Summary
Cellular heterogeneity represents one of the greatest challenges in cancer therapeutics. In many malignancies, this heterogeneity is generated during tumor evolution through a combination of genetic alterations and epigenetic events that recapitulate normal developmental processes including stem cell self-renewal and differentiation. Many, if not most, tumors display similar hierarchal organization, at the apex of which are “stem-like cells” that drive tumor growth, mediate metastasis and contribute to treatment resistance. Using breast cancer as a model, we discuss how an improved understanding of tumor cellular heterogeneity and plasticity may lead to development of more effective therapeutic strategies.
Introduction
Generation of Cellular Heterogeneity During Carcinogenesis and Tumor Evolution
Several models have been proposed to explain cellular heterogeneity within tumors. The clonal evolution model has focused on random mutation and clonal selection to generate cellular heterogeneity. Elegant mutational analysis has demonstrated the existence of numerous sub-clones within solid tumors including breast cancer (Kandoth et al., 2013). Cells within these sub-clones may interact through paracrine interactions (Zhang et al., 2015; Rajaram et al., 2015; Fillmore et al., 2010). Furthermore, several studies have demonstrated both genetically similar, as well as genetically diverse, sub-clones in primary tumors and metastases in individual patients (Ding et al., 2010; Vignot et al., 2013) consistent with clonal evolution models. The cancer stem cell (CSC) model posits that tumors are hierarchically organized and, at the apex of the hierarchy, are cells that display stem cell properties. These properties include self-renewal, as well as the ability to generate more differentiated cells forming the bulk of the tumor. Hierarchical tumor organization was first demonstrated in human leukemia (Bonnet and Dick, 1997). This was followed by the demonstration that solid tumors, including those of the breast (Al-Hajj et al., 2003), colon (O’Brien et al., 2007; Ricci-Vitiani et al., 2007), brain (Singh et al., 2004), ovaries (Zhang et al., 2008), lungs (Eramo et al., 2008), prostate (Collins et al., 2005), and pancreas (Li et al., 2007) display a similar hierarchical organization The existence of CSCs in melanoma remains controversial, since the ability of human melanoma cells to initiate tumors in immunosuppressed mice was shown to be dependent on the mouse model system utilized (Quintana et al., 2008). Nevertheless, the preponderance of evidence suggests that the vast majority of both hematologic and solid tumors contain cells that display stem cell properties (Visvader and Lindeman, 2008). In these tumors, cells displaying protein markers including CD44, CD133 or the enzyme aldehyde dehydrogenase were able to generate tumors when transplanted into immunocompromised mice (Bonnet and Dick, 1997; Al-Hajj et al., 2003; Ginestier et al., 2007). These transplanted cells generated tumors that recapitulated the phenotypic heterogeneity of the initial tumor. Although CSC markers have proven useful for the enrichment of CSC populations, their utility is limited by variability of expression and regulation by environmental factors. As a result, definitive identification of CSCs requires functional assays demonstrating the capacity to initiate tumors in mouse models.
As has been emphasized by others, stochastic clonal evolution and stem cell models are not mutually exclusive. CSC’s may serve as the unit of selection in the genetic evolution of tumors while also being genetically unstable, generating multiple clones consisting of genetically altered CSCs and their differentiated progeny (Figure 1). Furthermore, as discussed below, cancer cells display a remarkable degree of phenotypic plasticity, and there is evidence that more differentiated tumor cells may acquire “stem-cell” properties through a “dedifferentiation” process; a phenomenon with significant clinical implications.
Figure 1.
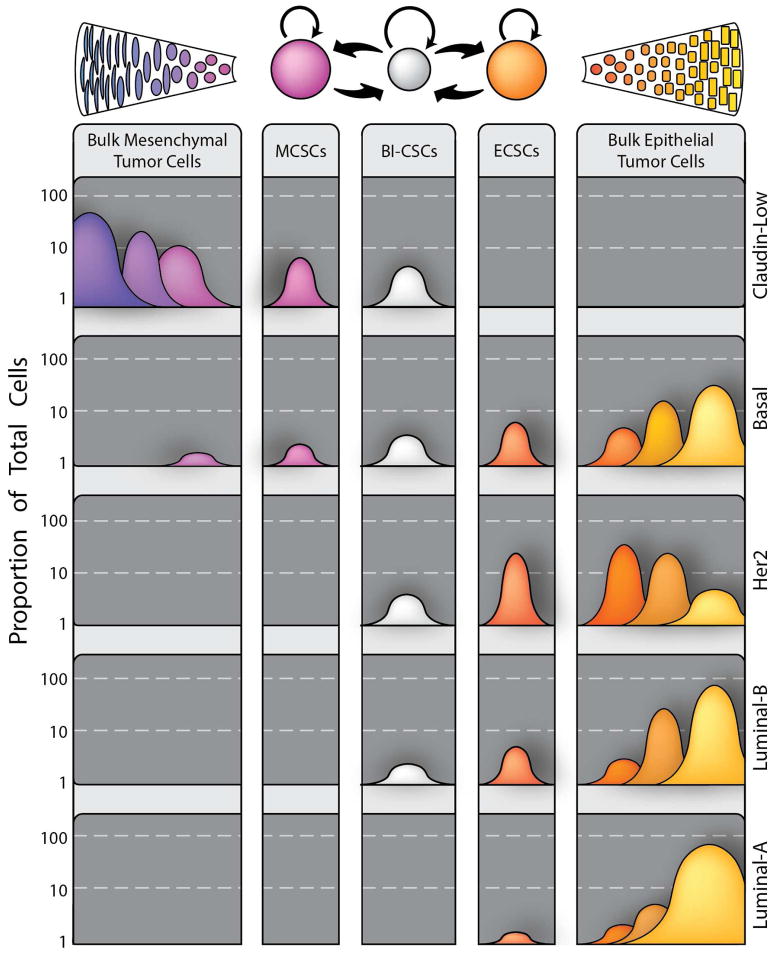
Cellular Heterogeneity and Plasticity in Breast Cancer. Recent work has identified two distinct types of cancer stem cells in breast cancer, a mesenchymal, quiescent type marked by being CD44+/CD24− (EMT-CSCs) and an epithelial, proliferative type identified as ALDH+ (MET-CSCs). A double positive CD44+/CD24−/ALDH+ population with even higher tumorigenicity exists, but it is yet to be determined if this is a stable or transient population. The transition between these states is common in vitro and is likely to be regulated by multiple cell types in the tumor microenvironment through IL8, Notch, and GH (growth hormone) signaling as well as paracrine interactions between CSCs and their more differentiated progeny. Derived from these two CSC states are two differentiated bulk tumor cell types; a epithelial type produced by MET-CSCs (common in most of the molecular subtypes) and a group of mesenchymal bulk tumor cells derived from EMT-CSCs (rare in the majority of breast cancer cases, but common in the claudin-low molecular subtype). Among the corollaries of this model is the idea that the probability of dedifferentiation is not uniform, but is instead inversely proportional to the number of cellular divisions removed from being a cancer stem cell. Furthermore, mutation in CSCs generate new CSCs as well as clones of differentiated progeny generating cellular heterogeneity during tumor evolution.
Interpretations of mouse transplantation studies are limited by the environmental perturbations associated with these approaches. To circumvent these limitations, a number of groups have employed lineage tracing to demonstrate the generation and propagation of CSCs. For example, Parada’s group has demonstrated that a Nestin-driven GFP reporter marks neural stem cells in the brain subventricular zone as well as a corresponding population of glioma stem cells. These Nestin/GFP+ cells repopulate the tumor after treatment with chemotherapy or genetic ablation. (Chen et al., 2012). Similar lineage tracing studies utilized stage-specific Cre-recombinase labeling to demonstrate the presence of stem cells in a squamous carcinoma model system (Driessens et al., 2012). Elegant lineage tracing has been reported by the Clevers group who employed this method to follow the fate of colon stem cells during carcinogenesis. Using a confetti reporter, in which Cre randomly activates one of four fluorescent reporters, it was shown that intestinal adenomas originate in cells expressing the stem cell marker Lgr5 (Schepers et al., 2012). These studies, and others, have provided support to hierarchal models of tumor development in which cells that display “stem-like” properties drive tumor initiation and propagation. In this review, we will discuss the mechanisms that generate cellular heterogeneity during carcinogenesis. Since the role of genetic clonal heterogeneity in the mediation of therapeutic resistance has been the subject of excellent recent reviews (Bedard et al., 2013; Burrell et al., 2013), we will focus on the role of epigenetically generated heterogeneity and cellular plasticity in generating tumors driven by cells that display stem cell properties. We will also discuss the therapeutic implications of this epigenetic heterogeneity across the molecular subtypes of breast cancer.
Breast Cancer Stem Cells (BCSCs): Defining the Cell of Origin, Cancer Stem Cells, and their Relationships
The cancer stem cell hypothesis is composed of two important, but separable, components: the first concerns the cellular origin of cancer, and the second addresses the nature of cells that are responsible for tumor maintenance and progression.
Cellular Origin of Breast Cancer
Multiple cells within the breast have been proposed as the “cell of origin” in the development of human breast cancer. An understanding of the relationship between cellular hierarchies in the normal breast is critical to elucidating the cells of origin that generate the diverse molecular subtypes of breast cancer. Unfortunately, studies aimed at understanding this hierarchy in normal tissue have been complicated by the choice of markers, plasticity between cell states, and differences between human and mouse tissue. Early studies utilizing the protein marker CD24 suggested that the CD24low population (myoepithelial) had the greatest repopulation capacity compared to CD24high (luminal) or CD24neg (non-epithelial) cells (Sleeman et al., 2006). Further studies using additional markers, however, revealed that both CD24+/CD29hi (Shackleton et al., 2006) and CD24+/CD49fhi (Stingl et al., 2006) cells retained the greatest capacity to reconstitute mammary glands, containing both lineages when transplanted into the cleared mammary fat pads of syngeneic mice. In addition to the controversies surrounding the selection of stem cell markers, there has been debate concerning the relative importance of bipotent and unipotent luminal and myoepithelial stem cells during normal development and carcinogenesis.
