Yì-Xiáng J. Wáng
Pancreatic cancer is a lethal disease because incidence and mortality rates are nearly identical. The 5-year survival rate in Western countries is 4%, the lowest among all types of cancer (1). In China, pancreatic cancer is the sixth leading cause of death from malignant disease, with an overall cumulative 5-year survival rate of 1% to 3% (2,3). The indentified reported risk factors for pancreatic cancer in China are considered to be similar to those in Western countries (2,3). Patients with pancreatic cancer discovered by chance through imaging seem to have increased survival (4-6). Further more, recent evidence from genomic sequencing indicates a 15-year interval for genetic progression of pancreatic cancer from initiation to the metastatic stage, suggesting a sufficient window for early detection (7,8). Prior to the 1990’s, it was not widely appreciated how often pancreas cancer can be an inherited disease. Now we know that individuals with abnormalities in certain genes, such as BRCA2, p16, hereditary non-polyposis colorectal cancer, and individuals with histories of familial pancreatitis and Peutz-Jeghers syndrome are all predisposed to pancreatic cancer (9-14). These are important discoveries. However, pancreatic cancer is still diagnosed in only 10% of patients with known risk factors, the other 90% percent are considered sporadic cancers with no currently known risk factors (4,12).
An ideal pancreatic cancer screening test should be a safe, inexpensive, highly accurate test that reliably diagnoses pancreas cancer at a stage when it is not causing symptoms in the patient. This would provide that person the opportunity to take appropriate and effective action to treat and potentially cure the disease. Currently, a serum screening test for pancreas cancer to meet these demands is not available. Serum CA 19-9 is the most extensively validated pancreatic cancer biomarker. CA 19-9 serum levels have a sensitivity and specificity of 79-81% and 82-90% respectively for the diagnosis of pancreatic cancer in symptomatic patients; but are not useful as a screening marker because of low positive predictive value (0.5-0.9%) (15). Due to the inability of CA 19-9 to identify early potentially curable disease, several other markers have been studied, including SPAN-1, CA-50, DUPAN-2, elastase-1, tissue polypeptide antigen and tissue polypeptide-specific antigen. These markers have not performed nearly as well as the CA 19-9 (16). Population screening with radiographic imaging or endoscopic procedures makes no clinical or economic sense for a cancer that represents only 3% of estimated new cancers each year (4). However it may be reasonable to screen high-risk cohorts of patients using imaging; including patients with chronic pancreatitis, individuals with a family history of pancreatic cancer, patients with hereditary pancreatitis, Peutz-Jeghers syndrome, cystic fibrosis or familial atypical multiple mole melanoma. Precursor lesions include pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasia. The yield of high-risk cohorts patients screening programs reported in the literature ranges from 1.3% to 50% (4,8). Intraductal papillary mucinous neoplasia has consensus-guideline indications for resection (17). All symptomatic pancreas cysts should undergo resection, and mucinous cystic neoplasms should be resected (17).
Recently Del Chiaro et al. of the Karolinska Institute, Stockholm, Sweden, and coauthors analyzed short-term results from a magnetic resonance imaging (MRI)-based screening program for individuals at high risk of pancreatic cancer (18). The study included 40 patients (24 women and 16 men with an average age of nearly 50). Inclusion criteria met the current recommendations of the International Cancer of the Pancreas Screening Consortium for high-risk patients, namely, individuals from a familial pancreatic cancer kindred with at least 2 affected first degree relatives; patients with Peutz-Jeghers syndrome; and p16, BRCA2, or BRCA1 mutation carriers. In 38 of the patients, increased risk of the disease was based on family history of pancreatic cancer. BRCA2, BRCA1 and p16 gene mutations were identified in some patients. The average study follow-up was 12.9 months, with MRI screening repeated after 1 year if the initial screen was negative or at 6 months if there were unspecific findings or findings that did not indicate surgery. MRI revealed a pancreatic lesion: intraductal papillary mucinous neoplasia (14 patients, 35%) and pancreatic ductal adenocarcinoma (2 patients, 5%). One patient had a synchronous intraductal papillary mucinous neoplasia and pancreatic ductal adenocarcinoma. Five patients (12.5%) required surgery (3 for pancreatic ductal adenocarcinoma and 2 for intraductal papillary mucinous neoplasia), while the remaining 35 are under continued surveillance.
