Abstract
Silencing Amblyomma americanum insulin-like growth factor binding protein-related protein 1 (AamIGFBP-rP1) mRNA prevented ticks from feeding to repletion. In this study, we used recombinant (r)AamIGFBP-rP1 in series of assays to obtain further insight into role(s) of this protein in tick feeding regulation. Our results suggest that AamIGFBP-1 is an antigenic protein that is apparently exclusively expressed in salivary glands. We found that both, males and females, secrete AamIGFBP-rP1 into the host during feeding and confirmed that female ticks secrete this protein from within 24–48h after attachment. Our data suggest that native AamIGFBP-rP1 is a functional insulin binding protein in that both yeast- and insect cell-expressed rAamIGFBP-rP1 bound insulin, but not insulin-like growth factors. When subjected to anti-blood clotting and platelet aggregation assays, rAamIGFBP-rP1 did not have any effect. Unlike human IGFBP-rP1, which is controlled by trypsinization, rAamIGFBP-rP1 is resistant to digestion, suggesting that the tick protein may not be under mammalian host control at the tick-feeding site. Majority of tick-borne pathogens are transmitted 48 hours after the tick has attached. Thus, demonstrated antigenicity and secretion into the host within 24–48 hours of the tick starting to feed makes AamIGFBP-rP1 an attractive target for anti-tick vaccine development.
Keywords: Amblyomma americanum, insulin-like growth factor binding protein-related protein 1, tick saliva
Introduction
Ticks are obligate hematophagous ectoparasites and competent vectors of numerous animal and human disease agents including bacteria, viruses, protozoa, and helminths (Sonenshine & Roe, 2013). Without successful feeding, ticks cannot transmit the majority of disease agents. Consequently, interruption of the tick feeding process can prevent pathogen exchange between ticks and their hosts, affecting maintenance of their natural enzootic cycles. Current tick control methods are largely dependent on acaricide use. However sustainability of this strategy is threatened by numerous limitations, most notably: resistance development and environmental pollution (Wharton, 1967; Wang et al., 1994; Lovis et al., 2013; Coles & Dryden, 2014). Anti-tick vaccination is a feasible alternative (Willadsen, 2005). In this respect, identifying and characterizing molecules important for the tick feeding process is necessary.
Amblyomma americanum ticks have an important role in the transmission of several tick-borne pathogens (Childs & Paddock, 2003; Mixson et al., 2006). This tick transmits causative agents of human ehrlichiosis, Ehrlichia chaffeensis, and Ehrlichia ewingii (Anderson et al., 1993; Ewing et al., 1995; Wolf et al., 2000). Recently, A. americanum was also associated with deadly Heartland virus (Savage et al., 2013). In addition, this tick is associated with transmission of Francisella tularensis and Coxiella burnetii in humans and animals (Parker & Kohls, 1943; Hopla & Downs, 1953; Philip & White, 1955), as well as Theileria cervi to deer (Laird et al., 1988). Reported geographical expansion from the south and southeastern United States toward northern states up to Maine (Keirans & Lacombe, 1998; Merten & Durden, 2000) makes A. americanum an important consideration in medical health planning.
We are interested in the identification of tick saliva proteins that have the potential to be good targets for anti-tick immunizations. A suppressive subtractive hybridization approach identified 40 genes that were found to be up-regulated in A. americanum that were exposed to tick feeding stimuli (Mulenga et al., 2007). Interestingly, two insulin-like growth factor binding proteins (IGFBP) were identified provoking a follow up study, in which three related sequences were detected after cloning, and designated according to similarity to human sequences as insulin-like growth factor binding protein-related proteins (IGFBP-rP) (Mulenga & Khumthong, 2010). Based on comparison with human IGFBP-rPs the first, 248 amino acids long sequence, was identified as AamIGFBP-rP1, while the remaining two sequences, 152 and 168 amino acids long, were respectively identified as AamIGFBP-rP6 short and AamIGFBP-rP6 long. Real-time RT-PCR analysis showed that AamIGFBP-rP6 short and AamIGFBP-rP6 long were expressed in tick salivary glands (SG), midgut (MG), ovaries (OV), and the remaining tick body parts, so-called carcass (CA), with their transcript abundances decreasing with tick feeding (Mulenga et al., 2007). On the other side, Mulenga & Khumthong (2010) semi-quantitative RT-PCR analysis revealed that AamIGFBP-rP1 is expressed in SG, MG, CA, and six fold less in OV in comparison to other tissues, but with transcript abundance increasing within the first five days of the feeding process. In the same study Mulenga & Khumthong (2010) indicated that AamIGFBP-rPs amino acid sequences are conserved among other hard tick species, which makes them potential targets for universal anti-tick vaccine development. RNAi-mediated silencing of AamIGFBP-rPs caused ticks to take smaller blood meals (Mulenga & Khumthong, 2010) demonstrating the importance of these proteins in tick feeding physiology and prompting this study.
In vertebrates, IGFBP-rPs are included in the IGF system, and are primarily responsible for growth and differentiation of cells (Hwa et al., 1999). The role of IGFBP-rPs in invertebrates is poorly explored. Limited evidence shows that invertebrate IGFBP-rP1 bound insulin-like peptides in Spodoptera frugiperda and Drosophila melanogaster (Sloth Andersen et al., 2000; Honegger et al., 2008). The possible role(s) of AamIGFBP-rP1 for successful tick feeding revealed by RNAi silencing (Mulenga & Khumthong, 2010) is intriguing. In this study we demonstrate that native AamIGFBP-rP1 is an immunogenic tick saliva protein that is injected into the host by both male and female ticks, and that it is a functional insulin binding protein, but does not show anti-hemostatic functions.
Results
Expression of recombinant AamIGFBP-rP1
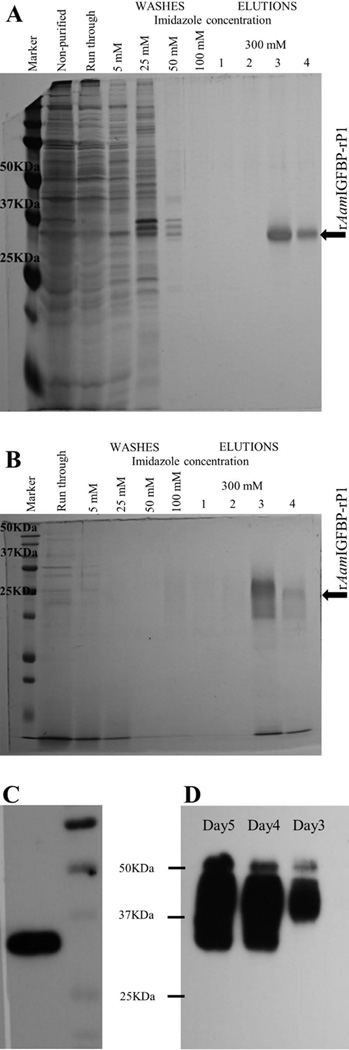
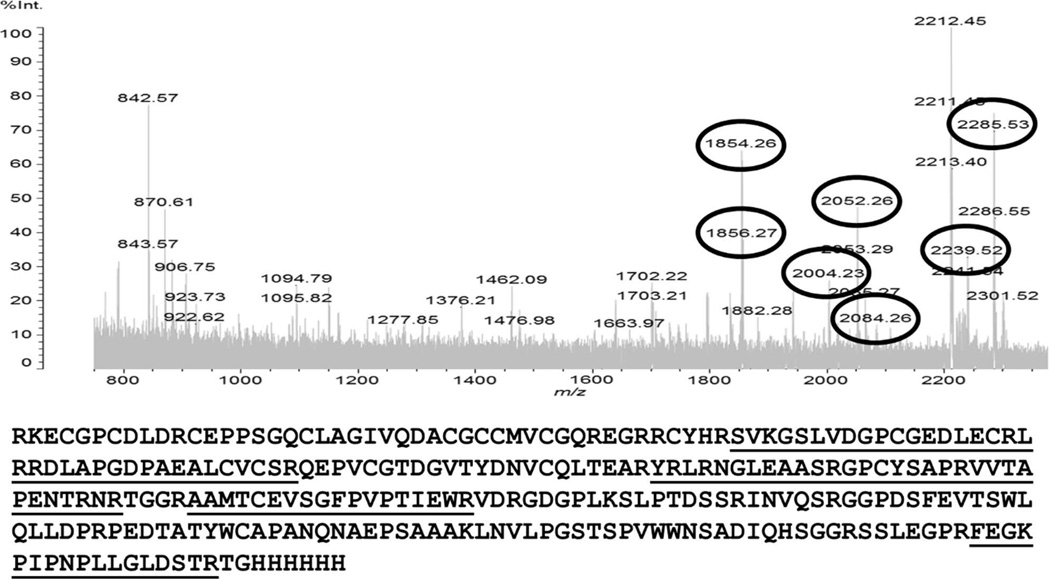
Recombinant (r) AamIGFBP-rP1 was expressed in both, lepidopteran insect and Pichia pastoris yeast expression systems (Fig. 1). Affinity purification of insect- and yeast-expressed rAamIGFBP-rP1 was verified by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with silver and Coomassie blue staining of gels (Fig. 1A and 1B) and western blotting analysis using the antibody to hexa-histidine tag (Fig. 1C and 1D). In addition, mass spectrometry analysis of insect cell-expressed rAamIGFBP-rP1 yielded seven peptides that matched the total sequence with 37% coverage (Fig. 2).
