Abstract
Laparoscopic pancreaticoduodenectomy (LPD) is an extremely challenging surgery. First described in 1994, it has been slow to gain in popularity. Recently, however, we have seen an increase in the number of centers performing this operation, including our own institution, as well as an increase in the quantity of published data. The purpose of this review is to describe the current status of LPD as described in the literature. We performed a literature search in the PubMed database using MeSH terms “laparoscopy” and “pancreaticoduodenectomy”. We then identified articles in the English language with over 20 patients that focused on LPD only. Review articles were excluded and only one article per institution was used for descriptive analysis in order to avoid overlap. There were a total of eight articles meeting review criteria, consisting of 492 patients. On descriptive analysis we found that percent of LPD due to high-grade malignancy averaged 47% over all articles. Average operative time was 452 minutes, blood loss 369 cc’s, pancreatic leak rate 15%, delayed gastric emptying 8.6%, length of hospital stay 9.4 days, and short term mortality 2.3%. Comparison studies between open pancreaticoduodenectomy (OPD) and LPD suggested decreased blood loss, longer operative time, similar post-operative complication rate, decreased pain, and shorter hospital length of stay for LPD. There was also increased number of lymph nodes harvested and similar margin free resections with LPD in the majority of studies. LPD is a safe surgery, providing many of the advantages typically associated with laparoscopic procedures. We expect this operation to continue to gain in popularity as well as be offered in increasingly more complex cases. In future studies, it will be beneficial to look further at the oncologic outcome data of LPD including survival.
Keywords: Laparoscopic, laparoscopy, pancreaticoduodenectomy, whipple, review, laparoscopic vs. open

Justin Merkow

Barish H. Edil
Introduction
Pancreatic cancer is the 12th most common cancer in the world, with 338,000 new cases diagnosed in 2012 (1). In the United States in 2014, it affected over 46,000 people resulting in a mortality 39,590 individuals (2). The treatment—pancreaticoduodenectomy (PD) has seen improved perioperative outcomes and complication rates over the last few decades (3-6). Nevertheless, it continues to be a morbid operation with complications ranging from 24-59% (7-9). Laparoscopic surgery reduces surgical morbidity in various operations, however laparoscopic pancreaticoduodenectomy (LPD) is a relatively new procedure which lacks a clear consensus regarding its benefits (10-14). Although the first published case was described in 1994, it has been slow to gain popularity (15). This is likely in part due to the challenging technical aspect of the procedure including the retroperitoneal location of the pancreas, close vicinity to the superior mesenteric artery and vein, portal vein and hepatic arteries and the technical difficulty of three anastamosis. In recent years, however, we have seen an increasing number of studies examining LPD. Initial research evaluated feasibility and outcomes, assessing whether LPD could be done with adequate safety (16-23). The question then moved from is LPD safe to how does it compare to the open approach? Will it appreciate the same benefits of other laparoscopic surgeries? Partially enabled by higher volumes at specialized centers, studies began comparing LPD with open pancreaticoduodenectomy (OPD). Although there are a handful of pancreaticoduodenectomy review articles evaluating LPD in the literature, many include papers with limited sample sizes and case reports. Our goal with this review was to examine the larger sampled articles available and evaluate the present state of LPD.
Methods
A literature search was performed in the PubMed database using MeSH terms “laparoscopy” and “pancreaticoduodenectomy”. The final search was completed on February 20, 2015 and revealed 180 articles. We identified only those in English involving total LPD with over 20 patients in the study. Irrelevant articles, review articles, those with less than 20 patients, laparoscopic assist, robotic, and hybrid focused studies were excluded. Those involving colon, spleen, biliary resections, porcine models, and articles published prior to 2005 were also excluded from the study. Two researchers (JM and AP) worked through these criteria independently and identified 12 studies deemed suitable. For our descriptive analysis we used only one article per institution when multiple publications originated from a single center to avoid overlap. In these instances we chose the most recent article. Following this exclusion, we were left with eight articles. See Figure 1.
Figure 1.
