Abstract
Background
Pre-clinical evidence supports the clinical investigation of inhibitors to the insulin-like growth factor receptor (IGFR) and the epidermal growth factor receptor (EGFR) alone and in combination in patients with NSCLC.
Patients and Methods
Patients with chemotherapy-naïve, advanced NSCLC and an ECOG performance status (PS) 0/1 were eligible. Patients were randomized to receive: carboplatin AUC 6 iv + paclitaxel 200 mg/m2 iv on day 1 every 3 weeks combined with either cetuximab (CET) iv weekly (arm A), cixutumumab (CIX) iv every 2 weeks (arm B), or both (arm C). Patients with non-progressive disease (PD) after 12 weeks of therapy were permitted to continue on maintenance antibody therapy until PD. The primary endpoint was progression-free survival (PFS). The design required 180 eligible patients and had an 88% power to detect a 60% increase in median PFS for either comparison (arm A vs C or arm B vs C) using the log-rank test.
Results
From 9/09 until 12/10, 140 patients were accrued. The study was closed to accrual early because of excessive number of grade 5 events reported on arms A and C. Thirteen patients died during treatment (A=6; B=2; C=5), including 9 within approximately 1 month of starting therapy. The estimated median PFS for arms A/B/C were similar at 3.4, 4.2, and 4 months, respectively.
Conclusions
Based upon the apparent lack of efficacy and excessive premature deaths, this study does not support the continued investigation of carboplatin + paclitaxel + CIX alone or in combination with CET in patients with advanced NSCLC.
Introduction
Molecularly targeted agents are becoming an important component of therapy in the management of some patients with advanced non-small cell lung cancer (NSCLC). One of the hallmarks of cancer is the dysregulation of growth signaling pathways (1). Inhibition of the epidermal growth factor receptor (EGFR) pathway has been validated in some patients with advanced NSCLC (2). In one phase III study, patients with advanced NSCLC randomized to receive chemotherapy with or without cetuximab, a monoclonal antibody to EGFR, demonstrated a modest improvement survival with the addition of cetuximab, although this was not sufficient to lead to regulatory approval (3). Another study of carboplatin plus a taxane with and without cetuximab demonstrated a numerically higher overall survival (p=NS) for the cetuximab-containing arm (4).
In recent years the insulin-like growth factor (IGF) has been studied to understand its’ role in the development, pathogenesis, and progression of lung cancer. Signaling through the IGF receptor (IGFR) pathway occurs primarily through the phosphatidylinositol-3 kinase (PI3K)-Akt pathway and the mitogen-activated protein kinase (MAPK) pathway, resulting in increased cell proliferation and inhibition of apoptosis (5–7). Deregulation of the IGF pathway appears to result in an increased risk of lung cancer, decreased survival in patients with stage I disease, and facilitation of malignant transformation (8–11). Synergistic activity of IGF-1R inhibitors and cytotoxic agents has been described, establishing the rationale for combining IGFR inhibitors with chemotherapy (12). In addition, outcomes of nude mice bearing A549 NSCLC tumors treated with the anti-IGF-1R antibody h7C10 combined with chemotherapy or the anti-EGFR antibody, cetuximab, were superior to those of mice treated with either agent alone (12). The rationale for combining EGFR and IGFR inhibitors is further supported by additional studies which implicate the expression of IGF-1R with a reduced efficacy of anti-EGFR targeting, including resistance to gefitinib (13–15). Other pathway activation, including AKT signaling, has been associated with resistance to EGFR inhibitors (16). Combining IGF-1R inhibition with EGFR inhibition may reduce this AKT pathway activity (16).
Monoclonal antibodies, including the fully human IgG1 antibody Cixutumumab, have been developed to deregulate the IGF pathway. In vitro, treatment with Cixutumumab induces apoptosis in human xenograft models and demonstrates increased cytotoxicity when combined with EGFR inhibition (17). A randomized phase II study comparing patients treated with chemotherapy +/− IGFR inhibition originally reported higher response rates (although subsequently retracted in 2012) for those receiving the anti-IGFR therapy (18). Therefore, based upon pre-clinical and early clinical information, we conducted this randomized phase II study to evaluate the efficacy of chemotherapy combined with EGFR inhibition or IGFR inhibition or both in patients with advanced NSCLC who are not receiving bevacizumab-based therapy.
