Abstract
Adenine phosphoribosyltransferase (APRT) deficiency is a rare autosomal recessive enzyme defect of purine metabolism that usually manifests as 2,8-dihydroxyadenine (2,8-DHA) nephrolithiasis and more rarely chronic kidney disease. The disease is most often misdiagnosed and can recur in the renal allograft. We analyzed 9 patients with recurrent 2,8-DHA crystalline nephropathy, in all of whom the diagnosis had been missed prior to renal transplantation. The diagnosis was established for a median of 5 (range, 1.5–312) weeks following the transplant procedure. Patients had delayed graft function (n=2), acute-on-chronic (n=5) or acute (n=1) allograft dysfunction, whereas one patient had normal graft function at the time of diagnosis. Analysis of allograft biopsies showed birefringent 2,8-DHA crystals in renal tubular lumens, within tubular epithelial cells and interstitium. Fourier transformed infrared microscopy confirmed the diagnosis in all cases, which was further supported by 2,8-DHA crystalluria, undetectable erythrocyte APRT enzyme activity, and genetic testing. With allopurinol therapy, the allograft function improved (n=7), remained stable (n=1), or worsened (n=1). At last follow-up, 2 patients had experienced allograft loss and 5 had persistent chronic allograft dysfunction. 2,8-DHA nephropathy is a rare but underdiagnosed and preventable disorder that can recur in the renal allograft and may lead to allograft loss.
INTRODUCTION
Adenine phosphoribosyltransferase (APRT) deficiency is a rare autosomal recessive inherited disorder of purine metabolism. In the absence of APRT, adenine is oxidized by xanthine dehydrogenase to 2,8-dihydroxyadenine (2,8-DHA), which is excreted in the urine (Figure 1). Because 2,8-DHA is poorly soluble at any physiological pH, 2,8-DHA crystals form in the urine, resulting in recurrent 2,8-DHA nephrolithiasis, and less commonly, crystalline nephropathy (1–4). APRT deficiency is frequently misdiagnosed, owing to the absence of specific manifestations and lack of awareness of the disease among physicians. When untreated, the disease can result in chronic kidney disease (CKD) that can progress to end-stage renal disease (ESRD), and may recur after renal transplantation. To date, only a few cases of recurrent 2,8-DHA nephropathy have been reported (5–13). In the present retrospective study, we analyzed the presenting clinical features and outcome of 9 patients who displayed 2,8-DHA nephropathy following renal transplantation.
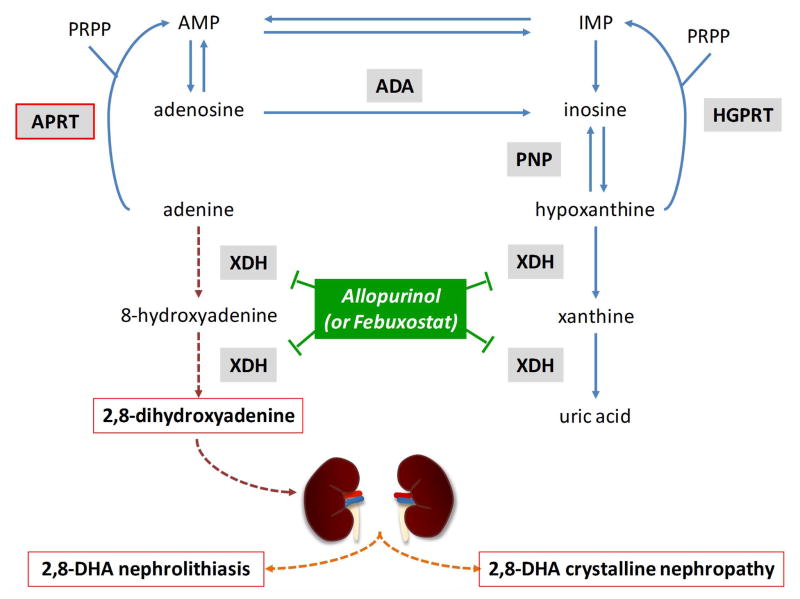
Figure 1. Metabolic pathways for the disposal of adenine in humans.
Adenine phosphoribosyltransferase (APRT) deficiency causes 2,8-dihydroxyadenine (2,8-DHA) accumulation, leading to nephrolithiasis and crystalline nephropathy. In the absence of APRT activity, adenine cannot be converted to adenosine. Adenine is metabolized through an alternative pathway where it is oxidized by xanthine dehydrogenase (XDH) to 2,8-DHA via the generation of an intermediate compound, 8-hydroxyadenine. Because 2,8-DHA is insoluble at any physiological urine pH, it forms 2,8-DHA crystals eventually leading to 2,8-DHA nephrolithiasis and/or crystalline nephropathy.
ADA, adenosine deaminase; AMP, adenosine monophosphate; HGPRT, hypoxanthine-guanine phosphoribosyltransferase; IMP, inosine monophosphate; PNP, purine nucleoside phosphorylase; PRPP, 5-phosphoribosyl-1-pyrophosphate.
METHODS
Study population
Nine patients from 7 different institutions and with documented recurrent 2,8-DHA allograft crystalline nephropathy were identified through search of the Necker Hospital database (Paris, France), which is a referral center for nephrolithiasis and purine metabolic disorders, including 2 previously reported patients (14,15). Patient care and conduct of the study complied with good clinical practice and the Declaration of Helsinki and Istanbul guidelines.
Baseline characteristics of patients
Clinical and laboratory data at the time of diagnosis and during follow-up were obtained from the medical records. Glomerular filtration rate was estimated according to the four-variable Modification of Diet in Renal Disease formula (16).