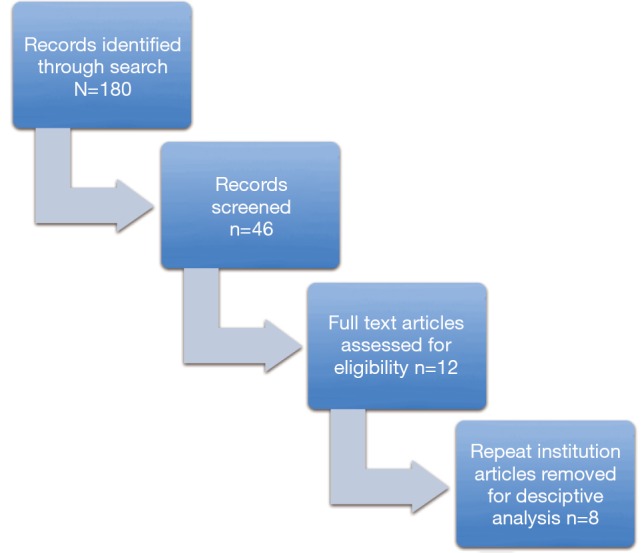
Literature search.
In the literature review, both descriptive and comparative studies were found. We extracted technical, perioperative and intraoperative data. This included conversion rate, operative time, and intraoperative blood loss. We also collected information on hospital length of stay, pancreatic leak, delayed gastric emptying, post-operative bleeding, abscess formation and short term mortality. Oncologic data including proportion of patients with invasive malignancy, number of lymph nodes removed, and margin status was also recorded. Five-year overall survival was not available in most studies and the diversity of malignant etiologies in patients made this more difficult to interpret collectively. In our descriptive analysis, we used a weighted average to calculate our various rates based on the number of subjects in each study.
Results
Descriptive analysis
A total of eight articles were included that met our inclusion and exclusion criteria. Year of publication ranged from 2009 to 2015. There were a total of 492 patients who underwent LPD included in our review. All of the studies were retrospective. Three studies were purely descriptive in nature and the remaining five articles compared laparoscopic and OPD. Regarding article country of origin there were 4 from USA, 1 from Korea, 1 from India, 1 from Japan, and 1 from Italy (19,24-30). See Table 1.
Table 1. Articles reporting on 20 or more laparoscopic pancreaticoduodenectomies.
| Author | Year | Number of cases | Country |
|---|---|---|---|
| Asbun | 2012 | 268 | USA |
| Croome | 2014 | 322 | USA |
| Speicher | 2014 | 56 | USA |
| Song | 2015 | 2,192 | Korea |
| Palanivelu | 2009 | 75 | India |
| Mesleh | 2013 | 123 | USA |
| Honda | 2013 | 26 | Japan |
| Corcione | 2013 | 22 | Italy |
Purpose for PD ranged from treatment of benign and low-grade malignancies to high-grade malignancies such as pancreatic ductal adenocarcinoma, ampullary adenocarcinoma, cholangiocarcinoma, and metastatic renal cell carcinoma. The percent of LPD for high-grade malignancy in studies reviewed ranged from 10.1% to 100%, with an average of 47% over all cases.
Although documented in only four articles, rate of laparoscopic pylorus preserving pancreaticoduodenectomy was found to be the technique of choice in 63% of cases, ranging from 0 to 100% per article. Additionally, five studies discussed pancreatic duct anastomosis technique, of which four used an end to side anastomosis, and one used both end-to-end and end-to-side technique. Conversion rate to open was noted in 7 of the 8 articles. The average rate of conversion ranged from 0-15%, with an average over all cases of 13%. Average operating time among patients undergoing LPD was 452 minutes, ranging from 357 to 551 minutes. There did appear to be a significant improvement in operating time depending on the experience of the surgeon. Average blood loss for LPD was 369 cc’s, ranging from 74 to 592 cc’s. This also improved considerably based on surgeon experience.
Pancreatic leak information was available in all eight papers, and ranged from 6.7% to 29.9% of cases per article. The average pancreatic leak proportion was 15%. Over all, the average delayed gastric emptying rate was 8.6%, ranging from 3.2% to 13% over included studies. The average length of hospital stay for LPD patients was 9.4 days, ranging from 6 to 20 days per article. This data was reported in 7 of the 8 articles. Finally, short-term mortality, defined as all cause mortality less than 100 days from surgery, was 2.3% over all studies. See Table 2.