Patients and Methods
Eligibility
To be eligible, patients must have had a diagnosis of NSCLC, measurable (as defined by RECIST version 1.1) stage IV disease (including M1a and M1b according to the 7th edition of the TNM classification system) or T4NX (stage IIIB) defined by a nodule in the ipsilateral lung lobe, if not a candidate for combined chemotherapy and radiation or surgery. Patients must have not received prior systemic therapy, including bevacizumab, anti-EGFR or anti-IGFR therapy, for advanced disease. Patients receiving neo-adjuvant or adjuvant chemotherapy were eligible if more than 1 year had passed prior to randomization to this trial. All patients had an ECOG PS of 0 or 1. Patients were not eligible if they had untreated or symptomatic central nervous system (CNS) metastases. Patients with a history of CNS metastases that were both definitively treated and stably controlled were eligible. Patients were also excluded if they had major surgery within 4 weeks prior to randomization, history of interstitial pneumonitis or pulmonary fibrosis, uncontrolled hypertension or cardiac disease, synchronous malignancy within the last 3 years or those thought to be of a low risk for recurrence definitively treated < 3 years prior to randomization, serum fasting glucose of > 120 mg/dL or above the institutional upper limits of normal (ULN) within 2 weeks prior to randomization, poorly controlled diabetes mellitus, history of allergic reactions attributed to compounds of similar chemical or biological composition to cixutumumab, history of arterial thrombosis, pulmonary embolus, deep venous thrombosis or hemorrhagic disorders <28 days prior to randomization, or peripheral neuropathy > grade 1 as per the CTCAE version 4 grading scale. Within 2 weeks prior to randomization, partial thromboplastin time (PTT) < 1.2 × ULN and international normalized ratio (INR) ≤ 1.5 was required. Patients must have had normal hepatic, renal, and bone marrow function. Written informed consent was obtained from all participants.
Study Design, Endpoints, and Treatment
This was a multi-center, randomized trial conducted by ECOG. The primary objective was to evaluate the progression-free survival (PFS) of patients with NSCLC randomized to carboplatin plus paclitaxel plus either cetuximab (arm A), cixutumumab (arm B), or both (arm C). The secondary objectives included evaluation of response rate, disease control rate (complete response plus partial response plus stable disease), overall survival, and toxicities. Additional secondary objectives included evaluating EGFR by immunohistochemistry (IHC), mutation, and gene copy number, IGF-1R and IGF-2R expression, and KRAS mutation. Plasma-based biomarkers were also evaluated for total and free IGF-1 and IGF-2, and IGF-growth factor binding protein 3 (IGFBP3).
The treatment schema for this trial is displayed in Figure 1. Each treatment cycle lasted 6 weeks (42 days). Patients were randomized to receive carboplatin AUC=6 i.v. plus paclitaxel 200 mg/m2 i.v. on days 1 and 22 of each cycle in combination with either cetuximab 250 mg/m2 i.v. days 1, 8, 15, 22, 29, and 36 of each cycle (Arm A) or cixutumumab 10 mg/kg i.v. on days 1, 15, and 29 of each cycle (Arm B), or the combination of cetuximab and cixutumumab (Arm C) at the dose and schedule specified for these therapies on arms A and B, respectively. The loading dose for patients receiving cetuximab was 400 mg/m2 i.v. on day 1 of cycle 1 only. After two 6-week cycles of induction therapy, patients who had not progressed continued onto maintenance therapy of cetuximab, cixutumumab, or both corresponding to their treatment assignment per randomization until disease progression or unacceptable toxicity. Imaging studies were conducted at baseline and at each 6 week treatment interval.
Figure 1. E4508 Schema.
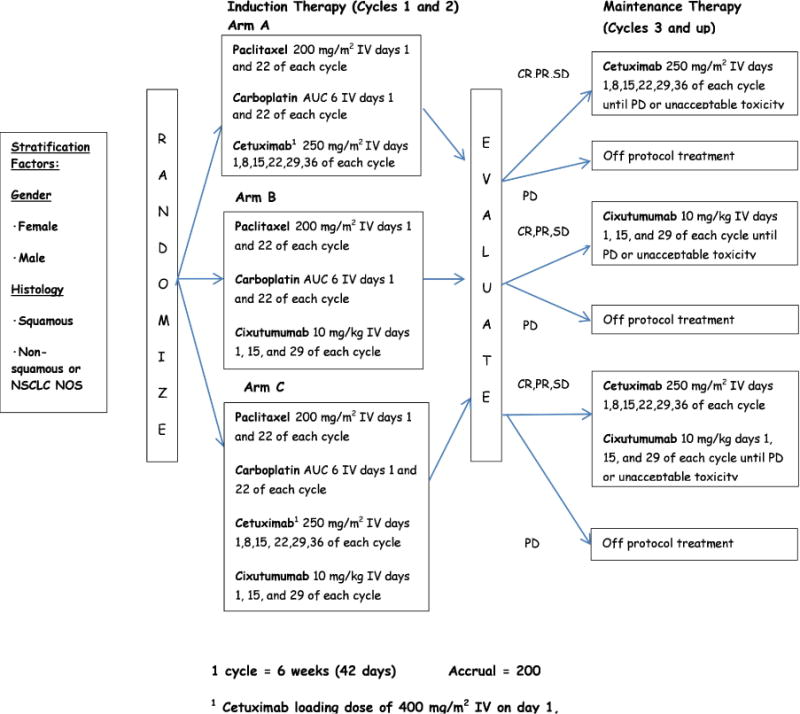
This figure provides the treatment regimen, schedule, and stratification factors
Tumor samples were analyzed by immunohistochemistry (IHC; EGFR, IGF-1R and IGF-2R expression), fluorescent in-situ hybridization (FISH; EGFR gene copy number) and DNA sequencing (EGFR, KRAS gene mutations). FISH scores were interpreted according to the Colorado Scoring Criteria (19).