Laboratory methods and genetic testing
Kidney biopsy specimens were processed according to standard techniques, stained with hematoxylin and eosin and Masson’s trichrome, and analyzed by light and polarized light microscopy. Crystals in the renal tissue were further characterized using Fourier transformed infrared microscopy, as described previously (17). The diagnosis of 2,8-DHA crystalline nephropathy was established in all patients by the detection of 2,8-DHA crystals in the renal allograft and/or urine. APRT enzyme activity assay and/or genetic testing were performed to confirm APRT deficiency in most patients. Crystalluria assessment was performed as previously reported (18,19). APRT enzyme activity was measured in erythrocyte lysates using radiolabeled 14C-adenine in a chromatographic assay (3). Mutation analysis was performed using PCR amplification and sequencing of the APRT gene after obtaining written informed consent from the patients (3).
Statistical analysis
Descriptive analyses are provided as median values and range for continuous variables, and percentages for categorical variables.
RESULTS
Nine patients with recurrent 2,8-DHA crystalline nephropathy were identified, including 4 women and 5 men, all of whom were of European ancestry. Patients’ clinical and laboratory characteristics are detailed in Table 1. Median age at the onset of ESRD was 43 (range, 25–65) years, and 49 (range, 28–67) years at the diagnosis of APRT deficiency. All 9 patients had a past history of CKD, which had been attributed to obstructive uropathy and nephrolithiasis-related chronic tubulointerstitial nephritis in 3 (33%) cases, to hypertensive nephrosclerosis in one (11%), and to CKD of unknown cause in 5 (56%) patients. None had been diagnosed with APRT deficiency before the recurrence in the renal allograft. The diagnosis was made following the second renal transplant in 2 patients. One had lost the first allograft because of an acute torsion of the graft vein, shortly after the transplant surgery. The other one had allograft loss because of disease recurrence which had been initially missed. Five (55.6%) patients had a past history of nephrolithiasis, with the first episode occurring before the age of 20 years in 4 cases. However, none had analysis of kidney stone. The median delay between the first stone event and diagnosis of APRT deficiency was 30 (range, 11–52) years.
Table 1.
Clinical and laboratory characteristics at diagnosis.
| Pt | Demographic data | Order of Renal Tx | History of nephrolithiasis* | Suspected cause of CKD | Delay of diagnosis after Tx (weeks) | Renal manifestations | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Gender | Origin | HTN | Pu/Hu/Lu | sCr at diagnosis (μmol/L) | Graft dysfunction | |||||
| 1 | 28 | F | France | 1st | + (11) | CTIN/nephrolithiasis, solitary kidney | 5 | + | − / − / NA | 150 | AGD |
| 2 | 41 | M | France | 1st | − | Undetermined | 144 | + | + / + / − | 248 | A/CGD |
| 3 | 48 | M | Italy | 2nd | + (30) | Undetermined | 72 | − | − / + / − | 283 | A/CGD |
| 4 | 48 | M | France | 2nd | − | Oxalate nephropathy | 1.5 | + | + / + / + | 676/Hemodialysis | DGF |
| 5 | 51 | M | Canada | 1st | + (43) | Undetermined | 312 | − | − / − / NA | 366 | A/CGD |
| 6 | 49 | F | Italy | 1st | − | Undetermined | 156 | − | − / + / − | 430 | A/CGD |
| 7 | 58 | F | France | 1st | + (13) | CTIN/nephrolithiasis | 1.5 | + | − / + / − | 109 | - |
| 8 | 64 | M | France | 1st | − | Undetermined | 4 | + | + / − / − | 600/Hemodialysis | DGF |
| 9 | 67 | F | Italy | 1st | + (52) | Hypertensive nephrosclerosis | 3 | + | − / − / NA | 442/Hemodialysis | A/CGD |
Pt, patient; F, female; M, male; Tx, transplant; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; HTN, hypertension; Pu/Hu/Lu, proteinuria, hematuria, leukocyturia; sCr, serum creatinine; NA, not available; AGD, acute graft dysfunction; A/CGD, acute-on-chronic graft dysfunction; DGF, delayed graft function.
Delay between the first kidney stone episode and diagnosis (years). Conversion factor from μmol/L to mg/dL = 0.0113.
Age at kidney transplantation was 46 (range, 28–67) years. All patients, except one, received a deceased donor kidney. After induction therapy, maintenance immunosuppression included prednisone, a calcineurin inhibitor, and mycophenolate mofetil, or azathioprine in one case. APRT deficiency was diagnosed with a median delay of 5 (range, 1.5–312) weeks posttransplant. The median serum creatinine and estimated glomerular filtration rate (eGFR) at diagnosis were 366 μmol/L (range, 109–676) and 14 mL/min per 1.73 m2 (range, 8–45), respectively. Two patients experienced delayed graft function and underwent early allograft biopsy. One patient with normal graft function had urine microscopy shortly after the transplantation because of a past history of nephrolithiasis. Crystalluria showed 2,8-DHA crystals. Renal allograft biopsy then confirmed the recurrence of 2,8 DHA nephropathy. Four patients had a long diagnostic delay, ranging from 72 to 312 weeks after transplantation. Two had experienced delayed graft function, but no early biopsy had been performed because of spontaneous and partial improvement of allograft function. All 4 patients then developed chronic allograft dysfunction. They were initially diagnosed with oxalate, urate or undetermined crystalline nephropathy. The diagnosis of 2,8-DHA nephropathy was later established in the context of acute deterioration of allograft function.