Figure 1.
Expression and affinity purification of recombinant (r) Amblyomma americanum (Aam) IGFBP-rP1. Affinity purification fractions of rAamIGFBP-rP1 expressed in insect cells (A) and in Pichia pastoris (B) were resolved on 12.5% SDS-PAGE and visualized by silver or Coomassie brilliant blue staining. Western blotting analysis validating insect cell (C) and yeast (D) expression rAamIGFBP-rP1 using the antibody to the c-terminus hexa-histidine tag antibody. Please note in figure 1D: lanes marked day 3–5 = samples were taken at days 3, 4, and 5.
Figure 2.
MALDI-TOF analysis of the rAamIGFBP-rP1 expressed in insect cells. In-gel trypsin digestion was performed and analysis of mass spectrum identified seven peptides (underlined) that match obtained and predicted masses (circled numbers) with 37% of sequence coverage.
In Figure 1B, yeast-expressed rAamIGFBP-rP1 protein is diffused and migrates at high molecular weight than insect cell expressed suggesting massive posttranslational modifications. In silico analysis of AamIGFBP-rP1 sequence predicted for one putative N-linked glycosylation site (asparagine at position 134 of mature protein) and five O-linked glycosylation sites (serines at positions 16, 46, 50, 115, and 121) (Fig. 3A). Treatment with Peptide-N-Glycosidase F (PNGase F) enzyme, which cleaves N-linked oligosaccharides, or with deglycosylation enzyme mix that cleaves both N- and O-linked oligosaccharides, caused comparable downward shifts in molecular weight for both insect cell- (Fig. 3B) and yeast- (Fig. 3C) expressed recombinant proteins. This may suggest that predicted N- but not O-linked glycosylation sites were functional in both insect- and yeast-expressed versions of rAamIGFBP-rP1.
Figure 3.
Deglycosylation analysis of the rAamIGFBP-rP1. Recombinant AamIGFBP-rP1 amino acid sequence was scanned for prediction of N- (underlined with one line) and O-linked (underlined with double lines) glycosylation sites (A). Affinity purified, insect cell- (B) and yeast- (C) expressed rAamIGFBP-rP1 were treated with deglycosylation enzymes, resolved on 12.5% SDS-PAGE, and visualized by western blot analysis using antibody to hexa-histidine tag or silver staining as indicated. Lines 1 and 4 = non-treated rAamIGFBP-rP1, 2 = rAamIGFBP-rP1 treated with PNGase F, and 3 = rAamIGFBP-rP1 treated with enzyme deglycosylation mix.
AamIGFBP-rP1 is immunogenic, exclusively expressed in tick SGs and secreted into the host through saliva
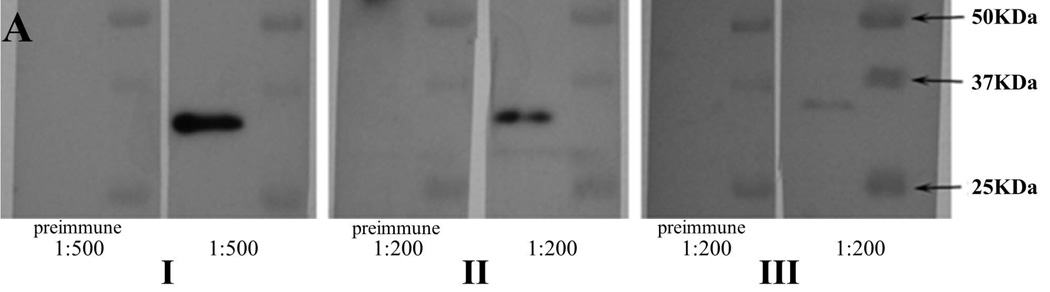
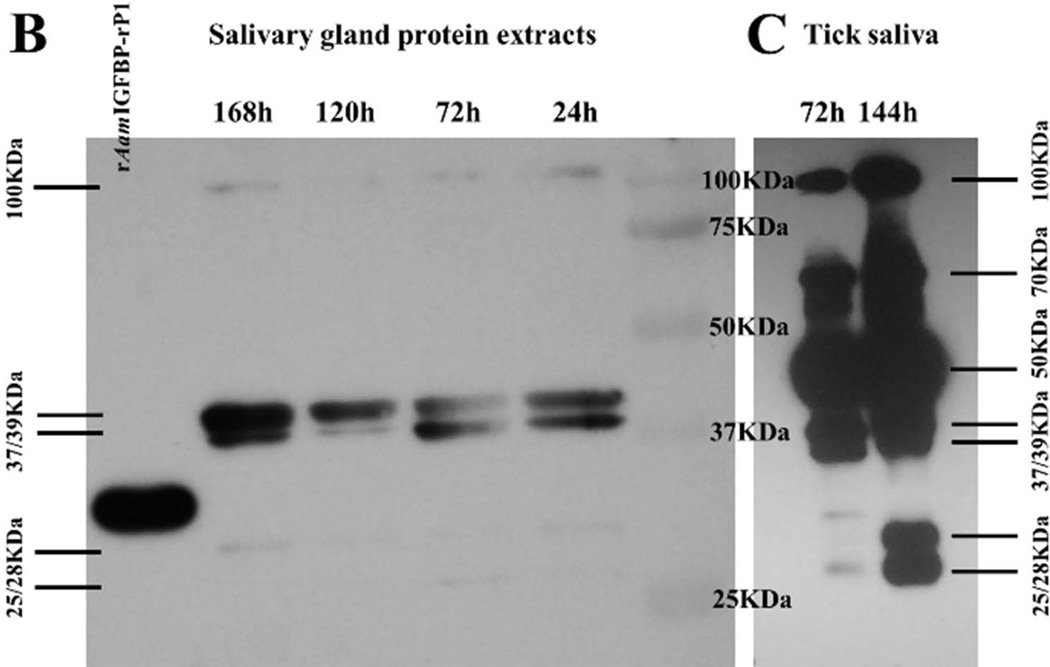
Figure 4 summarizes the native AamIGFBP-rP1 protein profile during tick feeding. In the previous studies we produced antibodies to tick saliva proteins (TSP) of fed male (Radulović et al., 2014) and 24–48h fed female (Mulenga et al., 2013b) A. americanum ticks. In Figure 4A, rAamIGFBP-rP1 specifically bound antibodies to TSP, suggesting that native AamIGFBP-rP1 was injected into the host during tick feeding by both male and female A. americanum ticks. To investigate if the observed antibody binding to rAamIGFBP-rP1 in figure 4 was specific, a mono-specific antibody to rAamIGFBP-rP1 was eluted. Eluted mono-specific antibody to rAamIGFBP-rP1 was used in western blotting analysis of 24–168h fed A. americanum tick SG protein extracts, as well as 72 and 120h fed tick saliva proteins (Fig. 4B). The eluted monospecific antibody bound to doublet ~25 and 28 kDa protein bands on both blots. This is within the range of the calculated 24.42 kDa molecular weight for mature AamIGFBP-rP1 277 amino acid residue long protein. Additionally, the eluted monospecific antibody bound higher molecular weight bands including the doublet 37/39 and the 100 kDa protein bands on both, SG and saliva blots. Furthermore, 50 and ~70 kDa bands were detected on saliva blot only (Fig. 4B). AamIGFBP-rP1 was not detected in any of the other tested A. americanum tick tissues, including MG, OV, Malpighian tubules (MT), synganglion (SY), and CA (not shown).
Figure 4.