Lineage tracing experiments using a series of cytokeratins driving Cre-Recombinase demonstrated that while cytokeratin 14 (K14) marks a multipotent progenitor capable of generating all of the mammary lineages during development, unipotent basal and luminal stem cells are responsible for lineage maintenance (Van Keymeulen et al., 2011). However, subsequent cytokeratin 5 driven lineage-tracing studies reported that both bipotent MaSCs and unipotent luminal and myoepithelial progenitor cells are involved in morphogenesis and homeostasis (Rios et al., 2014). Two important recent publications have shed further light on questions concerning the cellular origin of breast cancers as well as the relative roles of particular oncogenic drivers versus the cell of origin in determining their phenotype. Reports by two independent groups using mouse mammary models (Koren et al., 2015; Van Keymeulen et al., 2015) revealed that introduction of oncogenic PIK3CAH1047R, a common mutation found in human breast cancer, into either luminal or basal restricted mammary progenitor cells, resulted in dedifferentiation, endowing the cells with multilineage potential. Furthermore, the phenotype of the transduced cell determined the phenotype of the resulting tumors. These studies suggest the combined importance of particular genetic alterations, as well as the cell of origin in determining molecular characteristics and clinical behavior of breast cancers. Although it remains to be determined if similar mechanisms are involved in generation of human breast cancer phenotypes, the marked similarities in gene expression profiles in these mouse models and human breast cancers suggests that this may be the case (Van Keymeulen et al., 2015).
In parallel to studies in murine mammary development, elucidation of stem cells in the human mammary gland have relied on marker expression coupled with functional assays including mammosphere formation (Dontu et al., 2003) or transplantation into humanized mouse mammary fat pads (Kuperwasser et al., 2004). Quantification of mammary repopulating units in these mouse models demonstrated that the markers CD49f+CD29+CD24low identified a cell population capable of generating human mammary structures composed of luminal and myoepithelial lineages. (Eirew et al., 2008). Additional studies utilizing the markers CD49f, EpCAM, CD10 demonstrated that immature mammary lobules contained the greatest proportion of stem cells with bi-lineage differentiation potential (Arendt et al., 2014). This important spatial distribution was validated by a separate study using a different set of markers including ALDH1A1, SSEA4, CD44, and CK14+/CK19+ (Honeth et al., 2015). However, given the significant differences in anatomy and cellular complexity between human and mouse mammary glands, the exact relationship between stem cells in human and rodent models remains to be definitively determined.
Guided by the information gathered from the studies on the mouse and human hierarchies, efforts have focused on those cells within normal breast tissue that may be the cell of origin for breast tumors. Although it has been postulated that breast cancers may arise from normal breast stem cells, it is more likely that the various molecular subtypes of breast cancer arise from cells at different levels within the mammary hierarchy. Expression profiling revealed strong similarities between the claudin-low subtype and MaSCs, while the basal subtype aligns with luminal progenitor cells (Shehata et al., 2012). In concordance with these findings, it was shown that breast tumors in women with germline BRCA1 mutations contain enriched populations of luminal progenitor cells. These tumors are most often characterized as the basal molecular subtype (Molyneux et al., 2010). Pre-clinical studies have suggested that BRCA1 plays an important role in mammary stem/progenitor cell differentiation, and that the loss of BRCA1 function in mouse models expands the population of cells expressing aldehyde dehydrogenase 1 (ALDH1), a marker of both normal and cancer stem-like cells (Molyneux et al., 2010). These and other studies have led to the suggestion that basal breast cancers arise from luminal progenitor cells defined as CD49+/ESA+ (Lim et al., 2009). However this population itself is heterogeneous and includes a sub-population defined by expression of aldehyde dehydrogenase (ALDH), a population recently shown to have properties of epithelial like multi-potent stem cells (Liu et al., 2014). In addition to being the cell of origin of basal tumors, ALDH1 expressing stem/luminal progenitor cells may also be the cell of origin of other molecular subtypes of breast cancer. A more differentiated luminal progenitor may give rise to the most highly differentiated luminal tumors.
The studies designed to elucidate the cell of origin of the different molecular subtypes of breast cancer have largely relied on transduction of activated oncogenes into mammary cells whose differentiation state is defined by marker expression followed by transplantation into the mammary fat pads of immunosuppressed mice. These studies have suggested that both the cell of origin and the specific oncogenic events contribute the diversity of molecular subtypes of breast cancer. However, these studies are limited by potential artifacts introduced by transplantation. As previously noted Rios et al. have demonstrated that normal mouse mammary stem cells maintain the capacity for multi lineage upon transplantation but also maintain unipotent lineage differentiation in their normal unperturbed microenvironment as accessed by lineage tracing. Application of similar lineage tracing technologies to mammary carcinogenesis may provide a more definitive characterization for the cell of origin of murine mammary cancers. Extrapolation to human disease will necessitate the utilization of transplantation technologies. The elucidation of the cell of origin of human breast cancers is of considerable importance in developing effective cancer prevention strategies.
Cancer Stem Cell State Plasticity and the Tumor Microenvironment
Recent work has demonstrated that BCSCs maintain the plasticity to transition between two different phenotypic states: a more proliferative epithelial-like state characterized by expression of the CSC marker ALDH, as well as a more quiescent and invasive, mesenchymal-like state characterized by the expression CD44+/CD24−. These states share the molecular characteristics of both luminal and basal stem cells in the normal breast (Liu et al., 2014). Each of these CSC states is also capable of generating their respective epithelial or mesenchymal bulk cell progeny, which can secrete signals to positively reinforce CSC self-renewal (Zhang et al., 2015; Fillmore et al., 2010). Transition between the two cancer stem cell states is mediated by epigenetic alterations regulated by the tumor microenvironment, either through cytokine and chemokine signaling and/or transcriptional regulation, including differential expression of microRNAs (Pal et al., 2015) (Figure 1). These concepts expand upon previous work that suggested an important relationship between CSCs and the process of epithelial mesenchymal transition (EMT) (Mani et al., 2008). In the expanded model, EMT-like cancer stem cells are localized at the tumor invasive edge where they are capable of entering the circulation and forming micro metastases at distant sites. Transition back to the proliferative epithelial state at distant sites is mediated by factors such as ID1, which, through downregulation of TWIST, induce this phenotypic switch (Stankic et al., 2013).
A thorough understanding of cancer stem cells requires delineation of how the associated tumor microenvironment mediates the balance between the two cancer stem cell states. Unfortunately, while recent experiments using syngeneic mouse models and transplantation into immunocompetent hosts have confirmed the existence of CSCs in the presence of immune cells, most CSC experiments have so far been done in immunocompromised mouse models. These and other studies suggest that CSCs may deploy specific mechanisms to thwart immune surveillance (Pan et al., 2015). Immune cells are critical components of the tumor microenvironment, and rapidly accumulating evidence suggests that the immune system plays a role in carcinogenesis and tumor development. This emphasizes the importance of utilizing immunocompetent models to more fully explore the mechanisms by which the tumor microenvironment regulates CSC’s.
An important issue in CSC biology is whether bulk tumor cells are capable of “de-differentiating” into CSCs. If this were a common occurrence, then the concept of “CSC states” rather than fixed populations would be applicable. However, in normal cells, the process of differentiation is very tightly controlled by epigenetic events involving histone and DNA modifications. As a result, the differentiation process is largely unidirectional (Cantone and Fisher, 2013). However, the ability to reprogram fully differentiated cells into pluripotent stem cells through the addition of the four Yamanaka factors (Oct3/4, Sox2, Klf4, c-Myc) clearly indicates that dedifferentiation can be driven to occur in normal cells. It is important to note, that in in vitro systems, this process is extremely inefficient. Furthermore, the frequency that such dedifferentiation events occur during carcinogenesis is a matter of conjecture. Guo et al. demonstrated that differentiated breast cancer cells could be converted back to stem-like cells though combined expression of the transcription factors SLUG and SOX9 (Guo et al., 2012). Dedifferentiation has also been shown in gliomas with loss of P16/Inc4A and P19ARF in astrocytes (Friedmann-Morvinski et al., 2012). In intestinal tumorigenesis, overexpression of NFκB and β-catenin causes both mitotic intestinal epithelial cells to regain stem cell properties (Schwitalla et al., 2013). However, many of these studies suggesting dedifferentiation have been performed in vitro (Guo et al., 2012), and the actual contribution of dedifferentiation during carcinogenesis remains unknown. Lineage tracing studies involving stem cells as well as differentiation stage-specific promoters in mouse models will be required to definitively demonstrate the importance of “dedifferentiation” in the generation of CSCs. Once a thorough understanding of how dedifferentiation contributes to cancer initiation and progression has been achieved, subsequent studies can begin to analyze how this information can be used to facilitate better therapeutic treatments. Mathematical modeling has shown that dedifferentiation and plasticity will substantially reduce the effectiveness of CSC directed therapies and increase rates of resistance across a range of dedifferentiation rates (Leder et al., 2010).
An extreme version of cancer cellular plasticity is represented by the phenomenon of “vascular mimicry”, where there is evidence that CSCs from a number of tumor types undergo transdifferentiation to form vascular-like networks (Maniotis et al., 1999; Soda et al., 2011; Ricci-Vitiani et al., 2010). In breast cancer, recent studies have implicated two secretory proteins, SERPINE2 and SLPI, which are involved in this phenomenon (Wagenblast et al., 2015). These vascular mimicry networks have been associated with increased levels of metastasis. However, a more impactful role could be the generation of an expanded niche for cancer stem cells, as endothelial cells have been shown to be important for both normal and cancer stem cell regulation.