The imaging methodology for pancreas cancer screening is mainly MRI with magnetic resonance cholangiopancreatography (MRCP), or biopsy or fine-needle aspiration using endoscopic ultrasound (EUS) (19-24). EUS also has the unique ability to obtain specimens for histopathological diagnosis using EUS-guided FNA. MRCP allows non-invasive delineating the pancreatic ductal system, and detecting subtle ductal narrowing that may suggest the presence of a small mass (23). MRI with MRCP allows more successful tumor detection at an early stage than multi-detector CT (23). However, small or non-contour-deforming pancreatic adenocarcinomas may lack classic imaging features and thus may not be detected on conventional MRI (25). The use of functional imaging methods such as diffusion-weighted imaging may allow earlier detection of pancreatic adenocarcinoma (26,27).
Currently, the challenge remains to validate strategies for early diagnosis and show that they reduce pancreatic cancer-specific mortality rates. This issue has been highly controversial in other cancers, including prostate cancer, breast cancer, and lung cancer (4,28-32). Surgical and radiation interventions are associated with morbidities that are sometime significant in many patients. In the US Prostate, Lung, Colorectal, and Ovarian Cancer study, a large number of excess tumors were detected in the screening group but without a reduction in mortality (28,32). Screening programs all carry potential biases that could overestimate the benefits of the screening intervention (Figure 1), including among others, lead-time and length biases (Figures 2,3). Another disadvantage of imaging of screening programs is the cost and high rate of clinically irrelevant false-positive findings (30). Poruk et al. (4) summarized seven studies for pancreatic cancer screening. Of the total 410 high-risk patients, 43 patients underwent surgical resection because of suspicion for malignancy. Eight cases of invasive pancreatic ductal adenocarcinoma were identified, resulting in a diagnostic yield for malignancy of 1.95% (8/410) (4). It is clear that a limiting feature of imaging-based screening programs is the relatively high rate of identification of benign lesions that subsequently require additional invasive evaluation.
Figure 1.
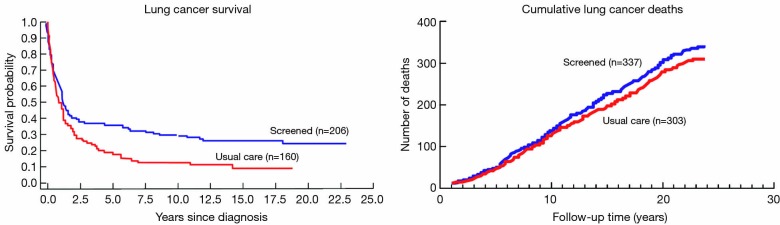
The Mayo Lung Project (MLP) was a randomized, controlled clinical trial of lung cancer screening that was conducted in 9,211 male smokers between 1971 and 1983 (intervention arm, n=4,618; usual-care arm, n=4,593). The intervention arm was offered chest X-ray and sputum cytology every 4 months for 6 years. Extended follow-up of MLP participants did not reveal a lung cancer mortality reduction for the intervention arm. The median follow-up time was 20.5 years. Lung cancer mortality was 4.4 [95% confidence interval (CI), 3.9-4.9] deaths per 1,000 person-years in the intervention arm and 3.9 (95% CI, 3.5-4.4) in the usual-care arm (two-sided P: for difference =0.09). Similar mortality but better survival for individuals in the intervention arm indicates that some lesions with limited clinical relevance may have been identified in the intervention arm. Data from Marcus PM, Bergstralh EJ, Fagerstrom RM, Williams DE, Fontana R, Taylor WF, and Prorok PC. Lung cancer mortality in the Mayo Lung Project: Impact of extended follow-up. J Natl Cancer Inst 2000;92:1308-16.
Figure 2.
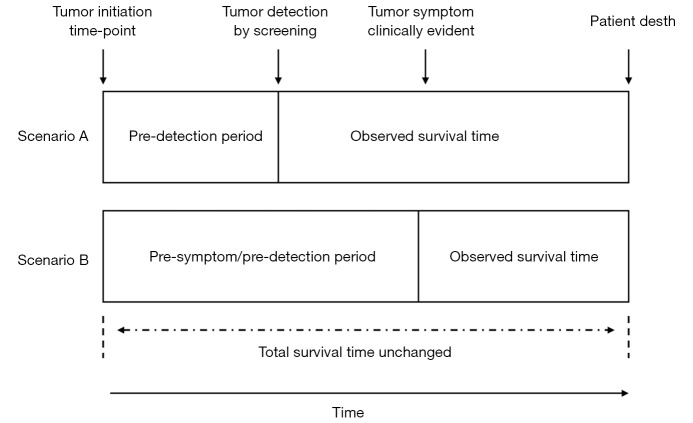
Screening programs have potential biases that make demonstration of benefit difficult. In lead-time bias, earlier detection of tumors via screening (scenario A) may seem to result in longer survival than control subjects identified by clinical symptoms (scenario B); however, the natural history of the tumor may not have been altered. Modified from reference (29).
