Abstract
BACKGROUND
Pericardial effusion (PE) is common in cancer patients but the optimal therapeutic approach is not well defined. Percutaneous pericardiocentesis is less invasive than surgery, but its long-term effectiveness and safety are not well documented.
OBJECTIVES
We evaluated outcomes of cancer patients undergoing percutaneous pericardiocentesis for PE and assessed the procedure’s safety in patients with thrombocytopenia.
METHODS
Cancer patients who underwent percutaneous pericardiocentesis for PE between November 2009 and October 2014 at MD Anderson Cancer Center were included. Procedure-related complications, effusion recurrence rate, and overall survival were analyzed.
RESULTS
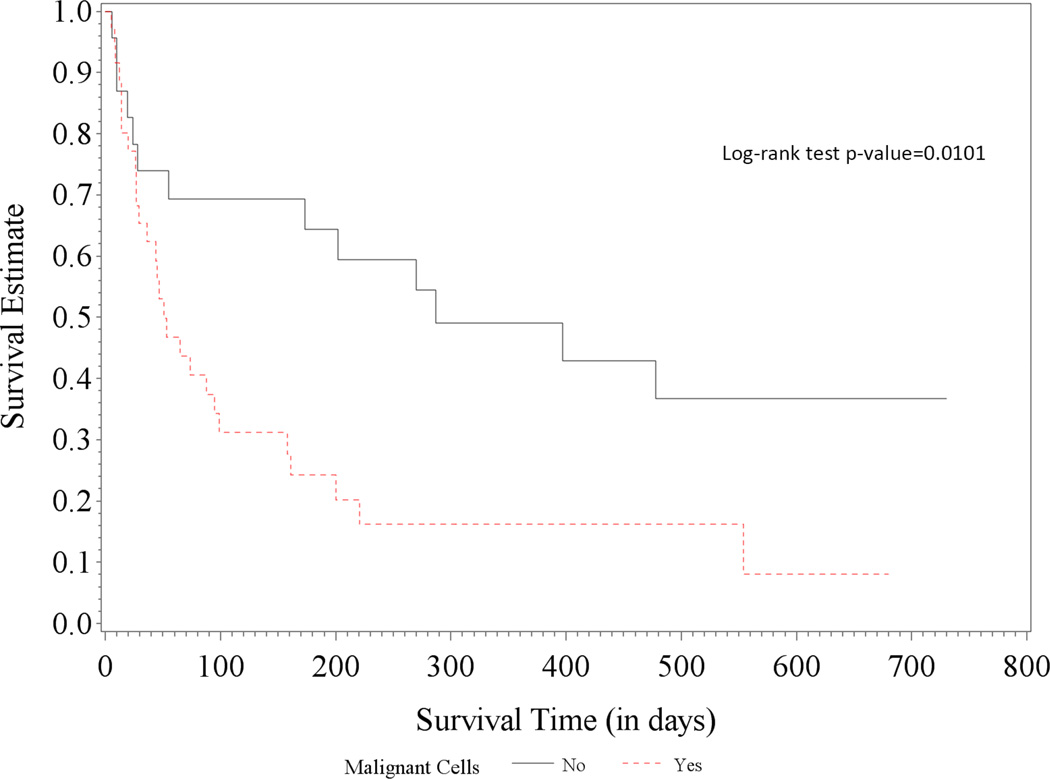
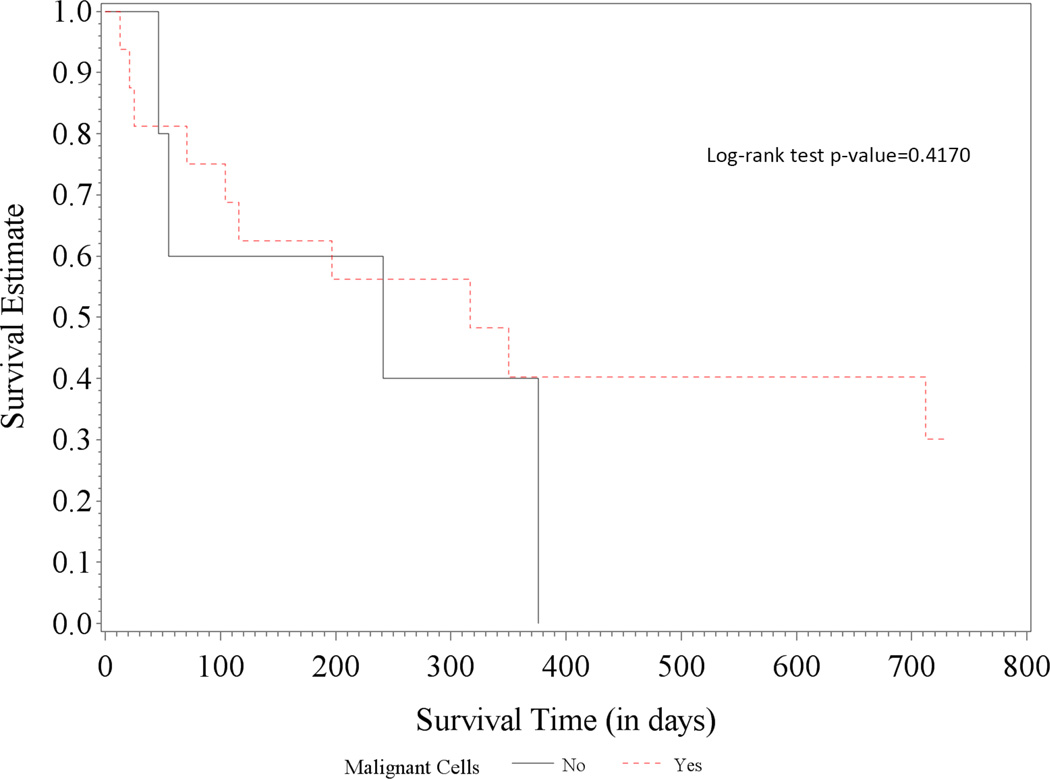
Of 1,645 cancer patients referred for PE, 212 (13%) underwent percutaneous pericardiocentesis. The procedure was successful in 99% of the cases with no procedure-related deaths. Four patients had major procedure-related bleeding that did not vary by platelet count <50,000/µl or ≥50,000/µl (p = 0.1281). Patients with catheter drainage for 3 to 5 days had the lowest recurrence rate (10%). Median overall survival was 143 days with age >65 years, lung cancer, platelet count <20,000/µl, and malignant pericardial fluid independently associated with poor prognosis. Lung cancer patients with proven malignant effusions had a significantly shorter median 1-year survival compared to those with nonmalignant effusions (16.2% vs. 49.0%, respectively; log-rank test p value = 0.0101). A similar difference in 1-year survival was not observed in breast cancer patients (40.2% vs. 40.0%, respectively; log-rank test p = 0.4170).