Cancer Stem Cells and the Molecular Subtypes of Breast Cancer
Data generated over the past several decades suggests that, rather than being a single disease, breast cancers represent multiple diseases that can be subdivided into clinical subtypes based on cellular expression of estrogen and progesterone receptor, as well as the growth factor receptor HER2 (Erb-B2). By this classification, tumors are classified as hormone-receptor positive (those that express ER and/or PR), HER2 positive, or triple-negative (those that do not express ER, PR, or HER2). Gene expression profiling has expanded upon this classification, defining tumors as luminal-like (further divided into luminal A and luminal B); HER2 positive, characterized by amplification of the HER2 gene; and basal-like (Perou et al., 2000; Sorlie et al., 2001). The development of specific therapeutic strategies for each of the major pathologic subtypes of breast cancer has improved outcomes for many breast cancer patients. In these tailored approaches, ER positive breast cancers are treated with hormonal therapies, cancers with HER2 gene amplification are treated with HER2 targeting agents and, in the absence of specific targeted therapeutics, the TN collection of breast cancers is treated with cytotoxic chemotherapy. These therapeutic approaches have emphasized genomic and phenotypic differences between the subtypes of breast cancer and have been designed to target bulk tumor cell populations.
In addition to having different cells of origin, the different molecular subtypes of breast cancer are characterized by different frequencies of mesenchymal and epithelial CSC types as well as differentiation states of bulk cell populations. These differences are illustrated in Figure 2. The clinically aggressive claudin-low subtype, which is typically a triple-negative breast cancer, is characterized by a high proportion of CD44+/CD24low and ALDH1 expressing CSCs as well as mesenchymal-like bulk tumor cells. In contrast the basal subtype, also commonly triple negative and often associated with BRCA1 loss of function, contains epithelial bulk cells along with a subcomponent of mesenchymal bulk tumor cells and a higher proportion of epithelial ALDH1 positive CSCs. The CSCs give rise to bulk tumor cells that are characterized by an epithelial morphology and lack of expression of the steroid hormone receptors ER or PR (Perou, 2011). The HER2 positive subtype of breast cancer is characterized by a high proportion of ALDH1 positive CSCs that give rise to epithelial bulk populations that may or may not express ER and PR (Korkaya and Wicha, 2013). Luminal B breast cancers generally express a lower proportion of cells expressing CSC markers than either HER2 positive or triple-negative breast cancers. The bulk cells in these tumors are highly epithelial in morphology and a proportion express ER and PR. Luminal A tumors, which have the best prognosis, display the lowest proportion of cells expressing CSC markers. These tumors also display the highest proportion of ER and PR expressing cells.
Figure 2.
Model illustrating variations in CSCs and their differentiated progeny across the spectrum of breast cancer subtypes. In this model, the different molecular subtypes of breast cancer are characterized by varying proportions of CSCs in mesenchymal (EMT) versus epithelial (MET) states as well as differential blocks in the differentiation hierarchy seen in normal mammary development.
Plasticity between cell types is an important part of normal biology, both in development and in adult homeostasis to respond to stimuli and deal with cellular stress; but one hallmark of cancer is its ability to lower the activation barrier and increase cellular plasticity. The processes of EMT and mesenchymal-epithelial transition (MET) have been well studied in the context of cancer biology and have profound implications for the plasticity between CSC types and their relation to the molecular subtypes of breast cancer. As previously mentioned, work from the Weinberg lab has shown that EMT, driven by overexpression of mesenchymal transcription factors such as Snail, Twist, Slug and/or Sox9 (Mani et al., 2008; Guo et al., 2012), can induce an EMT phenotypic shift, resulting in increased numbers of cancer stem cells. This plasticity between cell types results in significant challenges to therapy as EMT increases invasion and dissemination of cells, while also decreasing proliferation and avoiding cytotoxic chemotherapy agents (Creighton et al., 2009). Alternatively, MET allows cells to return to a highly proliferative state and mediates tumor relapse at sites of distant metastases after cessation of treatment (Yao et al., 2011). The development of drugs capable of blocking this cellular plasticity, such as inhibitors of c-Met (Sylvester, 2014) and TGFB (Bhola et al., 2013) by preventing transition between CSC states, may increase the efficacy of CSC targeting agents. In addition, as described below, strategies aimed at simultaneous targeting CSC’s and bulk tumor cells may be useful in overcoming the therapeutic challenges posed by tumor cell plasticity.
Mutation Profile and CSC’s Across the Molecular Spectrum of Breast Cancer
Genetic analysis of breast cancers has demonstrated different mutational profiles across the subtypes of breast cancer. For example, the most frequent genetic alteration found in luminal breast cancers is mutational activation of PI3K signaling (Creighton et al., 2010). In contrast, triple-negative breast cancers almost always contain mutations in p53 and also frequently display deletions or epigenetic silencing of the PTEN tumor suppressor gene. In addition to HER2 gene amplification, HER2 positive breast cancers frequently display deletions in PTEN, and indeed this is a likely cause of resistance to HER2 targeted therapies (Dave et al., 2011; Fabi et al., 2010; Park et al., 2014). BRCA1 germline mutations or epigenetic silencing of the Brca1 locus are most frequently associated with triple-negative breast cancers (Foulkes et al., 2003). Interestingly, all of these molecular alterations have been demonstrated to increase CSC frequency in preclinical models, as well as in patient samples. The different molecular subtypes of breast cancer are also characterized by distinct profiles of microRNA expression, a number of which regulate cancer stem cell states (Pal et al., 2015). In addition to their effects on CSCs, genetic and epigenetic events produce blocks in normal differentiation pathways that differ across the molecular subtypes of breast cancer. It is notable that despite the diversity of genetic changes driving the different molecular subtypes of breast cancer, CSCs within these subtypes have similar patterns of gene expression (Liu et al., 2014) suggestive of common, shared regulatory pathways. For example, the Notch (Al-Hussaini et al., 2011), and Wnt (King et al., 2012) developmental pathways, as well as Her2-Akt (Korkaya et al., 2008), and STAT3-NFκB signal (Iliopoulos et al., 2009) transduction pathways, may play important roles in the regulation of self-renewal and differentiation of CSCs across the molecular subtypes of breast cancer. This observation has important implications for understanding the mechanisms underlying therapeutic resistance, as well as the development of more effective therapeutic strategies capable of producing sustained effects.
Therapeutic Implications of CSC Models
Approaches involving more specific targeted therapeutics have led to improved outcomes, particularly when they are administered in the adjuvant setting after surgical removal of the primary tumor to lessen the probability of tumor recurrence. However despite these new options, metastatic breast cancer remains largely incurable (Ruiterkamp et al., 2011), primarily due to the emergence of treatment resistance during therapy. Cellular heterogeneity, generated through genetic and epigenetic mechanisms, contributes to development of treatment resistance. EZH2, which acts as a transcriptional repressor via control of the repressive histone modification mark H3K27me3, has been linked to aggressiveness in breast cancer (Kleer et al., 2003; Holm et al., 2012). In ER positive breast cancer, methylation events have been associated with resistance to both tamoxifen and aromatase inhibitors (Magnani et al., 2013), and H3K27me3 marks are correlated with worse outcome during aromatase inhibitor therapy (Jansen et al., 2013); preclinical data suggests that treatment of ER positive breast tumors with histone deacetylase (HDAC) inhibitors can improve responses to Tamoxifen (Hodges-Gallagher et al., 2007; Thomas et al., 2011; Bicaku et al., 2008). Epigenetic silencing may also drive tumor cells toward a CSC-like state (Ohm et al., 2007). For example, in basal breast cancers, activation of Zeb1 via epigenetic modulation of its promoter leads to transition of non-CSCs to CSCs (Chaffer et al., 2013). Clinically, however, results of trials utilizing HDAC inhibitors have been mixed. Promising results have been demonstrated in using these agents to reverse endocrine resistance, however HDAC inhibitors have not shown single-agent efficacy in breast cancer (Munster et al., 2011; Luu et al., 2008).
The CSC hypothesis has important clinical implications. CSCs display relative resistance to cytotoxic chemotherapy, both in preclinical models and neoadjuvant clinical trials (Creighton et al., 2009). In fact, both chemotherapy and radiation therapy may actually stimulate CSC self-renewal through cytokine production and DNA repair mechanisms (Maugeri-Sacca et al., 2012; Li et al., 2008; Phillips et al., 2006). The development of individualized therapies for each molecular subtype of breast cancer was largely based on strategies to target bulk cell populations. However, patients inevitably develop resistance to these therapeutic approaches. The evolution of clones of cells with “driver”mutations, undoubtedly contribute to this resistance, providing a substantial therapeutic challenge (McGranahan et al., 2015). Genetic mutations as well as micro-environmentally driven epigenetic diversity at metastatic sites further limit the effectiveness of agents targeting genetic lesions of the primary tumor (Oskarsson et al., 2014).
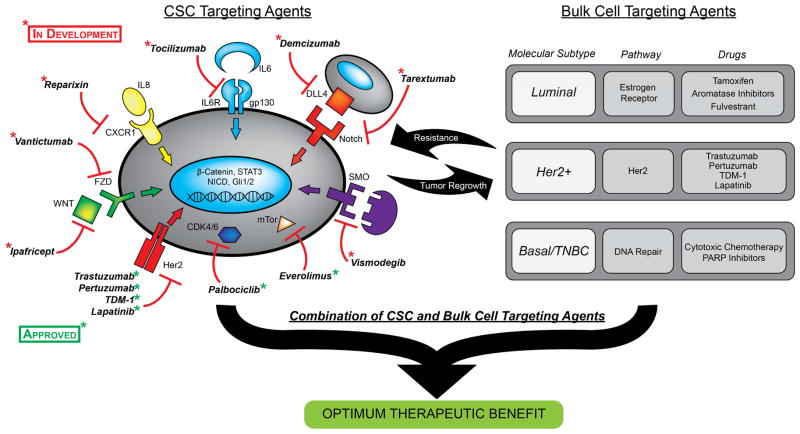
Although mutations are shared between CSC and their clonal progeny (Marusyk et al., 2014) CSC may be dependent on alternate pathways many of which are shared across the molecular subtypes of breast cancer. This suggests that addition of CSC targeting agents to molecular subtype specific therapies may improve treatment efficacy. This approach is illustrated in Figure 3 and discussed in more detail below.
