Abstract
Background: An ongoing epidemic of thyroid carcinoma (TC) has affected Americans since 1975. Understanding the contribution of subclinical disease and the mechanism of such disease detection may help to alter the course of this epidemic.
Methods: We used Rochester Epidemiology Project resources to examine the incidence of TC cases, disease specific mortality, and method of diagnosis during 1935 through 2012. During 2000–2012, we also extracted the mechanism of detection of clinically occult tumors.
Results: The age-adjusted incidence (AAI) for TC increased from 7.1 [95% confidence interval (CI) 5.5–8.8] per 100,000 person-years (p-y) during 1990–1999 to 13.7 [CI 11.8–15.6] per 100,000 p-y during 2000–2012, with no change in disease-specific mortality since 1935. The incidence trend analysis stratified by the mechanism of detection revealed the AAI of clinically recognized TC was 5.5 per 100,000 p-y [CI 3.4–7.5] in 1960–1969, a rate similar to the incidence seen during 2000–2012. However, AAI of clinically occult TC increased from 0.2 per 100,000 p-y [CI 0.0–0.6] in 1935–1949 to 1.9 per 100,000 p-y [CI 1.2–2.9] in 1990–1999 and to 7.4 per 100,000 p-y [CI 6.0–8.8] in 2000–2012. During 2000–2012, the most frequent reasons for recognition of “occult” tumors were (1) incidental discovery during diagnostic neck imaging in 40 (19%), (2) pathology review of specimens from thyroid surgery for benign conditions in 29 (14%), and (3) investigations of patients with symptoms or palpable nodules that were clearly not associated with coexistent but occult TC but triggered the use of diagnostic neck imaging in 37 (27%).
Conclusions: In this population-based study conducted in Olmsted County, Minnesota, the rapid increased incidence of TC during 2000–2012 can be completely attributed to the increased diagnosis of occult TCs, which are mainly found through the use of diagnostic neck imaging. The incidence of clinical TC and disease-specific TC mortality remains stable since 1970, implying that the observed increased incidence is due to the increased detection of subclinical lesions.
Introduction
There is currently an epidemic of thyroid cancer (TC) in the United States. The Surveillance, Epidemiology, and End Results (SEER) program data suggests that the incidence of TC increased from 4.9 in 1975, to 14.3 cases per 100,000 individuals in 2009 (1). Currently, TC has the most rapid increase in incidence of any cancer, such that it has been estimated that by 2019 TC will become the third most common cancer in women (2). This surge of TC cases has been especially pronounced over the last decade, leading to a substantial impact on the cost of care. In 2013, the aggregate national cost of care for well-differentiated TC in the United States was $1.6 billion, and by 2030 it will likely exceed $3.55 billion (3). This economic burden also impacts individuals, since patients with TC are 2.5-fold more likely to file for bankruptcy than those without cancer, a rate that is higher than seen for any other malignancy (4).
The rapid change of TC incidence has been attributed to the detection of small lesions. Many of these small lesions are found in asymptomatic patients who may not receive significant benefit from thyroid surgery and adjuvant therapies (5). Brito et al. (6) have suggested that the main mechanism of detection for these lesions is the increasing use of imaging technology, but likely it is not the only one. For instance, a retrospective study found that about 47% of incidental TC lesions were found in the course of histologic review of thyroid glands removed for apparently benign conditions (7).
To the extent that clinically “silent” lesions may contribute significantly to the increased incidence of TC, one approach to a better understanding of the rapidly increasing incidence of TC is to identify the mechanism of detection of these lesions and to design interventions to manage them. To date, administrative datasets, such as SEER, have contributed to our understanding of TC incidence trends. However, these data sources cannot provide information regarding how patients were diagnosed. This limitation can be overcome using population-based clinical datasets.
The Rochester Epidemiology Project (REP) medical records linkage system was utilized to study disease epidemiology and patterns of health care among the residents of Olmsted County, Minnesota (8). The data set includes patients from all medical care facilities used by residents of Rochester, Minnesota (estimated population of 164,129 in 2012) and encompasses the Mayo Clinic, the Olmsted Medical Center and individual private practices across Olmsted County.