CONCLUSION
Percutaneous pericardiocentesis with extended catheter drainage, as primary treatment for PE in cancer patients, is safe and effective, including in those with thrombocytopenia. Malignant PE significantly shortens the survival outcome of lung, but not breast, cancer patients.
Keywords: catheter drainage, prothrombin time, safety, thrombocytopenia
Commonly found in cancer patients, pericardial effusion (PE) has been reported in up to 21% of patients with underlying malignancy (1) and has been shown to affect patient survival (2–5). The clinical presentation may range from absence of any symptoms to life-threatening tamponade/shock. The best approach for draining effusion is controversial, with procedure selection often dependent on patient characteristics and local hospital expertise. Surgery is the most studied modality, with different approaches including pericardial window construction, pericardio-peritoneal shunt creation, and/or pericardiectomy. The other well-established approach, which is less invasive, is percutaneous pericardiocentesis with or without extended catheter drainage. Sclerosing agents and balloon pericardiectomy have also been used and are reported to reduce the risk for PE recurrence.
Several studies have suggested that the surgical method provides more definitive primary treatment of malignant PE compared to pericardiocentesis (6–8); however, this approach is associated with significant morbidity (4,9–12). As instruments and techniques have improved – especially the use of echography guidance for percutaneous and catheter-based procedures – clinical application of minimally invasive techniques has often outpaced the published data regarding their safety and efficacy. The purpose of our study was to evaluate the outcomes of cancer patients with PE who underwent percutaneous pericardiocentesis.
Furthermore, thrombocytopenia is a common finding in cancer patients and traditionally has been considered a relative contraindication to pericardiocentesis (13). However, there are limited data regarding the safety of the procedure in these patients; therefore, we assessed its safety in patients with thrombocytopenia.
METHODS
We conducted a retrospective study of cancer patients undergoing percutaneous pericardiocentesis for PE at The University of Texas MD Anderson Cancer Center from November 2009 to October 2014. The study protocol was reviewed and approved by the Institutional Review Board, and a waiver of informed consent was obtained. Patients were selected by searching the main institutional database for hospital discharge diagnostic codes and matching the selected patients with records in the cardiac catheterization laboratory database; the patients were included in the study if they had undergone primary percutaneous pericardiocentesis. Patients were excluded if they had primary surgical pericardial window placement.
PATIENT ANALYSIS
Patient clinical characteristics, including age, sex, type of malignancy, prior cancer therapy (chemotherapy, radiation therapy), and usage of anticoagulant agents at the time of procedure were collected. Clinical and echocardiographic findings of patients presenting with PEs were also documented. These included clinical symptoms (e.g., dyspnea, syncope, chest pain, and palpitations) and clinical signs (tachycardia, hypotension, shock, or presence or absence of pulsus paradoxus). Echocardiographic findings documented included PE size and absence or presence of chamber collapse, mitral and tricuspid valve inflow variation on Doppler images, and inferior vena caval size and respiratory variation. A large PE was defined as ≥2 cm (14). Also reviewed were the effusion pathology and microbiology results obtained at the time of pericardiocentesis.
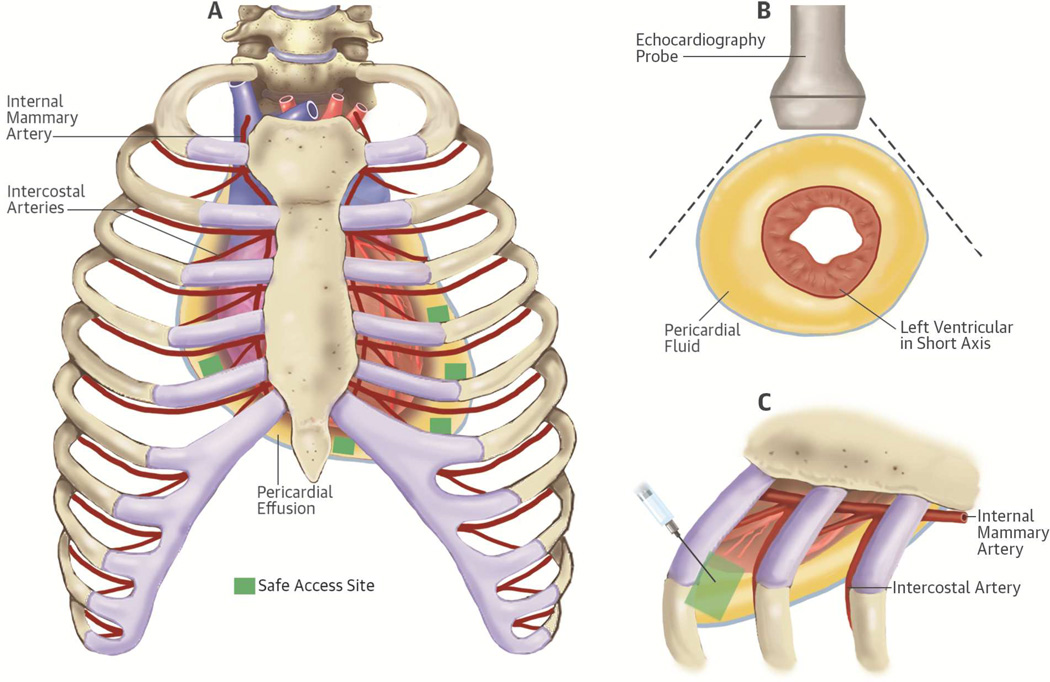
Patients underwent primary percutaneous pericardiocentesis (guided by echocardiography, computerized tomography [CT] scan, or fluoroscopy) for therapeutic or diagnostic purposes. Percutaneous pericardiocentesis was performed using the shortest distance to the pericardial cavity from the subxiphoid or intercostal space (Figure 2). To reduce bleeding, a 5-F micropuncture kit (Cook Medical Micropuncture Introducer Kit, Silhouette Tansitionless Push-Plus Design, Bloomington, Indiana) was typically used in patients with higher bleeding risk (15). Both needle and catheter positions were confirmed using saline contrast injection when echocardiography guidance was used. After accessing the pericardial space, the needle was exchanged over a wire to a dilator, followed by a multihole pigtail catheter. The catheter was then sutured and affixed to the chest wall, where it was kept for several days. A sample (80 ml) of aspirated fluid was sent for pathology, chemistry, and microbiology testing. Post-procedure chest X-rays were performed regularly to assess catheter position and any immediate complication (e.g., pneumothorax). Rarely maintained beyond 7 days, the catheter was removed earlier if fluid drainage dropped below 25 to 50 ml with no residual effusion seen by follow-up echocardiography. Any use of sclerosing agents was documented.