Figure 3.
Strategy for combining CSC targeting agents with approved drugs that target the bulk tumor populations in each molecular breast cancer subtype. The diagram on left illustrates CSC targeting drugs either approved or in development and, on the right, FDA approved drugs to treat each of the molecular subtypes of breast cancer.
ER Positive Breast Cancer
Breast cancers that express estrogen and/or progesterone receptors have been subdivided into luminal A and luminal B based on molecular profiling. The proliferation marker Ki67 has served as a surrogate for molecular profiling, where a Ki67 index of 14 divides the less proliferative luminal A breast cancers from the more highly proliferative luminal B breast cancers (Goldhirsch et al., 2011). Although both of these luminal breast cancers are treated with hormonal therapy, the clinical outcome is significantly more favorable for patients with luminal A tumors compared to luminal B tumors (Sorlie et al., 2001). It is likely that many luminal A breast cancers may lack a true CSC and instead, consist of a relatively uniform population of luminal progenitor/differentiated cells that express steroid hormone receptors; in contrast, luminal B breast cancers contain a proportion of CSCs that do not express ER or PR (Harrison et al., 2013; Horwitz et al., 2008). Of interest, it was recently reported that despite the fact that ALDH+ CSCs do not express the classical 66 kilodalton ERα (ERα66), they may express ERα36, an alternative estrogen receptor that is localized in the plasma membrane and is capable of directly activating mitogenic signaling (Zhang et al., 2012a; Deng et al., 2014). In luminal breast cancers, CSCs have been shown to be regulated through paracrine loops, involving estrogen-induced FGF 9 (Fillmore et al., 2010) and progesterone-induced RANKL (Schramek et al., 2010). These growth factors, produced by ER/PR expressing differentiated cells, regulate the self-renewal of CSC populations (Fillmore et al., 2010; Rajaram et al., 2015). In vitro, development of resistance to hormonal therapy is accompanied by an increase in the CSC population (Piva et al., 2014; Wang et al., 2012). The clinical relevance of these findings was evidenced in a study that demonstrated an increase in the proportion of cells expressing the CSC phenotype CD44+/CD24− following neoadjuvant hormone therapy (Creighton et al., 2009). This suggests that simultaneous targeting of CSC and ERα expressing differentiated cells might increase the clinical efficacy of hormonal therapy.
In addition to direct mitogenic effects of estrogen, luminal subtype cancer stem cells may be regulated by growth factor pathways including PI3K, mTOR and HER2 (Ithimakin et al., 2013; Korkaya et al., 2009; Zhang et al., 2012b; Zhou et al., 2007). It has been demonstrated that the combination of the mTOR inhibitor everolimus and hormonal therapy significantly delayed the time to tumor progression in ER positive breast cancer (Baselga et al., 2012). Similarly in a randomized Phase II trial, the addition of the cyclin-dependent kinase (CDK) 4/6 CDK4/6 inhibitor palbociclib to endocrine therapy resulted in a near doubling of the time to tumor progression (Finn et al., 2015). This clinical benefit has been attributed to the ability of CDK4/6 inhibitors to inhibit the downstream target of estrogen mitogenic signaling, Cyclin D. However, it has recently been reported that the CyclinD-CDK4/6 complex also plays a role in the regulation of self-renewal in breast CSCs and that palbociclib targets this cell population (Finn et al., 2009); (Lamb et al., 2013). This raises the intriguing possibility that inhibition of CSCs by targeting mTOR or CDK 4/6 may contribute to the clinical benefit of these compounds, supporting the strategy of combining CSC targeting agents with endocrine therapies that target the ER positive bulk tumor populations as illustrated in Figure 3.
HER2+ Breast Cancer
The development of HER2-targeted therapeutics for the treatment of HER2 positive breast cancer has been one of the greatest advances in breast cancer therapeutics. When used in the adjuvant setting, the HER2-targeting antibody trastuzumab dramatically reduces breast cancer recurrence (Slamon et al., 2011). In addition to trastuzumab, other HER2-targeting agents, including the monoclonal antibody pertuzumab and the immunotoxin conjugate ado-trastuzumab emtansine (TDM-1), have further improved the efficacy of HER2-targeting (Swain et al., 2013; Verma et al., 2012). The importance of HER2 signaling in breast cancer pathogenesis and treatment may relate to the observation that HER2 is an important regulator of BCSC self-renewal (Giordano et al., 2012; Korkaya et al., 2008). HER2 over expression through gene amplification increases the CSC population, whereas HER2-blocking agents such as trastuzumab reduce this cell population. Although the use of HER2-targeting agents has been limited to patients whose tumors display HER2 gene amplification, retrospective analysis of large randomized adjuvant trials have suggested that the benefit of adjuvant trastuzumab may extend to patients with tumors that did not display HER2 amplification (Paik et al., 2008). The recent demonstration of HER2 expression in CSCs, in the absence of HER2 gene amplification, may provide a biological explanation for these surprising results (Ithimakin et al., 2013). The utility of HER2 blockade in patients classified as HER2 negative is being tested in a large randomized clinical trial.
Despite the clinical efficacy of HER2-targeting agents, the vast majority of patients with metastatic HER2 amplified breast cancer eventually develop resistance to these therapies. The most frequent genetic abnormality associated with this resistance is deletion of the PTEN gene (Dave et al., 2011). It has been demonstrated that PTEN deletion in HER2 over expressing cells activates cytokine-signaling pathways including IL-6, STAT3 and NFκB (Korkaya et al., 2012). This pathway generates EMT-like CSCs. The existence of CSCs in multiple states, each dependent on alternative signal transduction pathways, may necessitate the development of strategies to target these multiple pathways. A clinical trial adding the IL-6 receptor blocking antibody tocilizumab to HER2 blocking agents is in development (Figure 3).
Triple-Negative Breast Cancer (TNBC)
TNBC is characterized by lack of expression of ER, PR, and HER2, and these tumors typically have the highest proportion of cells that express CSC markers compared to the other subtypes (Idowu et al., 2012). Recent molecular profiling of TNBC has further demonstrated the heterogeneity of this group of tumors. Unlike the other subtypes of breast cancer, there are currently no targeted therapies that improve patient outcome, and the only established treatments involve the use of cytotoxic chemotherapy. Although these therapies are clearly beneficial, patients invariably develop resistance. This resistance has been shown to be associated with an increase in CSCs. In fact, cytotoxic chemotherapy has been demonstrated to actually increase CSCs through activation of cytokine loops involving IL-6 and IL-8 (Tsavaris et al., 2002; Samanta et al., 2014). It appears that a limited repertoire of signal transduction pathways regulate CSCs of this subtype. Recently, Junankar, et al., reported that the inhibitor of differentiation-4 (ID4) is a key regulator of mammary stem cell renewal and marks a subset of TNBCs cases with a particularly poor prognosis. Interestingly, ID4 expression is unrelated to previous molecular triple-negative classifications, and was found to control mammary stem cell self-renewal acting upstream of Notch signaling to repress luminal fate commitment (Junankar et al., 2015). Other pathways that play important roles in the regulation of CSCs within this triple-negative subtype include Wnt (King et al., 2012), Notch (Al-Hussaini et al., 2011), Hedgehog (Liu et al., 2006), and IL8-CXCR1 (Rody et al., 2011). Early stage clinical trials utilizing inhibitors of these pathways to target CSCs in women with TNBC are in progress (Figure 3). The increased efficacy of such combinations may also result from the induction of cell differentiation in CSCs by the targeted agents, subsequently rendering the CSCs susceptible to chemotherapy. Recently, it has been demonstrated that defects in DNA repair in TNBC associated with BRCA1 mutation or loss of activity are susceptible to inhibition of poly-ADP-polymerase PARP (Dent et al., 2013; Shen et al., 2013). The sensitivity of BCSC in these tumors to PARP inhibition remains to be determined. Another approach to treat TNBC utilizes the targeting of tumor angiogenesis using VEGF inhibitors, such as bevacizumab. Use of this agent resulted in delayed progression time of patients with TNBC. However, post-treatment monitoring of this patient group revealed that, although treatment delayed tumor progression, once the tumors had progressed they displayed a more aggressive phenotype resulting in little, if any, difference in ultimate patient survival (Wagner Anna et al., 2012). It has recently been demonstrated that these anti-angiogenic agents stimulate CSCs through generation of tissue hypoxia, with resultant stimulation of HIF-1α, a potent regulator of breast CSCs (Conley et al., 2012). Addition of HIF-1α inhibitors were able to block this effect, causing a decrease in CSCs and increasing the efficacy the anti-angiogenic agents in preclinical models (Conley et al., 2015).
Most recently, preliminary data suggests that immunotherapy utilizing an immune checkpoint blocker against PD-L1 benefited a subset of women with TNBC (Emens et al., 2015). There is evidence that CSC may utilize multiple mechanisms to evade immune surveillance. Specific strategies for immune targeting of CSCs are in development.
Implications of CSCs for Clinical Trial Design
The existence of CSCs in the vast majority of breast cancers has important clinical implications for development strategies, as well as for the design, of clinical trials to assess treatment efficacy. As described above, pre-clinical models provide strong support for combining molecular subtype-specific agents with CSC targeting drugs. This combined approach also applies to molecular targeted therapies, the selection of which is based on molecular profiling of bulk tumor cells. It is critical to consider that, despite their genetic similarities, CSCs and the bulk tumor cells derived from them may be dependent on different signaling pathways. These differential sensitivities, presumably due to epigenetic differences in these cell populations, may have important implications for the selection of the appropriate therapeutic regimen and for selection of appropriate endpoints to access treatment efficacy. The existence of multiple CSCs in a given tumor presents an additional therapeutic challenge, which may require the combination of multiple CSC targeting drugs.