Figure 3.
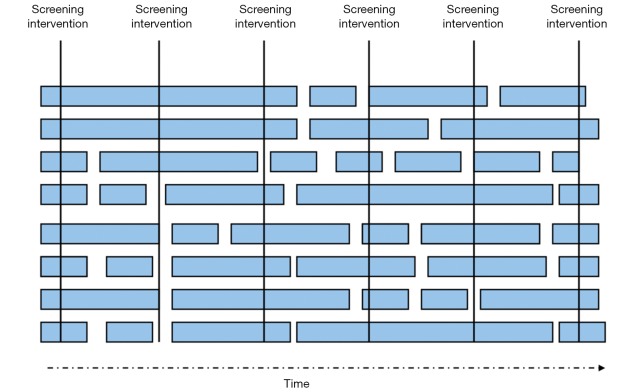
In this schematic, a screening tool has been applied to a hypothetical population of patients at intervals. Each patient is represented by a bar. The length of the bar represents the cancer-related survival of the patient. Length bias refers to the tendency of screening programs to identify patients with more favorable tumor biology (i.e., slower growth rates or less risk of metastasis), whereas patients with tumors with aggressive biology (rapidly growing tumors or those with a high risk of metastasis) may not be identified because of a short natural history until death. The finding of longer survival in patients identified through screening may not be related to the screening intervention—the benefit could lie in the natural history of these tumors. Modified from reference (29).
One principal obstacle to effective surveillance is the lack of knowledge about the natural history of premalignant lesions of the pancreas, as well as the lack of criteria for reliable prediction of progression and outcome of these lesions in individual high-risk patients (Figure 4). In a retrospective cohort study, Chernyak et al. (33) demonstrated lack of increase in overall mortality in patients over 65 years with pancreatic cysts. Despite elevated risk of ductal adenocarcinoma and cyst related malignancies (mucinous cystic neoplasm and intraductal papillary mucinous carcinoma), there was no increase in all-cause mortality in patients over 65 years. Incidental pancreatic cysts were associated with increased all-cause mortality only in patients younger than 65 years. There is also a risk of over-treating an indolent condition in those patients where pancreatic intraepithelial neoplasias and intraductal papillary mucinous neoplasms do not lead to pancreatic cancer. In 2007 Rubenstein et al. (34) created a model for 45-year-old male first-degree relatives from patients with familial pancreatic cancer and chronic pancreatitis on EUS. A “do-nothing”-strategy provided the best life expectancy for patients with increased risk and the lowest cost compared with prophylactic total pancreatectomy and annual surveillance by EUS with or without fine-needle aspiration. Owing to the high mortality of even localized pancreatic cancer, efforts with regards to early diagnosis only have a minimal impact on life expectancy, even with 100% test accuracy.
Figure 4.
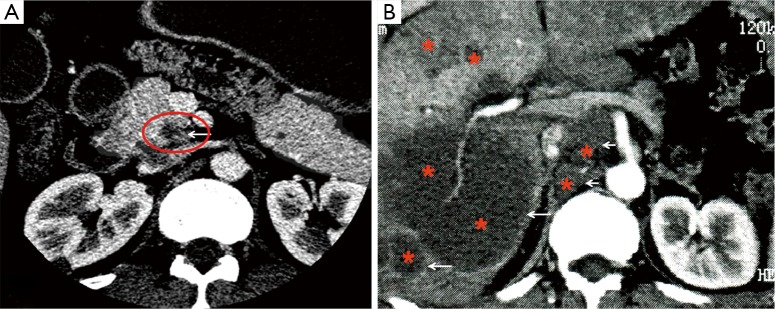
Axial contrast enhanced-CT image shows a small hypo-attenuation focus measured 0.8 cm at the pancreatic head (A) in a 42-year-old man. The small tumor was completely resected and proved to be a pancreatic adenocarcinoma pathologically. However, a 1-year follow-up CT image (B) reveals multiple hepatic metastases (long arrows) and enlarged retroperitoneal lymph nodes (short arrows). In this case, an early detection of small tumor did not ensure improved survival.
