD11c (clone HL-3, BD Bioscience), rat anti-CD11b (clone ICRF4, BD Biosystems), rat anti-F4/80 (clone BM8, eBioscience), goat anti-MRC1/CD206 (R&D Systems), rat anti-Tie2 (clone TEK4, eBioscience), and rabbit anti-NG2 (Millipore). Sections were washed with PBS-T and the primary antibodies were detected with AlexaFluor488, 555 or 647 secondary antibody conjugates (Molecular Probes/Invitrogen) and nuclei stained with 4,6-diamidino-2-phenylindole (DAPI). All fluorescently labeled samples were mounted with Prolong Gold antifade reagent (Molecular Probes/Invitrogen) and analyzed with a compound fluorescent microscope (Zeiss Axio Observer; 40X objective with numerical aperture 1.3). Images of tumor sections were acquired using mosaic tiling of 40X images in AxioRel version 4.8. All images were acquired as 16-bit TIFF images and all quantitative analysis was performed on the raw 16-bit TIFF images in ImageJ.
TMEM immunohistochemistry
Mouse tumor sections were fixed overnight in 10 % neutral buffered saline prior to embedding in paraffin. 5 μm tumor sections were deparaffinized and stained for H&E or TMEM using anti-Iba-1 (macrophages), anti-endomucin (blood vessels) and anti-Mena (tumor cells) (36) and TMEM quantified as previously described (8).
Human immunohistochemistry and immunofluorescence
Formalin-fixed paraffin embedded patient tissue from 5 invasive ductal carcinomas was collected under the Montefiore-Einstein IRB approval. Paraffin embedded human breast cancer tumors were cut to 5 μm sections, deparaffinized and stained for H&E or TMEM. The sequence was anti-CD31 (clone JC70A, DAKO, Carpinteria, CA) and Vector Blue chromogen (for endothelial cells); anti CD-68 (clone PG-M1; DAKO) with and DAB chromogen (for macrophages); and anti-pan-Mena with Fast Red chromogen (for carcinoma cells) (8, 11). Sequential sections were cut for tyramide signal amplification (TSA) for quantitative immunofluorescence using the Opal 3-plex Kit (Perkin Elmer) according to manufacturer’s directions. The sequence was rabbit anti-VEGF (1:2000, Rb 9031-P0-A, Thermo) with TSA Plus Cy3; rabbit anti-Tie2 (1:3000, clone C-20, Santa Cruz Biotech) with TSA Plus Cy5; goat anti-VE-cadherin (1:200, clone C-19, Santa Cruz Biotech) with TSA Plus fluorescein and nuclei stained with 4,6-diamidino-2-phenylindole (DAPI). All quantitative analysis was performed on the raw 16-bit TIFF images and images of TMEM were validated independently by a pathologist.
Exponentially Modified Gaussian function fitting
Vascular leakage is composed of two competing processes, an increase in extravascular signal due to leakage from the vasculature, as well as a diffusive clearance. The Exponentially Modified Gaussian (EMG) asymetic function (37) is composed of the product of a sigmoidal error function with an exponential decay. Least squares curve fitting was performed utilizing a nonlinear Generalized Reduced Gradient (GRG2) solver and values directly compared (38).
Statistical analysis
Individual animals are presented as individual points, a horizontal line indicates the mean and error bars represent the standard error of the mean. One-way or two-way ANOVA was performed for data sets with more than two groups to determine significance. Statistical significance was determined by the comparison of the means of two groups using an unpaired, two-sided t-test using Prism (Graph Pad Inc.). Data sets were checked for normality (D’Agostino & Pearson omnibus normality test or Shapiro-Wilk normality test) and unequal variance using Prism (Graph Pad Inc.). Welch’s correction was applied to t-tests as needed. P values of less than 0.05 were deemed significant.
Supplementary Material
SIGNIFICANCE.
Tumor vasculature is abnormal with increased permeability. Here we show that VEGFA signaling from Tie2Hi TMEM macrophages results in local, transient vascular permeability and tumor cell intravasation. These data provide evidence for the mechanism underlying the association of TMEM with distant metastatic recurrence, offering a rationale for therapies targeting TMEM.
Acknowledgments
For technical assistance we thank H. Guziak at the Analytical Imaging Facility of the Albert Einstein College of Medicine and the NCI cancer center support grant (P30CA013330). We thank A. Smith (UIUC) for Quantum dots and F. Gerlter (MIT) for the gift of the pan-Mena antibody. We thank Jiufeng Li for assistance in generating the MMTV-Dendra mouse. This research was supported by the Department of Defense Breast Cancer Research Program under award number (W81XWH-13-1-0010), NIH CA100324 and the Integrated Imaging Program. Views and opinions of, and endorsements by, the authors do not reflect those of the US Army or the Department of Defense.
List of abbreviations
- TAM
tumor-associated macrophage
- MMTV
mouse mammary tumor virus
- PyMT
polyoma middle T antigen
- TMEM
tumor microenvironment of metastasis
- IVM
intravital microscopy
- LC
late carcinoma
- EC
early carcionoma
- TEM
Tie2-expressing macrophage
- TMR
tetramethylrhodamine
- FOV
field of view
- IHC
immunohistochemisty
- FITC
fluorescein isothiocynate
Footnotes
Conflicts of interest: The authors acknowledge the following financial relationships: Consultant and stockholder of MetaStat, Inc. (Boston, MA) (JGJ and JSC); Consultant of MetaStat (MHO, DE); Consultant of Deciphera Pharmaceuticals, LLC (Cambridge, MA) (JSC); (ASH, and YW) Sponsored research agreement with Deciphera. Neither MetaStat nor Deciphera contributed financial resources and reagents to this paper. The other authors disclose no potential conflicts of interest.