Since CSCs constitute only a minor fraction of tumor cells, classical clinical endpoints, such as tumor shrinkage as accessed by Response Evaluation Criteria for Solid Tumors (RECIST), are inadequate to access the efficacy of CSC targeting agents. The assessment of CSC markers in serial tumor biopsies may prove valuable in accessing these effects (Schott et al., 2013). Alternately, circulating tumor cells (CTC’s) have been shown to be enriched in CSCs (Aktas et al., 2009) and may provide more readily available liquid biopsies (Pantel and Alix-Panabieres, 2013). Neoadjuvant clinical trial designs, in which primary therapy is given before surgical removal of tumors, afford the opportunity to directly assess the effects of therapy on both CSC and bulk tumor populations. Indeed, such studies have demonstrated that persistence of CSCs, as assessed by expression of ALDH, is associated with increased development of subsequent metastatic disease (Alamgeer et al., 2014).
A number of CSC targeting drugs are now being tested in early stage clinical trials (Figure 3). Following the demonstration of safety, these drugs are being combined with more traditional therapies that target bulk cell populations. Ultimately, randomized clinical trials will be required to ascertain the benefit of these combined approaches for treatment of advanced, metastatic disease. Furthermore, since pre-clinical studies suggest that the benefit of CSC-targeting agents is greatest when they are deployed in the adjuvant setting, use of these agents in earlier stage disease needs to be tested. The use of rational combinations of agents to target both differentiated tumor cells and cancer stem cells holds great promise for improved therapeutics in all subtypes of breast cancer.
Acknowledgments
We would like to thank Jill Granger for her help editing the manuscript. The Wicha laboratory is supported, in part, by NIH grants R01-CA129765-06A1 and R01-CA101860-06 The Breast Cancer Research Foundation, Komen For the Cure Foundation and the Alfred Taubman Research Institute.
Footnotes
Financial conflict of interest: MSW has financial holdings and is a scientific advisor for OncoMed Pharmaceuticals, is a scientific advisor for Verastem, Paganini and MedImmune and receives research support from Dompe Pharmaceuticals, MedImmune, and Cormorant Pharmaceuticals. MLB and MDB have no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AKTAS B, TEWES M, FEHM T, HAUCH S, KIMMIG R, KASIMIR-BAUER S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Research. 2009;11:R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-HAJJ M, WICHA MS, BENITO-HERNANDEZ A, MORRISON SJ, CLARKE MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-HUSSAINI H, SUBRAMANYAM D, REEDIJK M, SRIDHAR SS. Notch signaling pathway as a therapeutic target in breast cancer. Mol Cancer Ther. 2011;10:9–15. doi: 10.1158/1535-7163.MCT-10-0677. [DOI] [PubMed] [Google Scholar]
- ALAMGEER M, GANJU V, KUMAR B, FOX J, HART S, WHITE M, HARRIS M, STUCKEY J, PRODANOVIC Z, SCHNEIDER-KOLSKY ME, WATKINS DN. Changes in aldehyde dehydrogenase-1 expression during neoadjuvant chemotherapy predict outcome in locally advanced breast cancer. Breast Cancer Res. 2014;16:R44. doi: 10.1186/bcr3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARENDT LM, KELLER PJ, SKIBINSKI A, GONCALVES K, NABER SP, BUCHSBAUM RJ, GILMORE H, COME SE, KUPERWASSER C. Anatomical localization of progenitor cells in human breast tissue reveals enrichment of uncommitted cells within immature lobules. Breast Cancer Res. 2014;16:453. doi: 10.1186/s13058-014-0453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASELGA J, CAMPONE M, PICCART M, BURRIS HA, RUGO HS, SAHMOUD T, NOGUCHI S, GNANT M, PRITCHARD KI, LEBRUN F, BECK JT, ITO Y, YARDLEY D, DELEU I, PEREZ A, BACHELOT T, VITTORI L, XU Z, MUKHOPADHYAY P, LEBWOHL D, HORTOBAGYI GN. Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer. New England Journal of Medicine. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEDARD PL, HANSEN AR, RATAIN MJ, SIU LL. Tumour heterogeneity in the clinic. Nature. 2013;501:355–64. doi: 10.1038/nature12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHOLA NE, BALKO JM, DUGGER TC, KUBA MG, SANCHEZ V, SANDERS M, STANFORD J, COOK RS, ARTEAGA CL. TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Invest. 2013;123:1348–58. doi: 10.1172/JCI65416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BICAKU E, MARCHION DC, SCHMITT ML, MUNSTER PN. Selective inhibition of histone deacetylase 2 silences progesterone receptor-mediated signaling. Cancer Res. 2008;68:1513–9. doi: 10.1158/0008-5472.CAN-07-2822. [DOI] [PubMed] [Google Scholar]
- BONNET D, DICK JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- BURRELL RA, MCGRANAHAN N, BARTEK J, SWANTON C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501:338–45. doi: 10.1038/nature12625. [DOI] [PubMed] [Google Scholar]
- CANTONE I, FISHER AG. Epigenetic programming and reprogramming during development. Nat Struct Mol Biol. 2013;20:282–9. doi: 10.1038/nsmb.2489. [DOI] [PubMed] [Google Scholar]
- CHAFFER CHRISTINEL, MARJANOVIC NEMANJAD, LEE T, BELL G, KLEER CELINAG, REINHARDT F, D’ALESSIO ANAC, YOUNG RICHARDA, WEINBERG ROBERTA. Poised Chromatin at the ZEB1 Promoter Enables Breast Cancer Cell Plasticity and Enhances Tumorigenicity. Cell. 2013;154:61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN J, LI Y, YU TS, MCKAY RM, BURNS DK, KERNIE SG, PARADA LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–6. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLINS AT, BERRY PA, HYDE C, STOWER MJ, MAITLAND NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- CONLEY SJ, BAKER TL, BURNETT JP, THEISEN RL, LAZARUS D, PETERS CG, CLOUTHIER SG, ELIASOF S, WICHA MS. CRLX101, an investigational camptothecin-containing nanoparticle-drug conjugate, targets cancer stem cells and impedes resistance to antiangiogenic therapy in mouse models of breast cancer. Breast Cancer Res Treat. 2015;150:559–67. doi: 10.1007/s10549-015-3349-8. [DOI] [PubMed] [Google Scholar]
- CONLEY SJ, GHEORDUNESCU E, KAKARALA P, NEWMAN B, KORKAYA H, HEATH AN, CLOUTHIER SG, WICHA MS. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc Natl Acad Sci U S A. 2012;109:2784–9. doi: 10.1073/pnas.1018866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREIGHTON CJ, FU X, HENNESSY BT, CASA AJ, ZHANG Y, GONZALEZ-ANGULO AM, LLUCH A, GRAY JW, BROWN PH, HILSENBECK SG, OSBORNE CK, MILLS GB, LEE AV, SCHIFF R. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010;12:R40. doi: 10.1186/bcr2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREIGHTON CJ, LI X, LANDIS M, DIXON JM, NEUMEISTER VM, SJOLUND A, RIMM DL, WONG H, RODRIGUEZ A, HERSCHKOWITZ JI, FAN C, ZHANG X, HE X, PAVLICK A, GUTIERREZ MC, RENSHAW L, LARIONOV AA, FARATIAN D, HILSENBECK SG, PEROU CM, LEWIS MT, ROSEN JM, CHANG JC. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–5. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVE B, MIGLIACCIO I, GUTIERREZ MC, WU MF, CHAMNESS GC, WONG H, NARASANNA A, CHAKRABARTY A, HILSENBECK SG, HUANG J, RIMAWI M, SCHIFF R, ARTEAGA C, OSBORNE CK, CHANG JC. Loss of Phosphatase and Tensin Homolog or Phosphoinositol-3 Kinase Activation and Response to Trastuzumab or Lapatinib in Human Epidermal Growth Factor Receptor 2–Overexpressing Locally Advanced Breast Cancers. Journal of Clinical Oncology. 2011;29:166–173. doi: 10.1200/JCO.2009.27.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENG H, ZHANG XT, WANG ML, ZHENG HY, LIU LJ, WANG ZY. ER-alpha36-mediated rapid estrogen signaling positively regulates ER-positive breast cancer stem/progenitor cells. PLoS One. 2014;9:e88034. doi: 10.1371/journal.pone.0088034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENT RA, LINDEMAN GJ, CLEMONS M, WILDIERS H, CHAN A, MCCARTHY NJ, SINGER CF, LOWE ES, WATKINS CL, CARMICHAEL J. Phase I trial of the oral PARP inhibitor olaparib in combination with paclitaxel for first- or second-line treatment of patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2013;15:R88. doi: 10.1186/bcr3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DING L, ELLIS MJ, LI S, LARSON DE, CHEN K, WALLIS JW, HARRIS CC, MCLELLAN MD, FULTON RS, FULTON LL, ABBOTT RM, HOOG J, DOOLING DJ, KOBOLDT DC, SCHMIDT H, KALICKI J, ZHANG Q, CHEN L, LIN L, WENDL MC, MCMICHAEL JF, MAGRINI VJ, COOK L, MCGRATH SD, VICKERY TL, APPELBAUM E, DESCHRYVER K, DAVIES S, GUINTOLI T, LIN L, CROWDER R, TAO Y, SNIDER JE, SMITH SM, DUKES AF, SANDERSON GE, POHL CS, DELEHAUNTY KD, FRONICK CC, PAPE KA, REED JS, ROBINSON JS, HODGES JS, SCHIERDING W, DEES ND, SHEN D, LOCKE DP, WIECHERT ME, ELDRED JM, PECK JB, OBERKFELL BJ, LOLOFIE JT, DU F, HAWKINS AE, O’LAUGHLIN MD, BERNARD KE, CUNNINGHAM M, ELLIOTT G, MASON MD, THOMPSON DM, JR, IVANOVICH JL, GOODFELLOW PJ, PEROU CM, WEINSTOCK GM, AFT R, WATSON M, LEY TJ, WILSON RK, MARDIS ER. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONTU G, ABDALLAH WM, FOLEY JM, JACKSON KW, CLARKE MF, KAWAMURA MJ, WICHA MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRIESSENS G, BECK B, CAAUWE A, SIMONS BD, BLANPAIN C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–30. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIREW P, STINGL J, RAOUF A, TURASHVILI G, APARICIO S, EMERMAN JT, EAVES CJ. A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat Med. 2008;14:1384–9. doi: 10.1038/nm.1791. [DOI] [PubMed] [Google Scholar]