Table 2. Descriptive data of laparoscopic pancreaticoduodenectomy.
| Author | Malignant etiology (%) | Conversion rate (%) | Operative time (min) | Blood loss (cc) | Hospital stay (days) | Pancreatic leak (%) | Delayed gastric emptying (%) | Short-term mortality (%) |
|---|---|---|---|---|---|---|---|---|
| Asbun | 64 | 15 | 541 | 195 | 8 | 16.7 | 11.3 | 5.7 |
| Croome | 100 | 7 | 379.4 | 492.4 | 6 | 11 | 9 | 2 |
| Speicher | NR | 0 | 381 | 200 | 8.5 | 16 | NR | 4 |
| Song | 10.1 | NR | 480.4 | 592 | 14.1 | 29.9 | 3.2 | 0 |
| Palanivelu | 96 | 0 | 357 | 74 | 8.2 | 6.7 | NR | 1.3 |
| Mesleh | 79 | 10 | 551 | NR | 7 | 9 | 13 | NR |
| Honda | 46 | 2 | 519 | 303 | NR | 23 | 11.5 | 0 |
| Corcione | 100 | 2 | 450 | NR | 20 | 27 | NR | 4.5 |
NR, not recorded.
Although survival data was rarely available and difficult to interpret with varying malignant etiologies, we did record two surrogates for oncologic outcomes—number of LNs removed and margin free resection. Firstly, the average number of lymph nodes removed was recorded in 6 of the 8 articles, ranging from 14 to 23.4 nodes. Margin free resection ranged from 77% to 100% and was available in six studies.
This data, although not directly comparing LPD to OPD, does show that LPD is safe and feasible with acceptable outcomes. Descriptive studies such as these have led to more acceptance in the surgical community of this complex laparoscopic surgery. One hindrance to the utilization of more surgeons performing this technique is likely the technical difficulty and the lack of formalization of education in this technique. Interestingly, some studies have specifically looked at this learning process with encouraging findings.
Learning curve
A number of the studies we include in our review address the learning curve required for LPD with promising findings. Surgeons performing LPD do indeed improve significantly over time, with decreased operative times, blood loss, pancreatic leak rates, and length of hospital stay. For example, Kim et al. (22), in a study of 100 consecutive cases of laparoscopic pylorus preserving pancreaticoduodenectomy performed by the same surgeon found that when they divided these patients into three chronological periods, there were significant outcome improvements. For example, operative times went from 9.8 hours in period one to 6.6 hours in period three. Length of hospital stay went from 20.4 to 11.5 days, and complication rate (including pancreatic fistula, ileus, bleeding, delayed gastric emptying) went from 33.3% to 17.6% in period one and three, respectively. A study by Speicher et al. (28) divided LPD into three cohorts of ten patients (last cohort had six patients) based on order performed, and found that operative time as well as blood loss decreased. Additionally, they proposed a staged learning process, with separate performance measures that progressed in difficulty as the operator’s skill improved. These authors found the learning curve for LPD involved a slow difficult beginning phase, a precipitous acceleration in improvement phase, and finally a plateau phase with slow but continued improvement over time. Finally, Song et al. (24) performed a matched cohort analysis comparing LPD vs. OPD. They found that when dividing their LPD patients into early and late groups consisting of 47 and 50 patients respectively, the late group had significantly shorter operative times (399.4 vs. 566.5 minutes, P<0.001), less EBL (503 vs. 685 cc’s, P=0.018), and shorter length of hospital stay (11.2 vs. 17.3 days, P<0.001).
Although these improvements may be intuitive as surgeons move along the learning curve, the significant progress observed by these authors, including the decreased rate of complications is encouraging. With appropriate guidance, we expect more surgeons to move to LPD.
Open vs. LPD
As initial studies have showed the feasibility and safety of LPD, more recent studies are directly comparing OPD to LPD. In our review, we found 6 articles that met our inclusion and exclusion criteria that compared these surgeries. Articles were published between 2012 and 2015, and were all retrospective in nature. Study subjects ranged from 56 to 680 individuals per study, and five papers originated from the USA. The remaining study was from Korea. We will examine these on a study-by-study basis in order of publication.