Figure 2. Access site selection for percutaneous pericardiocentesis.
Access site selection for percutaneous pericardiocentesis is based on the detection of the shortest distance to the largest fluid pocket within the pericardial space detected by echocardiography. This site selection can be achieved after careful bedside scanning from multiple directions. Needle and catheter insertion are performed while avoiding the intercostal and internal mammary arteries structures. Figure 2A represent the anterior view of the rib cage with the internal mammary arteries and intercostal arteries in red and pericardial fluid in yellow. The small green squares mark the safe access sites location. Figure 2B shows sample of short axis echocardiographic views to help select the shortest distance to the largest fluid pocket. Figure 2C shows the needle and catheter insertion site performed while avoiding the intercostal and the internal mammary arteries.
Recurrence of PE and its subsequent management were reviewed. Recurrent PE was defined as reaccumulation of pericardial fluid within 3 months post-procedure, documented by echocardiography. Management of such recurrence included repeated simple percutaneous pericardiocentesis, use of sclerosing agents, or placement of a surgical pericardial window.
DATA ANALYSIS
Complications (type and rate) were reviewed and divided into major and minor categories. Procedural bleeding complications were divided into 4 grades: minor or no bleeding (grade 1), major bleeding requiring blood transfusion (grade 2), major bleeding requiring emergent surgery (grade 3), and major bleeding leading to the patient’s death (grade 4). The source of bleeding was recorded only if identified. The need to transfuse platelets, fresh frozen plasma (FFP), or red blood cell (RBC) units was also recorded. Patients with thrombocytopenia were classified into 4 subgroups: <20,000/µl, 20,000/µl to 49,999/µl, 50,000/µl to 99,999/µl, and 100,000/µl to 150,000/µl. Prophylactic platelet transfusion refers to platelet transfusion given to prevent spontaneous bleeding. A therapeutic platelet transfusion is given usually to patients with active bleeding or to those with platelet levels lower than 50,000/µl before an invasive procedure is performed (16). The incidence of bleeding related to pericardiocentesis and odds ratio (OR) of bleeding for platelet count <50,000/µl relative to platelet count ≥50,000/µl were estimated. Fisher exact test was used to compare the incidences of bleeding for platelet counts <50,000/µl and ≥50,000/µl. Bleeding was defined as a documented bleeding event related to the procedure or transfusion of RBCs for unexplained acute anemia. Bleeding was attributed directly to the procedure when no other source of bleeding was identified. Transfusions given routinely because of low hemoglobin related to chemotherapy or hematologic malignancies were excluded and not considered associated with procedural complications.
All continuous variables were described as mean (± SD), and categorical variables were described in terms of counts and percentages. Since patients may have had more than 1 pericardiocentesis during the study period, we considered only the first procedure in the study; the second one was classified as treatment for recurrence and excluded from further analysis.
We studied overall survival, defined as the time from date of procedure to date of death or last follow-up, all within 2 years post-procedure. Univariate Cox proportional hazards regression analysis was done to assess any relationship between clinical variables and overall survival. A multivariable model initially included variables with a p value < 0.15, then a final multivariable model was chosen using a backward elimination process. The parameters included in this analysis were age, sex, platelet count subgroups, tumor type, stem cell transplantation, presence or absence of malignancy in the PE, chest radiation within 1 year before the procedure, clinical or echocardiographic tamponade, PE size (<, =, or >2 cm), hemorrhagic tamponade, use of blood thinners, and duration of drainage. Hazard ratios (HRs) were estimated and 95% confidence intervals (CI) provided as appropriate. All p values were considered statistically significant if < 0.05. SAS 9.4 (SAS Institute Inc, Cary, North Carolina) was used for data analysis.
RESULTS
PATIENT CHARACTERISTICS
From a total of 1,645 cancer patients with PE documented by echocardiography and seen by the cardiology service, we identified 217 patients who needed drainage. Of those, 5 patients had primary surgical pericardial window placement and were excluded from the study; 212 patients underwent percutaneous pericardiocentesis and were analyzed.
Patient clinical characteristics are summarized in Table 1. Lung cancer was the most common malignancy (61 patients [29% of all cases]), followed by acute myeloblastic leukemia and lymphoma. Breast cancer was seen in 10% of the cases with the remainder being cancers of the gastrointestinal tract, cancers of the genitourinary tract, other hematologic disorders (acute lymphoblastic leukemia, chronic myeloblastic leukemia, multiple myeloma, and myelodysplastic syndrome), mesothelioma, osteogenic sarcoma, thymoma, ovarian cancer, prostate cancer, squamous cell carcinoma of the head and neck, and adenocarcinoma of unknown origin. One hundred eighty-six patients (88%) were exposed to chemotherapy, including 122 patients (58%) who received chemotherapy within 6 weeks prior to pericardiocentesis. The most commonly used chemotherapeutic agents were doxorubicin (30%), cyclophosphamide (29%), and carboplatin (27%). Thirty-three patients (16%) were receiving different types of tyrosine kinase inhibitors at the time of PE diagnosis, the most common of which were pazopanib and erlotinib (6 patients each) and dasatinib and sorafenib (3 patients each). Thirty-three patients (16%) received radiation therapy to the mediastinum within 1 year before the procedure.
Table 1.