Examination of the renal allograft biopsy specimens revealed tubulointerstitial injury with no obvious glomerular lesions and only mild vascular lesions. No patient had evidence of acute or chronic allograft rejection, nor of drug toxicity. The diagnosis of oxalate nephropathy was initially suggested in 4 patients (44.4%), whereas that of 2,8-DHA crystalline nephropathy was suspected in only one patient. Examination by light microscopy revealed varying degrees of interstitial fibrosis, tubular atrophy, and acute tubular necrosis. Interstitial inflammatory infiltrate was a common feature (Figure 2 A). The appearance of the crystal deposits for each patient is detailed in Table 2. Yellow-brownish crystals were predominantly located within the tubular lumens and in the cytoplasm of tubular epithelial cells (Figure 2 B–D). Intraluminal crystals formed spherical aggregates of different sizes, plugging tubular lumens (Figure 2 B and D). A foreign-body type reaction surrounding the crystal aggregates was observed in the biopsies from 3 patients (Figure 2 B). Polarized light microscopy showed the crystals to be strongly birefringent, demonstrating a radial orientation with a variable appearance, including needle-, ring- and spherically-shaped aggregates (Figure 3 A–D). The so-called Maltese cross pattern was observed within the tubular lumens in only 2 cases (Figure 3 A, inset). All renal allograft biopsy specimens were analyzed by Fourier transformed infrared spectroscopy, which confirmed the presence of 2,8-DHA crystals. Native kidney or previous allograft biopsies were available for 6 patients, and showed similar findings, particularly crystal deposits.
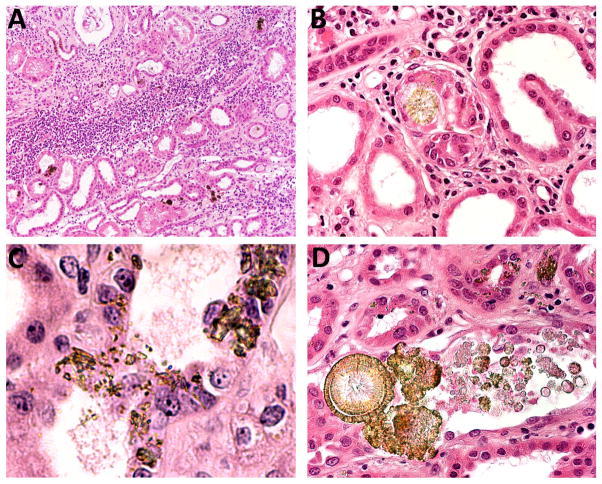
Figure 2. Renal allograft biopsy findings viewed by conventional light microscopy.
Low magnification view showing focal deposition of crystals in the allograft parenchyma together with diffuse inflammatory interstitial infiltrates and varying degrees of interstitial fibrosis and tubular atrophy (A). Deposits of 2,8-DHA crystals within tubular lumens forming spherical aggregates, causing tubular obstruction with foreign-body type reaction (B). Small needle-shaped and irregular crystals located within the tubular epithelial cells (C). Small spherical to large crystal aggregates in the tubular lumen and within tubular epithelial cells (D).
Table 2.
Biopsy findings and diagnostic methods.
| Pt | Crystals on previous biopsy |
Diagnosis initially evoked on the current biopsy |
Tubulointerstitial lesions | Distribution of crystal deposits | Foreign body reaction |
Crystal appearance by regular light microscopy |
Crystal appearance by polarized light microscopy |
Diagnostic methods | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATN | IF/TA | Interstitial infiltrates |
Tubular lumens |
Tubular cells |
Interstitium | Irregular aggregates |
Ring formations |
Spherical | Birefringence | Needle shape |
Radial orientation |
Maltese Cross |
FTIR | Crystalluria | APRT activity |
||||
| 1 | +/GB | Oxalate CN | − | − | + | + | + | − | − | + | − | + | + | + | + | − | +/GB | NA | 0% |
| 2 | +/GB | Oxalate CN | − | + | − | + | + | − | − | + | − | − | + | + | − | − | +/GB | NA | 0% |
| 3 | +/GB | Undetermined CN | + | + | + | + | + | + | − | + | + | + | + | − | + | + | +/GB | + | NA |
| 4 | NA | Oxalate CN | + | + | − | + | + | + | − | + | − | − | + | + | − | − | +/GB | NA | 0% |
| 5 | +/NK | Oxalate CN | + | + | + | + | + | + | + | + | − | + | + | − | − | − | +/GB | NA | 0% |
| 6 | +/GB | Urate CN | − | − | + | + | + | + | + | + | + | + | + | − | + | + | +/GB | + | NA |
| 7 | NA | 2,8-DHA CN | − | − | + | + | + | − | − | + | − | − | + | + | − | − | +/GB | + | 0% |
| 8 | +/NK | Undetermined CN | + | + | − | + | + | + | + | + | + | + | + | + | + | − | +/GB | + | 0% |
| 9 | NA | Undetermined CN | − | − | − | + | + | + | − | + | − | − | + | + | + | − | +/GB | NA | 0% |
Pt, patient; GB, graft biopsy; NK, native kidney; CN, crystalline nephropathy; ATN, acute tubular necrosis; IF/TA, interstitial fibrosis and tubular atrophy; NA, not available (not performed); FTIR, Fourier transformed infrared spectroscopy.
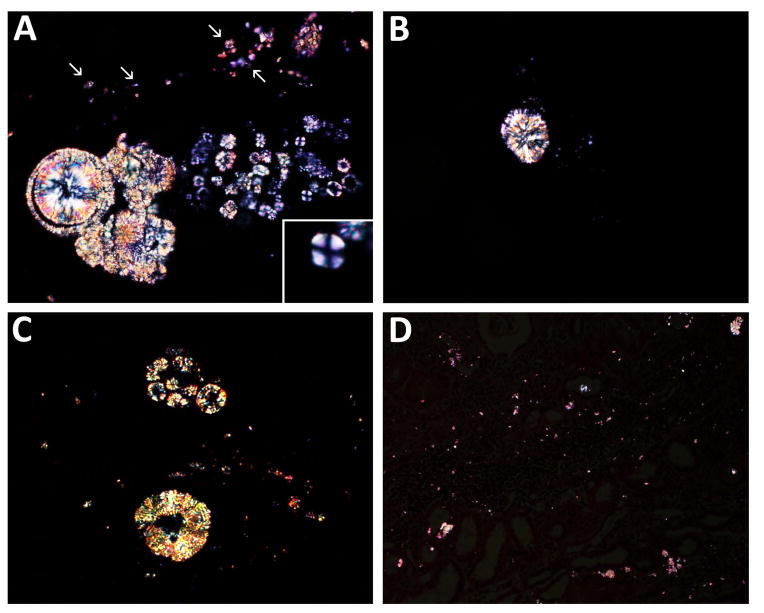
Figure 3. Renal allograft biopsy findings viewed by polarized light microscopy.