In 2012 Asbun et al. (25) published an article in JACS which compared 215 OPD with 53 LPD that underwent surgery between 2005 and 2011. These cohorts were well matched for gender, comorbidities, ASA score, BMI, and age. Authors state selection criteria was based mainly on patient preference and not clinical factors, although if major vascular resection was required or the abdomen was expected to be hostile either open or laparoscopic with a low threshold to convert to open was performed (these patients were analyzed on a non-intention to treat fashion). They found that the LPD group had less intraoperative blood loss (1,032 vs. 195 cc’s, P<0.001), PRBC transfusions (4.7 vs. 0.64 U, P<0.001), decreased ICU stay (3 vs. 1.1 days, P<0.001), and overall hospital stay (12.4 vs. 8 days, P<0.001). LPD patients did have increased operative time (401 vs. 541 minutes, P<0.001). Rate of complications, including pancreatic leak rate and delayed gastric emptying, were similar between the groups. In terms of oncologic data, numbers of lymph nodes removed as well as lymph node ratio were better for the LPD group (16.84 vs. 23.44, P<0.001 and 0.241 vs. 0.159, P=0.0072, respectively). Furthermore, margin status, number of patients utilizing adjuvant chemotherapy, and time to start adjuvant treatment was similar between groups. This article demonstrates possible benefits of the laparoscopic procedure over open. The finding of an improved LN resection with LPD is very interesting. However, as patients requiring major vascular resection and those with hostile abdomens were more likely to be in the open group, there is potential for selection bias that affected the results in favor of the LPD group.
Mesleh et al. (30) published an article 2013 which addressed the issue of cost of OPD vs. LPD. Their study included 48 OPD and 75 LPD who underwent operation between 2009 and 2012. Patients appear matched on demographic data and difficulty of the operation. There were ten patients requiring conversion to open. Analysis was completed on an intention to treat basis. Authors extracted cost information, divided into “admission” and “surgical” cost. They found that while “surgical” cost was higher for the laparoscopic group, “admission” cost was greater for the open group. The increased “surgical” cost was tied to the longer OR time as well as more expensive surgical equipment. On the other hand, “admission” cost was less for the laparoscopic group. These differences in part cancelled each other out and overall cost (converted from dollars to “units” for this publication) was similar between OPD vs. LPD groups (154 vs. 173 units, P=0.5). As a side note, the authors also found that the LPD group had increased lymph node retrieval as well as decreased blood loss compared to OPD. Although these cost findings may not be generalizable to other institutions, this is an important article as it shows LPD may not actually be more expensive overall, which is a common assumption. Furthermore, as the learning curve improves, surgical cost of LPD should decrease with operative times.
One criticism of many comparison studies is that there is inherent bias in favor laparoscopic approaches, as the more difficult resections are reserved for the open surgeries. In 2014, Croome et al. (31) in part addressed this issue by comparing only LPD vs. OPD with comparable vascular resections. Their study included 58 OPD and 31 LPD cases, all requiring major vascular resections. Patients were similar in demographic data with the exception that the LPD group was significantly older (63.6 vs. 69.5 years, P=0.01). There was no difference in the distribution or difficulty of vessels requiring resection between groups. Operative time was similar between the OPD vs. LPD groups (465 vs. 465 minutes, P>0.99), although clamp time was greater in the laparoscopic group (25.1 vs. 46.8 minutes, P<0.001). As seen previously, blood loss was less in the laparoscopic group (1,452.1 vs. 841.8 cc’s, P<0.001) as well as length of hospital stay (9 vs. 6 days, P=0.006). In terms of oncologic data, LPD group had more lymph nodes harvested (15.9 vs. 20 nodes, P=0.01), and greater R0 resection (75.9 vs. 93.5%, P=0.038). These improved oncologic variables did not translate to improved survival, as intention-to-treat analysis using Kaplan-Meier survival estimates were similar (P=0.14). In-hospital 30-day mortality was similar between groups as well (P=0.96). Although these authors admittedly have advanced technical expertise in LPD, the fact that they have similar and in some cases improved results even in the context of difficult laparoscopic cases involving major vascular resections underlines the future possibilities of LPD. Furthermore, the improved oncologic data begs the question—is there potential for a survival benefit with the laparoscopic approach?