- EMENS LA, BRAITEH FS, CASSIER P, DELORD J, EDER JP, FASSO M, XIAO Y, WANG Y, MOLINERO L, CHEN DS, KROP I. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer. Abstract presentation at American Association of Cancer Research Annual Meetin,; 2015. p. Abstract 2859. [Google Scholar]
- ERAMO A, LOTTI F, SETTE G, PILOZZI E, BIFFONI M, DI VIRGILIO A, CONTICELLO C, RUCO L, PESCHLE C, DE MARIA R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–14. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- FABI A, METRO G, DI BENEDETTO A, NISTICO C, VICI P, MELUCCI E, ANTONIANI B, PERRACCHIO L, SPERDUTI I, MILELLA M, COGNETTI F, MOTTOLESE M. Clinical significance of PTEN and p-Akt co-expression in HER2-positive metastatic breast cancer patients treated with trastuzumab-based therapies. Oncology. 2010;78:141–9. doi: 10.1159/000312656. [DOI] [PubMed] [Google Scholar]
- FILLMORE CM, GUPTA PB, RUDNICK JA, CABALLERO S, KELLER PJ, LANDER ES, KUPERWASSER C. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc Natl Acad Sci U S A. 2010;107:21737–42. doi: 10.1073/pnas.1007863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINN RS, CROWN JP, LANG I, BOER K, BONDARENKO IM, KULYK SO, ETTL J, PATEL R, PINTER T, SCHMIDT M, SHPARYK Y, THUMMALA AR, VOYTKO NL, FOWST C, HUANG X, KIM ST, RANDOLPH S, SLAMON DJ. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- FINN RS, DERING J, CONKLIN D, KALOUS O, COHEN DJ, DESAI AJ, GINTHER C, ATEFI M, CHEN I, FOWST C, LOS G, SLAMON DJ. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOULKES WD, STEFANSSON IM, CHAPPUIS PO, BEGIN LR, GOFFIN JR, WONG N, TRUDEL M, AKSLEN LA. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–5. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- FRIEDMANN-MORVINSKI D, BUSHONG EA, KE E, SODA Y, MARUMOTO T, SINGER O, ELLISMAN MH, VERMA IM. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science. 2012;338:1080–4. doi: 10.1126/science.1226929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINESTIER C, HUR MH, CHARAFE-JAUFFRET E, MONVILLE F, DUTCHER J, BROWN M, JACQUEMIER J, VIENS P, KLEER CG, LIU S, SCHOTT A, HAYES D, BIRNBAUM D, WICHA MS, DONTU G. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIORDANO A, GAO H, ANFOSSI S, COHEN E, MEGO M, LEE BN, TIN S, DE LAURENTIIS M, PARKER CA, ALVAREZ RH, VALERO V, UENO NT, DE PLACIDO S, MANI SA, ESTEVA FJ, CRISTOFANILLI M, REUBEN JM. Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Mol Cancer Ther. 2012;11:2526–34. doi: 10.1158/1535-7163.MCT-12-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDHIRSCH A, WOOD WC, COATES AS, GELBER RD, THÜRLIMANN B, SENN HJ, MEMBERS P. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Annals of Oncology. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUO WJ, KECKESOVA Z, DONAHER JL, SHIBUE T, TISCHLER V, REINHARDT F, ITZKOVITZ S, NOSKE A, ZURRER-HARDI U, BELL G, TAM WL, MANI SA, VAN OUDENAARDEN A, WEINBERG RA. Slug and Sox9 Cooperatively Determine the Mammary Stem Cell State. Cell. 2012;148:1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON H, SIMOES BM, ROGERSON L, HOWELL SJ, LANDBERG G, CLARKE RB. Oestrogen increases the activity of oestrogen receptor negative breast cancer stem cells through paracrine EGFR and Notch signalling. Breast Cancer Res. 2013;15:R21. doi: 10.1186/bcr3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGES-GALLAGHER L, VALENTINE CD, BADER SE, KUSHNER PJ. Inhibition of histone deacetylase enhances the anti-proliferative action of antiestrogens on breast cancer cells and blocks tamoxifen-induced proliferation of uterine cells. Breast Cancer Res Treat. 2007;105:297–309. doi: 10.1007/s10549-006-9459-6. [DOI] [PubMed] [Google Scholar]
- HOLM K, GRABAU D, LÖVGREN K, ARADOTTIR S, GRUVBERGER-SAAL S, HOWLIN J, SAAL LH, ETHIER SP, BENDAHL PO, STÅL O, MALMSTRÖM P, FERNÖ M, RYDÉN L, HEGARDT C, BORG Å, RINGNÉR M. Global H3K27 trimethylation and EZH2 abundance in breast tumor subtypes. Molecular Oncology. 2012;6:494–506. doi: 10.1016/j.molonc.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONETH G, SCHIAVINOTTO T, VAGGI F, MARLOW R, KANNO T, SHINOMIYA I, LOMBARDI S, BUCHUPALLI B, GRAHAM R, GAZINSKA P, RAMALINGAM V, BURCHELL J, PURUSHOTHAM AD, PINDER SE, CSIKASZNAGY A, DONTU G. Models of breast morphogenesis based on localization of stem cells in the developing mammary lobule. Stem Cell Reports. 2015;4:699–711. doi: 10.1016/j.stemcr.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORWITZ KB, DYE WW, HARRELL JC, KABOS P, SARTORIUS CA. Rare steroid receptor-negative basal-like tumorigenic cells in luminal subtype human breast cancer xenografts. Proc Natl Acad Sci U S A. 2008;105:5774–9. doi: 10.1073/pnas.0706216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDOWU MO, KMIECIAK M, DUMUR C, BURTON RS, GRIMES MM, POWERS CN, MANJILI MH. CD44(+)/CD24(−/low) cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum Pathol. 2012;43:364–73. doi: 10.1016/j.humpath.2011.05.005. [DOI] [PubMed] [Google Scholar]
- ILIOPOULOS D, HIRSCH HA, STRUHL K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITHIMAKIN S, DAY KC, MALIK F, ZEN Q, DAWSEY SJ, BERSANO-BEGEY TF, QURAISHI AA, IGNATOSKI KW, DAIGNAULT S, DAVIS A, HALL CL, PALANISAMY N, HEATH AN, TAWAKKOL N, LUTHER TK, CLOUTHIER SG, CHADWICK WA, DAY ML, KLEER CG, THOMAS DG, HAYES DF, KORKAYA H, WICHA MS. HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab. Cancer Res. 2013;73:1635–46. doi: 10.1158/0008-5472.CAN-12-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSEN MPHM, KNIJNENBURG T, REIJM EA, SIMON I, KERKHOVEN R, DROOG M, VELDS A, VAN LAERE S, DIRIX L, ALEXI X, FOEKENS JA, WESSELS L, LINN SC, BERNS EMJJ, ZWART W. Hallmarks of Aromatase Inhibitor Drug Resistance Revealed by Epigenetic Profiling in Breast Cancer. Cancer Research. 2013;73:6632–6641. doi: 10.1158/0008-5472.CAN-13-0704. [DOI] [PubMed] [Google Scholar]
- JUNANKAR S, BAKER LA, RODEN DL, NAIR R, ELSWORTH B, GALLEGO-ORTEGA D, LACAZE P, CAZET A, NIKOLIC I, TEO WS, YANG J, MCFARLAND A, HARVEY K, NAYLOR MJ, LAKHANI SR, SIMPSON PT, RAGHAVENDRA A, SAUNUS J, MADORE J, KAPLAN W, ORMANDY C, MILLAR EKA, O’TOOLE S, YUN K, SWARBRICK A. ID4 controls mammary stem cells and marks breast cancers with a stem cell-like phenotype. Nat Commun. 2015:6. doi: 10.1038/ncomms7548. [DOI] [PubMed] [Google Scholar]
- KANDOTH C, MCLELLAN MD, VANDIN F, YE K, NIU B, LU C, XIE M, ZHANG Q, MCMICHAEL JF, WYCZALKOWSKI MA, LEISERSON MD, MILLER CA, WELCH JS, WALTER MJ, WENDL MC, LEY TJ, WILSON RK, RAPHAEL BJ, DING L. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–9. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING TD, SUTO MJ, LI Y. The Wnt/beta-catenin signaling pathway: a potential therapeutic target in the treatment of triple negative breast cancer. J Cell Biochem. 2012;113:13–8. doi: 10.1002/jcb.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEER CG, CAO Q, VARAMBALLY S, SHEN R, OTA I, TOMLINS SA, GHOSH D, SEWALT RGAB, OTTE AP, HAYES DF, SABEL MS, LIVANT D, WEISS SJ, RUBIN MA, CHINNAIYAN AM. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proceedings of the National Academy of Sciences. 2003;100:11606–11611. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOREN S, REAVIE L, DO COUTO JP, DE SILVA D, STADLER MB, ROLOFF T, BRITSCHGI A, EICHLISBERGER T, KOHLER H, AINA O, CARDIFF RD, BENTIRES-ALJ M. PIK3CA induces multipotency and multi-lineage mammary tumours. Nature. 2015 doi: 10.1038/nature14669. [DOI] [PubMed] [Google Scholar]