Western blotting analysis to validate native AamIGFBP-rP1 secretion into host and expression profile in tick tissues. (A) Western blot detection of the rAamIGFBP-rP1 using antibodies to Amblyomma americanum tick saliva proteins (TSP), I – antibodies to 48h fed female TSP, II – antibodies to 24h fed female TSP, and III – antibodies to 4 days fed male TSP. Western blot detection of native AamIGFBP-rP1 in A. americanum tick salivary gland protein extracts (B) and saliva (C) collected from different time feeding points using mono-specific anti-rAamIGFBP-rP1 antibody.
AamIGFBP-rP1 has no anti-hemostatic or anti-microbial functions
Given that native AamIGFBP-rP1 is injected into the host during tick feeding, we were prompted to investigate its role(s) at the tick-feeding site. When subjected to anti-hemostatic function assays, affinity purified rAamIGFBP-rP1 did not have any effect on blood clotting time in recalcification time (RT), thrombin time (TT), activated partial thromboplastin time (aPTT), and prothrombin time (PT) assays to measure effects on the entire blood coagulation cascade, the extrinsic, intrinsic, and the common blood coagulation pathways respectively (not shown). Likewise, rAamIGFBP-rP1 did not have any effect on platelet aggregation (not shown). When subjected to anti-microbial function assays, a clear antimicrobial effect of rAamIGFBP-rP1 could not be confirmed.
AamIGFBP-rP1 is not controlled by trypsinization
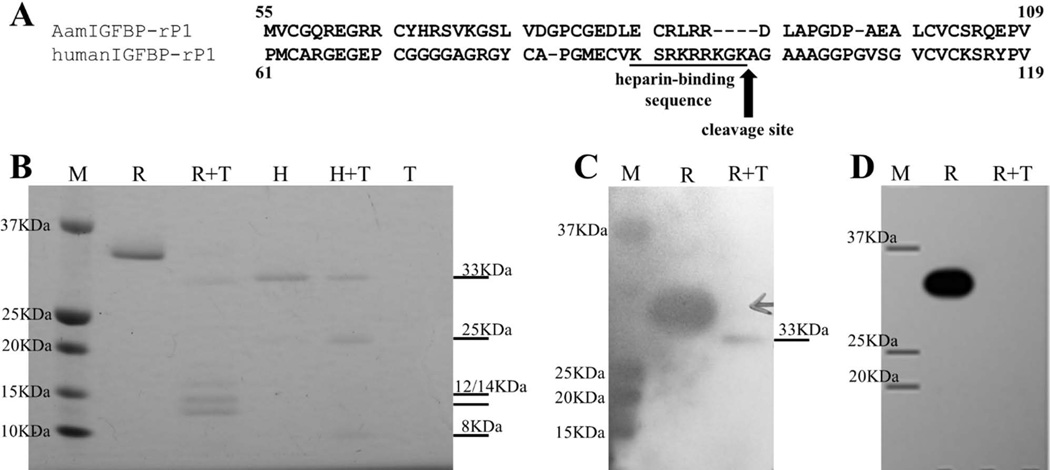
Previous studies have shown that human IGFBP-rP1 activity is regulated by a trypsin-like serine protease, which cleaves the protein between lysine and alanine at positions 97 and 98, respectively (Ahmed et al., 2003). Sequence alignment showed a significant difference between AamIGFBP-rP1 and its human homolog in the region predicted to be recognized by trypsin (Fig. 5A). This indicates that control mechanism for host IGFBP-rP1, does not affect AamIGFBP-rP1 which A. americanum ticks secrete at the feeding site. Consistently, trypsin treatment digested human IGFBP-rP1 into expected 25 and 8 kDa protein bands (Ahmed et al., 2003), but not rAamIGFBP-rP1 (Fig. 5B). Trypsinization of rAamIGFBP-rP1 produced a protein band migrating approximately at the same level as non-treated human IGFBP-rP1, and two additional bands: ~12 and 14 kDa (Fig. 5B). Western blot analyses revealed that trypsin cleaved off a small peptide containing the C-terminal histidine tag of rAamIGFBP-rP1 as confirmed in Figure 5C where both non-treated and treated rAamIGFBP-rP1 bound antibodies to 48h fed A. americanum tick saliva proteins, while in Figure 5D non-treated rAamIGFBP-rP1 bound the antibody to C-terminal hexa-histidine tag, but not the trypsin treated from which the histidine tag was cleaved. It is important to note that 10 µg/mL trypsin was sufficient to cleave off the C-terminal recombinant fusion tag, while the remaining part of rAamIGFBP-rP1 resisted digestion even up to 100 µg/mL of enzyme (not shown).
Figure 5.
Comparative sequence analysis and trypsinization of Amblyomma americanum and human IGFBP-rP1: A. Alignment of A. americanum and human IGFBP-rP1 amino acid sequences in the region where human protein is cleaved by trypsin. B. Products of 15 min trypsin digestion of rAamIGFBP-rP1 and its human homologue performed at 37°C, separated on 12.5% SDS-PAGE and stained with Coomassie brilliant blue. C) Western blot detection of products of rAamIGFBP-rP1 trypsinization using antibody to 48h fed A. americanum female tick saliva proteins. D) Western blot detection of products of rAamIGFBP-rP1 trypsinization using an antibody to hexa-histidine tag. Lane M – marker, lane R – non digested rAamIGFBP-rP1, lane R+T – rAamIGFBP-rP1 digested with trypsin, lane H – non digested human IGFBP-rP1, lane H+T – human IGFBP-rP1 digested with trypsin, and lane T – control containing trypsin only.
AamIGFBP-rP1 binds insulin but not IGFs
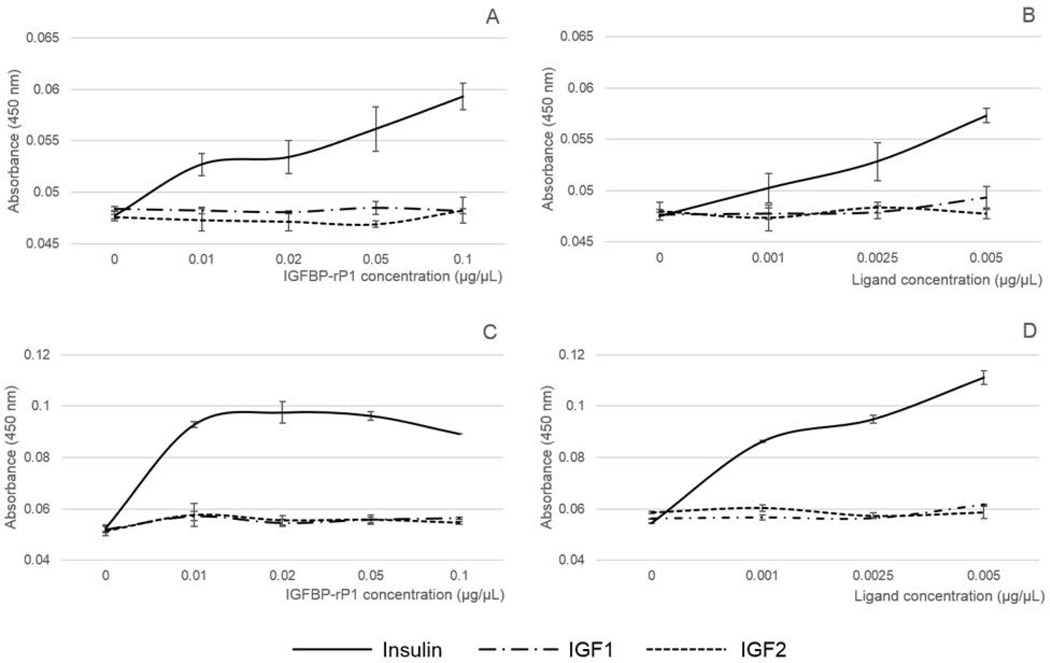
Published molecular analysis revealed that AamIGFBP-rP1 secondary structure was similar to mammalian homologs (Mulenga & Khumthong, 2010) which prompted us to investigate if functions were also conserved. The ability of AamIGFBP-rP1 to bind insulin and IGFs was tested by incubation of biotinylated ligands in ELISA plate wells pre-coated with rAamIGFBP-rP1. Our results show that neither insect cell- nor yeast-expressed rAamIGFBP-rP1 bound IGF I and II, however, both show the ability to bind insulin in a dose-responsive manner (Fig. 6). In Figure 6C, the observed apparent plateauing of the line graph from 0.01 µg/µL of rAamIGFBP-rP1 may suggest that the insulin binding capacity of yeast-expressed protein far exceeded the 0.25 µg of insulin used in this assay. Comparison of the observed optical densities (OD, A450nm) indicated that yeast-expressed rAamIGFBP-rP1 might have a higher binding capacity for insulin in comparison to those expressed in insect cells (Fig. 6). As a negative control, A. americanum tick saliva serine protease inhibitor (AAS) 8, expressed in insect cells (unpublished), did not bind insulin or IGFs (not shown).