In an attempt to answer this, Croome et al. (27) performed another study looking specifically at patients undergoing PD for pancreatic ductal adenocarcinoma (PDA) only, and compared open vs. laparoscopic surgery to assess whether there were oncologic differences. They compared 214 OPD and 108 LPD patients who underwent surgery from 2008 to 2013. They not only compared the typical perioperative variables, but also looked at proportion of patients undergoing chemotherapy, time to start chemotherapy, and delay of chemotherapy. Firstly, they found similar operative times, tumor characteristics, margin status, number of nodes resected, and perioperative complications (including pancreatic fistula, delayed gastric emptying, short term mortality) between groups. LPD was associated with decreased blood loss (866.7 vs. 492.4 cc’s, P<0.001), blood transfusion (33% vs. 19%, P=0.01), and length of hospital stay (9 vs. 6 days, P<0.001). By looking solely at patients with PDA, the authors were able to more precisely compare oncologic outcomes between LPD and OPD groups. Interestingly, they found that not only was time to adjuvant therapy less for the LPD group (59 vs. 48 days, P<0.001), but delay beyond 8 weeks and number not receiving treatment (or delay beyond 3 months) was also less for the LPD group (41% vs. 27%, P=0.01 and 12% vs. 5%, P=0.04, respectively). In their survival analysis, they found that progression free survival was superior in the LPD group compared to the OPD (P=0.02) but overall survival was similar (P=0.12). Although no overall survival difference was appreciated, the fact that progression free survival improved is encouraging. Further studies should be done with larger sample size to further analyze survival.
A study by Speicher et al. (28), as discussed previously, primarily studied the learning curve for LPD. However, they also compared LPD vs. OPD. Their overall findings were consistent with most other studies, in that LPD was associated with less blood loss, higher lymph node harvest, and similar post op morbidity. They found that the early laparoscopic cases had worse outcomes compared to open, but over time these variables improved substantially and overall results were as stated.
Finally, the most recent article, published by Song et al. (24) in 2015 comprised 576 OPD and 104 LPD after exclusions. They performed a matched analysis with the benign and low-grade malignancy patients that consisted of 93 OPD controls and 93 LPD cases. Exclusion criteria for the LPD group were vascular involvement, severe pancreatitis, trauma or injury, and history of major abdominal surgery. They also analyzed patients with carcinoma in a separate analysis, comprising 483 OPD and 11 LPD patients. Exclusion criteria were similar for matched analysis but also included patients with severe cardiopulmonary morbidity. Results found that in the matched comparison, LPD had longer operative times (347.9 vs. 482.5 minutes, P<0.001), similar blood loss (570 vs. 609 cc’s, P=0.5), shorter length of hospital stay (19.2 vs. 14.3 days, P<0.001), and decreased analgesic injection requirement. Major complications, including pancreatic fistula and delayed gastric emptying were similar. In terms of the oncologic outcomes for those patients with high-grade malignancy, they found no difference in lymph nodes removed or 5-year overall survival. Margins were also similar.
Comparison of LPD and OPD suggest that although the laparoscopic approach has increased operative times, complication rate and mortality are similar. Additionally blood loss, length of hospital stay, and oncologic outcomes appear better in most studies. Although many of these papers had similar demographic characteristics between groups, selection bias favoring LPD continues to be a problem. Many studies excluded patients with vascular involvement or higher risk surgical candidates. It is promising, however, that when surgical difficulty was similar, as shown by Croome et al., the LPD group continued to have good outcomes. Although a randomized controlled trial is needed to best evaluate differences between these groups it would be quite difficult to set up, especially as many LPD are done at centers specializing in this procedure with patients going to them specifically for laparoscopic surgery. However, as further studies are performed the evidence illustrating the benefits of LPD will likely strengthen. Furthermore, it will be an important topic in future research to evaluate how LPD affects oncologic outcomes, especially survival. Any meaningful improvement in survival would be a great advancement in the treatment in periampullary cancer.