The REP maintains a database of all of the TC cases diagnosed from 1935 to 1999. Based on this database, several analyses of the incidence trends in TC cases during this period of time have been published elsewhere (9,10). This database includes several variables including which TC cases were found clinically with physical exam. Here we present an update to this database to include mortality data and in addition used the REP to identify all cases of TC from 2000 to 2012 and explore the mechanism of diagnosis of TC during this period.
Methods
Patient identification and data collection
Our study was approved by the institutional review boards at the Mayo Clinic and Olmsted Medical Center. The REP links and archives the medical records of virtually all persons residing in Olmsted County, Minnesota. The linkage process primarily involves matching records by using computer algorithms and is augmented by routine manual verification of questionable matches (11). The linkage method has been validated showing high sensitivity and excellent specificity (12).
From this comprehensive medical records system from all health care providers in the county, and following the methodology used to identify TC cases from 1935–1999 (9), an experienced retrieval specialist created a cohort of 566 cases by using the Rochester Medical Index database and the World Health Organization's International Classification of Diseases, ninth edition, billing codes for thyroid malignancy. However, this list also included many cases of neck lymphomas and other neck malignancies (reflecting the high sensitivity of the approach). Thus, from these 566 cases, we searched for pathology reports to confirm the presence of one of the following diagnoses: papillary, follicular, Hürthle cell, medullary, anaplastic TC or TC not otherwise specified. This left 213 confirmed cases of TC. Two reviewers independently extracted information for the first 10 charts. This information was then compared for accuracy. Any discrepancy was resolved by discussion and further review of medical records. Then, another 50 charts were reviewed independently. Chance-adjusted inter-rater agreement for this stage was high (kappa statistic=0.81), and discrepancies were resolved by discussion. Reviewers then proceeded with abstraction of the rest of the charts independently. At the end of the abstraction, several charts were randomly chosen to verify accuracy.
From each chart we abstracted the date of initial diagnosis of TC, demographic data (including residency based on zip codes), histological type of the tumor, the staging of the tumor, the triggers of TC diagnosis, and which patients may have died because of TC (disease-specific mortality).
Mechanism of detection
For the time period 1935–1999, we extracted from the available database the cases that were palpable or symptomatic at presentation and labeled clinically recognized. Cases that were not palpable or symptomatic at presentation were labeled as clinically occult. In order to confirm the accuracy of this extraction, we reviewed the records of 20 patients from the period 1990–1999 and confirmed the accuracy of extraction. For the period 2000–2012, the majority of records are available electronically, including results of imaging studies and pathology reports. This allowed us to explore the mechanism of detection. For this period, the triggers of TC diagnosis were classified following a prespecified framework (Table 1). For screening cases, we further determined whether the nodule palpated by the clinician corresponded with the nodule harboring cancer or if the nodule harboring cancer was found as part of imaging studies triggered by a palpated nodule; for instance, a nodule harboring cancer found in the contralateral lobe of the palpated nodule was deemed not associated with a palpated nodule and thus classified under the category of incidental imaging. When TC was diagnosed incidentally by imaging, we recorded the modality of imaging used. Similarly, we defined the indication for thyroid surgery for incidental cases found histologically. For patients in whom the work-up was initiated for symptoms, we determined whether or not the symptoms were likely related to the malignant nodule. For instance, patients were classified as “unlikely” if the symptom was difficulty swallowing but the nodule harboring cancer was less than 1 cm in maximum diameter. Finally, for the “unrelated test” category, we recorded the condition that initiated the workup. A useful framework to describe the triggers of diagnosis has been described previously (13). However, we do not use this framework because we have used different definitions for symptomatic nodules and for the evaluation of tests unrelated to symptomatic nodules and use a category of “incidental histological” not included in this other framework.
Table 1.