Figure 6.
Affinity purified rAamIGFBP-rP1 binding of insulin and IGF I and II. rAamIGFBP-rP1 immobilized on ELISA plates was incubated with biotinylated ligands. Bound ligands were detected using HRP-labeled avidin. OD (A450nm) value is proportional to the binding capacity of rAamIGFBP-rP1. A and C = Constant amount of ligand binding to variable amounts of insect- and yeast cell-expressed rAamIGFBP-rP1 respectively; B and D = Variable amounts of ligands. binding to constant amount of insect- and yeast cell-expressed rAamIGFBP-rP1 respectively.
Discussion
AamIGFBP-rP1 encoding cDNA was found among genes that were up-regulated in ticks that were stimulated to start feeding (Mulenga et al., 2007), and RNAi silencing analysis later demonstrated that this protein was important to A. americanum tick feeding success (Mulenga & Khumthong, 2010). Western blotting analysis data in this study indicates that native AamIGFBP-rP1 is immunogenic, exclusively expressed in salivary glands, and secreted into the host during tick feeding. In a separate tick saliva proteome study (unpublished), we have found AamIGFP-rP1 in A. americanum tick saliva further confirming data in this study. It is interesting to note that both male and female ticks secrete native AamIGFBP-rP1 into the host provoking an antibody response. From the perspective of anti-tick vaccine development, our observation that native AamIGFBP-rP1 is injected into the host from within 24h after tick attachment is significant. The majority of tick-borne pathogens are transmitted into the host 24–48h after tick attachment (McQuiston et al., 2000). Thus, targeting proteins such as AamIGFBP-rP1 for anti-tick immunizations could confer protective immunity that prevents tick-borne pathogen transmission.
Given that native AamIGFBP-rP1 is injected into the host during tick feeding, we sought to gain insight into the functions of this protein at the tick-host interface. Ticks are pool feeders and accomplish feeding by lacerating host tissue and sucking up blood that bleeds into the feeding site (Mehlhorn, 2008). This feeding style provokes innate host defenses to injury such as inflammation and blood clotting. The tick accomplishes feeding by secreting hundreds of proteins to counter host defenses. Towards understanding how ticks feed, several tick saliva proteins with functions against host hemostasis, inflammation, complement activation, and immune response processes have been identified (Jaworski et al., 2001; Anguita et al., 2002; Prevot et al., 2006; Tyson et al., 2007; Mans & Ribeiro, 2008; Déruaz et al., 2008; Mulenga et al., 2013a; Ibelli et al., 2014). The observation in this study that both yeast- and insect cell-expressed rAamIGFBP-rP1 did not have antimicrobial effects, and did not affect plasma clotting and platelet aggregation suggest that this protein may play a role(s) not associated with mediating tick evasion of anti-hemostatic functions or the tick’s ability to protect the feeding site from bacterial contamination.
Comparative tertiary structure modeling revealed that AamIGFBP-rP1 retained a secondary structure fold similar to human IGFBP-rP1 (Mulenga & Khumthong, 2010). One of the goals of this study was to determine if rAamIGFBP-rP1 was functionally similar to mammalian homologs. One of the main characteristics of IGFBP-rP1 is its ability to bind insulin and IGFs (Hwa et al., 1999). Our data shows that rAamIGFBP-rP1 apparently binds insulin, but not IGFs, implying that the native tick protein probably displays similar characteristics. The significance of AamIGFBP-rP1 binding of insulin, but not IGFs in tick feeding remains to be investigated. It is interesting to note that exogenous insulin improves wound healing (Chen et al., 2012; Hrynyk & Neufeld, 2014). Ticks accomplish feeding by sucking up blood that bleeds into the wounded area. Thus, preventing wound healing is essential for ticks to complete feeding. Demonstrated ability of rAamIGFBP-rP1 to bind insulin points to possibility of insulin sequestration at the feeding site by tick native protein, which consecutively interfere with hosts’ tissue repair mechanisms. It is also notable that mammalian IGFBP-rP1 functions as a tumor suppressor factor that inhibits cell proliferation (Murphy et al., 1993; Landberg et al., 2001; Tennant et al., 2003). Whether or not native AamIGFBP-rP1 inhibits cell proliferation remains to be determined. Prevention of cell proliferation is a desirable pro-tick feeding function as it will affect the host immune response to tick feeding, as well as wound healing. There is evidence that cell growth and proliferation in humans is accompanied by a decreased level of IGFBP-rP1 (Wandji et al., 2000). Human IGFBP-rP1 is controlled by trypsin-like protease cleavage between lysine and alanine at positions 97 and 98 of the original protein sequence within the heparin binding site motif (Sato et al., 1999; Ahmed et al., 2003). Data in this study indicates that AamIGFBP-rP1 is not under mammalian host control. From the perspective of the yet unknown role(s) of AamIGFBP-rP1 in tick feeding regulation, these findings are significant. AamIGFBP-rP1 might be able to mimic the presence of the host’s homolog and possibly keep active signal pathways that suppress cell proliferation and affect the host’s tissue repair mechanisms.
Human IGFBP-rP1 has also been shown to stimulate secretion of prostacyclin in culture of bovine aortic endothelial cells (Yamauchi et al., 1994). Prostacyclin is reported in the saliva of several tick species, including Ixodes scapularis (Ribeiro et al., 1988) and A. americanum (Aljamali et al., 2002), with proposed anti-inflammatory and vasodilator functions (Hyman & Kadowitz 1979; Smith et al., 1980). Furthermore, prostacyclin inhibits platelet function (Kelton & Blajchman, 1980; Kozek-Langenecker et al., 2003) preventing blood from clotting, which is a pro-tick feeding function. The mechanisms of how prostacyclin is secreted into tick saliva have not been investigated. Our finding that AamIGFBP-rP1 is exclusively expressed in tick salivary glands is interesting. There is a possibility that AamIGFBP-rP1 stimulates secretion of prostacyclin from tick salivary glands that is released in tick saliva.
Although it was previously accepted that IGFBP-rP1 is ubiquitously expressed in all human tissues based on transcription profiling (Oh et al., 1996), immunohistochemistry analysis has shown that this protein is restricted to specific cell types in certain organs (Degeorges et al., 2000). In the same manner, AamIGFBP-rP1 gene expression was detected in multiple tick tissues (Mulenga & Khumthong, 2010), while data in this study demonstrated that AamIGFBP-rP1 protein is exclusively expressed in SGs and secreted into tick saliva. Our observation could be explained by the fact that transcript abundance is not always in proportion to protein level, primarily because of the influence of regulatory processes occurring after transcription, including post-transcriptional, translational, and protein degradation regulation (Vogel & Marcotte, 2012). Additionally, in accordance with the aforementioned human IGFBP-rP1 expression pattern, AamIGFBP-rP1 could be present in a very limited number of cells among tick organs other than SGs. In this way, the concentration of AamIGFBP-rP1 in protein extracts from these tissues could be below the detectable level for the western blot analysis we performed.
Our western blotting analysis results showed the presence of more protein bands on tick saliva blots than salivary gland blot. This could be explained by the possibility of AamIGFBP-rP1 forming complexes with yet unknown molecules that it transports from the tick into the host. There is evidence that human IGFBP-rP1 forms complexes with a variety of different molecules including neuroendocrine factor protein, collagen IV, activin, as well as several chemokines (Wilson et al., 2001; Nagakubo et al., 2003; Burger et al., 2005). Insulin-like peptides were reported in several invertebrate species including Dermacentor variabilis ticks (Bissinger et al., 2011). These peptides in insects serve as neurotransmitters, hormones, and growth factors (Wu & Brown, 2006). Thus, tick IGFBP-rP1 could be a part of the system that is similar to IGF system of vertebrates, which maintain some basic tick homeostatic processes.