- KORKAYA H, KIM GI, DAVIS A, MALIK F, HENRY NL, ITHIMAKIN S, QURAISHI AA, TAWAKKOL N, D’ANGELO R, PAULSON AK, CHUNG S, LUTHER T, PAHOLAK HJ, LIU S, HASSAN KA, ZEN Q, CLOUTHIER SG, WICHA MS. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell. 2012;47:570–84. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORKAYA H, PAULSON A, CHARAFE-JAUFFRET E, GINESTIER C, BROWN M, DUTCHER J, CLOUTHIER SG, WICHA MS. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORKAYA H, PAULSON A, IOVINO F, WICHA MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–30. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORKAYA H, WICHA MS. HER2 and breast cancer stem cells: more than meets the eye. Cancer Res. 2013;73:3489–93. doi: 10.1158/0008-5472.CAN-13-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUPERWASSER C, CHAVARRIA T, WU M, MAGRANE G, GRAY JW, CAREY L, RICHARDSON A, WEINBERG RA. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101:4966–71. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMB R, LEHN S, ROGERSON L, CLARKE RB, LANDBERG G. Cell cycle regulators cyclin D1 and CDK4/6 have estrogen receptor-dependent divergent functions in breast cancer migration and stem cell-like activity. Cell Cycle. 2013;12:2384–94. doi: 10.4161/cc.25403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDER K, HOLLAND EC, MICHOR F. The therapeutic implications of plasticity of the cancer stem cell phenotype. PLoS One. 2010;5:e14366. doi: 10.1371/journal.pone.0014366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C, HEIDT DG, DALERBA P, BURANT CF, ZHANG L, ADSAY V, WICHA M, CLARKE MF, SIMEONE DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- LI X, LEWIS MT, HUANG J, GUTIERREZ C, OSBORNE CK, WU MF, HILSENBECK SG, PAVLICK A, ZHANG X, CHAMNESS GC, WONG H, ROSEN J, CHANG JC. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- LIM E, VAILLANT F, WU D, FORREST NC, PAL B, HART AH, ASSELIN-LABAT ML, GYORKI DE, WARD T, PARTANEN A, FELEPPA F, HUSCHTSCHA LI, THORNE HJ, KCONFAB FOX SB, YAN M, FRENCH JD, BROWN MA, SMYTH GK, VISVADER JE, LINDEMAN GJ. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–13. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- LIU S, CONG Y, WANG D, SUN Y, DENG L, LIU Y, MARTIN-TREVINO R, SHANG L, MCDERMOTT SP, LANDIS MD, HONG S, ADAMS A, D’ANGELO R, GINESTIER C, CHARAFE-JAUFFRET E, CLOUTHIER SG, BIRNBAUM D, WONG ST, ZHAN M, CHANG JC, WICHA MS. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports. 2014;2:78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU S, DONTU G, MANTLE ID, PATEL S, AHN NS, JACKSON KW, SURI P, WICHA MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–71. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUU TH, MORGAN RJ, LEONG L, LIM D, MCNAMARA M, PORTNOW J, FRANKEL P, SMITH DD, DOROSHOW JH, WONG C, APARICIO A, GANDARA DR, SOMLO G. A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: a California Cancer Consortium study. Clin Cancer Res. 2008;14:7138–42. doi: 10.1158/1078-0432.CCR-08-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGNANI L, STOECK A, ZHANG X, LÁNCZKY A, MIRABELLA AC, WANG TL, GYORFFY B, LUPIEN M. Genome-wide reprogramming of the chromatin landscape underlies endocrine therapy resistance in breast cancer. Proceedings of the National Academy of Sciences. 2013;110:E1490–E1499. doi: 10.1073/pnas.1219992110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANI SA, GUO W, LIAO MJ, EATON EN, AYYANAN A, ZHOU AY, BROOKS M, REINHARD F, ZHANG CC, SHIPITSIN M, CAMPBELL LL, POLYAK K, BRISKEN C, YANG J, WEINBERG RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANIOTIS AJ, FOLBERG R, HESS A, SEFTOR EA, GARDNER LM, PE’ER J, TRENT JM, MELTZER PS, HENDRIX MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–52. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARUSYK A, TABASSUM DP, ALTROCK PM, ALMENDRO V, MICHOR F, POLYAK K. Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity. Nature. 2014;514:54–8. doi: 10.1038/nature13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUGERI-SACCA M, BARTUCCI M, DE MARIA R. DNA damage repair pathways in cancer stem cells. Mol Cancer Ther. 2012;11:1627–36. doi: 10.1158/1535-7163.MCT-11-1040. [DOI] [PubMed] [Google Scholar]
- MCGRANAHAN N, FAVERO F, DE BRUIN EC, BIRKBAK NJ, SZALLASI Z, SWANTON C. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci Transl Med. 2015;7:283ra54. doi: 10.1126/scitranslmed.aaa1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLYNEUX G, GEYER FC, MAGNAY FA, MCCARTHY A, KENDRICK H, NATRAJAN R, MACKAY A, GRIGORIADIS A, TUTT A, ASHWORTH A, REIS-FILHO JS, SMALLEY MJ. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell. 2010;7:403–17. doi: 10.1016/j.stem.2010.07.010. [DOI] [PubMed] [Google Scholar]
- MUNSTER PN, THURN KT, THOMAS S, RAHA P, LACEVIC M, MILLER A, MELISKO M, ISMAIL-KHAN R, RUGO H, MOASSER M, MINTON SE. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br J Cancer. 2011;104:1828–1835. doi: 10.1038/bjc.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’BRIEN CA, POLLETT A, GALLINGER S, DICK JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- OHM JE, MCGARVEY KM, YU X, CHENG L, SCHUEBEL KE, COPE L, MOHAMMAD HP, CHEN W, DANIEL VC, YU W, BERMAN DM, JENUWEIN T, PRUITT K, SHARKIS SJ, WATKINS DN, HERMAN JG, BAYLIN SB. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet. 2007;39:237–242. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSKARSSON T, BATLLE E, MASSAGUE J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell. 2014;14:306–21. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAIK S, KIM C, WOLMARK N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358:1409–11. doi: 10.1056/NEJMc0801440. [DOI] [PubMed] [Google Scholar]
- PAL B, CHEN Y, BERT A, HU Y, SHERIDAN JM, BECK T, SHI W, SATTERLEY K, JAMIESON P, GOODALL GJ, LINDEMAN GJ, SMYTH GK, VISVADER JE. Integration of microRNA signatures of distinct mammary epithelial cell types with their gene expression and epigenetic portraits. Breast Cancer Res. 2015;17:85. doi: 10.1186/s13058-015-0585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAN Q, LI Q, LIU S, NING N, ZHANG X, XU Y, CHANG AE, WICHA MS. Concise Review: Targeting Cancer Stem Cells Using Immunologic Approaches. Stem Cells. 2015;33:2085–92. doi: 10.1002/stem.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANTEL K, ALIX-PANABIÈRES C. Real-time Liquid Biopsy in Cancer Patients: Fact or Fiction? Cancer Research. 2013;73:6384–6388. doi: 10.1158/0008-5472.CAN-13-2030. [DOI] [PubMed] [Google Scholar]
- PARK YH, JUNG HA, CHOI MK, CHANG W, CHOI YL, DO IG, AHN JS, IM YH. Role of HER3 expression and PTEN loss in patients with HER2-overexpressing metastatic breast cancer (MBC) who received taxane plus trastuzumab treatment. Br J Cancer. 2014;110:384–391. doi: 10.1038/bjc.2013.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEROU CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2011;16(Suppl 1):61–70. doi: 10.1634/theoncologist.2011-S1-61. [DOI] [PubMed] [Google Scholar]
- PEROU CM, SORLIE T, EISEN MB, VAN DE RIJN M, JEFFREY SS, REES CA, POLLACK JR, ROSS DT, JOHNSEN H, AKSLEN LA, FLUGE O, PERGAMENSCHIKOV A, WILLIAMS C, ZHU SX, LONNING PE, BORRESEN-DALE AL, BROWN PO, BOTSTEIN D. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- PHILLIPS TM, MCBRIDE WH, PAJONK F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–85. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- PIVA M, DOMENICI G, IRIONDO O, RABANO M, SIMOES BM, COMAILLS V, BARREDO I, LOPEZ-RUIZ JA, ZABALZA I, KYPTA R, VIVANCO M. Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO Mol Med. 2014;6:66–79. doi: 10.1002/emmm.201303411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUINTANA E, SHACKLETON M, SABEL MS, FULLEN DR, JOHNSON TM, MORRISON SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAJARAM RD, BURIC D, CAIKOVSKI M, AYYANAN A, ROUGEMONT J, SHAN J, VAINIO SJ, YALCIN-OZUYSAL O, BRISKEN C. Progesterone and Wnt4 control mammary stem cells via myoepithelial crosstalk. EMBO J. 2015;34:641–52. doi: 10.15252/embj.201490434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICCI-VITIANI L, LOMBARDI DG, PILOZZI E, BIFFONI M, TODARO M, PESCHLE C, DE MARIA R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- RICCI-VITIANI L, PALLINI R, BIFFONI M, TODARO M, INVERNICI G, CENCI T, MAIRA G, PARATI EA, STASSI G, LAROCCA LM, DE MARIA R. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–8. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- RIOS AC, FU NY, LINDEMAN GJ, VISVADER JE. In situ identification of bipotent stem cells in the mammary gland. Nature. 2014;506:322–7. doi: 10.1038/nature12948. [DOI] [PubMed] [Google Scholar]
- RODY A, KARN T, LIEDTKE C, PUSZTAI L, RUCKHAEBERLE E, HANKER L, GAETJE R, SOLBACH C, AHR A, METZLER D, SCHMIDT M, MULLER V, HOLTRICH U, KAUFMANN M. A clinically relevant gene signature in triple negative and basal-like breast cancer. Breast Cancer Res. 2011;13:R97. doi: 10.1186/bcr3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUITERKAMP J, ERNST MF, DE MUNCK L, VAN DER HEIDEN-VAN DER LOO M, BASTIAANNET E, VAN DE POLLFRANSE LV, BOSSCHA K, TJAN-HEIJNEN VC, VOOGD AC. Improved survival of patients with primary distant metastatic breast cancer in the period of 1995–2008. A nationwide population-based study in the Netherlands. Breast Cancer Res Treat. 2011;128:495–503. doi: 10.1007/s10549-011-1349-x. [DOI] [PubMed] [Google Scholar]