Definitions for the Methods of Detection
| Methods of detection before thyroid surgery | |
| Screening | When a thyroid nodule harboring thyroid cancer is found during a physical exam (thyroid palpation) of an asymptomatic patient. |
| Symptomatic nodule | When a thyroid nodule harboring thyroid cancer is found during a physical exam (thyroid palpation) or imaging study in a symptomatic patient. |
| Unrelated tests | When a thyroid nodule harboring thyroid cancer is found during the work up of non-nodular thyroid disease (e.g., patient with hyperthyroidism who has a thyroid ultrasound positive for a nodule). |
| Incidental imaging | When a thyroid nodule harboring thyroid cancer is found during an imaging test requested for reasons unrelated to a thyroid disorder or symptom. This category, however, also includes patients who had an imaging test for possible palpable or symptomatic nodule but the nodule harboring thyroid cancer is not related to any of these symptoms. |
| Method of detection after thyroid surgery | |
| Incidental histological | Thyroid cancer is found incidentally in the histological examination of the thyroid gland removed for a benign condition (e.g., Graves disease). |
To maintain concordance with the wording and classification of the mechanism of detection already used in the period 1935–1999, TC lesions that did not cause any symptoms or were clinically silent were considered “occult” cases, and the remaining cases were considered “clinically recognized.”
Mortality
To assess mortality and cause of death for all TC cases (1935–2012), we used the Rochester Epidemiology Death Data System. This death data source includes, but is not limited to, the State of Minnesota Electronic Death Certificates, Olmsted County Electronic Death Certificates, and National Death Index. Additionally, each chart was reviewed to search for the death certificate and cause of death.
Analytic plan
We summarized continuous variables using means and standard deviations and categorical variables using percentages. We used two-sample t-tests and Pearson's chi-squared tests to test hypotheses of differences between groups in continuous and categorical variables, respectively. For incidence analysis of TC and disease-specific mortality, age- and sex-adjusted incidence rates were based on direct standardization against the 1990 data for the 1935–1999 cohort and against the 2010 U.S. white population for the 2000–2012 cohort, with the corresponding denominators derived from annual census figures for Olmsted County, assuming that the entire population was at risk (12). We estimated 95% confidence intervals [CIs] assuming the Poisson distribution for number of cases.
Results
From 1935 to 2012, 476 cases of TC were diagnosed in Olmsted County, Minnesota. The mean age at diagnosis was 46.3 years (standard deviation [SD] 16.6 years); 72% were female, 86% were papillary thyroid cancers (PTC) and the mean tumor size was 1.88 cm (SD 1.5 cm). The mean MACIS (metastasis, age, completeness, invasion, and size) score was 4.78 (SD 1.8) with 86% of the TCs having MACIS score less than 6. Finally, the most frequent type of thyroid surgery for TCs was total/near total thyroidectomy, 53% (Table 2).
Table 2.
Demographic Variables and Comparison Between Two Time Periods
| Entire cohort 1935–2012(n=476) | 1935–1999(n=263) | 2000–2012(n=213) | p Value | |
|---|---|---|---|---|
| Age at diagnosis in years, mean (SD) | 46.2 (16.6) | 46 (17.8) | 46.3 (14.9) | 0.84 |
| Female, n (%) | 342 (72) | 193 (73) | 149 (79) | 0.04 |
| Size of tumor, mean (SD) | 1.88 (1.5) | 1.98 (1.6) | 1.76 (1.2) | 0.09 |
| MACIS score, mean (SD)* | 4.78 (1.6) | 4.6 (1.5) | 4.7 (1.3) | 0.44 |
| MACIS score<6, n (%)* | 398 (85) | 184 (86) | 175 (88) | 0.38 |
| Papillary, n (%) | 413 (86) | 216 (82) | 199 (93) | <0.01 |
| Follicular, n (%) | 21 (4) | 15 (6) | 6 (3) | 0.48 |
| Hürthle, n (%) | 20 (4) | 19 (7) | 1 (0.5) | – |
| Medullary, n (%) | 12 (3) | 8 (2) | 4 (2) | – |
| Metastatic, n (%) | 1 (0) | 0 (0) | 1 (0.5) | – |
| Anaplastic, n (%) | 6 (1) | 6 (2) | 0 (0) | – |
| Lymphoma, n (%) | 2 (0) | 0 (0) | 2 (1) | – |
| Total/near total thyroidectomy, n (%) | 236 (50) | 60 (23) | 176 (83) | <0.01 |
| Hemithyroidectomy, n (%) | 176 (37) | 152 (56) | 24 (11) | <0.01 |
| Subtotal thyroidectomy, n (%) | 30 (6) | 21 (8) | 9 (4) | 0.41 |
| Other, n (%) | 4 (1) | 3 (1) | 2 (1) | – |
| Not able to assess, n (%) | 30 (6) | 30 (12) | 0 (0) | – |
Only cases of papillary thyroid cancer were used for this analysis.