Statistical Considerations
This 3-arm study planned to randomize 180 eligible and treated patients (200 total patients after 10% inflation for ineligibility) over 12 months with 9 additional months of follow-up to compare the combination of cixutumumab and cetuximab (Arm C) to each of the single agents (Arms A and B) without a formal comparison between the cetuximab alone (Arm B) and cixutumumab alone (Arm C) arms. This design had a yield of 88% power to detect a 60% increase in median PFS (5.6 months vs. 3.5 months) for either comparison at the 1-sided 0.10 significance level using the log rank test. Full information would be reached at 108 PFS events in each comparison. There was no plan to compare the experimental arms to each other.
Safety data on patients and a comparison of toxicity rates of each arm was collected and an interim safety analysis was planned among the first 20 patients who completed the initial 3 cycles of treatment. Early stopping rules were defined by 6 or more events of any grade 3 or higher non-hematological toxicity, any grade 4 or higher hematological toxicity, or grade 3 or higher febrile neutropenia or infection with neutropenia.
Archived tissue and serum was obtained from all patients who consented to participate in the exploratory laboratory research studies. Assuming 75% of patients would participate in the correlative studies and have analyzable samples for study, the estimated sample size for the correlative study was 135 patients. For the correlative endpoints to cetuximab (EGFR and K-Ras) we anticipated that the mutation rate of this population would be 15%. Among the 135 expected samples, 90 of these patients would have received cetuximab and 45 would have been randomized to the cixutumumab alone arm. Therefore, 14 of the 90 patients receiving cetuximab were expected to have a mutation. Assuming a one-sided type I error rate of 0.10, this sample size provided 81% power to detect a 100% improvement in the median PFS from 3 months in the mutation negative group to 6 months in the mutation positive group among those patients treated with cetuximab. For the correlative endpoints specific to cixutumumab (IGF-1R and IGF-2R) the anticipated expression rate was assumed to be 50%. Among the 135 expected samples, 90 of these patients will have received cixutumumab. Therefore, 45 of 90 patients receiving cixutumumab were expected to have positive expression levels. Assuming a one-sided type I error rate of 0.10, this sample size provided 96% power to detect a 100% improvement in the median PFS from 3 months in the zero expressing group to 6 months in the expression positive group among those treated with cixutumumab.
Overall survival was defined at the time from randomization to death from any cause, with follow-up censored at the date of last contact. Objective response was evaluated using RECIST 1.1 criteria. PFS was defined to be the time from randomization to death or documented disease progression, whichever occurred first. Patients that were alive at the time of analysis were censored at the date at which they were last known to be alive and progression-free.
Kaplan-Meier curves were used to estimate event-time distributions. Cox proportional hazards model, stratified on gender and histology (squamous vs. non-squamous) were used to estimate hazard ratios and test for significance for PFS. PFS and OS were compared using logrank tests. Adverse events, patient demographics, disease characteristics and response rates were compared using Fisher’s exact tests. All p-values are two-sided and confidence intervals are at the 95% level. Correlative studies were conducted using similar analysis methodology.
Results
From September 11, 2009 through December 17, 2010, one-hundred and forty patients were accrued. The study was subsequently terminated on April 11, 2011 due to excessive grade 5 events within 30 days of registration. Table 1 summarizes the patient demographics and disease characteristics. There were no substantial differences between the arms. The majority of patients had male gender and white race, as well as ECOG PS 0 and < 5% weight loss in the previous 6 months. Similar rates of squamous and non-squamous histology were represented. The percentage of patients receiving at least 2 cycles (12 weeks) of therapy were 51%, 69%, and 60% for arms A, B, and C, respectively. The percentage of patients receiving at least 4 cycles (24 weeks) of therapy were 26%, 26%, and 17% for Arms A, B, and C, respectively.
Table 1.