The crystals are highly birefringent and of variable size and appearance. Crystals precipitates within the tubular lumens (A), forming spherical and irregular aggregates. Very small needle-shaped crystal deposits located within the tubular epithelial cells (A, arrows). Crystals exhibiting a typical birefringent Maltese cross pattern are rarely observed within the tubular lumens (A, inset). Spherical (B) and ring-like (C) crystal aggregates composed of radially-oriented crystals. Lower magnification showing small birefringent crystals in only some foci of the graft parenchyma, and very small crystals diffusely interspersed within the renal interstitium yielding a stardust-like appearance (D).
The diagnosis of APRT deficiency was further supported by the detection of 2,8-DHA crystals in urine samples from 4 patients. Crystalluria revealed round and reddish-brown crystals when examined by light microscopy, with a characteristic central Maltese cross pattern observed by polarized light. Erythrocyte APRT activity was undetectable in 7 tested patients. Genetic analysis was carried out in 6 patients (Table 3) and revealed homozygous or compound heterozygous APRT mutations in most cases. A single allelic mutation was identified in 3 patients despite sequencing of the entire coding region and the intron-exon junctions of the APRT gene.
Table 3.
Results of the genetic testing.
| Pt | Demographic data | 1st allele | 2nd allele | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Gender | Origin | Exon | Transcript | Protein | Exon | Transcript | Protein | |
| 1 | 28 | F | France | 4 | c.400+2dup | p.Ala108Glufs*3 | 4 | c.400+2dup | p.Ala108Glufs*3 |
| 2 | 41 | M | France | 3 | complex rearrangement | undetermined | 3 | complex rearrangement | undetermined |
| 3 | 48 | M | Italy | - | NA | - | NA | - | |
| 4 | 48 | M | France | 1 | c.1A>G | p.Met1? | 4 | c.352G>C | p.Glu118Gln |
| 5 | 51 | M | Canada | - | NA | - | - | NA | - |
| 6 | 49 | F | Italy | - | NA | - | - | NA | - |
| 7 | 58 | F | France | 5 | c.541T>C | p.*181Argext*121 | undetermined | No mutation found | undetermined |
| 8 | 64 | M | France | 4 | c.400+2dup | p.Ala108Glufs*3 | undetermined | No mutation found | undetermined |
| 9 | 67 | F | Italy | 4 | c.400+2dup | p.Ala108Glufs*3 | undetermined | No mutation found | undetermined |
Pt, patient; F, female; M, male; NA, not available (because not performed).
All patients received allopurinol as first-line therapy with an initial dose ranging from 100 to 300 mg/day. The allopurinol dose was increased in 4 patients, and up to 400 mg/day in one case, in order to achieve the complete disappearance of 2,8-DHA crystals from the urine. One patient received febuxostat (80 mg/day), a non-purine selective inhibitor of xanthine dehydrogenase, as a maintenance therapy. Patients were also advised to increase water intake and to avoid purine-rich diet. The treatment and outcome are outlined in Table 4 and Figure 4.
Table 4.
Treatment and outcome.
| Pt | Allopurinol therapy* (mg/d) | Follow-up after diagnosis (months) | sCr at diagnosis (μmol/L) | sCr at last follow-up (μmol/L) | Renal outcome | |
|---|---|---|---|---|---|---|
| initial dosage | maintenance | |||||
| 1 | 200 | 400 | 132 | 150 | 173 | Chronic graft dysfunction |
| 2 | 100 | 200 | 6 | 248 | 220 | Chronic graft dysfunction |
| 3 | 300 | 300 | 40 | 283 | 107 | Normal graft function |
| 4 | 100 | 200# | 24 | 676/Hemodialysis | 185 | Chronic graft dysfunction |
| 5 | 100 | 100 | 6 | 366 | Hemodialysis | Graft loss |
| 6 | 150 | 150 | 30 | 430 | 160 | Chronic graft dysfunction |
| 7 | 300 | 300 | 24 | 109 | 105 | Normal graft function |
| 8 | 100 | 200 | 8 | 600/Hemodialysis | Hemodialysis | Graft loss |
| 9 | 300 | 300 | 12 | 442/Hemodialysis | 168 | Chronic graft dysfunction |
Pt, patient; sCr, serum creatinine.
Allopurinol was initiated shortly after diagnosis.
Febuxostat (80 mg/d) was then administered as maintenance therapy.
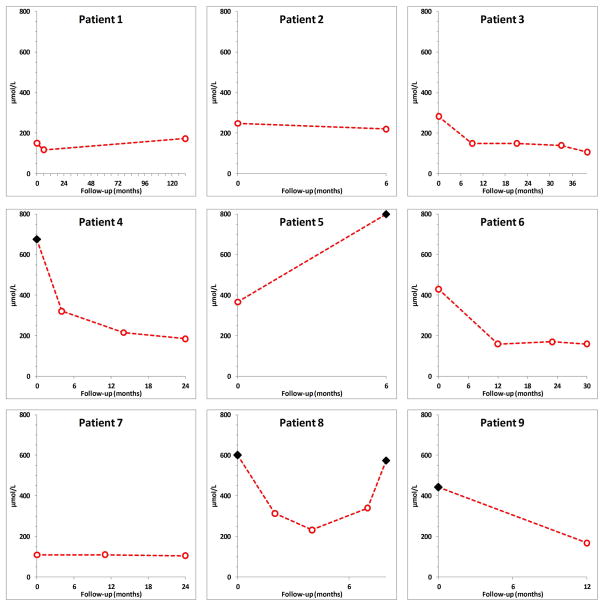
Figure 4. Evolution of graft function from diagnosis to last follow-up.