MACIS, metastasis, age, completeness, invasion, and size (14); SD, standard deviation.
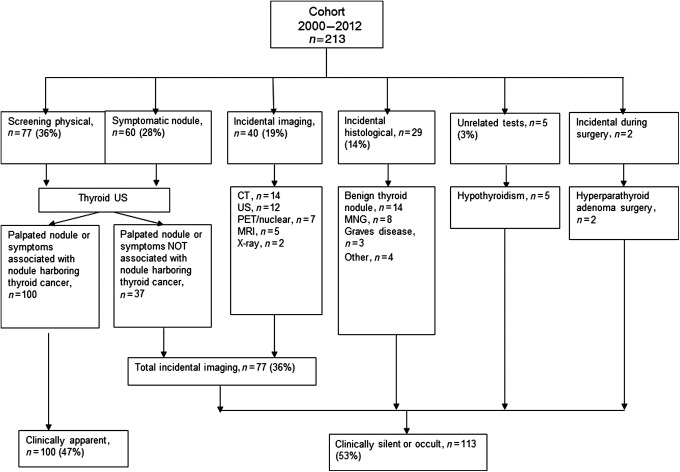
From 1935 to 1999, 59 (23%) were clinically occult and 203 (77%) were clinically recognized TC cases. From 2000 to 2012, the period in which we were able to extract the triggers of diagnosis in more detail, the mechanisms that ultimately led to the discovery of a thyroid nodule harboring malignancy were: (a) physical examination in an asymptomatic patient (77; 36%); and (b) evaluation of patients with symptomatic nodules (60; 28%). Within these two groups (137 cases), 37 of the nodules that harbored cancer were neither the palpated nodule nor the nodule that was deemed to be causing the patient's symptoms, but instead a separate nodule found during the evaluation.
Forty cases (19%) were found incidentally during an imaging procedure. The most frequent imaging modalities were computed tomography (14; 7%) and ultrasound (12; 6%). The reasons for imaging in these 40 patients were: (a) work-up for other cancer (10; 5%); (b) neuromuscular complaints (8; 4%); (c) ear/nose or throat discomfort (5; 2%); (d) discovery strategy—to understand the origin of a systemic (5; 2%), endocrine (5; 2%), infection (4; 2%), or other (3; 1%) type of symptom.
Finally, 29 cases (14%) were found in the histology of thyroid glands that were removed for benign indications, 5 (2.3%) cases were found as a consequence of unrelated tests as part of the work-up of hypothyroidism, and 2 cases (1%) of TC were found when the surgeon observed, intra-operatively, a suspicious thyroid nodule during the removal of a parathyroid adenoma.
Of the 213 cases from 2000 to 2012, 100 were cases in whom the palpated nodule or symptoms were triggers of diagnosis and classified as clinically recognized, whereas 113 were clinically occult (Fig. 1). Taken together, from 1935 to 2012, 303 (64%) were clinically recognized and 172 (36%) were clinically occult TC cases. The occult cases, when compared with clinically recognized cases, were significantly older at diagnosis (mean age 51 vs. 44 years, p<0.01), had smaller lesions (mean tumor size 1.2 cm vs. 2.3 cm, p<0.01) included a similar distribution of low-risk (14) (MACIS score <6) papillary cancers (82% vs. 87%, p=0.08), and had a higher proportion of papillary histology (94 vs. 83, p<0.01) (Table 3).
FIG. 1.
Methods of thyroid cancer detection for the 2000–2012 cohort. CT, computed tomography; MNG, multinodular goiter; MRI, magnetic resonance imaging; PET, positron emission tomography; US, ultrasound.
Table 3.