Patient Demographics and Disease Characteristics by Treatment Arm
| Variable | Category | Arm A | Arm B | Arm C | Total |
|---|---|---|---|---|---|
| Total (n) | 39 | 41 | 47 | 127 | |
| Sex | Male Female |
20(51) 19(49) |
24(59) 17(41) |
25(53) 22(47) |
69(54) 58(46) |
| Race, n (%) | White Black |
36(92) 3(8) |
34(83) 6(15) |
44(94) 2(4) |
114(90) 11(9) |
| Age | Median (Min, Max) |
60 (42, 89) |
60 (43, 81) |
60 (44, 76) |
60 (42, 89) |
| PS | 0 1 |
19(49) 20(51) |
22(54) 19(46) |
28(60) 19(40) |
69(54) 58(46) |
| Weight Loss | < 5 5– < 10 10– < 20 > = 20 |
29(74) 8(21) 2(5) 0(0) |
31(76) 5(12) 5(12) 0(0) |
35(74) 7(15) 4(9) 1(2) |
95(75) 20(16) 11(9) 1(1) |
| Smoking Status | Current Smoker Former Smoker Never Smoked |
17(44) 21(54) 1(3) |
19(46) 19(46) 3(7) |
31(66) 15(32) 1(2) |
67(53) 55(43) 5(4) |
| Stage | IIIB, not recurrent IV, not recurrent Recurrent |
1(3) 34(87) 4(10) |
1(2) 34(83) 6(15) |
1(2) 44(94) 2(4) |
3(2) 112(88) 12(9) |
| Histology | Squamous Adenocarcinoma Large cell BAC NOS Combined/Mixed Other |
11(28) 17(44) 1(3) 2(5) 4(10) 2(5) 2(5) |
19(46) 11(27) 0(0) 0(0) 7(17) 2(5) 2(5) |
21(45) 20(43) 0(0) 0(0) 6(13) 0(0) 0(0) |
51(40) 48(38) 1(1) 2(2) 17(13) 4(3) 4(3) |
| Liver | No Yes Unknown |
29(74) 9(23) 1(3) |
30(73) 11(27) 0(0) |
39(83) 7(15) 1(2) |
98(77) 27(21) 2(2) |
| Adrenal | No Yes Unknown |
33(85) 5(13) 1(3) |
30(73) 11(27) 0(0) |
34(72) 12(26) 2(4) |
97(76) 28(22) 2(2) |
| Bone | No Yes Unknown |
23(59) 14(36) 2(5) |
29(71) 12(29) 0(0) |
28(60) 17(36) 2(4) |
80(63) 43(34) 4(3) |
| Brain | No Yes |
36(92) 3(8) |
33(80) 8(20) |
40(85) 7(15) |
109(86) 18(14) |
An interim safety analysis was conducted in November 2010 after approximately 20 patients were randomized to each arm and had completed 3 cycles of treatment. At that time, the study was accruing more rapidly than anticipated and a follow-up call was schedule for December 2010, after more toxicity data had been submitted and further analyses conducted. The study was suspended for excessive grade 5 events within 30 days of registration at that time, on December 17, 2010. A full review of all available data was then conducted and reviewed, resulting in termination to accrual on April 19, 2011. At the time of the safety analysis, there was found a higher-than-expected rate of adverse events on Arm C, which included deaths for which a treatment-related attribution could not be excluded. In addition, there was a higher-than-expected rate of early deaths on arm A. However, a review of these deaths identified that disease, not treatment-related complications, was the likely cause of death in most of the cases. Therefore, all patients on Arm C discontinued therapy. All patients on Arms A and B may have continued therapy according to the protocol at the discretion of the study participant and the treating physician.
All toxicities, regardless of attribution and including grade 5 events, are summarized in Table 2. In total, 6 deaths were reported on arm A, 3 on arm B, and 5 on arm C. Deaths on Arm A were attributed to asystole in 1 patient, lung infection or respiratory failure in 2 patients, colitis in 1 patient, and unspecified reasons in the other 2. On Arm B, 2 deaths were for unspecified reasons and 1 death was due to cancer. On arm C, 3 deaths were attributed to pulmonary disease (hypoxia, respiratory failure, acute respiratory distress syndrome), and 2 were unspecified. Grade 3/4 neutropenia was reported in 29%, 30%, and 42% for arms A/B/C, respectively; however, grade 3/4 febrile neutropenia was reported in only 7%, 4.5%, and 4.2% for arms A/B/C, respectively. Grade 3/4 hyperglycemia was more common on the cixutumumab-containing arms (14–17%) compared with arm A (2%). In addition, more patients on arm C experienced grade 3/4 hyponatremia (17%) compared with arms A or B (2% and 4.5%, respectively).
Table 2.