Experimental procedures
Tick feeding, dissection, and protein extraction
A. americanum ticks used in this experiment were purchased from the tick laboratory at Oklahoma State University (Stillwater, OK, USA). Tick handling and feeding on New Zealand white rabbits according to an animal use protocol approved by Texas A & M University IACUC, were performed as previously described (Mulenga et al., 2013a; Kim et al., 2014). Ticks were fed on the outer surface of rabbit ears, restricted by orthopedic stockinets glued to the skin with Kamar Adhesive (Kamar Products Inc., Zionsville, IN, USA). Six males per ear, which were pre-fed for three days, were accompanied with 15 females per ear. Female ticks were manually detached from rabbits at four feeding time points, 24h, 72h, 120h, and 168h after attachment. Five ticks were collected per time point and dissected in diethylpyrocarbonate (DEPC)-treated water, as previously described (Mulenga et al., 2013a). Collected tissues were stored in RNAlater (Life Technologies) at −80°C. After total RNA isolation from dissected SG, MG, OV, MT, SY, and CA using the Trizol method (Kim et al., 2014), total proteins were extracted from the organic phase according to Trizol manufacturer’s instructions.
Collection of tick saliva
Tick saliva was collected from individuals detached at two feeding time points, 72h and 144h after attachment. After washing of partially engorged ticks in DEPC-treated water, salivation was induced by injection of ~2 µL of 2% pilocarpine hydrochloride in phosphate buffered saline (PBS, pH 7.4) into tick hemocoel at the ventral basis of the fourth coxa using a 33 gauge needle. To collect saliva, tick mouthparts were washed in 2 µL of PBS and then saliva was collected using 10 µL pipette tips.
Expression and affinity purification of rAamIGFBP-rP1
Expression of rAamIGFBP-rP1 was performed in InsectSelect BSD System (Life Technologies) using pIB/V5-His expression vector and Spodoptera frugiperda Sf9 lepidopteran insect cell line, as well as in yeast P. pastoris expression system using pPICZ α A expression vector. The open reading frame sequence of AamIGFBP-rP1 was amplified from A. americanum cDNA (Chalaire et al., 2011) using PCR primers with added restriction enzyme sites (lower case): 5′aagcttGTAATGGGAGCCGTCCACTGCAC3′ and 5′ggatccGGCCAGCACGTTGAGCTTGG3′ for insect cell expression, and 5′gaattcCGCAAGGAGTGCGGGCCTTGCGACCT3′ and 5′gcggccgcGGGCAGCACGTTGAGCTTGGCGGCGG3′ for yeast expression. Amplified sequences were sub-cloned into expression vectors using the pGEM-T vectors (Promega, Madison, WI, USA) for sequencing.
The pIB/V5-His-AamIGFBP-rP1 expression plasmid was purified using S.N.A.P Miniprep Kit (Life Technologies). Sf9 insect cells was transfected with pIB/V5-His-AamIGFBP-rP1 expression plasmid using Cellfectin II Reagent (Life Technologies). A culture was grown in Sf-900 II SFM (Life Technologies) containing 100 µg/mL each of penicillin and streptomycin. Following verification of expression, transfected cells were initially selected with blasticidin at a concentration of 50 µg/mL, and grown to confluence under blasticidin at 10 µg/mL. Cultures were grown in 75 cm2 tissue culture flasks at 27°C and spent media was collected every second day and stored at −80°C. The presence of rAamIGFBP-rP1 in collected media was confirmed by western blot analysis using an antibody to the C-terminus histidine tag. The positive signal was detected using chromogenic Metal Enhanced DAB Substrate Kit (Thermo Scientific, Rockford, IL, USA) or BioFX chemiluminescent reagent (SurModics, Eden Prairie, MN, USA). Prior to affinity purification, spent media was concentrated ~100 fold and dialyzed against PBS using Jumbosep Centrifugal Devices with 10kDa molecular weight cut off (MWCO) filters (Pall Life Sciences, Ann Arbor, MI, USA). Affinity purification of rAamIGFBP-rP1 was performed under native conditions using Hi-Trap Chelating HP Columns (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Further concentration and/or dialysis of purified protein was performed with 10 kDa MWCO Microsep Advance Centrifugal Devices (Pall Life Sciences) and Slide-A-Lyzer Dialysis Cassettes (Thermo Scientific).
In the case of yeast expression, the pPICZα A-AamIGFBP-rP1 plasmid was linearized with SacI restriction enzyme and transformed into Pichia pastoris X-33 strain cells (Life Technologies) by using ECM600 electroporator (BTX Harvard Apparatus Inc., Holliston, MA, USA). The transformation mix was plated onto Yeast Extract Peptone Dextrose Medium with Sorbitol (YPDS) agar plates with zeocin (100 µg/µL) and incubated at 28°C. Positive transformants were inoculated and grown overnight in Buffered Glycerol-Complex Medium (BMGY) at 28°C with shaking ~250 rpm. Subsequently cells were pelleted by centrifugation 5 min at 3000g and used for inoculation of Buffered Methanol-Complex Medium (BMMY) up to OD (A600) ~1. Protein expression was induced by daily introduction of methanol up to 0.5% of the final concentration. After four days, cells were pelleted by centrifugation and spent media was used for protein concentration by ammonium-sulfate precipitation. Pelleted proteins were re-suspended and dialyzed in PBS using SnakeSkin Dialysis Tubing 10 kDa MWCO (Thermo Scientific). Verification of rAamIGFBP-rP1 expression, affinity purification, and further concentration and/or dialysis was performed as it was described for insect cell expressed protein.
Mass spectrometry analysis
Insect cell-expressed rAamIGFBP-rP1 was subjected to matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry analysis. Protein was run on 12.5% SDS-PAGE and the gel was stained according to mass spectrometry compatible silver stain protocol (Blum et al., 1987). The rAamIGFBP-rP1 protein band was excised from the gel and de-stained as previously described (Gharahdaghi et al., 1999). In-gel digestion with trypsin was performed as previously published (Shevchenko et al., 2007). Analysis was performed on Shimadzu/Kratos MALDI-TOF mass spectrophotometer and results were interpreted using Scaffold 4 software (Proteome Software, Portland, OR, USA).
Deglycosylation assay
Prediction of glycosylation sites in AamIGFBP-rP1 sequence was performed using online NetNGlyc 1.0 and NetOGlyc 4.0 servers (Blom et al., 2004; Steentoft et al., 2013). Approximately 1 µg of affinity purified recombinant protein was treated with PNGaseF (New England BioLabs, Ipswich, MA, USA) or Enzyme Deglycosylation Mix (New England BioLabs) according to manufacturer’s protocols. Treated samples accompanied with non-treated controls were run on 12.5% SDS-PAGE and visualized by silver staining or western blot analysis using HRP-labeled anti C-terminal His-tag antibody (Life Technologies) and Metal Enhanced DAB Substrate Kit (Thermo Scientific).
Determination if native AamIGFBP-rP1 is secreted into the host during the tick feeding process
Affinity purified rAamIGFBP-rP1 was subjected to routine western blotting analysis using antibodies to A. americanum tick saliva proteins. Antibodies to A. americanum tick saliva proteins used in this experiment were generated as previously described (Mulenga et al., 2013a; Radulović et al., 2014). The rAamIGFBP-rP1 was resolved on 12.5% SDS-PAGE, transferred to a PVDF membrane, incubated in antibodies to 24 and 48h fed A. americanum female and fully fed male tick saliva proteins, followed by exposure to HRP-labeled goat anti-rabbit antibody (EMD Millipore, Billerica, MA, USA). Antibody binding was detected using BioFX chemiluminescent reagent (SurModics). As a negative control we used pre-immune serum obtained from rabbits prior to tick feeding.
Detection of native AamIGFBP-rP1 in different tick tissues
A mono-specific anti-AamIGFBP-rP1 antibody was eluted as previously described (Mulenga et al., 1999; Sambrook et al., 2001). Briefly, insect cell-expressed rAamIGFBP-rP1 was resolved on SDS-PAGE and transferred on PVDF membrane. Amido black-stained protein band was excised and membrane pieces was incubated in 5% skim milk in PBS-Tween-20 (blocking solution) at room temperature for 1h. After washing in PBS/Tween, membrane pieces were exposed overnight at 4°C to antibodies to A. americanum tick saliva proteins diluted 1:10 in blocking solution. Following washing, the mono-specific antibody to rAamIGFBP-rP1 was eluted from the membrane by application of 0.1M glycine buffer, pH 2.8 for 5 min. Acidic conditions were neutralized by mixing eluted antibody with the same volume of 1M Tris and subsequently with 0.1 volume of PBS. A mono-specific antibody was used in western blot analysis for the detection of native AamIGFBP-rP1 in organic phase protein extracts from different tick tissues. The secondary antibody: HRP-labeled goat anti-rabbit antibody (EMD Millipore) diluted 1:2000 in blocking solution. The positive signal was detected using BioFX chemiluminescent reagent (SurModics).