Comparison Between Triggers of Diagnosis
| 1935–1999 | 2000–2012 | 1935–1999 | 2000–2012 | ||||
|---|---|---|---|---|---|---|---|
| Clinically occult(n=59) | Clinically occult(n=113) | Total clinically occult(n=172) | Clinically recognized(n=203) | Clinically recognized(n=100) | Total clinically recognized(n=303) | p Value (occult vs. clinically recognized) | |
| Age at diagnosis in years, mean (SD) | 52.3 (20) | 49.6 (14.9) | 50.6 (17) | 44.2 (16) | 42.7 (14.14) | 43.7 (15.8) | 0.01 |
| Female, n (%) | 37 (63) | 79 (70) | 116 (68) | 157 (77) | 70 (70) | 226 (74) | 0.09 |
| Size of tumor, mean (SD) | 0.98 (1) | 1.3 (1) | 1.19 (1) | 2.3 (1.6) | 2.26 (1.2) | 2.3 (1.5) | 0.01 |
| MACIS score, mean (SD)* | 5 (1.7) | 4.6 (1.2) | 4.8 (1.5) | 4.8 (1.8) | 4.7 (1.4) | 4.8 (1.7) | 0.86 |
| MACIS score<6, n (%)* | 41 (70) | 94 (87) | 135 (82) | 177 (87) | 81 (89) | 258 (87) | 0.08 |
| Papillary, n (%) | 53 (90) | 108 (96) | 161 (94) | 162 (80) | 91 (91) | 253 (83) | 0.001 |
| Follicular, n (%) | 2 (3) | 3 (2) | 5 (3) | 13 (6) | 3 (3) | 16 (5) | 0.22 |
| Hürthle, n (%) | 1 (2) | 1 (1) | 2 (1) | 17 (8) | 0 (0) | 17 (6) | – |
| Medullary, n (%) | 3 (5) | 1 (1) | 4 (2) | 5 (2) | 3 (3) | 8 (3) | – |
| Metastatic, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (0) | – |
| Anaplastic, n (%) | 0 (0) | 0 (0) | 0 (0) | 6 (3) | 0 (0) | 6 (2) | – |
| Lymphoma, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 2 (1) | – |
| Total/near total thyroidectomy, n (%) | 11 (19) | 88 (78) | 99 (52) | 48 (23) | 90 (90) | 138 (45) | 0.01 |
| Hemithyroidectomy, n (%) | 22 (37) | 19 (17) | 41 (20) | 130 (64) | 5 (5) | 135 (45) | 0.01 |
| Subtotal thyroidectomy, n (%) | 5 (8) | 4 (4) | 9 (5) | 16 (8) | 5 (5) | 21 (7) | 0.46 |
| Other, n (%) | 1 (2) | 2 (2) | 3 (2) | 0 (0) | 0 (0) | 0 (0) | – |
| Not able to assess, n (%) | 20 (34) | 0 (0) | 20 (21) | 10 (5) | 0 (0) | 10 (3) | 0.01 |
Incidence of TC from 1935 to 2012.
The age-adjusted incidence of TC progressively increased from the period 1935–1949 (1.8 per 100,000 p-y [CI 0.6–4.7]) through 1950–1959 (5.3 per 100,000 p-y [CI 2.8–7.8]) to 1960–1969, (8.1 per 100,000 p-y [CI 5.5–10.5]). The incidence remained stable from 1970 to 1990. However, the age-adjusted incidence for TC increased from 7.1 per 100,000 p-y [CI 5.5–8.8] during 1990–1999 to 13.7 per 100,000 p-y [CI 11.8–15.6] during 2000–2012. Furthermore, 11 out of 476 patients (2.3%) died from thyroid cancer from 1935 to 2012 (Appendix Table A1), with no significant change in the age-adjusted specific disease mortality—0.3 per 100,000 p-y [CI 0.1–1] in 1935–1945 and 0.2 per 100,000 p-y [CI 0.1–0.45] in 2000–2012 (Fig. 2).
FIG. 2.