The American Cancer Society strongly recommends that anyone considering genetic testing talk with a genetic counselor, nurse, or doctor qualified to interpret and explain the test results before they proceed with testing (35). Talking to someone with experience in hereditary cancer syndromes such as a genetic counselor, geneticist, or an oncologist is often helpful. However, most of the changes in the DNA and other molecules in the pancreas cells that give rise to pancreatic cancer are not inherited and occur as the result of other factors such as smoking, diet, and age (4). It is hoped that in the future, specific and more sensitive screening serum biomarker based tests can be developed, and such screening test will be able to detect pancreatic cancer at an early stage when it still cannot be visualized even using state-of-the-art diagnostic imaging techniques.
In conclusion, the key question for pancreas cancer screening remains unresolved as to whether or not the survival of afflicted patients is actually increased with early detection, and whether the population death rate from pancreatic cancer is decreased. While several international societies recommend a surveillance program for individuals at risk for developing pancreatic cancer, the actual efficacy of such preventive programs has not been validated so far. Del Chiaro et al.’s current study (18), with high positive yield and using non-invasive MR imaging protocol, add valuable information for future synthetic analysis. In addition, with further development of MRI data acquisition and image reconstruction (36-38), MR imaging is becoming faster and the cost-effectiveness is likely to further improve.
Acknowledgements
None.
Biography
Author’s introduction: Dr. Yi-Xiang Wang read medicine and trained in diagnostic and interventional radiology in China. He did post-doctoral fellowships in Sheffield Medical School, UK, and Rotterdam School of Medicine (Erasmus), The Netherlands. Prior to his current post, Dr. Wang was with AstraZeneca Pharmaceuticals R & D (UK) as a senior scientist and associate team leader. He joined the Faculty of Medicine at the Chinese University of Hong Kong in late 2006, where he is currently an associate professor, the division head for graduate fairs, as well as an associate Professor (by courtesy) at the School of Biomedical Sciences of the University. In principle investigator or co-principle investigator capacity, he discovered magnetic resonance T1rho relaxation time is increased in fibrotic liver, and established the normal magnetic resonance T1rho relaxation time in healthy livers. He found the spine osteoporotic fracture rate (prevalence) in elderly people is the same in Chinese, Korean, Japanese, and Latin American. He discovered in elderly subjects intervertebral disc degeneration is more common and more severe in women than men, and estrogen is beneficial for intervertebral disc. He established the prevalence and progression rate of spondylolisthesis in elderly Chinese men and women. He also discovered the regression pattern of chronic radiation induced brain injury. He pioneered Chemical Exchange Saturation Transfer magnetic resonance technique in liver application. He is the founding editor of the journal Quantitative Imaging in Medicine and Surgery. Dr. Wang also has strong interests in history and classics, and is passionate in educating healthcare to the general public. Dr. Wang received a number of international awards, and delivered a number of international invited lectures. Dr. Wang published 200 papers in international journals, and his work has been cited over 2,500 times according to ISI web of science. He is a regular reviewer of a number of European grant funding agencies.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [DOI] [PubMed] [Google Scholar]
- 2.Guo X, Cui Z. Current diagnosis and treatment of pancreatic cancer in China. Pancreas 2005;31:13-22. [DOI] [PubMed] [Google Scholar]
- 3.Long J, Luo GP, Xiao ZW, et al. Cancer statistics: current diagnosis and treatment of pancreatic cancer in Shanghai, China. Cancer Lett 2014;346:273-7. [DOI] [PubMed] [Google Scholar]
- 4.Poruk KE, Firpo MA, Adler DG, et al. Screening for pancreatic cancer: why, how, and who? Ann Surg 2013;257:17-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishikawa O, Ohigashi H, Imaoka S, et al. Minute carcinoma of the pancreas measuring 1 cm or less in diameter--collective review of Japanese case reports. Hepatogastroenterology 1999;46:8-15. [PubMed] [Google Scholar]
- 6.Ariyama J, Suyama M, Satoh K, et al. Imaging of small pancreatic ductal adenocarcinoma. Pancreas 1998;16:396-401. [DOI] [PubMed] [Google Scholar]
- 7.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:1114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gemmel C, Eickhoff A, Helmstädter L, et al. Pancreatic cancer screening: state of the art. Expert Rev Gastroenterol Hepatol 2009;3:89-96. [DOI] [PubMed] [Google Scholar]