Determination of anti-hemostatic function of rAamIGFBP-rP1
The effects of AamIGFBP-rP1 on blood clotting were tested using RT, aPTT, TT, and PT assays. Assays were performed in two replicates using the Infinite M200Pro plate reader (Tecan US, Morrisville, NC, USA) to create a kinetic measurement protocol for OD (A650nm). Four different final concentrations of rAamIGFBP-rP1 in 20 mM Tris-HCl, 150 mM Nacl, pH 7.4 were tested as follows: 10, 5, 2, and 1 µM, accompanied with controls containing buffer only. In all assays Universal Coagulation Reference Human Plasma (UCRP) was used (Thermo Scientific).
In the RT assay 50 µL of UCRP was incubated with indicated amounts of rAamIGFBP-rP1 for 15 min at 37°C, followed by adding 10 µL of pre-warmed 150 mM CaCl2 which triggered plasma clotting. OD was monitored every 20 s for 30 min.
In the aPTT assay, 50 µL of UCRP was combined with the same volume of 20 mM Tris-HCl, 150 mM NaCl, pH 7.4 and indicated amounts of rAamIGFBP-rP1, and incubated 15 min at 37°C. Subsequently, 50 µL of the aPTT reagent (Thermo Scientific) was added and incubation was continued for 5 more minutes before addition of 50 µL of 25 mM CaCl2 triggered plasma clotting, which is monitored every 10 s for 5 min.
In the TT assay, 25 µL of the TT reagent (Thermo Scientific) was combined with the same amount of 20 mM Tris-HCl, 150 mM Nacl, pH 7.4 buffer and pre-incubated with indicated amounts of rAamIGFBP-rP1 for 15 min at 37°C. Subsequently, 50 µL of UCRP was added and clotting was monitored every 10 s for 5 min.
In the PT assay, 100 µL of the PT reagent (Thermo Scientific) was combined with 50 µL of 20 mM Tris-HCl, 150 mM Nacl, pH 7.4 buffer and pre-incubated with indicated amounts of rAamIGFBP-rP1 for 15 min at 37°C, followed by addition of 50 µL of UCRP. Plasma clotting was monitored every 10 s for 5 min.
Determination of anti-platelet aggregation function of rAamIGFBP-rP1
The effect of AamIGFBP-rP1 on platelet aggregation function was assessed. Recombinant protein in three different final concentrations as follows: 5, 2, and 1 µM, in 20 mM Tris-HCl, pH 7.4 buffer was used. Controls containing buffer only were included in assay. The rAamIGFBP-rP1 was pre-incubated at 37°C for 15 min with platelet aggregation agonist, ADP (20 µM) or thrombin (10 NIH units), in a 50 µL reaction filled up with 20 mM Tris-HCl, pH 7.4 buffer. Subsequently, 100 µL of platelet-rich plasma, prepared from citrated whole bovine blood as described elsewhere (Horn et al., 2000; Berger et al., 2010), was added and platelet aggregation was monitored by measuring OD (A650nm) every 20 s over 30 min using the Infinite M200Pro plate reader (Tecan). In addition, we repeated this assay in the way that rAamIGFBP-rP1 was pre-incubated with platelet-rich plasma, while the agonists were added afterwards.
Determination of antimicrobial effect of rAamIGFBP-rP1
We tested bactericidal and/or bacteriostatic effects of rAamIGFBP-rP1 on E. coli BL21 strain transformed with green fluorescent protein (GFP) plasmid. In 100 µL of super optimal broth with catabolite repression (SOC media), different concentrations of rAamIGFBP-rP1 (0, 10, 30, 50, 70, 100, and 300 µg/mL) were co-incubated with approximately the same amount of BL21 cells at 37°C for 1h with shaking ~230 rpm. BL21 cells were plated on LB agar plates with ampicillin (75 µg/mL) and arabinose (200 µg/mL), and overnight incubation at 37°C. As a control, instead of rAamIGFBP-rP1 we used kanamycin at the same concentrations. Subsequently, 50 µL of culture was plated on pre-warmed LB agar plates with ampicillin and arabinose and incubated overnight at 37°C. In order to detect eventual bactericidal effect of rAamIGFBP-rP1, additional control cultures without recombinant protein and kanamycin were prepared and plated before 1h incubation in SOC media. Plates with formed colonies were exposed to UV light for visualization of GFP and documentation.
Trypsinization of rAamIGFBP-rP1
Comparative analysis of insect cell-expressed rAamIGFBP-rP1 and human recombinant IGFBP-rP1 (Fitzgerald, Acton, MA, USA) trypsin digestion was performed. Around 0.5 µg of protein in 20 µL of PBS was subjected to digestion with bovine trypsin in the final concentrations of 5–100 µg/mL for 15 min at 37°C. Products of digestion were separated on 12.5% SDS-PAGE and visualized with standard Coomassie brilliant blue staining. In addition, western blot analysis was performed with digested samples using HRP-labeled anti C-terminal His-tag antibody (Life Technologies), as well as previously generated antibodies to A. americanum tick saliva proteins (Mulenga et al., 2013b), to detect separated fragments. Positive signal was detected by using BioFX chemiluminescent reagent (SurModics).
Determination of rAamIGFBP-rP1 ability to bind insulin and IGFs
Insulin and IGFs labeled with biotin (Eagle Biosciences, Nashua, NH, USA), with preserved ligand function, were used in determination of the binding affinity of both insect cell- and yeast-expressed rAamIGFBP-rP1. Variable rAamIGFBP-rP1 amounts: 0, 0.01, 0.02, 0.05, and 0.1 µg/µL, in 100 µL of ELISA Coating Buffer (BioLegend, San Diego, CA, USA) were coated onto EIA/RIA Costar plates (Corning, Corning, NY, USA) overnight. Following appropriate washing, plates were incubated with 5% skim milk in PBS/Tween overnight at 4°C to block. Subsequently, washing was performed 5 times using 300 µL of PBS/Tween per well, and 0.25 µg of ligand in 100 µL of blocking solution was applied in each well. The plate was incubated 3h at room temperature and washed 5 times with PBS/Tween. Detection of bound ligands was performed using HRP-labeled streptavidin (BioLegend), which was applied in 100 µL of blocking solution, in dilution 1:3000. After 30 min incubation at room temperature, wells were washed 5 times with PBS/Tween and 100 µL of 1-step Ultra TMB Reagent (Termo Scientific) was applied. Color was developed 30 min at room temperature and stopped by application of 100 µL of 2M sulfuric acid. OD (A450nm) was measured using the Infinite M200Pro plate reader (Tecan). This assay was performed in duplicate and values were presented as mean ± standard error. Additionally, this assay was repeated with all wells coated with 2 µg of rAamIGFBP-rP1, while different concentrations of ligands were used, as follows: 0, 0.001, 0.0025, and 0.005 µg/µL in 100 µL of blocking solution. Control binding assays were performed using A. americanum serpin 8 expressed in insect cells, as well as using A. americanum serpin 2 expressed in yeast.
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Diseases/National Institutes of Health (NIAID/NIH) grants (AI081093 and AI093858) to AM.