Key Grade 3–5 Adverse Event Incidence, Any Attribution
| A (n=42) | B (n=44) | C (n=48) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | Grade | |||||||
| 3 | 4 | 5 | 3 | 4 | 5 | 3 | 4 | 5 | |
| Adverse Event Type | (n) | (n) | (n) | (n) | (n) | (n) | (n) | (n) | (n) |
| Anemia | 7 | 0 | 0 | 3 | 1 | 0 | 5 | 1 | 0 |
| Febrile neutropenia | 3 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| Fatigue | 6 | 0 | 0 | 9 | 0 | 0 | 5 | 0 | 0 |
| Rash acneiform | 2 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 1 | 0 | 0 | 4 | 0 | 0 |
| Nausea | 5 | 0 | 0 | 3 | 0 | 0 | 4 | 0 | 0 |
| Vomiting | 3 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 |
| Lung Infection | 3 | 0 | 1 | 2 | 0 | 0 | 3 | 0 | 0 |
| Neutrophil count decreased | 3 | 9 | 0 | 5 | 8 | 0 | 6 | 14 | 0 |
| Platelet count decreased | 1 | 2 | 0 | 1 | 5 | 0 | 2 | 4 | 0 |
| Anorexia | 3 | 0 | 0 | 4 | 0 | 0 | 2 | 0 | 0 |
| Dehydration | 1 | 0 | 0 | 2 | 0 | 0 | 3 | 0 | 0 |
| Hyperglycemia | 1 | 0 | 0 | 6 | 0 | 0 | 5 | 3 | 0 |
| Hyponatremia | 1 | 0 | 0 | 2 | 0 | 0 | 8 | 0 | 0 |
| Arthralgia | 3 | 0 | 0 | 4 | 0 | 0 | 3 | 0 | 0 |
| Myalgia | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| Dyspnea | 10 | 1 | 0 | 6 | 0 | 0 | 4 | 2 | 0 |
| Thromboembolic event | 0 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 0 |
At the time of data analysis, 111 of 127 eligible and treated patients had died and a total of 123 patients experienced a PFS event. The median follow-up on patients still alive (n=18) was 31 months. The estimated median PFS and corresponding 95% CI on each treatment arm was 3.4 months (2.6–5.8), 4.2 months (3.5–5.3), and 4 months (3.2–5.4), for arms A, B, and C, respectively (Figure 2). Arm C did not improve PFS compared to arm A (HR 1.12, 95% CI (0.71–1.78), p=0.62) or arm B (HR 1.10, 95% CI (0.71–1.72), p=0.67). The estimated median OS and corresponding 95% CI on each treatment arm was 9.8 months (7.4–17.2), 7.7 months (5.8–11.5), and 8.8 months (7.2–14.9) for arms A, B, and C, respectively (Figure 3). The response rate for arm A, B, and C, were 11%, 22%, and 22%, respectively. The disease control rate for arms A, B, and C, were 47%, 63%, and 63%, respectively.
Figure 2. Profression-free survival.
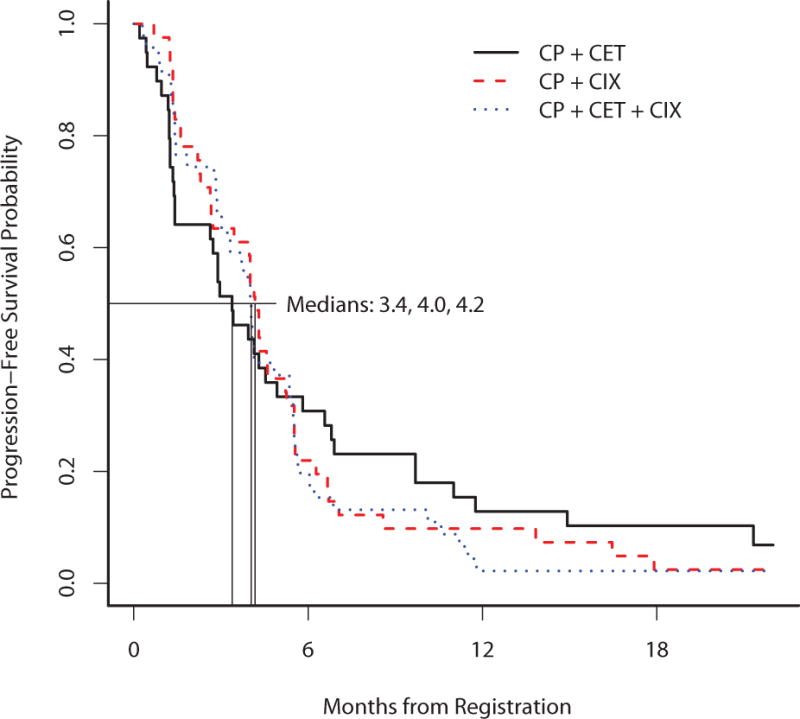
This figure provides the Kaplan-Meier curves for progression-free survival of the 3 arms
Figure 3. Overall survival.