Conclusions
LPD is a safe operation that provides many of the benefits associated with laparoscopic surgery. We expect the prevalence of this operation will continue to grow in the future and will also likely be utilized in increasingly more difficult cases. Future studies should minimize selection bias and also focus on further evaluating oncologic outcome differences between LPD and OPD.
Acknowledgements
None.
Biographies
Authors’ introduction: Dr. Justin Merkow is a General Surgery resident at the University of Colorado and is currently pursuing a research fellowship under the mentorship of Dr. Barish Edil, a surgical oncologist and Dr. Yuwen Zhu, an immunologist at the University of Colorado focusing on novel gene pathways shown to be involved in the immunogenicity of various cancers, including pancreatic cancer. Dr. Merkow attended undergraduate school at the University of Wisconsin, graduating Summa Cum Laude and then obtained his medical degree at the University of Colorado, winning the Owens-Swan outstanding promise in surgery award upon graduation. Since starting General Surgery training he has been involved in multiple research projects focusing mainly on oncology, specifically melanoma and pancreatic cancer. He has presented his work nationally.
Barish H. Edil, M.D., F.A.C.S. is an Associate Professor of Surgery, Director of Surgical Oncology and Chief of the section of pancreas and biliary surgery at the University of Colorado. He has authored more than a 100 peer reviewed articles as well as authoring multiple chapters in the leading textbooks in surgery. He has performed over 100 laparoscopic pancreaticoduodenectomies in his career and performed the first laparoscopic pancreaticoduodenectomy at Johns Hopkins and the University of Colorado. In addition he has traveled China many times to lecture and teach his techniques in laparoscopic pancreas surgery.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.World Cancer Research Fund International. Pancreatic cancer statistics. Available online: http://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/pancreatic-cancer-statistics [cited 2015 Mar 3].
- 2.Available online: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf
- 3.Basson JJ, Du Toit RS, Nel CJ. Carcinoma of the head of the pancreas. Morbidity and mortality of surgical procedures. S Afr J Surg 1994;32:9-12. [PubMed] [Google Scholar]
- 4.Ishikawa O, Ohigashi H, Eguchi H, et al. Survival and Late Morbidity after Resection of Pancreatic Cancer. The Pancreas: An Integrated Textbook of Basic Science, Medicine, and Surgery, Second Edition. 2008. [Google Scholar]
- 5.Zovak M, Mužina Mišić D, Glavčić G. Pancreatic surgery: evolution and current tailored approach. Hepatobiliary Surg Nutr 2014;3:247-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun H, Ma H, Hong G, et al. Survival improvement in patients with pancreatic cancer by decade: a period analysis of the SEER database, 1981-2010. Sci Rep 2014;4:6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Addeo P, Delpero JR, Paye F, et al. Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French Surgical Association. HPB (Oxford) 2014;16:46-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Outcomes comparing a pancreaticogastrostomy (PG) and a pancreaticojejunosto...: EBSCOhost. Available online: http://web.b.ebscohost.com.hsl-ezproxy.ucdenver.edu/ehost/pdfviewer/pdfviewer?sid=c4932221-8b88-4023-a557-3ce49450c19f%40sessionmgr114&vid=1&hid=116 [cited 2015 Mar 2].