References
- Ahmed S, Yamamoto K, Sato Y, Ogawa T, Herrmann A, Higashi S, Miyazaki K. Proteolytic processing of IGFBP-related protein-1 (TAF/angiomodulin/mac25) modulates its biological activity. Biochem Biophys Res Comm. 2003;310:612–618. doi: 10.1016/j.bbrc.2003.09.058. [DOI] [PubMed] [Google Scholar]
- Aljamali M, Bowman AS, Dillwith JW, Tucker JS, Yates GW, Essenberg RC, Sauer JR. Identity and synthesis of prostaglandins in the lone star tick, Amblyomma americanum (L.), as assessed by radio-immunoassay and gas chromatography/mass spectrometry. Insect Biochem Mol Biol. 2002;32:331–341. doi: 10.1016/s0965-1748(01)00113-8. [DOI] [PubMed] [Google Scholar]
- Anderson BE, Sims KG, Olson JG, Childs JE, Piesman JF, Happ CM, Maupin GO, Johnson BJ. Amblyomma americanum: a potential vector of human ehrlichiosis. Am J Trop Med Hyg. 1993;49:239–244. doi: 10.4269/ajtmh.1993.49.239. [DOI] [PubMed] [Google Scholar]
- Anguita J, Ramamoorthi N, Hovius JWR, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincón M, Kantor FS, Fikrig E. Salp15, an Ixodes scapularis salivary protein, inhibits CD4+ T cell activation. Immunity. 2002;16:849–859. doi: 10.1016/s1074-7613(02)00325-4. [DOI] [PubMed] [Google Scholar]
- Berger M, Reck J, Jr, Terra RMS, Pinto AFM, Termignoni C, Guimarães JA. Lonomia obliqua caterpillar envenomation causes platelet hypoaggregation and blood incoagulability in rats. Toxicon. 2010;55:33–44. doi: 10.1016/j.toxicon.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Bissinger BW, Donohue KV, Khalil SMS, Grozinger CM, Sonenshine DE, Zhu J, Roe RM. Synganglion transcriptome and developmental global gene expression in adult females of the American dog tick, Dermacentor variabilis (Acari: Ixodidae) Insect Mol Biol. 2011;20:465–491. doi: 10.1111/j.1365-2583.2011.01086.x. [DOI] [PubMed] [Google Scholar]
- Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
- Burger AM, Leyland-Jones B, Banerjee K, Spyropoulos DD, Seth AK. Essential roles of IGFBP-3 and IGFBP-rP1 in breast cancer. Eur J Cancer. 2005;41:1515–1527. doi: 10.1016/j.ejca.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Chalaire KC, Kim TK, Garcia-Rodriguez H, Mulenga A. Amblyomma americanum (L.) (Acari: Ixodidae) tick salivary gland serine protease inhibitor (serpin) 6 is secreted into tick saliva during tick feeding. J Exp Biol. 2011;214:665–673. doi: 10.1242/jeb.052076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Liu Y, Zhang X. Topical insulin application improves healing by regulating the wound inflammatory response. Wound Repair Regen. 2012;20:425–434. doi: 10.1111/j.1524-475X.2012.00792.x. [DOI] [PubMed] [Google Scholar]
- Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Ann Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- Coles TB, Dryden MW. Insecticide/acaricide resistance in fleas and ticks infesting dogs and cats. Parasit Vectors. 2014;7:8. doi: 10.1186/1756-3305-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déruaz M, Frauenschuh A, Alessandri AL, Dias JM, Coelho FM, Russo RC, Ferreira BR, Graham GJ, Shaw JP, Wells TNC, Teixeira MM, Power CA, Proudfoot AEI. Ticks produce highly selective chemokine binding proteins with antiinflammatory activity. J Exp Med. 2008;205:2019–2031. doi: 10.1084/jem.20072689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degeorges A, Wang F, Frierson HF, Seth A, Sikes RA. Distribution of IGFBP-rP1 in Normal Human Tissues. J Histochem Cytochem. 2000;48:747–754. doi: 10.1177/002215540004800603. [DOI] [PubMed] [Google Scholar]
- Ewing S, Dawson J, Kocan A, Barker R, Warner C, Panciera R, Fox J, Kocan K, Blouin E. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 1995;32:368–374. doi: 10.1093/jmedent/32.3.368. [DOI] [PubMed] [Google Scholar]
- Gharahdaghi F, Weinberg CR, Meagher DA, Imai BS, Mische SM. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis. 1999;20:601–605. doi: 10.1002/(SICI)1522-2683(19990301)20:3<601::AID-ELPS601>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Honegger B, Galic M, Kohler K, Wittwer F, Brogiolo W, Hafen E, Stocker H. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J Biol. 2008;7:179–192. doi: 10.1186/jbiol72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopla CE, Downs CM. The isolation of Bacterium tularense from the tick, Amblyomma americanum . J Kansas Entomol Soc. 1953;26:72–73. [Google Scholar]
- Horn F, Coutinho dos Santos P, Termignoni C. Boophilus microplus anticoagulant protein: an antithrombin inhibitor isolated from the cattle tick saliva. Arch Biochem Biophys. 2000;384:68–73. doi: 10.1006/abbi.2000.2076. [DOI] [PubMed] [Google Scholar]
- Hrynyk M, Neufeld RJ. Insulin and wound healing. Burns. 2014;40:1433–1446. doi: 10.1016/j.burns.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily 1. Endocrine Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- Hyman AL, Kadowitz PJ. Pulmonary vasodilator activity of prostacyclin (PGI2) in the cat. Circulation Res. 1979;45:404–409. doi: 10.1161/01.res.45.3.404. [DOI] [PubMed] [Google Scholar]
- Ibelli AM, Kim TK, Hill CC, Lewis LA, Bakshi M, Miller S, Porter L, Mulenga A. A blood meal-induced Ixodes scapularis tick saliva serpin inhibits trypsin and thrombin, and interferes with platelet aggregation and blood clotting. Int J Parasitol. 2014;44:369–379. doi: 10.1016/j.ijpara.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski DC, Jasinskas A, Metz CN, Bucala R, Barbour AG. Identification and characterization of a homologue of the pro-inflammatory cytokine macrophage migration inhibitory factor in the tick, Amblyomma americanum. Insect Mol Biol. 2001;10:323–331. doi: 10.1046/j.0962-1075.2001.00271.x. [DOI] [PubMed] [Google Scholar]
- Keirans JE, Lacombe EH. First Records of Amblyomma americanum Ixodes (Ixodes) dentatus, and Ixodes (Ceratixodes) uriae (Acari: Ixodidae) from Maine. J Parasitol. 1998;84:629–631. [PubMed] [Google Scholar]
- Kelton JG, Blajchman MA. Prostaglandin I2 (prostacyclin) Can Med Assoc J. 1980;122:175–179. [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Curran J, Mulenga A. Dual silencing of long and short Amblyomma americanum acidic chitinase forms weakens the tick cement cone stability. J Exp Biol. 2014;217:3493–3503. doi: 10.1242/jeb.107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozek-Langenecker SA, Spiss CK, Michalek-Sauberer A, Felfernig M, Zimpfer M. Effect of prostacyclin on platelets, polymorphonuclear cells, and heterotypic cell aggregation during hemofiltration. Critical Care Med. 2003;31:864–868. doi: 10.1097/01.CCM.0000055374.77132.4D. [DOI] [PubMed] [Google Scholar]
- Laird JS, Kocan AA, Kocan KM, Presley SM, Hair JA. Susceptibility of Amblyomma americanum to natural and experimental infections with Theileria cervi . J Wildlife Dis. 1988;24:679–683. doi: 10.7589/0090-3558-24.4.679. [DOI] [PubMed] [Google Scholar]
- Landberg G, Ostlund H, Nielsen NH, Roos G, Emdin S, Burger AM, Seth A. Downregulation of the potential suppressor gene IGFBP-rP1 in human breast cancer is associated with inactivation of the retinoblastoma protein, cyclin E overexpression and increased proliferation in estrogen receptor negative tumors. Oncogene. 2001;20:3497–3505. doi: 10.1038/sj.onc.1204471. [DOI] [PubMed] [Google Scholar]
- Lovis L, Reggi J, Berggoetz M, Betschart B, Sager H. Determination of acaricide resistance in Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) field populations of Argentina, South Africa, and Australia with the larval tarsal test. J Med Entomol. 2013;50:326–335. doi: 10.1603/me12127. [DOI] [PubMed] [Google Scholar]
- Mans BJ, Ribeiro JM. A novel clade of cysteinyl leukotriene scavengers in soft ticks. Insect Biochem Mol Biol. 2008;38:862–870. doi: 10.1016/j.ibmb.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston JH, Childs JE, Chamberland ME, Tabor E. Transmission of tick-borne agents of disease by blood transfusion: a review of known and potential risks in the United States. Transfusion. 2000;40:274–284. doi: 10.1046/j.1537-2995.2000.40030274.x. [DOI] [PubMed] [Google Scholar]
- Mehlhorn H. Encyclopedia of Parasitology: AM. 2008 Springer [Google Scholar]
- Merten HA, Durden LA. A state-by-state survey of ticks recorded from humans in the United States. J Vector Ecol. 2000;25:102–113. [PubMed] [Google Scholar]