- SAMANTA D, GILKES DM, CHATURVEDI P, XIANG L, SEMENZA GL. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc Natl Acad Sci U S A. 2014;111:E5429–38. doi: 10.1073/pnas.1421438111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- SCHEPERS AG, SNIPPERT HJ, STANGE DE, VAN DEN BORN M, VAN ES JH, VAN DE WETERING M, CLEVERS H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–5. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- SCHOTT AF, LANDIS MD, DONTU G, GRIFFITH KA, LAYMAN RM, KROP I, PASKETT LA, WONG H, DOBROLECKI LE, LEWIS MT, FROEHLICH AM, PARANILAM J, HAYES DF, WICHA MS, CHANG JC. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin Cancer Res. 2013;19:1512–24. doi: 10.1158/1078-0432.CCR-11-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRAMEK D, LEIBBRANDT A, SIGL V, KENNER L, POSPISILIK JA, LEE HJ, HANADA R, JOSHI PA, ALIPRANTIS A, GLIMCHER L, PASPARAKIS M, KHOKHA R, ORMANDY CJ, WIDSCHWENDTER M, SCHETT G, PENNINGER JM. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468:98–102. doi: 10.1038/nature09387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWITALLA S, FINGERLE AA, CAMMARERI P, NEBELSIEK T, GOKTUNA SI, ZIEGLER PK, CANLI O, HEIJMANS J, HUELS DJ, MOREAUX G, RUPEC RA, GERHARD M, SCHMID R, BARKER N, CLEVERS H, LANG R, NEUMANN J, KIRCHNER T, TAKETO MM, VAN DEN BRINK GR, SANSOM OJ, ARKAN MC, GRETEN FR. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- SHACKLETON M, VAILLANT F, SIMPSON KJ, STINGL J, SMYTH GK, ASSELIN-LABAT ML, WU L, LINDEMAN GJ, VISVADER JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–8. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- SHEHATA M, TESCHENDORFF A, SHARP G, NOVCIC N, RUSSELL IA, AVRIL S, PRATER M, EIREW P, CALDAS C, WATSON CJ, STINGL J. Phenotypic and functional characterisation of the luminal cell hierarchy of the mammary gland. Breast Cancer Res. 2012;14:R134. doi: 10.1186/bcr3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN Y, REHMAN FL, FENG Y, BOSHUIZEN J, BAJRAMI I, ELLIOTT R, WANG B, LORD CJ, POST LE, ASHWORTH A. BMN 673, a Novel and Highly Potent PARP1/2 Inhibitor for the Treatment of Human Cancers with DNA Repair Deficiency. Clinical Cancer Research. 2013;19:5003–5015. doi: 10.1158/1078-0432.CCR-13-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH SK, HAWKINS C, CLARKE ID, SQUIRE JA, BAYANI J, HIDE T, HENKELMAN RM, CUSIMANO MD, DIRKS PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- SLAMON D, EIERMANN W, ROBERT N, PIENKOWSKI T, MARTIN M, PRESS M, MACKEY J, GLASPY J, CHAN A, PAWLICKI M, PINTER T, VALERO V, LIU MC, SAUTER G, VON MINCKWITZ G, VISCO F, BEE V, BUYSE M, BENDAHMANE B, TABAH-FISCH I, LINDSAY MA, RIVA A, CROWN J. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. New England Journal of Medicine. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLEEMAN KE, KENDRICK H, ASHWORTH A, ISACKE CM, SMALLEY MJ. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res. 2006;8:R7. doi: 10.1186/bcr1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SODA Y, MARUMOTO T, FRIEDMANN-MORVINSKI D, SODA M, LIU F, MICHIUE H, PASTORINO S, YANG M, HOFFMAN RM, KESARI S, VERMA IM. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci U S A. 2011;108:4274–80. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SORLIE T, PEROU CM, TIBSHIRANI R, AAS T, GEISLER S, JOHNSEN H, HASTIE T, EISEN MB, VAN DE RIJN M, JEFFREY SS, THORSEN T, QUIST H, MATESE JC, BROWN PO, BOTSTEIN D, LONNING PE, BORRESEN-DALE AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANKIC M, PAVLOVIC S, CHIN Y, BROGI E, PADUA D, NORTON L, MASSAGUE J, BENEZRA R. TGF-beta-Id1 signaling opposes Twist1 and promotes metastatic colonization via a mesenchymal-to-epithelial transition. Cell Rep. 2013;5:1228–42. doi: 10.1016/j.celrep.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STINGL J, EIREW P, RICKETSON I, SHACKLETON M, VAILLANT F, CHOI D, LI HI, EAVES CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–7. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- SWAIN SM, KIM SB, CORTES J, RO J, SEMIGLAZOV V, CAMPONE M, CIRUELOS E, FERRERO JM, SCHNEEWEISS A, KNOTT A, CLARK E, ROSS G, BENYUNES MC, BASELGA J. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–71. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYLVESTER PW. Targeting met mediated epithelial-mesenchymal transition in the treatment of breast cancer. Clinical and Translational Medicine. 2014;3:30. doi: 10.1186/s40169-014-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS S, THURN KT, BICAKU E, MARCHION DC, MUNSTER PN. Addition of a histone deacetylase inhibitor redirects tamoxifen-treated breast cancer cells into apoptosis, which is opposed by the induction of autophagy. Breast Cancer Res Treat. 2011;130:437–47. doi: 10.1007/s10549-011-1364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSAVARIS N, KOSMAS C, VADIAKA M, KANELOPOULOS P, BOULAMATSIS D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87:21–7. doi: 10.1038/sj.bjc.6600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN KEYMEULEN A, LEE MY, OUSSET M, BROHEE S, RORIVE S, GIRADDI RR, WUIDART A, BOUVENCOURT G, DUBOIS C, SALMON I, SOTIRIOU C, PHILLIPS WA, BLANPAIN C. Reactivation of multipotency by oncogenic PIK3CA induces breast tumour heterogeneity. Nature. 2015 doi: 10.1038/nature14665. [DOI] [PubMed] [Google Scholar]
- VAN KEYMEULEN A, ROCHA AS, OUSSET M, BECK B, BOUVENCOURT G, ROCK J, SHARMA N, DEKONINCK S, BLANPAIN C. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–93. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- VERMA S, MILES D, GIANNI L, KROP IE, WELSLAU M, BASELGA J, PEGRAM M, OH DY, DIERAS V, GUARDINO E, FANG L, LU MW, OLSEN S, BLACKWELL K. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIGNOT S, FRAMPTON GM, SORIA JC, YELENSKY R, COMMO F, BRAMBILLA C, PALMER G, MORO-SIBILOT D, ROSS JS, CRONIN MT, ANDRE F, STEPHENS PJ, LAZAR V, MILLER VA, BRAMBILLA E. Next-generation sequencing reveals high concordance of recurrent somatic alterations between primary tumor and metastases from patients with non-small-cell lung cancer. J Clin Oncol. 2013;31:2167–72. doi: 10.1200/JCO.2012.47.7737. [DOI] [PubMed] [Google Scholar]
- VISVADER JE, LINDEMAN GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- WAGENBLAST E, SOTO M, GUTIERREZ-ANGEL S, HARTL CA, GABLE AL, MACELI AR, ERARD N, WILLIAMS AM, KIM SY, DICKOPF S, HARRELL JC, SMITH AD, PEROU CM, WILKINSON JE, HANNON GJ, KNOTT SR. A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature. 2015;520:358–62. doi: 10.1038/nature14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER ANNA D, THOMSSEN C, HAERTING J, UNVERZAGT S. Vascular-endothelial-growth-factor (VEGF) targeting therapies for endocrine refractory or resistant metastatic breast cancer. Cochrane Database of Systematic Reviews. 2012 doi: 10.1002/14651858.CD008941.pub2. [Online]. Available: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD008941.pub2/abstract. [DOI] [PMC free article] [PubMed]
- WANG X, WANG G, ZHAO Y, LIU X, DING Q, SHI J, DING Y, WANG S. STAT3 mediates resistance of CD44(+)CD24(−/low) breast cancer stem cells to tamoxifen in vitro. Journal of Biomedical Research. 2012;26:325–335. doi: 10.7555/JBR.26.20110050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAO D, DAI C, PENG S. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res. 2011;9:1608–20. doi: 10.1158/1541-7786.MCR-10-0568. [DOI] [PubMed] [Google Scholar]
- ZHANG M, TSIMELZON A, CHANG CH, FAN C, WOLFF A, PEROU CM, HILSENBECK SG, ROSEN JM. Intratumoral heterogeneity in a Trp53-null mouse model of human breast cancer. Cancer Discov. 2015;5:520–33. doi: 10.1158/2159-8290.CD-14-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG S, BALCH C, CHAN MW, LAI HC, MATEI D, SCHILDER JM, YAN PS, HUANG TH, NEPHEW KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG X, DING L, KANG L, WANG ZY. Estrogen Receptor-Alpha 36 Mediates Mitogenic Antiestrogen Signaling in ER-Negative Breast Cancer Cells. PLoS ONE. 2012a;7:e30174. doi: 10.1371/journal.pone.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG X, ZHANG S, LIU Y, LIU J, MA Y, ZHU Y, ZHANG J. Effects of the combination of RAD001 and docetaxel on breast cancer stem cells. European Journal of Cancer. 2012b;48:1581–1592. doi: 10.1016/j.ejca.2012.02.053. [DOI] [PubMed] [Google Scholar]
- ZHOU J, WULFKUHLE J, ZHANG H, GU P, YANG Y, DENG J, MARGOLICK JB, LIOTTA LA, PETRICOIN E, 3RD, ZHANG Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A. 2007;104:16158–63. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]