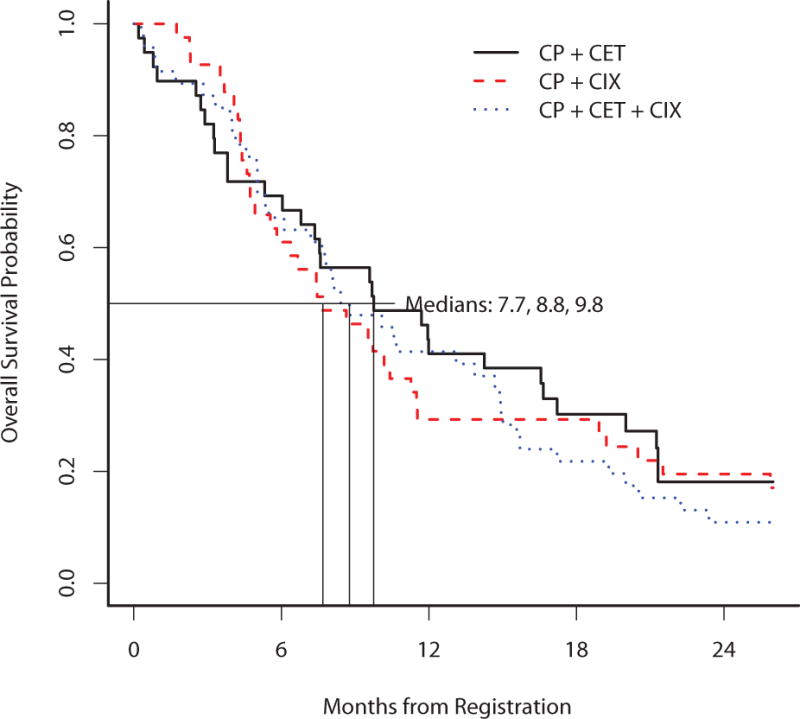
This figure provides the Kaplan-Meier curves for overall survival of the 3 arms
Results of Exploratory Correlative Studies
This study was underpowered to detect differences in subsets defined by biomarker studies. When EGFR was analyzed by FISH, there were 5 failures, while 39 tested negative and 30 tested positive according to criteria described previously (20). The median OS for EGFR negative and positive (by FISH) was 8.6 months (95% CI 6.4–12) and 9.5 months (95% CI 6.8–26.7), respectively, log rank p=0.06. There was no difference in these groups’ PFS (log rank p=0.18); similarly, no differences were observed by whether or not cetuximab was received.
For EGFR mutation status, exons 18–21 were evaluated but no mutations were found except in 5 patients harboring an exon 19 mutation. No significant differences were observed in their outcomes compared to those without mutation.
KRAS genotyping was conducted, and the numbers of patients tested was too small for statistical testing. For codon 12.34, 72 patients had the GG genotype, while 7 had the GT genotype, 1 had GC, 4 had GA, and 1 had TT. For codon 12.35, 8 patients had the GC genotype, while 73 had the GG genotype; 2 had the GA genotype and 1 had the GT genotype. For codon 13.38, 5 patients had the GA genotype, while 79 had the GG genotype. For codons 61.182 and 61.183, all patients had the AA genotype. A total of 23 patients (among 85) had a mutation in any of the above mentioned codons, but comparisons of OS and PFS were not significant.
IHC studies reported no significant differences when compared by treatment arm or by group when the protein expression was dichotomized as positive (≥ 200) and negative using the H-score system (21). Median IGF-1R score was 190 (range 30–390); median IGF-2R score was 145 (range 20–350); median EGFR membrane and cytoplasm H score was 190 (range 0–380); and median EGFR membrane H scores was 160 (range 0–390).
Discussion
Results from this randomized, open-label, multi-institutional, phase II study do not support continued evaluation of cixutumumab in combination with carboplatin plus paclitaxel with or without cetuximab in the patient population studied. The study did not meet its primary endpoint of demonstrating improved PFS with the cixutumumab-containing regimens compared with carboplatin plus paclitaxel plus cetuximab regimen. While response rates and disease control rates trended higher with the 2 cixutumumab-containing arms compared with the carboplatin/paclitaxel/cetuximab arm, neither PFS nor OS appeared superior with the cixutumumab-containing arms. Patient characteristics were similar between 3 arms with the exception of more active smokers in the cetuximab + cixutumumab-containing arm. Disease characteristics were also similar between each arm. A few toxicity differences were noted between the arms. More grade 3 hyponatremia was observed in the cetuximab-cixutumumab-containing arm and, as expected, more grade 3 and 4 hyperglycemia was reported in the cixutumumab-containing arms. While more grade 3/4 neutropenia was reported on the cetuximab-cixutumumab-containing arm there was no difference in febrile neutropenia or infection rates amongst the arms.
The trial was closed prior to planned accrual goals due to concerns about excessive grade 5 events reported for arm A and C. A detailed analysis of each death did not demonstrate a clear pattern of causality. Excessive grade 5 events had not been previously reported in larger phase III trials of chemotherapy with cetuximab, including in combination with carboplatin and paclitaxel (3, 4). While excessive grade 5 events were not reported on arm B, accrual was halted based on a consensus opinion of the leaders of the study, including ECOG members as well as members of NCI/CTEP. Reasons for this decision included the recently reported negative phase III study with figitumumab (monoclonal antibody targeting the insulin-like growth factor-1 receptor), which failed to confirm the promising results reported in the randomized phase II study (22). Our study was designed prior to knowing the results of the phase III trial with figitumumab (22). In that trial, the DSMC recommended early closure due to futility, but also excessive toxicity, including 5% grade 5 events in the figitumumab arm.