- Mixson TR, Campbell SR, Gill JS, Ginsberg HS, Reichard MV, Schulze TL, Dasch GA. Prevalence of Ehrlichia, Borrelia, and rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J Med Entomol. 2006;43:1261–1268. doi: 10.1603/0022-2585(2006)43[1261:poebar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Blandon M, Khumthong R. The molecular basis of the Amblyomma americanum tick attachment phase. Exp Appl Acarol. 2007;41:267–287. doi: 10.1007/s10493-007-9064-3. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Khumthong R. Silencing of three Amblyomma americanum (L.) insulin-like growth factor binding protein-related proteins prevents ticks from feeding to repletion. J Exp Biol. 2010;213:1153–1161. doi: 10.1242/jeb.035204. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Kim T, Ibelli AMG. Amblyomma americanum tick saliva serine protease inhibitor 6 is a cross-class inhibitor of serine proteases and papain-like cysteine proteases that delays plasma clotting and inhibits platelet aggregation. Insect Mol Biol. 2013a;22:306–319. doi: 10.1111/imb.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga A, Kim TK, Ibelli AM. Deorphanization and target validation of cross-tick species conserved novel Amblyomma americanum tick saliva protein. Int J Parasitol. 2013b;43:439–451. doi: 10.1016/j.ijpara.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga A, Sugimoto C, Sako Y, Ohashi K, Musoke A, Shubash M, Onuma M. Molecular characterization of a Haemaphysalis longicornis tick salivary gland-associated 29-kilodalton protein and its effect as a vaccine against tick infestation in rabbits. Infection Immun. 1999;67:1652–1658. doi: 10.1128/iai.67.4.1652-1658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Pykett MJ, Harnish P, Zang KD, George DL. Identification and characterization of genes differentially expressed in meningiomas. Cell Growth Different. 1993;4:715–722. [PubMed] [Google Scholar]
- Nagakubo D, Murai T, Tanaka T, Usui T, Matsumoto M, Sekiguchi K, Miyasaka M. A high endothelial venule secretory protein, Mac25/Angiomodulin, interacts with multiple high endothelial venule-associated molecules including chemokines. J Immunol. 2003;171:553–561. doi: 10.4049/jimmunol.171.2.553. [DOI] [PubMed] [Google Scholar]
- Oh Y, Nagalla SR, Yamanaka Y, Kim H, Wilson E, Rosenfeld RG. Synthesis and characterization of insulin-like growth factor-binding protein (IGFBP)-7: recombinant human mac25 protein specifically binds IGF-I and -II. J Biol Chem. 1996;271:30322–30325. doi: 10.1074/jbc.271.48.30322. [DOI] [PubMed] [Google Scholar]
- Parker R, Kohls GM. American Q fever: the occurrence of Rickettsia diaporica in Amblyomma americanum in eastern Texas. Pub Health Rep. 1943;58:1510–1511. [Google Scholar]
- Philip CB, White JS. Disease agents recovered incidental to a tick survey of the Mississippi Gulf Coast. J Economic Entomol. 1955;48:396–400. [Google Scholar]
- Prevot P, Adam B, Boudjeltia KZ, Brossard M, Lins L, Cauchie P, Brasseur R, Vanhaeverbeek M, Vanhamme L, Godfroid E. Anti-hemostatic effects of a serpin from the saliva of the tick Ixodes ricinus . J Biol Chem. 2006;281:26361–26369. doi: 10.1074/jbc.M604197200. [DOI] [PubMed] [Google Scholar]
- Radulović Ž, Kim T, Porter L, Sze S, Lewis L, Mulenga A. A 24–48h fed Amblyomma americanum tick saliva immuno-proteome. BMC Genomics. 2014;15:518. doi: 10.1186/1471-2164-15-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J, Makoul G, Robinson D. Ixodes dammini: evidence for salivary prostacyclin secretion. J Parasitol. 1988;74:1068–1069. [PubMed] [Google Scholar]
- Sambrook J, Russell DW, Russell DW. Molecular cloning: a laboratory manual. 3-ume. New York: Cold spring harbor laboratory press Cold Spring Harbor; 2001. Set. [Google Scholar]
- Sato J, Hasegawa S, Akaogi K, Yasumitsu H, Yamada S, Sugahara K, Miyazaki K. Identification of cell-binding site of angiomodulin (AGM/TAF/Mac25) that interacts with heparan sulfates on cell surface. J Cellular Biochem. 1999;75:187–195. [PubMed] [Google Scholar]
- Savage HM, Godsey MS, Jr, Lambert A, Panella NA, Burkhalter KL, Harmon JR, Lash RR, Ashley DC, Nicholson WL. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg. 2013;89:445–452. doi: 10.4269/ajtmh.13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Henrik Tomas JH, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nature Protocols. 2007;6:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- Sloth Andersen A, Hertz Hansen P, Schäffer L, Kristensen C. A new secreted insect protein belonging to the immunoglobulin superfamily binds insulin and related peptides and inhibits their activities. J Biol Chem. 2000;275:16948–16953. doi: 10.1074/jbc.M001578200. [DOI] [PubMed] [Google Scholar]
- Smith JB, Araki H, Lefer AM. Thromboxane A2, prostacyclin and aspirin: effects on vascular tone and platelet aggregation. Circulation. 1980;62:V19–V25. [PubMed] [Google Scholar]
- Sonenshine DE, Roe RM. Biology of ticks. Oxford: University Press; 2013. [Google Scholar]
- Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KTG, Lavrsen K, Dabelsteen S, Pedersen NB, Marcos-Silva L, Gupta R, Paul Bennett E, Mandel U, Brunak S, Wandall HH, Levery SB, Clausen H. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32:1478–1488. doi: 10.1038/emboj.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant MK, Vessella RL, Sprenger CC, Sikes RA, Hwa V, Baker LD, Plymate SR. Insulin-like growth factor binding protein-related protein 1 (IGFBP-rP1/mac 25) is reduced in human prostate cancer and is inversely related to tumor volume and proliferation index in Lucap 23.12 xenografts. Prostate. 2003;56:115–122. doi: 10.1002/pros.10223. [DOI] [PubMed] [Google Scholar]
- Tirloni L, Reck J, Terra RMS, Martins JR, Mulenga A, Sherman NE, Fox JW, Yates III JR, Termignoni C, Pinto AFM, da Silva Vaz I., Jr Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: A comparison between partially and fully engorged females. PLoS One. 2014;9:e94831. doi: 10.1371/journal.pone.0094831. IGFBP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson K, Elkins C, Patterson H, Fikrig E, De Silva A. Biochemical and functional characterization of Salp20, an Ixodes scapularis tick salivary protein that inhibits the complement pathway. Insect Mol Biol. 2007;16:469–479. doi: 10.1111/j.1365-2583.2007.00742.x. [DOI] [PubMed] [Google Scholar]
- Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandji S-, Gadsby JE, Barber JA, Hammond JM. Messenger ribonucleic acids for MAC25 and connective tissue growth factor (CTGF) are inversely regulated during folliculogenesis and early luteogenesis. Endocrinology. 2000;141:2648–2657. doi: 10.1210/endo.141.7.7576. [DOI] [PubMed] [Google Scholar]
- Wang YS, Chen SW, Yen JH, Chen YL. Dissipation and movement of acaricide chlorobenzilate in the environment. Ecotoxicol Environ Safety. 1994;28:193–200. doi: 10.1006/eesa.1994.1045. [DOI] [PubMed] [Google Scholar]
- Wharton RH. Acaricide resistance and cattle tick control. Aus Vet J. 1967;43:394–398. doi: 10.1111/j.1751-0813.1967.tb04892.x. [DOI] [PubMed] [Google Scholar]
- Willadsen P. Vaccination against ticks and the control of ticks and tick-borne disease. In: Makkar HS, Viljoen G, editors. Application of Gene-Based Technologies for Improving Animal Production and Health in Developing Countries. Netherlands: Springer; 2005. pp. 313–321. [Google Scholar]
- Wilson EM, Oh Y, Hwa V, Rosenfeld RG. Interaction of IGF-binding protein-related protein 1 with a novel protein, neuroendocrine differentiation factor, results in neuroendocrine differentiation of prostate cancer cells. J Clin Endocrin Metabolism. 2001;86:4504–4511. doi: 10.1210/jcem.86.9.7845. [DOI] [PubMed] [Google Scholar]
- Wolf L, McPherson T, Harrison B, Engber B, Anderson A, Whitt P. Prevalence of Ehrlichia ewingii in Amblyomma americanum in North Carolina. J Clin Microbiol. 2000;38:2795–2795. doi: 10.1128/jcm.38.7.2795-2795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Annu Rev Entomol. 2006;51:1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Umeda F, Masakado M, Isaji M, Mizushima S, Nawata H. Purification and molecular cloning of prostacyclin-stimulating factor from serum-free conditioned medium of human diploid fibroblast cells. Biochem J. 1994;303:591–598. doi: 10.1042/bj3030591. [DOI] [PMC free article] [PubMed] [Google Scholar]