Each dot (○) represents the value of a measured serum creatinine. ◆ indicates the need for hemodialysis.
The median duration of follow-up was 24 (range, 6–132) months. Renal function remained normal and stable in one patient, worsened in one, and initially improved in 7. The 3 patients who were hemodialyzed at diagnosis recovered enough allograft function to discontinue dialysis. However, one of them finally returned to dialysis despite allopurinol therapy as the consequence of disease recurrence. Another patient also progressed towards ESRD because of disease recurrence. The remaining patients had normal graft function (n=2) or persistent chronic allograft dysfunction (n=5) with a median serum creatinine and eGFR of 168 (range, 105–220) μmol/L and 31 (range, 26–61) mL/min per 1.73m2, respectively.
DISCUSSION
We analyzed herein 9 patients with recurrent 2,8-DHA crystalline nephropathy, in all of whom the diagnosis had been missed prior to renal transplantation. Only a few similar cases have been reported worldwide (Tables 5 and 6) (5–15,20–22). The present study thus provides a valuable characterization of this rare disease in the context of renal transplantation, underscoring the need for a greater awareness of APRT deficiency among physicians.
Table 5.
Literature review of cases of recurrent 2,8-DHA crystalline nephropathy – characteristics at diagnosis and diagnostic methods.
| First Author (ref.) | Year | Age at diagnosis |
Gender | Order of Renal Tx |
History of nephrolithiasis ** |
Suspected cause of CKD |
Delay of diagnosis after Tx (weeks) |
sCr at diagnosis (μmol/L) |
Graft dysfunction |
FTIR | Diagnostic methods | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystalluria | APRT activity |
APRT gene analysis |
|||||||||||
| De Jong (5) | 1996 | 56 | M | 1st | + (40) | CTIN/Urate stones | 0,3 | Hemodialysis | CGD# | NA | + | 0% | NA |
| Brown (6) | 1998 | 49 | F | 1st | − | Undetermined CN | 4 | Hemodialysis | PGNF | +/GB | NA | NA | NA |
| Benedetto (7) | 2001 | 48 | M | 1st | − | Undetermined CN | 19 | 248 | AGD | NA | NA | 0% | NA |
| Cassidy (8) | 2004 | 23 | M | 1st | − | Undetermined CN | 2 | 361 | CGD | +/NL | NA | 0% | NA |
| Eller (9) | 2007 | 11* | M | 4th | + (9.5) | 2,8-DHA nephropathy | 1 | Hemodialysis | DGF## | +/NL | + | 0% | hom. c.400+2dup |
| Nasr (10) | 2010 | 42 | F | 2nd | − | Oxalate nephropathy | 6 | 486 | CGD | +/GB | NA | 0% | NA |
| Nasr (10) | 2010 | 54 | M | 1st | + (36) | 2,8-DHA nephropathy | 1,3 | 398 | AGD# | NA | NA | 0% | NA |
| Bertram (11) | 2010 | 56 | M | 3rd | + (21) | CTIN/Urate stones | 0,3 | Hemodialysis | DGF | +/NL | NA | NA | NA |
| Sharma (12) | 2012 | 80 | M | 1st | + (30) | CTIN/nephrolithiasis | 1 | Hemodialysis | DGF | NA | NA | 0% | NA |
| Kaartinen (13) | 2014 | 63 | M | 2nd | + (from childhood) | CTIN/nephrolithiasis | 0,3 | Hemodialysis | PGNF | NA | + | hom. c.188G>A | |
ref., reference number; F, female; M, male; Tx, transplant; CKD, chronic kidney disease; NA, not available; Nx, nephropathy; CN, crystalline nephropathy; CTIN, chronic tubulointerstitial nephropathy; sCr, serum creatinine; CGD, chronic graft dysfunction; AGD, acute graft dysfunction; DGF, delayed graft function; PGNF, primary graft non-function; GB, graft biopsy; NL, nephrolithiasis; hom., homozygous mutation.
before 1st renal transplantation;
delay between first episode of kidney stone and diagnosis (years);
history of DGF;
in the context of acute rejection.
Table 6.
Literature review of cases of recurrent 2,8-DHA crystalline nephropathy – treatment and outcome.
| First Author (ref.) | Allopurinol therapy | Follow-up (months) | sCr at diagnosis (μmol/L) | sCr at last follow-up (μmol/L) | Renal outcome | |
|---|---|---|---|---|---|---|
| Initial dose | Maintenance dose | |||||
| De Jong (5) | - | - | 6 | Hemodialysis | Hemodialysis | Graft loss |
| Brown (6) | - | - | 4 | Hemodialysis | Hemodialysis | Graft loss |
| Benedetto (7) | 10 mg/kg | NA | NA | 248 | 177 | Chronic graft dysfunction |
| Cassidy (8) | 100 mg/d | 300 mg/d | 7 | 361 | 262 | Chronic graft dysfunction |
| Eller (9) | 150 mg x2/wk | 100 mg/d | 7 | Hemodialysis | 240 | Chronic graft dysfunction |
| Nasr (10) | 200 mg/d | 200 mg/d | 12 | 486 | Hemodialysis | Graft loss* |
| Nasr (10) | 300 mg/d ** | 600 mg/d | 18 | 398 | 141 | Chronic graft dysfunction |
| Bertram (11) | 150 mg/d | 300 mg/d | 9 | Hemodialysis | NA | Chronic graft dysfunction |
| Sharma (12) | NA | NA | NA | Hemodialysis | NA | NA |
| Kaartinen (13) | 300 mg/d | 500 mg/d | 11 | Hemodialysis | Hemodialysis | Graft loss |
ref., reference number; NA, not available (because not performed); hom, homozygous mutation; d, day; wk, week; sCr, serum creatinine.