Patient Characteristics*
| Parameter | Category | Frequency |
|---|---|---|
| Age, years | 54.9 ± 16.4 | |
| Sex | Male | 111 (52) |
| Female | 101 (48) | |
| Cancer type | Lung | 61 (29) |
| Acute myeloblastic leukemia | 34 (16) | |
| Lymphoma | 27 (13) | |
| Breast | 22 (10) | |
| Gastrointestinal | 16 (8) | |
| Other | 52 (25) | |
| Chemotherapy within 6 weeks | 122 (58) | |
| Mediastinal radiation therapy | ≤1 year | 33 (16) |
| >1 year | 35 (17) | |
| Stem cell transplantation | 39 (18) | |
| Blood thinners | Heparin derivative | 76 (36) |
| Antiplatelet | 39 (18) | |
| Oral anticoagulant | 10 (5) | |
| Baseline abnormal coagulation profile | Prothrombin time >16 seconds | 91 (43) |
| Thrombocytopenia (<150,000/µl) | 79 (37) | |
| Access wire size | 5-F micropuncture kit | 187 (88) |
| >5-F | 25 (12) |
Values are mean ± SD or n (%).
N = 212
Three iatrogenic effusions were the consequences of endomyocardial biopsies for cardiomyopathy (2 cases) or cardiac tumor biopsy (1 case). Evidence of tamponade was seen clinically in 83patients (39%) and via echocardiography in 144 patients (68%). Of all patients, 18 (8.5%) had small-to-moderate PE (<2 cm); 6 of them underwent pericardiocentesis for diagnostic purposes and 12 to manage tamponade. Of 194 patients (91.5%) with large PEs, two-thirds had clinical and/or echocardiographic evidence of tamponade.
Within 2 days before the procedure, 37% of patients were taking steroids or nonsteroidal anti-inflammatory drugs and 29% of patients were receiving antiplatelet or anticoagulant agents. Seventy-nine patients (37%) had a low platelet level (<150,000/µl) at the time of the procedure. Thirty-five patients (17%) had platelet counts <50,000/µl and 9 cases (4%) had counts <10,000/µl; the lowest level reported was 3,000/µl. All 35 patients had platelet transfusion to minimize bleeding risk. Post-transfusion platelet counts, obtained within 24 h after transfusion, demonstrated a mean platelet count increase of 20,700/µl. Ninety-one patients (43%) had a prothrombin time (PT) higher than 16 seconds, and 20 patients had both a platelet count <150,000/µl and a PT >16 seconds. The highest PT reported was 56.4 seconds. Eighteen patients (9%) needed prophylactic and/or therapeutic FFP transfusion.
PROCEDURES
Our success rate for pericardiocentesis (percentage of patients who had successful initial drainage of pericardial fluid clinically and echocardiographically) was 99%. In 2 cases, the procedure failed; 1 necessitated surgical window placement and the other was clinically observed. Pericardiocentesis was usually performed by cardiologists (91%) using echocardiographic guidance (93%) or combined echocardiographic and fluoroscopic guidance (7%); 16 procedures (8%) were performed by interventional radiologists with CT-scan guidance. A subxiphoid method was used in 63% of cases; intercostal site entry in 37%. Most patients had extended catheter drainage for 3 to 5 days (119 patients, 56%); 40 patients (19%) required 2 or fewer days; 36 (17%) required 6 to 7 days; 7 (3%) continued catheter drainage >8 days; and 10 (5%) had no catheter placement. A 5-F micropuncture kit was used in 187 patients (88%), and 36% of these patients had thrombocytopenia.
The mean initial volume of pericardial fluid drained was 590 ± 270 ml (range: 2.5 to 1,800 ml). The most common type of initial pericardial fluid aspirated was macroscopically hemorrhagic (48%). One case had initial purulent drainage secondary to lung abscess with pneumo-pericardial fistula. Of 52 pericardial fluid samples sent for viral analysis, 1 sample came back positive for adenovirus (500 copies/ml). Of 204 samples sent for pathology evaluation, 84 (41%) were malignant with positive cytology, while 120 (59%) were nonmalignant.
CLINICAL OUTCOMES
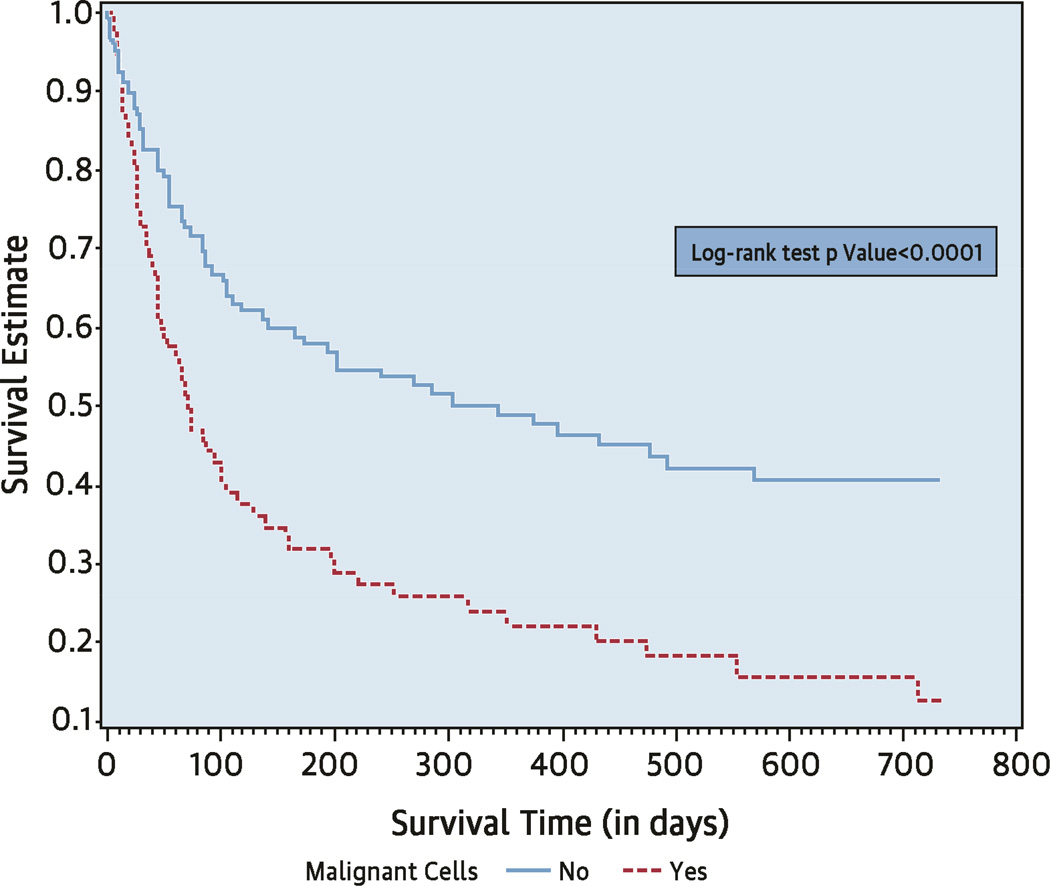
Thirty-eight patients (18%) died within a month after the procedure and 130 patients (61%) died within 2 years of the procedure. For the entire group, the median overall survival time from the procedure date was 143 days (95% CI: 95 to 221). In a multivariable analysis model, older age, lung cancer compared to lymphoma, platelet count <20,000/µl versus ≥100,000/µl, and the presence of malignant cells in the pericardial fluid were independently associated with poor prognosis (Table 2). Median overall survival was 344 days (95% CI: 166 to not estimable) for patients with nonmalignant PE and 71 days (95% CI: 45 to 104) for patients with malignant PE (Central Illustration). One-year survival post-procedure was 50% for patients with PE without malignant cells compared to only 12% of patients with malignant effusions.