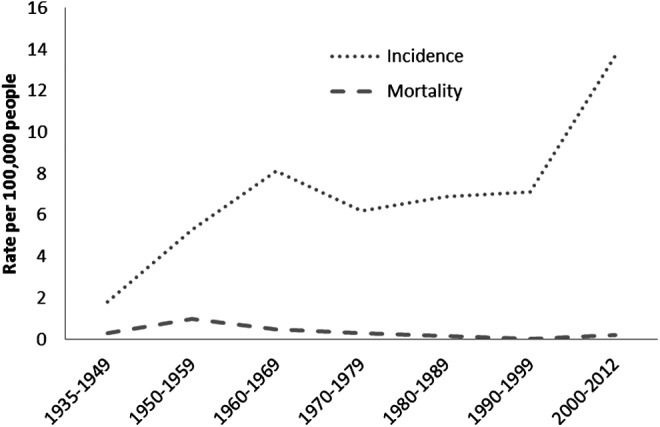
Age-adjusted incidence of thyroid cancer and thyroid cancer mortality in Olmsted County, Minnesota, from 1935 to 2012.
The age-adjusted incidence in women has been consistently higher than in men. This difference was more pronounced between 2000 and 2012 where the age-adjusted incidence in women during this period was 18.9 [CI 15.8–21.9] per 100,000 p-y, and in men, 8.6 [CI 6.4–12.8] per 100,000 p-y (p<0.001) (Fig. 2). The size of the primary tumor has decreased with time. From 1935–1949 the mean size of the primary tumor was 2.5 cm, yet during 2000–2012 the mean size of the primary tumor was 1.7 cm, (r2 0.008, p=0.04) (Fig. 3).
FIG. 3.
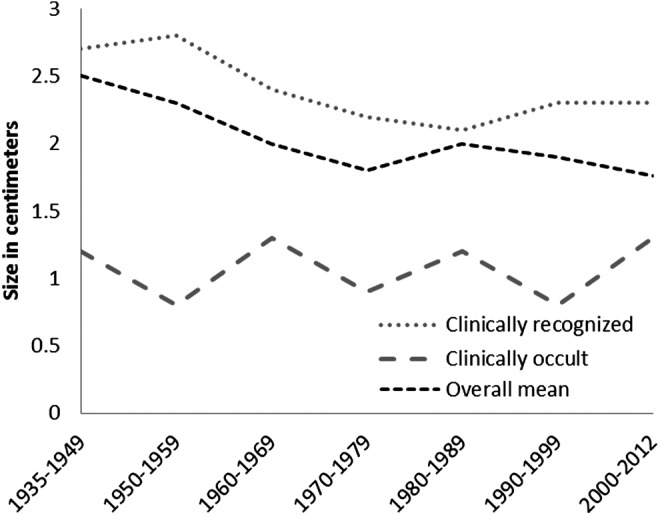
Mean of the size of primary tumor, in centimeters, from 1935 to 2012. Dotted line shows the size of the tumor during the 2000–2012 time period of the clinically apparent cases only.
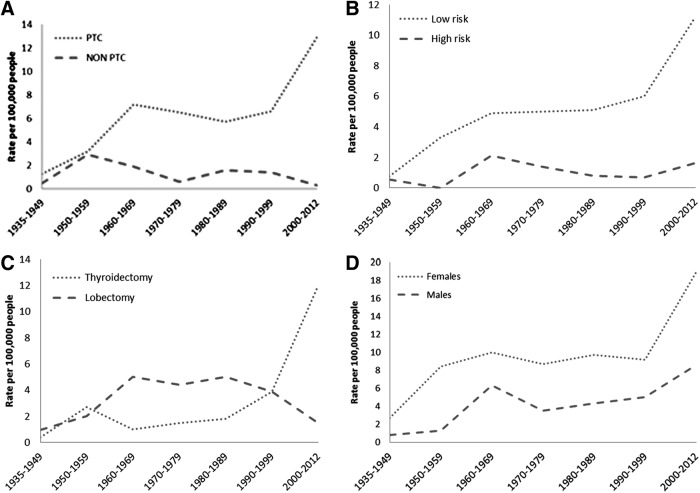
The incident cases of papillary thyroid cancer (PTC) and PTC with a MACIS score less than 6 (14) (low-risk thyroid cancer) are shown in Figure 4. The proportion of thyroid cancer cases treated with bilateral lobar resection (total, near, or subtotal thyroidectomy), as against lesser degrees of thyroid resection, significantly increased after 1999 (Fig. 4).