References
- 1.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–6. [PubMed] [Google Scholar]
- 2.Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues. Microvasc Res. 1986;31:288–305. doi: 10.1016/0026-2862(86)90018-x. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular Normalization as an Emerging Strategy to Enhance Cancer Immunotherapy. Cancer Research. 2013;73:2943–8. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dvorak HF, Dvorak AM, Manseau EJ, Wiberg L, Churchill WH. Fibrin gel investment associated with line 1 and line 10 solid tumor growth, angiogenesis, and fibroplasia in guinea pigs. Role of cellular immunity, myofibroblasts, microvascular damage, and infarction in line 1 tumor regression. Journal of the National Cancer Institute. 1979;62:1459–72. [PubMed] [Google Scholar]
- 5.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–40. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–46. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 7.De Palma M, Venneri MA, Galli R, Sergi LS, Politi LS, Sampaolesi M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–26. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Robinson BD, Sica GL, Liu YF, Rohan TE, Gertler FB, Condeelis JS, et al. Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res. 2009;15:2433–41. doi: 10.1158/1078-0432.CCR-08-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roh-Johnson M, Bravo-Cordero JJ, Patsialou A, Sharma VP, Guo P, Liu H, et al. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene. 2014;33:4203–12. doi: 10.1038/onc.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–56. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 11.Rohan TE, Xue X, Lin H-M, D’Alfonso TM, Ginter PS, Oktay MH, et al. Tumor Microenvironment of Metastasis and Risk of Distant Metastasis of Breast Cancer. Journal of the National Cancer Institute. 2014:106. doi: 10.1093/jnci/dju136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monsky WL, Fukumura D, Gohongi T, Ancukiewcz M, Weich HA, Torchilin VP, et al. Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 1999;59:4129–35. [PubMed] [Google Scholar]
- 13.Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–26. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. Journal of the National Cancer Institute. 2006;98:335–44. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 15.Gligorijevic B, Bergman A, Condeelis J. Multiparametric classification links tumor microenvironments with tumor cell phenotype. PLoS biology. 2014;12:e1001995. doi: 10.1371/journal.pbio.1001995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156:1363–80. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–9. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 18.Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, et al. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol. 2004;75:612–23. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- 19.Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–71. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pucci F, Venneri MA, Biziato D, Nonis A, Moi D, Sica A, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–14. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 21.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–26. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 22.De Palma M, Mazzieri R, Politi LS, Pucci F, Zonari E, Sitia G, et al. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell. 2008;14:299–311. doi: 10.1016/j.ccr.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Nakayama M, Berger P. Coordination of VEGF receptor trafficking and signaling by coreceptors. Experimental Cell Research. 2013;319:1340–7. doi: 10.1016/j.yexcr.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooke VG, LeBleu VS, Keskin D, Khan Z, O’Connell JT, Teng Y, et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell. 2012;21:66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–9. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 27.Ovchinnikov DA, van Zuylen WJM, DeBats CEE, Alexander KA, Kellie S, Hume DA. Expression of Gal4-dependent transgenes in cells of the mononuclear phagocyte system labeled with enhanced cyan fluorescent protein using Csf1r-Gal4VP16/UAS-ECFP double-transgenic mice. Journal of Leukocyte Biology. 2008;83:430–3. doi: 10.1189/jlb.0807585. [DOI] [PubMed] [Google Scholar]
- 28.Entenberg D, Wyckoff J, Gligorijevic B, Roussos ET, Verkhusha VV, Pollard JW, et al. Setup and use of a two-laser multiphoton microscope for multichannel intravital fluorescence imaging. Nat Protocols. 2011;6:1500–20. doi: 10.1038/nprot.2011.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Patel MR, Prescher JA, Patsialou A, Qian D, Lin J, et al. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci U S A. 2010;107:18115–20. doi: 10.1073/pnas.1006732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wyckoff J, Gligorijevic B, Entenberg D, Segall J, Condeelis J. High-Resolution Multiphoton Imaging of Tumors In Vivo. Cold Spring Harbor Protocols. 2011;2011 doi: 10.1101/pdb.top065904. pdb.top065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith AM, Nie S. Compact Quantum Dots for Single-molecule Imaging. Journal of Visual Experiments. 2012:e4236. doi: 10.3791/4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith AM, Nie S. Bright and compact alloyed quantum dots with broadly tunable near-infrared absorption and fluorescence spectra through mercury cation exchange. J Am Chem Soc. 2011;133:24–6. doi: 10.1021/ja108482a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith AM, Nie S. Minimizing the hydrodynamic size of quantum dots with multifunctional multidentate polymer ligands. J Am Chem Soc. 2008;130:11278–9. doi: 10.1021/ja804306c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wyckoff JB, Jones JG, Condeelis JS, Segall JE. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 2000;60:2504–11. [PubMed] [Google Scholar]
- 35.Roussos ET, Wang Y, Wyckoff JB, Sellers RS, Wang W, Li J, et al. Mena deficiency delays tumor progression and decreases metastasis in polyoma middle-T transgenic mouse mammary tumors. Breast Cancer Res. 2010;12:R101. doi: 10.1186/bcr2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lebrand C, Dent EW, Strasser GA, Lanier LM, Krause M, Svitkina TM, et al. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron. 2004;42:37–49. doi: 10.1016/s0896-6273(04)00108-4. [DOI] [PubMed] [Google Scholar]
- 37.Foley JP, Dorsey JG. A Review of the Exponentially Modified Gaussian (EMG) Function: Evaluation and Subsequent Calculation of Universal Data. Journal of Chromatographic Science. 1984;22:40–6. [Google Scholar]
- 38.Fylstra D, Lasdon L, Watson J, Waren A. Design and Use of the Microsoft Excel Solver. Interfaces. 1998;28:29–55. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.