Table 2.
Peri-treatment Variables and Their Association with Overall Survival
| Cox Regression Aanalysis | Survivors (n/N) |
2-year Kaplan- Meier Survival Estimate (%) |
Univariate | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Category | HR | 95% CI | p Value | HR | 95% CI | p Value | ||||
| Age, years | 82/212 | 28 | 1.02 | 1.00 | 1.03 | 0.0060 | 1.01 | 1.00 | 1.02 | 0.0473 | |
| Platelet group, /µl | 0.0460* | <0.0001* | |||||||||
| ≥100,000 | 64/156 | 31 | 1.00 | 1.00 | |||||||
| 50,000–100,000 | 10/21 | 34 | 1.09 | 0.58 | 2.04 | 0.7807 | 4.49 | 0.71 | 3.12 | 0.2880 | |
| 20,000–50,000 | 6/15 | 31 | 1.28 | 0.65 | 2.54 | 0.4774 | 1.58 | 0.78 | 3.23 | 0.2050 | |
| <20,000 | 2/20 | 5 | 2.06 | 1.24 | 3.42 | 0.0051 | 1.49 | 2.37 | 8.52 | <0.0001 | |
| Male | Yes | 48/111 | 36 | 0.89 | 0.63 | 1.25 | 0.4990 | ||||
| No | 34/101 | 20 | 1.00 | ||||||||
| Tumor type | 0.0040* | 0.0121* | |||||||||
| Lung cancer | 20/61 | 21 | 1.00 | 1.00 | |||||||
| Breast cancer | 7/22 | 23 | 0.71 | 0.40 | 1.29 | 0.2650 | 0.55 | 0.30 | 1.02 | 0.0581 | |
| Lymphoma | 20/27 | 67 | 0.23 | 0.11 | 0.52 | 0.0004 | 0.27 | 0.11 | 0.68 | 0.0053 | |
| Other | 35/102 | 24 | 0.89 | 0.60 | 1.32 | 0.5600 | 0.89 | 0.57 | 1.39 | 0.6124 | |
| Stem cell transplantation | Yes | 21/39 | 49 | 0.63 | 0.38 | 1.03 | 0.0650 | ||||
| No | 61/173 | 24 | 1.00 | ||||||||
| Malignant cells in effusion | Yes | 21/84 | 12 | 2.10 | 1.47 | 3.00 | <0.0001 | 2.71 | 1.73 | 4.24 | <0.0001 |
| No | 60/120 | 41 | 1.00 | 1.00 | |||||||
| Chest radiation | Yes | 12/33 | 24 | 1.31 | 0.82 | 2.09 | 0.2620 | ||||
| No | 70/179 | 29 | 1.00 | ||||||||
| Clinical tamponade | Yes | 26/82 | 24 | 1.38 | 0.98 | 1.96 | 0.0670 | ||||
| No | 56/130 | 31 | 1.00 | ||||||||
| Echocardiographic tamponade | Yes | 52/144 | 25 | 1.29 | 0.89 | 1.89 | 0.1820 | ||||
| No | 30/68 | 34 | 1.00 | ||||||||
| Pericardial effusion size, cm | <2 | 11/18 | 52 | 1.89 | 0.88 | 4.06 | 0.1020 | ||||
| ≥2 | 71/194 | 26 | 1.00 | ||||||||
| Macroscopic findings | Hemorrhagic | 40/102 | 31 | 1.02 | 0.72 | 1.44 | 0.9070 | ||||
| Nonhemorrhagic | 42/110 | 26 | 1.00 | ||||||||
| Blood thinner | Yes | 41/107 | 30 | 0.84 | 0.59 | 1.18 | 0.3080 | ||||
| No | 41/105 | 27 | 1.00 | ||||||||
| Drainage duration, days | 0.0198* | 0.0128* | |||||||||
| ≤2 | 16/50 | 25 | 1.00 | 1.00 | |||||||
| 3–5 | 55/119 | 33 | 0.70 | 0.46 | 1.06 | 0.0900 | 0.59 | 0.37 | 0.94 | 0.0268 | |
| ≥6 | 11/43 | 18 | 1.25 | 0.77 | 2.03 | 0.3690 | 1.08 | 0.63 | 1.86 | 0.7770 | |
Overall significance of the parameter.
CI = confidence interval; HR = hazard ratio.
Central Illustration. Pericardiocentesis in Cancer Patients: Overall Survival Based on Presence of Malignant Cells in Pericardial Effusion.
As seen in the Kaplan-Meier curve for the entire cohort, patients without malignant cells in pericardial effusion (PE) survived significantly longer than those with malignant cells: 344 days (95% CI: 166 to not estimatable) versus 71 days (95% CI: 45 to 104). CI = confidence interval.