FIG. 4.
Age-adjusted incidence of thyroid cancer in Minnesota, from 1935 to 2012. (A) Incidence rates of papillary thyroid cancer (PTC) vs. nonpapillary thyroid cancer. (B) Incidence rates of low-risk PTC [MACIS (metastasis, age, completeness, invasion, and size) score <6] vs. high-risk PTC (MACIS >6). (C) Incidence of thyroidectomies and lobectomies. (D) Incidence of thyroid cancer by sex.
The incidence trend analysis by the mechanism of detection revealed that the sex-adjusted incidence of clinically recognized TC was 5.5 per 100,000 p-y [CI 3.4–7.5], a rate similar to the rate seen during 1990–1999 and 2000–2012. In contrast, the sex-adjusted incidence of clinically occult TC increased from 1.9 per 100,000 p-y [CI 1.2–2.9] in 1990–1999 to 7.4 per 100,000 p-y [CI 6–8.8] in 2000–2012 (Figure 5). The average size of clinically recognized (r2 0.003, p=0.3) and clinically occult tumors (r2 0.008, p=0.22) were essentially unchanged over time (Fig. 3).
FIG. 5.
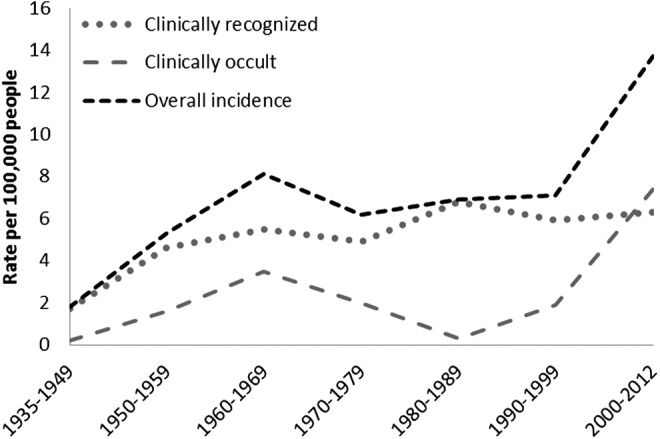
Age-adjusted incidence of thyroid cancer by method of detection in Olmsted County, Minnesota, from 1935 to 2012.
Discussion
We found that the incidence of TC has increased significantly among Olmsted County residents from 1935 to 2012. The more pronounced increase, however, happened during the last 12 years of the observation period. During this period, the incidence rate of thyroid cancer was 13. 7 per 100,000 p-y—almost double the incidence rate of 7.1 for1990–1999 and similar to the incidence of 14.3 observed in 2009 by the SEER analysis (1). The analysis including the mechanism of detection revealed that the rapid increase in incidence of TC during the last period, 2000–2012, is attributable to the increased diagnosis of occult TCs, whereas the incidence of clinically recognized TC has not changed over the last four decades.
Limitations and strengths
Our use of the REP gives us the ability to examine the health care of Olmsted County's residents regardless of age, sex, ethnicity, insurance, socioeconomic status, and setting of care delivery. Therefore, it allowed us to follow patients across the full spectrum of disease—from symptoms through the final diagnosis—without relying on administrative data (8). However, all types of epidemiological studies raise concerns about generalizability from the study sample to the target population and from the target population to other populations or to the entire United States. A study comparing the characteristics of the Olmsted County, Minnesota population with the characteristics of other populations revealed that age, sex, and ethnic characteristics of Olmsted County were similar to those of the state of Minnesota and the Upper Midwest from 1970 to 2000. However, Olmsted County was less ethnically diverse than the entire U.S. population (15). Thus, it is possible that the relationship of the mechanism of detection and the incidence of thyroid cancer varies depending on the source of data; however, the similarities between our thyroid cancer incidence rate in 2000–2012 and the nationally representative SEER cancer incidence rate incidence rate in 2009 suggest that the variation may be minimal. Likewise, results from other studies in Olmsted County have generally been consistent with national data (16,17). Thus, our findings are very likely relevant to the increasing incidence of thyroid cancer across most centers of medical care. Additionally, given the retrospective extraction of data, we could have misclassified patients into our categories of the methods of detection. To curtail this bias, the two extractors discussed the debatable cases to achieve consensus; when consensus was not achieved easily, we decided to err on the side of caution and classify these cases as “clinically apparent.” Finally, our analysis of the triggers of detection has limitations. The method for the extraction of diagnosis triggers during 1935–1999 differed from the framework used during 2000–2012. This different methodology did not allow us to understand how the occult tumors during 1935–1999 were found, and it may have led to misclassification of some of clinically recognized cases. However, the fact that the average size of the occult tumors during 1935–1999 is the same as the size of the occult tumors during 2000–2012 suggests that misclassification is unlikely.