Biomarker studies are an integral component of trials assessing molecularly targeted therapy. While unselected patient populations may not benefit from a given targeted therapy, correlation of outcomes with molecular targets may identify a subpopulation of patients likely to benefit. The best examples to date in NSCLC include the identification of EGFR mutations that predict response to EGFR tyrosine kinase inhibitors and ALK gene re-arrangements, which predict response to ALK tyrosine kinase inhibitors (23–25). Unfortunately, biomarker studies (which were exploratory and presumably compromised further due to early study closure) of the current trial did not provide additional insights into subgroups of patients who may benefit from cixutumumab-containing regimens.
In vitro and in vivo studies supported the clinical testing of combination studies of IGF-1R and EGFR dual inhibition (11, 12). Results from a phase I/II study of erlotinib in combination with cixutumumab in patients with advanced NSCLC (most had received 1 or more prior regimens) was reported by Weickhardt et al in 2012 (26). Patients were treated in a dose-escalation manner (3+3 design). The most frequent AE’s were fatigue, rash, diarrhea, anorexia, and nausea. Of 18 patients evaluated, 5 achieved stable disease, including 1 patient who had SD for > 14 months. There appeared to be little activity in an unselected EGFR-wild type patient population. Biomarker analysis on this small study demonstrated a trend towards benefit in PFS for patients with the highest quartile of baseline free IGF-1.
In the first line setting Karp et al initially reported promising results from a randomized phase II study of carboplatin plus paclitaxel plus or minus figitumumab (antibody to IGFR) (18). Since this initial report, results from this study were published in 2009, but subsequently retracted in 2012, when the sponsor learned that the overall response rate and PFS data was incorrect (27,28). Additionally, a phase III study of figitumumab in combination with carboplatin plus paclitaxel in a non-adenocarcinoma patient population, failed to demonstrate increased efficacy with the addition of figitumumab (22). In this trial, 681 patients were enrolled, most of whom had squamous cell cancer (86%). The HR crossed the pre-specified futility boundary of 1.1 favoring paclitaxel and carboplatin alone. In those with low baseline IGF levels, safety, tolerability, and survival appeared worse with the addition of figitumumab to carboplatin and paclitaxel.
In conclusion, treatment with cixutumumab in combination with carboplatin plus paclitaxel with or without cetuximab does not appear to improve PFS or OS in the unselected patient population tested in our study. Prior studies, as detailed above, hypothesize the level of circulating IGF levels may impact therapies that block IGFR activity. Future studies, if any are to be conducted in NSCLC, should only be conducted if a biomarker, such as high circulating IGF levels, is used to select potentially responsive patients.
Acknowledgments
This study was conducted by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA180820, CA180794, CA49883, CA180795, CA21076, CA180799, CA15488, CA180864, CA180834, CA180870, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services.
Footnotes
The following possible conflicts of interest are reported: Dr. Ramalingam reports serving on the advisory boards for Eli Lilly and Imclone as well as Amgen, Aveo, Boehringer-Ingelheim, Celgene, Genentech, Novartis, and AstraZeneca. He has received honoraria from Eli Lilly. Dr. Hirsch reports serving on advisory boards for Pfizer, Eli Lilly, Novartis, Amgen, Celgene, Roche-Genentech, and BMS and receiving funding for research from Eli Lilly, Genentech, Celgene, Ventana, Imclone, and Amgen. He has received consulting fees or honoraria from Genentech/Roche, Eli Lilly, Novartis, Bristol Myers Squibb, and Myriad. Dr. Schiller reports serving as a consultant on the advisory boards for Biodesix, AVEO, Eisai, Abb Vie, and Eli Lilly, and receiving grant support from Genentech, Synta, Astex, Sorono/EMD, Clovis, Pfizer, PUMA, and Abb Vie. Dr. Hanna reports having received grant support from Eli Lilly (for funding for this study) and AstraZeneca and has grant support pending from Merck. Suzanne Dahlberg reports receiving NIH grant support for the ECOG statistical center grant. The other co-authors report no conflicts of interest.