When stratified by cancer type, lung cancer patients had a median overall survival time of 95 days (95% CI: 45 to 202), whereas patients with other cancer types had a median overall survival time of 166 days (95% CI: 104 to 350). Lung cancer patients with proven malignant effusions had a significantly shorter median overall survival compared to those with nonmalignant effusions (24 patients or 39% of lung cancer patients): 1-year survival estimates of 16.2% (95% CI: 5.6% to −31.6%) versus 49.0% (95% CI: 26.7% to 68.0%), respectively; logrank test p value = 0.0101 (Figure 1A). No significant difference was observed in the subgroups of breast cancer patients: 1-year survival estimates of 40.2% (95% CI: 16.0% to 63.6%) versus 40.0% (95% CI: 5.2% to 75.3%), respectively; log-rank test p value = 0.4170 (Figure 1B). Of note, 80% of lung cancer patients and 91% of breast cancer patients had active disease, that is, chemotherapy within 6 weeks or newly diagnosed with de novo or recurrent lung or breast cancer. As for the hematologic malignancies, only 4 lymphoma patients and none of the leukemia patients had proven/confirmed malignant cells in their effusions.
Figure 1. Survival in Patients with Lung and Breast Cancer.
While the presence of malignant cells in PE in lung cancer patients (A) led to significantly worse survival than for those without (median overall survival: 51 [95% CI: 29 to 99] vs. 287 days [95% CI: 55 to not estimable]), there was no significant survival difference in patients with breast cancer (B) (317 [95% CI: 71 to not estimable] vs. 241 days [95% CI: 46 to 376]).
Among 212 patients, 4 patients experienced procedure-related bleeding; none died from bleeding (grade 4 bleeding). Of these 4 patients with procedure-related bleeding, 2 of 35 (5.7%) had a platelet count <50,000/µl and 2 of 177 (1.1%) had a platelet count ≥50,000/µl. The OR of bleeding for a platelet count <50,000/µl relative to ≥50,000/µl was 5.3. There was no strong evidence that procedure-related bleeding differs between platelet count <50,000/µl and ≥50,000/µl groups (p = 0.1281); however, the lack of a difference can be explained by the lack of statistical power to detect bleeding events of low incidence. The 2 patients with platelet counts <50,000/µl (14,000/µl and 29,000/µl) had grade 2 bleeding and both did well following RBC and platelet transfusions. The other 2 patients with platelets counts >50,000/µl, experienced grade 3 bleeding and underwent surgical intervention to stop the bleeding.
Major procedural complications occurred in 5 cases (2%): 1 patient had liver laceration requiring emergent surgical repair; 1 had intercostal artery laceration requiring emergent cauterization; 1 developed a pneumothorax requiring chest tube placement; and 2 cases of catheter-related infections were documented and occurred among the 7 patients whose catheter drainage extended beyond 7 days. They were treated by catheter withdrawal and antibiotics; the first patient fully recovered but the second patient was transferred to hospice care and died several weeks later from cancer progression. Minor procedural complications occurred in 72 patients (34%) (Table 3). Nonsustained supraventricular tachycardia was the most common complication (17%), with paroxysmal atrial fibrillation accounting for 60% of these arrhythmias. Pericardial catheter occlusion occurred in 20 cases (9%).
Table 3.
Complications Related to Percutaneous Pericardiocentesis
| Complication Severity |
Complication | Frequency |
|---|---|---|
| Minor | Nonsustained supraventricular tachycardia | 37 (17) |
| Pericardial catheter occlusion | 20 (9) | |
| Vasovagal response | 7 (3) | |
| Ventricular tachycardia | 6 (3) | |
| Small pneumothorax (on X-ray) | 1 (0.5) | |
| Transient chamber entry | 1 (0.5) | |
| Major | Bacteriema due to catheter placement | 2 (1) |
| Intercostal artery laceration requiring surgery | 1 (0.5) | |
| Pneumothorax requiring chest tube placement | 1 (0.5) | |
| Liver laceration requiring surgery | 1 (0.5) |
Values are n (%).
Among the 50 patients who had either simple pericardiocentesis without indwelling catheter (10 patients) or <3 days drainage (40 patients), the recurrence rate was 23%. In contrast, longer periods of drainage were associated with recurrence rates of 10%, 11%, and 14% when the catheter was left in place for 3 to 5 days, 5 to 7 days, or beyond 7 days, respectively. Overall, 26 patients (12%) had recurrent PE; 16 (62%) had subsequent repeated pericardiocentesis. Sclerosing agents were used in 5 of these cases (thiotepa in 4 and talc in 1). Two of these 16 patients had a second recurrence and subsequently underwent surgery. Surgical window placement was also performed after the first recurrence in 5 cases. The remaining 5 patients were observed clinically and recovered without any further treatment of their effusion.
DISCUSSION
Our results show that percutaneous pericardiocentesis with extended catheter drainage for 3 to 5 days is an effective treatment modality with a low recurrence rate. The low complication rates that we observed demonstrated that the procedure is well tolerated and safe, even in patients with thrombocytopenia. Additionally, we found that malignant PE appears to significantly affect the survival outcome of patients with lung, but not those with breast, cancer.
Previously published data showed that surgical approaches (pericardial window, pericardio-peritoneal shunt creation, and/or pericardiectomy) are associated with the highest success rate, ranging from 87% (17) to 100% (18), with a complication rate of 4.7% and rare mortality (1). In contrast, Jama et al. reported that percutaneous pericardiocentesis with or without extended catheter drainage had an overall low success rate of 60% and many patients requiring further intervention for effusion recurrence. They also noted a high procedure-related complication rate (8%) (1). While administration of sclerosing agents following pericardiocentesis was associated with an overall higher success rate (88%), their use was linked to a higher rate of complications and morbidities (20%). Our percutaneous pericardiocentesis success rate of 99%, low recurrence rate of 12%, and acceptable major complications rate of 2.5% all compare favorably with those of surgery.
CLINICAL CHARACTERISTICS
In our institution, similar to findings of other studies, lung cancer was the most common malignancy associated with PE, followed by hematologic malignancies, including leukemia and lymphoma (4,11,19). Unlike previously published data, breast cancer was responsible for only 10% of PEs in our patient population (4,10,11). These findings are possibly related to referral pattern; a very high number of leukemia and lymphoma patients are treated at our institution.