The patient initially improved but later developed acute deterioration of allograft function in the setting of toxic megacolon. Allopurinol was temporarily discontinued and renal allograft biopsy showed extensive crystal deposition. Despite resumption of allopurinol, the patient remained dialysis-dependent;
Allopurinol was started before renal transplantation.
The prevalence of APRT deficiency is estimated to be of 1/27,000 in the Japanese population and between 1/50,000 to 1/100,000 in white persons (23). The age at diagnosis varies greatly (1–15,20–22). Rarely, the disease can be diagnosed during childhood in patients with nephrolithiasis and obstructive uropathy (9,20). Most often, it remains unrecognized for years. Patients usually experience nephrolithiasis and almost one-third have slowly progressive CKD (5–9,11). Nearly 10% finally progress towards ESRD before the diagnosis (1–3,5–13,24). In case of renal transplantation, and in the absence of prophylactic treatment, 2,8-DHA crystalline nephropathy can recur in the renal allograft, leading to allograft loss in more than 25% of cases. Recurrence of nephrolithiasis has also been reported but is less common than in patients with native kidneys (5,24). 2,8-DHA crystals can be detected in the urine within the first few days after renal transplantation, leading to delayed graft function and primary graft non-function. 2,8-DHA nephropathy may also recur later and despite prophylactic treatment, responsible for chronic, acute-on-chronic, or acute allograft dysfunction. The diagnosis can be delayed because alternative diagnoses are most often considered, including rejection, drug nephrotoxicity, and acute tubular necrosis. Moreover, recurrence of the primary renal disease can also be accompanied by acute cellular or humoral rejection (5,6,9,11). The histopathological analysis thus represents a major challenge. At first evaluation, 2,8-DHA crystals may be missed. They can be easily confused with calcium oxalate because of their high birefringence under polarized light (15,25). A careful analysis shows 2,8-DHA crystal deposits to be of various shape and size, located in the tubular lumens, inside the renal tubular epithelial cells and in the interstitium. Various degrees of interstitial fibrosis, tubular atrophy, and interstitial inflammatory infiltrates may be observed. Several characteristic features are suggestive, including the yellow-brownish color, the presence of irregular crystal aggregates plugging tubular lumens, and ring-like formations of radially-oriented crystals (2,3,5–15,20–22,25). The Maltese cross pattern, generated by thinner and light permeable 2,8-DHA crystals, is exceptionally detected within the tubular lumens. The review of previous native or graft biopsies can also confirm the diagnosis. 2,8-DHA crystals, which had been initially missed, are most often obvious. The amount, distribution and shape of crystals may vary greatly from one patient to another and even in the same biopsy. The magnitude of urinary 2,8-DHA excretion, fluid intake, treatment dosage and factors promoting and inhibiting crystallization likely account for such variability (10). Alterations in the composition of the cell surface may also be necessary for crystal binding to the renal epithelial cells (26). Ischemia-reperfusion lesions and acute tubular necrosis, nephron mass reduction, infections and rejection may thus promote disease recurrence (5,9,10,23).
Because other causes of crystalline nephropathy tend to be harmful for the graft function (25), crystals in the renal allograft should never be dismissed. A panel of diagnostic tools is available, including microscopic techniques, enzyme assay and genetic testing (Table 7). In our experience, Fourier transformed infrared microscopy is a very sensitive and specific technique that should be performed whenever possible to determine the nature of crystal deposits in the kidney parenchyma (17,27). Analysis of kidney stones should also be performed in case of recurrence (18,19). Urine microscopy of a first-void morning specimen is also a very sensitive and reliable diagnostic tool (18,19). Nevertheless, crystals may be missed during routine microscopic examination (10), and crystalluria may be inconspicuous in oligo-anuric patients. Enzyme assay measuring APRT activity in erythrocyte lysates may thus be helpful. Undetectable enzyme activity confirms the diagnosis (1–3,23). Importantly, once the diagnosis has been established, the siblings should be screened using crystalluria and APRT enzyme assay. Genetic testing can be considered, particularly if enzyme assay is not available. Various germline mutations have been reported with no correlation between genotype and phenotype (3,28–32). Moreover, 10% of mutations remain undetermined despite sequencing of the entire coding region and intron-exon junctions of the APRT gene (3), as in 3 of our patients.
Table 7.
Recommended tests for the diagnosis of 2,8-DHA crystalline nephropathy and APRT deficiency.
| Diagnostic Tests | Advantages | Pitfalls | |
|---|---|---|---|
| First line investigations |
|
|
|
|
|
|
|
|
|
|
|
| Second line investigations |
|
|
|
|
|
|
|
APRT, adenine phosphoribosyltransferase; 2,8-DHA: 2,8-dihydroxyadenine; FTIR: Fourier transformed infrared microscopy.
The management of APRT deficiency is based on allopurinol (23), or the alternative agent febuxostat (33), in order to effectively reduce the generation of 2,8-DHA. High fluid intake and avoidance of purine-rich diet can also be advised, whereas urine alkalinization is not beneficial (23). The initial dose of allopurinol usually ranges from 100 to 300 mg/day in adults. Higher doses, in the range of 300 to 600 mg/day, are generally required to achieve complete inhibition of 2,8-DHA formation (10,13). Crystalluria can be used for monitoring treatment efficiency. The likelihood of complete regression of crystal deposition and recovery of allograft function depends on the extent of kidney damage at treatment initiation. In our series and previous reports, almost one third of patients had allograft loss at last follow-up, and most of the remaining patients had chronic allograft dysfunction (13,14,16,19,21,23). Nevertheless, if allopurinol is initiated early enough, graft function may remain stable or improve (7,11).