References
- 1.Hanahan D, Weinberg R. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd F, Rodrigues J, Ciuleanu T, et al. Erlotinib in previously treated non-small cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 3.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 4.Lynch T, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol. 2010;28(6):911–917. doi: 10.1200/JCO.2009.21.9618. [DOI] [PubMed] [Google Scholar]
- 5.Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- 6.Samani A, Yakar S, LeRoith D, et al. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 7.Kawauchi K, Ogasawara T, Yasuyama M, et al. Regulation and importance of the PI3K/Akt/mTOR signaling pathway in hematologic malignancies. Anticancer Agents Med Chem. 2009;9:1024–38. doi: 10.2174/187152009789377772. [DOI] [PubMed] [Google Scholar]
- 8.Yu H, Spitz M, Mistry J, et al. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst. 1999;91:151–156. doi: 10.1093/jnci/91.2.151. [DOI] [PubMed] [Google Scholar]
- 9.London S, Yuan J, Travlos G, et al. Insulin-like growth factor I, IGF-binding protein 3, and lung cancer risk in a prospective study of men in China. J Natl Cancer Inst. 2002;94:749–754. doi: 10.1093/jnci/94.10.749. [DOI] [PubMed] [Google Scholar]
- 10.Chang Y, Wang L, Liu D, et al. Correlation between insulin-like growth factor-binding protein-3 promoter methylation and prognosis of patients with stage I non-small cell lung cancer. Clin Cancer Res. 2002;8:3669–3675. [PubMed] [Google Scholar]
- 11.Moorehead R, Sanchez O, Baldwin R, et al. Transgenic overexpression of IGF-II induces spontaneous lung tumors: a model for human lung adenocarcinoma. Oncogene. 2003;22:853–857. doi: 10.1038/sj.onc.1206188. [DOI] [PubMed] [Google Scholar]
- 12.Goetsch L, Gonzalez A, Leger O, et al. A recombinant humanized anti-insulin-like growth factor receptor type I antibody (h7C10) enhances the antitumor activity of vinorelbine and anti-epidermal growth factor receptor therapy against human cancer xenografts. Int J Cancer. 2005;113:316–328. doi: 10.1002/ijc.20543. [DOI] [PubMed] [Google Scholar]
- 13.Huang F, Greer A, Hurlburt W, et al. The mechanisms of differential sensitivity to an insulin-like growth factor-1 receptor inhibitor (BMS-536924) and rationale for combining EGFR/HER2 inhibitors. Cancer Res. 2009;69:161–170. doi: 10.1158/0008-5472.CAN-08-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones H, Gee J, Hutcheson I, et al. Growth factor receptor interplay and resistance in cancer. Endocr Relat Cancer. 2006;13(suppl 1):S45–51. doi: 10.1677/erc.1.01275. [DOI] [PubMed] [Google Scholar]
- 15.van der Veeken V, Oliveira S, Schiffelers R, et al. Crosstalk between epidermal growth factor- and insulin-like growth factor-1 receptor signaling: implications for cancer therapy. Curr Cancer Drug Targets. 2009;9:748–760. doi: 10.2174/156800909789271495. [DOI] [PubMed] [Google Scholar]
- 16.Shin D, Min H, El-Naggar A, et al. Akt/mTOR counteract the antitumor activities of Cixutumumab, an anti-insulin-like growth factor receptor I receptor monoclonal antibody. Mol Cancer Ther. 2011;10(12):2437–2448. doi: 10.1158/1535-7163.MCT-11-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burtrum D, Zhu Z, Lu D, et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912–8921. [PubMed] [Google Scholar]
- 18.Karp D, Paz-Ares L, Blakely L, et al. Efficacy of the anti-insulin like growth factor I receptor (IGF-1R) antibody CP-751871 in combination with paclitaxel and carboplatin as first line treatment for advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2007;25(18S):7506. [Google Scholar]
- 19.Cappuzzo F, Hirsch F, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small cell lung cancer. J Natl Cancer Inst. 2005;97:643–55. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch F, Herbst R, Olsen C, et al. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small cell lung cancer patients treated with cetuximab and chemotherapy. J Clin Oncol. 2008;26:3351–7. doi: 10.1200/JCO.2007.14.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch F, Varella-Garcia M, Bunn P, et al. Epidermal growth factor receptor in non-small cell lung cancer: correlation between gene copy number and protein expression and implication for prognosis. J Clin Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 22.Langer C, Novello S, Park K, et al. Randomized, phase III trial of first-line Figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small cell lung cancer. J Clin Oncol. 2014;32:2059–2066. doi: 10.1200/JCO.2013.54.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi-Long W, Caicun Z, Cheng-Ping H, et al. Afatanib versus cisplatin plus gemcitabine for first line treatment of Asian patients with advanced non-small cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomized phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 24.Sequist L, Yang J, Yamamoto N, et al. Phase III study of Afatanib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 25.Camidge R, Bang Y, Kwak E, et al. Activity and safety of Crizotinib in patients with ALK-positive non-small cell lung cancer: updated results from a phase I study. Lancet Oncol. 2012;13:1011–1019. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weickhardt A, Doebele R, Oton A, et al. A phase I/II study of Erlotinib in combination with the anti-insulin-like growth factor-1 receptor monoclonal antibody IMC-A12 (Cixutumumab) in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2012;7:419–426. doi: 10.1097/JTO.0b013e31823c5b11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karp D, Paz-Ares L, Novello S, et al. Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol. 2009;27:2516–2522. doi: 10.1200/JCO.2008.19.9331. [DOI] [PubMed] [Google Scholar]
- 28.Karp D, Paz-Ares L, Novello S, et al. Retraction: Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol. 2012;30:4179. doi: 10.1200/JCO.2008.19.9331. [DOI] [PubMed] [Google Scholar]