- 9.He T, Zhao Y, Chen Q, et al. Pancreaticojejunostomy versus pancreaticogastrostomy after pancreaticoduodenectomy: a systematic review and meta-analysis. Dig Surg 2013;30:56-69. [DOI] [PubMed] [Google Scholar]
- 10.Schwenk W, Haase O, Neudecker JJ, et al. Short term benefits for laparoscopic colorectal resection. In: Schwenk W. editor. Chichester. UK: John Wiley & Sons, Ltd, 1996. [Google Scholar]
- 11.Antoniou SA, Antoniou GA, Koch OO, et al. Meta-analysis of laparoscopic vs open cholecystectomy in elderly patients. World J Gastroenterol 2014;20:17626-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zapf M, Denham W, Barrera E, et al. Patient-centered outcomes after laparoscopic cholecystectomy. Surg Endosc 2013;27:4491-8. [DOI] [PubMed] [Google Scholar]
- 13.Bracale U, Pignata G, Lirici MM, et al. Laparoscopic gastrectomies for cancer: The ACOI-IHTSC national guidelines. Minim Invasive Ther Allied Technol 2012;21:313-9. [DOI] [PubMed] [Google Scholar]
- 14.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med 2011;364:2128-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Zhou X, Ying D, et al. Laparoscopic pancreaticoduodenectomy. Hepatobiliary Surg Nutr 2014;3:421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu B, Cai X, Lu W, et al. Laparoscopic pancreaticoduodenectomy to treat cancer of the ampulla of Vater. JSLS 2006;10:97-100. [PMC free article] [PubMed] [Google Scholar]
- 18.Palanivelu C, Jani K, Senthilnathan P, et al. Laparoscopic pancreaticoduodenectomy: technique and outcomes. J Am Coll Surg 2007;205:222-30. [DOI] [PubMed] [Google Scholar]
- 19.Palanivelu C, Rajan PS, Rangarajan M, et al. Evolution in techniques of laparoscopic pancreaticoduodenectomy: a decade long experience from a tertiary center. J Hepatobiliary Pancreat Surg 2009;16:731-40. [DOI] [PubMed] [Google Scholar]
- 20.Pugliese R, Scandroglio I, Sansonna F, et al. Laparoscopic pancreaticoduodenectomy: a retrospective review of 19 cases. Surg Laparosc Endosc Percutan Tech 2008;18:13-8. [DOI] [PubMed] [Google Scholar]
- 21.Zureikat AH, Breaux JA, Steel JL, et al. Can laparoscopic pancreaticoduodenectomy be safely implemented? J Gastrointest Surg 2011;15:1151-7. [DOI] [PubMed] [Google Scholar]
- 22.Kim SC, Song KB, Jung YS, et al. Short-term clinical outcomes for 100 consecutive cases of laparoscopic pylorus-preserving pancreatoduodenectomy: improvement with surgical experience. Surg Endosc 2013;27:95-103. [DOI] [PubMed] [Google Scholar]
- 23.Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc 2006;20:1045-50. [DOI] [PubMed] [Google Scholar]
- 24.Song KB, Kim SC, Hwang DW, et al. Matched Case-Control Analysis Comparing Laparoscopic and Open Pylorus-preserving Pancreaticoduodenectomy in Patients With Periampullary Tumors. Ann Surg 2015;262:146-55. [DOI] [PubMed] [Google Scholar]
- 25.Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg 2012;215:810-9. [DOI] [PubMed] [Google Scholar]
- 26.Honda G, Kurata M, Okuda Y, et al. Laparoscopic pancreaticoduodenectomy: taking advantage of the unique view from the caudal side. J Am Coll Surg 2013;217:e45-9. [DOI] [PubMed] [Google Scholar]
- 27.Croome KP, Farnell MB, Que FG, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg 2014;260:633-8; discussion 638-40. [DOI] [PubMed] [Google Scholar]
- 28.Speicher PJ, Nussbaum DP, White RR, et al. Defining the learning curve for team-based laparoscopic pancreaticoduodenectomy. Ann Surg Oncol 2014;21:4014-9. [DOI] [PubMed] [Google Scholar]
- 29.Corcione F, Pirozzi F, Cuccurullo D, et al. Laparoscopic pancreaticoduodenectomy: experience of 22 cases. Surg Endosc 2013;27:2131-6. [DOI] [PubMed] [Google Scholar]
- 30.Mesleh MG, Stauffer JA, Bowers SP, et al. Cost analysis of open and laparoscopic pancreaticoduodenectomy: a single institution comparison. Surg Endosc 2013;27:4518-23. [DOI] [PubMed] [Google Scholar]
- 31.Croome KP, Farnell MB, Que FG, et al. Pancreaticoduodenectomy with major vascular resection: a comparison of laparoscopic versus open approaches. J Gastrointest Surg 2015;19:189-94; discussion 194. [DOI] [PubMed] [Google Scholar]