2,8-DHA crystalline nephropathy is a rare and underrecognized cause of CKD that can recur in the renal allograft. The presence of crystals in the renal parenchyma and urine sediment should not be overlooked. A high index of suspicion for disease recurrence should be maintained, regardless of the course and delay of allograft dysfunction and immunological risk. This is particularly important when the underlying cause of the CKD is unknown. Accurate diagnosis and prompt pharmacologic inhibition of xanthine dehydrogenase may allow the stabilization or even improvement of graft function, reducing the risk of allograft loss.
Acknowledgments
We thank all the nephrologists and pathologists who have contributed to the recruitment of patients, including Elodie Merieau (Tours, France), Eric Prinz (Strasbourg, France), Reda Sharobeem (Olivet, France), Christian Jacquot (Paris, France), Renato Demontis (Creil, France), Guillaume Bollée (Québec, Canada), Catterine Canavese (Novara, Italy), Gabriele Guglielmetti (Novara, Italy), Laure-Hélène Noël (Paris, France), Laurent Doucet (Brest, France), Marie-Christine Machet (Tours, France), Rémy Kerdraon (Orléans, France), Jérôme Olagne (Strasbourg, France), Dominique Bazin (Paris, France), and Christophe Sandt (Gif-sur-Yvette, France).
Runolfur Palsson and Vidar Orn Edvardsson, are supported by the Rare Kidney Stone Consortium (U54KD083908), a member of the NIH Rare Diseases Clinical Research Network (RDCRN), supported through a collaboration between the National Center for advancing Translational Sciences (NCATS), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)."
ABBREVIATIONS
- APRT
adenine phosphoribosyltransferase
- 2,8-DHA
2,8-dihydroxyadenine
- ESRD
end-stage renal disease
- eGFR
estimated glomerular filtration rate
- CKD
chronic kidney disease
Footnotes
DISCLOSURES
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Kamatani N, Terai C, Kuroshima S, Nishioka K, Mikanagi K. Genetic and clinical studies on 19 families with adenine phosphoribosyltransferase deficiencies. Hum Genet. 1987;75:163–8. doi: 10.1007/BF00591080. [DOI] [PubMed] [Google Scholar]
- 2.Edvardsson V, Palsson R, Olafsson I, Hjaltadottir G, Laxdal T. Clinical features and genotype of adenine phosphoribosyltransferase deficiency in iceland. Am J Kidney Dis. 2001;38:473–80. doi: 10.1053/ajkd.2001.26826. [DOI] [PubMed] [Google Scholar]
- 3.Bollée G, Dollinger C, Boutaud L, Guillemot D, Bensman A, Harambat J, et al. Phenotype and genotype characterization of adenine phosphoribosyltransferase deficiency. J Am Soc Nephrol. 2010;21:679–88. doi: 10.1681/ASN.2009080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harambat J, Bollée G, Daudon M, Ceballos-Picot I, Bensman A, Group AS. Adenine phosphoribosyltransferase deficiency in children. Pediatr Nephrol. 2012;27:571–9. doi: 10.1007/s00467-011-2037-0. [DOI] [PubMed] [Google Scholar]
- 5.de Jong DJ, Assmann KJ, De Abreu RA, Monnens LA, van Liebergen FJ, Dijkman HB, et al. 2,8-Dihydroxyadenine stone formation in a renal transplant recipient due to adenine phosphoribosyltransferase deficiency. J Urol. 1996;156:1754–5. doi: 10.1097/00005392-199611000-00057. [DOI] [PubMed] [Google Scholar]
- 6.Brown HA. Recurrence of 2,8-dihydroxyadenine tubulointerstitial lesions in a kidney transplant recipient with a primary presentation of chronic renal failure. Nephrol Dial Transpl. 1998;13:998–1000. doi: 10.1093/ndt/13.4.998. [DOI] [PubMed] [Google Scholar]
- 7.Benedetto B, Madden R, Kurbanov A, Braden G, Freeman J, Lipkowitz G. Adenine phosphoribosyltransferase deficiency and renal allograft dysfunction. Am J Kidney Dis. 2001;37:E37. doi: 10.1016/s0272-6386(05)90001-2. [DOI] [PubMed] [Google Scholar]
- 8.Cassidy MJ, McCulloch T, Fairbanks LD, Simmonds HA. Diagnosis of adenine phosphoribosyltransferase deficiency as the underlying cause of renal failure in a renal transplant recipient. Nephrol Dial Transpl. 2004;19:736–8. doi: 10.1093/ndt/gfg562. [DOI] [PubMed] [Google Scholar]
- 9.Eller P, Rosenkranz AR, Mark W, Theurl I, Laufer J, Lhotta K. Four consecutive renal transplantations in a patient with adenine phosphoribosyltransferase deficiency. Clin Nephrol. 2004;61:217–21. doi: 10.5414/cnp61217. [DOI] [PubMed] [Google Scholar]
- 10.Nasr S, Sethi S, Cornell L, Milliner D, Boelkins M, Broviac J, et al. Crystalline nephropathy due to 2,8-dihydroxyadeninuria: an under-recognized cause of irreversible renal failure. Nephrol Dial Transpl. 2010;25:1909–15. doi: 10.1093/ndt/gfp711. [DOI] [PubMed] [Google Scholar]
- 11.Bertram A, Broecker V, Lehner F, Schwarz A. Kidney transplantation in a patient with severe adenine phosphoribosyl transferase deficiency: obstacles and pitfalls. Transpl Int. 2010;23:e56–8. doi: 10.1111/j.1432-2277.2010.01128.x. [DOI] [PubMed] [Google Scholar]
- 12.Sharma SG, Moritz MJ, Markowitz GS. 2,8-dihydroxyadeninuria disease. Kidney Int. 2012;82:1036. doi: 10.1038/ki.2012.229. [DOI] [PubMed] [Google Scholar]