The mechanism of effusion was proven to be related to direct cancer involvement of the pericardium in only 41% of the cases. Beyond these, 4 (2%) patients had infectious etiologies (2 bacterial, 1 viral, and 1 fungal). PE etiology was undetermined in the remaining cases (57%). The etiologic mechanism of non-neoplastic and noninfectious effusions in cancer patients has been suggested to be related to obstruction of the mediastinal lymphatic system by tumor infiltration or radiotherapy-induced fibrosis. Other suggested mechanisms include inflammation or fluid retention triggered by certain chemotherapies (anthracyclines, dasatinib) (20–22).
Procedural indications included symptomatic PE with overt or impending tamponade (72%), large asymptomatic effusions (25%), and need for tissue diagnosis to guide cancer management (3%). Thus, most of the procedures were done under emergent circumstances. Draining large asymptomatic PE is part of the clinical practice algorithm at our cancer center and is based on clinical experience and extrapolated from published data (23) showing that up to one–third of large asymptomatic PEs can progress into tamponade without preceding symptoms. We commonly observed this in our patient population, where significant fluid shift often occurs with cancer therapy.
PROCEDURE-RELATED COMPLICATIONS
Similar to the findings of Lindenberger et al. (24), supraventricular arrhythmia was the most common procedure-related complication. This is likely related to mechanical irritation or inflammation triggered by the draining catheter. The 2 catheter-related infections observed were due to prolonged catheter placement (>7 days). Overall, 79 (37%) patients had thrombocytopenia (platelet counts <150,000/µl), including 35 (17%) patients with severe thrombocytopenia (platelet counts ≤50,000/µl). Following the standard transfusion guidelines (16,25–27), all 35 patients had platelet transfusion before, during, or immediately following pericardiocentesis. No major bleeding complications requiring surgery were noted in these patients while 2 other patients with platelet counts of 87,000/µl and 205,000/µl suffered grade 3 bleeding. While the low number of bleeding events did not allow meaningful comparison between the different platelets subgroups, it is clinically important to note the low incidence of bleeding complications in those with thrombocytopenia.
Recurrence rates vary by type of procedure and duration of follow-up. Simple pericardiocentesis without pericardial drainage has been shown to be associated with the highest rate of effusion recurrence, ranging from 33% to 55% (4,10,24) while extended catheter drainage has been reported to be associated with a recurrence rate of 13% to 45% (1,4,28). In contrast, chemical pericardiodesis was associated with a recurrence rate of 12% but was responsible for a much higher complication rate (21%). The surgical method has shown the best overall recurrence rate (7%) thus far reported (1). Of our patients, 95% had extended catheter drainage, while only 5% had simple pericardiocentesis without any drain placement. Of those without extended drainage, 23% had recurrence, in contrast to the lowest recurrence rate (10%) observed when the draining catheter was kept in place for 3 to 5 days. Thus, a catheter drainage duration of 3 to 5 days seems to be ideal, with optimal efficacy and lowest risk for pericardial infection. In cases of recurrence, a more definitive therapy is usually needed, such as repeated pericardiocentesis; of the 26 patients with recurrent PE in our study, 62% had malignant pericardial effusion.
Thirty-eight patients died within 30 days of the procedure, but their deaths were related to cancer disease progression or other severe comorbidities (sepsis, pulmonary embolism, multiple organ failure). No procedure-related deaths were reported. The overall median survival of the cohort was 143 days (95% CI: 95 to 221), and 130 patients died within 2 years from the procedure. Patients with malignant cells in their effusion specimens have been reported to have shorter overall survival compared with patients with nonmalignant findings (29). However, our data suggest that this observation varies by type of malignancy. The presence of pathologically-proven malignant effusion appears to significantly affect the survival of patients with lung cancer but not those with breast cancer. This difference was observed despite the fact that breast cancer was associated with a higher incidence (76%) of malignant effusions than was lung cancer (61%).
STUDY LIMITATIONS
There are several limitations to our study. Being a retrospective chart review, the choice of method and entry site, imaging guidance, and placement of catheter for extended drainage were all dependent on the operator and the patient’s general status (possible selection bias). Our patient population’s initial performance status could not be obtained from the data collected, and whether patients had a benefit of symptom relief or an improved short-term quality of life after pericardiocentesis could not be measured, especially in those with end-stage tumors.
CONCLUSIONS
Percutaneous pericardiocentesis with extended catheter drainage, as primary treatment of pericardial effusion in cancer patients, is safe and effective even in those with thrombocytopenia. It is associated with an acceptable recurrence rate and our data suggest that a catheter drainage duration of 3 to 5 days is associated with the most optimal efficacy/risk ratio. The presence of proven malignant cells in PE appears to significantly affect the survival of patients with lung cancer but not those with breast cancer.
Perspectives.
COMPETENCY IN PATIENT CARE
Percutaneous pericardiocentesis with continuous drainage for 3 to 5 days is generally a safe and effective strategy for PE management in patients with cancer, even when thrombocytopenia is present.
TRANSLATIONAL OUTLOOK
Further studies are needed to assess the safety and efficacy of continuing systemic anticoagulant therapy during extended catheter drainage of pericardial effusions in patients with cancer, given the concurrent risk of venous thromboembolism.