- 9.Naderi A, Couch FJ. BRCA2 and pancreatic cancer. Int J Gastrointest Cancer 2002;31:99-106. [DOI] [PubMed] [Google Scholar]
- 10.Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000;119:1447-53. [DOI] [PubMed] [Google Scholar]
- 11.Del Chiaro M, Boggi U, Presciuttini S, et al. Genetics of pancreatic cancer: where are we now? Where are we going? JOP 2005;6:60-7. [PubMed] [Google Scholar]
- 12.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res 2004;64:2634-8. [DOI] [PubMed] [Google Scholar]
- 13.Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013;62:339-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Chiaro M, Zerbi A, Falconi M, et al. Cancer risk among the relatives of patients with pancreatic ductal adenocarcinoma. Pancreatology 2007;7:459-69. [DOI] [PubMed] [Google Scholar]
- 15.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol 2012;3:105-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffy MJ, Sturgeon C, Lamerz R, et al. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol 2010;21:441-7. [DOI] [PubMed] [Google Scholar]
- 17.Fasanella KE, McGrath K. Cystic lesions and intraductal neoplasms of the pancreas. Best Pract Res Clin Gastroenterol 2009;23:35-48. [DOI] [PubMed] [Google Scholar]
- 18.Del Chiaro M, Verbeke CS, Kartalis N, et al. Short-term Results of a Magnetic Resonance Imaging-Based Swedish Screening Program for Individuals at Risk for Pancreatic Cancer. JAMA Surg 2015;150:512-8. [DOI] [PubMed] [Google Scholar]
- 19.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol 2006;4:766-81; quiz 665. [DOI] [PubMed] [Google Scholar]
- 20.Volmar KE, Vollmer RT, Jowell PS, et al. Pancreatic FNA in 1000 cases: a comparison of imaging modalities. Gastrointest Endosc 2005;61:854-61. [DOI] [PubMed] [Google Scholar]
- 21.Rösch T, Lorenz R, Braig C, et al. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc 1991;37:347-52. [DOI] [PubMed] [Google Scholar]
- 22.Maccioni F, Martinelli M, Al Ansari N, et al. Magnetic resonance cholangiography: past, present and future: a review. Eur Rev Med Pharmacol Sci 2010;14:721-5. [PubMed] [Google Scholar]
- 23.Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol 2014;20:7864-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol 2004;2:606-21. [DOI] [PubMed] [Google Scholar]
- 25.Yang MJ, Li S, Liu YG, et al. Common and unusual CT and MRI manifestations of pancreatic adenocarcinoma: a pictorial review. Quant Imaging Med Surg 2013;3:113-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozgeyik Z, Onur MR, Poyraz AK. The role of diffusion weighted magnetic resonance imaging in oncologic settings. Quant Imaging Med Surg 2013;3:269-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Miller FH, Chen ZE, et al. Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. Radiographics 2011;31:E47-64. [DOI] [PubMed] [Google Scholar]
- 28.Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA 2009;302:1685-92. [DOI] [PubMed] [Google Scholar]
- 29.Croswell JM, Ransohoff DF, Kramer BS. Principles of cancer screening: lessons from history and study design issues. Semin Oncol 2010;37:202-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang YX, Gong JS, Suzuki K, et al. Evidence based imaging strategies for solitary pulmonary nodule. J Thorac Dis 2014;6:872-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beinfeld MT, Wittenberg E, Gazelle GS. Cost-effectiveness of whole-body CT screening. Radiology 2005;234:415-22. [DOI] [PubMed] [Google Scholar]
- 32.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. PLCO Project Team . Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009;360:1310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chernyak V, Flusberg M, Haramati LB, et al. Incidental pancreatic cystic lesions: is there a relationship with the development of pancreatic adenocarcinoma and all-cause mortality? Radiology 2015;274:161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubenstein JH, Scheiman JM, Anderson MA. A clinical and economic evaluation of endoscopic ultrasound for patients at risk for familial pancreatic adenocarcinoma. Pancreatology 2007;7:514-25. [DOI] [PubMed] [Google Scholar]
- 35.Can pancreatic cancer be found early? Available online: http://www.cancer.org/cancer/pancreaticcancer/detailedguide/pancreatic-cancer-detection
- 36.Ji JX, Zhang X. Parallel and sparse MR imaging: methods and instruments-Part 2. Quant Imaging Med Surg 2014;4:68-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Ji JX. Parallel and sparse MR imaging: methods and instruments-Part 1. Quant Imaging Med Surg 2014;4:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang YX, Lo GG, Yuan J, et al. Magnetic resonance imaging for lung cancer screen. J Thorac Dis 2014;6:1340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]