- 13.Kaartinen K, Hemmilä U, Salmela K, Räisänen-Sokolowski A, Kouri T, Mäkelä S. Adenine Phosphoribosyltransferase Deficiency as a Rare Cause of Renal Allograft Dysfunction. J Am Soc Nephrol JASN. 2014;25:671–4. doi: 10.1681/ASN.2013090960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagné ER, Deland E, Daudon M, Noël LH, Nawar T. Chronic renal failure secondary to 2,8-dihydroxyadenine deposition: the first report of recurrence in a kidney transplant. Am J Kidney Dis. 1994;24:104–7. doi: 10.1016/s0272-6386(12)80168-5. [DOI] [PubMed] [Google Scholar]
- 15.Stratta P, Fogazzi G, Canavese C, Airoldi A, Fenoglio R, Bozzola C, et al. Decreased Kidney Function and Crystal Deposition in the Tubules After Kidney Transplant. Am J Kidney Dis. 2010;56:585–90. doi: 10.1053/j.ajkd.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Estepa-Maurice L, Hennequin C, Marfisi C, Bader C, Lacour B, Daudon M. Fourier transform infrared microscopy identification of crystal deposits in tissues: clinical importance in various pathologies. Am J Clin Pathol. 1996;105:576–82. doi: 10.1093/ajcp/105.5.576. [DOI] [PubMed] [Google Scholar]
- 18.Daudon M, Bader C, Jungers P. Urinary calculi: review of classification methods and correlations with etiology. Scanning Microsc. 1993;7:1081–104. discussion 1104–6. [PubMed] [Google Scholar]
- 19.Daudon M, Jungers P. Clinical value of crystalluria and quantitative morphoconstitutional analysis of urinary calculi. Nephron Physiol. 2004;98:31–6. doi: 10.1159/000080261. [DOI] [PubMed] [Google Scholar]
- 20.Greenwood MC, Dillon MJ, Simmonds HA, Barratt TM, Pincott JR, Metreweli C. Renal failure due to 2,8-dihydroxyadenine urolithiasis. Eur J Pediatr. 1982;138:346–9. doi: 10.1007/BF00442515. [DOI] [PubMed] [Google Scholar]
- 21.Gelb AB, Fye KH, Tischfield JA, Sahota AS, Sparks JW, Hancock DC, et al. Renal insufficiency secondary to 2,8-dihydroxyadenine urolithiasis. Hum Pathol. 1992;23:1081–5. doi: 10.1016/0046-8177(92)90273-6. [DOI] [PubMed] [Google Scholar]
- 22.Arnadottir M, Laxdal T, Hardarson S, Asmundsson P. Acute renal failure in a middle-aged woman with 2,8-dihydroxyadeninuria. Nephrol Dial Transpl. 1997;12:1985–7. doi: 10.1093/ndt/12.9.1985. [DOI] [PubMed] [Google Scholar]
- 23.Bollée G, Harambat J, Bensman A, Knebelmann B, Daudon M, Ceballos-Picot I. Adenine phosphoribosyltransferase deficiency. Clin J Am Soc Nephrol. 2012;7:1521–7. doi: 10.2215/CJN.02320312. [DOI] [PubMed] [Google Scholar]
- 24.Glicklich D, Gruber HE, Matas AJ, Tellis VA, Karwa G, Finley K, et al. 2,8-dihydroxyadenine urolithiasis: report of a case first diagnosed after renal transplant. Q J Med. 1988;68:785–93. [PubMed] [Google Scholar]
- 25.Herlitz LC, D’Agati VD, Markowitz GS. Crystalline nephropathies. Arch Pathol Lab Med. 2012;136:713–20. doi: 10.5858/arpa.2011-0565-RA. [DOI] [PubMed] [Google Scholar]
- 26.Verkoelen CF, Verhulst A. Proposed mechanisms in renal tubular crystal retention. Kidney Int. 2007;72:13–18. doi: 10.1038/sj.ki.5002272. [DOI] [PubMed] [Google Scholar]
- 27.Dessombz A, Bazin D, Dumas P, Sandt C, Sule-Suso J, Daudon M. Shedding light on the chemical diversity of ectopic calcifications in kidney tissues: diagnostic and research aspects. PLoS One. 2011;6:e28007. doi: 10.1371/journal.pone.0028007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Sahota A, Laxdal T, Scrine M, Bowman S, Cui C, et al. Identification of a single missense mutation in the adenine phosphoribosyltransferase (APRT) gene from five Icelandic patients and a British patient. Am J Hum Genet. 1991;49:1306–11. [PMC free article] [PubMed] [Google Scholar]
- 29.Gathof BS, Sahota A, Gresser U, Chen J, Stambrook PS, Tischfield JA, et al. A splice mutation at the adenine phosphoribosyltransferase locus detected in a German family. Adv Exp Med Biol. 1991;309B:83–6. doi: 10.1007/978-1-4615-7703-4_18. [DOI] [PubMed] [Google Scholar]
- 30.Hidaka Y, Tarlé SA, Fujimori S, Kamatani N, Kelley WN, Palella TD. Human adenine phosphoribosyltransferase deficiency. Demonstration of a single mutant allele common to the Japanese. J Clin Invest. 1988;81:945–50. doi: 10.1172/JCI113408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mimori A, Hidaka Y, Wu VC, Tarlé SA, Kamatani N, Kelley WN, et al. A mutant allele common to the type I adenine phosphoribosyltransferase deficiency in Japanese subjects. Am J Hum Genet. 1991;48:103–7. [PMC free article] [PubMed] [Google Scholar]
- 32.Kamatani N, Hakoda M, Otsuka S, Yoshikawa H, Kashiwazaki S. Only three mutations account for almost all defective alleles causing adenine phosphoribosyltransferase deficiency in Japanese patients. J Clin Invest. 1992;90:130–5. doi: 10.1172/JCI115825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becker MA, Schumacher HR, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–61. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]