Acknowledgments
Financial support: The statistical analysis work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
ABBREVIATIONS AND ACRONYMS
- CI
confidence interval
- CT
computerized tomography
- FFP
fresh frozen plasma
- HR
hazard ratio
- OR
odds ratio
- PE
pericardial effusion
- PT
prothrombin time
- RBC
red blood cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jama GM, Scarci M, Bowden J, Marciniak SJ. Palliative treatment for symptomatic malignant pericardial effusion. Interact Cardiovasc Thorac Surg. 2014;19:1019–1026. doi: 10.1093/icvts/ivu267. [DOI] [PubMed] [Google Scholar]
- 2.Mukai K, Shinkai T, Tomonaga K, Shimoto Y. The incidence of secondary tumors of the heart and pericardium: A ten-year study. Jpn J Clin Oncol. 1998;18:185–201. [PubMed] [Google Scholar]
- 3.Kim S-H, Kwak M, Park S. Clinical characteristics of malignant pericardial effusion associated with recurrence and survival. Cancer Res Treat. 2010;42:210–216. doi: 10.4143/crt.2010.42.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsang TS, Seward JB, Barnes ME, et al. Outcomes of primary and secondary treatment of pericardial effusion in patients with malignancy. Mayo Clin Proc. 2000;75:248–253. doi: 10.4065/75.3.248. [DOI] [PubMed] [Google Scholar]
- 5.Gornik H, Gerhard-Herman M, Beckman J. Abnormal cytology predicts poor prognosis in cancer patients with pericardial effusion. J Clin Oncol. 2005;23:5211–5216. doi: 10.1200/JCO.2005.00.745. [DOI] [PubMed] [Google Scholar]
- 6.Vaitkus P, Hermann H, LeWinter M. Treatment of malignant pericardial effusion. JAMA. 1994;272:59–64. [PubMed] [Google Scholar]
- 7.McDonald J, Meyers B, Guthrie T, et al. Comparison of open subxiphoid pericardial drainage with percutaneous catheter drainage for symptomatic pericardial effusion. Ann Thorac Surg. 2003;76:811–815. doi: 10.1016/s0003-4975(03)00665-9. [DOI] [PubMed] [Google Scholar]
- 8.Celik S, Cervesato E. Systemic chemotherapy in combination with pericardial window has better outcomes in malignant pericardial effusions. J Thorac Cardiovas Surg. 2014;148:2288–2293. doi: 10.1016/j.jtcvs.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Tsang T, Enriquez-Sarano M, Freeman W. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77:429–436. doi: 10.4065/77.5.429. [DOI] [PubMed] [Google Scholar]
- 10.Apodaca-Cruz A, Villarreal-Garza C, Torres-Avila B, et al. Effectiveness and prognosis of initial pericardiocentesis in the primary management of malignant pericardial effusion. Interact Cardiovasc Thorac Surg. 2010;11:154–161. doi: 10.1510/icvts.2010.232546. [DOI] [PubMed] [Google Scholar]
- 11.Patel N, Rafique AM, Eshaghian S, et al. Retrospective Comparison of Outcomes, Diagnostic Value, and Complications of Percutaneous Prolonged Drainage Versus Surgical Pericardiotomy of Pericardial Effusion Associated With Malignancy. Am J Cardiol. 2013;112:1235–1239. doi: 10.1016/j.amjcard.2013.05.066. [DOI] [PubMed] [Google Scholar]
- 12.Saltzman A, Paz Y, Rene A. Comparison of surgical pericardial drainage with percutaneous catheter drainage for pericardial effusion. J Invasive Cardiol. 2012;24:590–593. [PMC free article] [PubMed] [Google Scholar]
- 13.Maisch B, Seferovic P, Ristic A. Guidelines on the diagnosis and management of pericardial diseases executive summary: The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587–610. doi: 10.1016/j.ehj.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J. 2013;34:118–197. doi: 10.1093/eurheartj/ehs372. [DOI] [PubMed] [Google Scholar]
- 15.Murarka S, Movahed M. The use of micropuncture technique for vascular or body cavity access. Rev Cardiovasc Med. 2014;15:245–251. doi: 10.3909/ricm0709. [DOI] [PubMed] [Google Scholar]
- 16.Thiagarajan P, Afshar-Kharghan V. Platelet Transfusion Therapy. Hematol Oncol Clin N Am. 2013;27:629–643. doi: 10.1016/j.hoc.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Sarigül A, Farsak B, Ateş M, et al. Subxiphoid approach for treatment of pericardial effusion. Asian Cardiovasc Thorac Ann. 1999;7:297–300. [Google Scholar]
- 18.Toth I, Szucs G, Molnar T. Mediastinoscope-controlled parasternal fenestration of the pericardium: definitive surgical palliation of malignant pericardial effusion. J Cardiothorac Surg. 2012;7:56. doi: 10.1186/1749-8090-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maisch B, Ristic A, Pankuweit S. Evaluation and management of pericardial effusion in patients with neoplastic disease. Prog Cardiovasc Dis. 2010;53:157–163. doi: 10.1016/j.pcad.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Galderisi M, Marra F, Esposito R, et al. Cancer therapy and cardiotoxicity: The need of serial Doppler echocardiography. Cardiovasc Ultrasound. 2007;5:4. doi: 10.1186/1476-7120-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burazor I, Imazio M, Markel G, Adler Y. Malignant pericardial effusion. Cardiology. 2013;124:224–232. doi: 10.1159/000348559. [DOI] [PubMed] [Google Scholar]
- 22.Refaat M, Katz W. Neoplastic pericardial effusion. Clin Cardiol. 2011;34:593–598. doi: 10.1002/clc.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sagristà-Sauleda J, Angel J, Permanyer-Miralda G, Soler-Soler J. Long-term follow-up of idiopathic chronic pericardial effusion. N Engl J Med. 1999;341:2054–2059. doi: 10.1056/NEJM199912303412704. [DOI] [PubMed] [Google Scholar]
- 24.Lindenberger M, Kjellberg M, Karlsson E, Wranne B. Pericardiocentesis guided by 2-D echocardiography: the method of choice for treatment of pericardial effusion. J Intern Med. 2003;253:411–417. doi: 10.1046/j.1365-2796.2003.01103.x. [DOI] [PubMed] [Google Scholar]
- 25.Schiffer C, Anderson K, Bennett C. Platelet transfusion for patients with cancer: Clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1519–1538. doi: 10.1200/JCO.2001.19.5.1519. [DOI] [PubMed] [Google Scholar]
- 26.Slichter S. Evidence-based platelet transfusion guidelines. Hematol Am Soc Hematol Educ Program. 2007:172–178. doi: 10.1182/asheducation-2007.1.172. [DOI] [PubMed] [Google Scholar]
- 27.Kumar A, Mhaskar R, Grossman B. Platelet transfusion: a systematic review of the clinical evidence. Transfusion (Paris) 2015;55:1116–1127. doi: 10.1111/trf.12943. [DOI] [PubMed] [Google Scholar]
- 28.Buchanan CL, Sullivan VV, Lampman R, Kulkarni MG. Pericardiocentesis with extended catheter drainage: an effective therapy. Ann Thorac Surg. 2003;76:817–820. doi: 10.1016/s0003-4975(03)00666-0. [DOI] [PubMed] [Google Scholar]
- 29.Petcu C, Droc I. The efficiency of surgical subxiphoid pericardial drainage and percutaneous pericardial drainage in pericardial effusions associated with cardiac tamponade. Chirurgia (Bucur) 2013;108:226–233. [PubMed] [Google Scholar]