The results from this population study provide strong evidence that the increase in TC incidence is secondary to increased TC detection and not due to an increase in clinically apparent disease. Cadaveric studies several decades ago, from patients who died from nonthyroid-related causes, demonstrated that one-third of such patients harbored TC lesions without knowledge of them (18). Thus, a large pool of subclinical TC exists in the population. The discovery of this subclinical pool of TC lesions is directly associated with the access of individuals to health care, as has been demonstrated in a prior analysis of the SEER database (19). During the period 2000–2012, we have identified four important pathways for the detection of subclinical thyroid cancer, as shown in Figure 1. Two of these mechanisms might relate to the increased awareness from both patients and clinicians regarding thyroid cancer. As thyroid cancer incidence increases, patients might receive more information from the media or the community (e.g., “my neighbor was found to have thyroid cancer”) and become more aware of the risk. Clinicians, on the other hand, may also be aware of the risk, but as they find more thyroid nodules while palpating the neck, they may develop an increased awareness of benefit (e.g. “I found a thyroid cancer lesion in my patient that came with back pain”). While there is nothing wrong with going to the doctor for a check-up or to perform a physical exam, our analysis revealed that, of the 137 that initiated the work-up for TC for patient or clinical factors, 37 (27%) were found to have TC unrelated to these factors.
Another important mechanism is the number of TC lesions found in the histological examination of thyroid glands removed for benign thyroid conditions. In our analysis, 14% of all thyroid cancer lesions were found through this mechanism. Two retrospective studies conducted in tertiary care centers found that 47% (7) and 28% (20) of all incidental thyroid cancers were found in the histopathological review of these operative pathologic specimens. Another large Australian retrospective cohort found that the detection of these histological “incidentalomas” accounted for the entire increase of TC for the last 40 years in their institution (21). In the United States, the number of thyroidectomies for benign thyroid disease has increased from 43,946 in 1996 to 62,157 in 2006, and a recent report from Medicare insurance claims databases also shows that the choice of thyroidectomy versus lobectomy was associated with an almost three-fold increase in the diagnosis of thyroid cancer (22). This finding suggests that this mechanism is also contributing to the increased incidence of thyroid cancer at a national level.
Finally, the influence of imaging to the detection of the reservoir of TC is important. About 19% of TC cases were found incidentally by imaging technologies (mostly computed tomography and neck ultrasonography) not focused on the thyroid gland. Likewise, of 137 patients who had thyroid ultrasounds, 27% (17% of total TC cases) had thyroid cancers found incidentally as these thyroid cancer nodules did not relate to the clinical concern that triggered the use of the ultrasound. Therefore, of 113 clinically silent or occult TC cases, incidental findings in imaging accounted for a total of 77 cases, 68% (Fig. 1). This finding confirms that imaging plays a pivotal role in the increased detection of thyroid cancer.
These results support the prior findings of a retrospective study conducted in two tertiary care centers assessing the triggers of TC diagnosis (13). In this study, 279 patients underwent thyroidectomy and 95 were found to have TC. Among these 95 TC cases, 44 (46%) thyroid surgeries were performed as a result of screening, serendipity and the diagnostic cascade. Our study differs from this analysis in that we assessed the impact of the triggers of diagnosis on the incidence of TC; an aim that can only be explored in a population-based study like this one.
Biological significance
Papillary thyroid cancers are driving the increase in incident cases since 1935. In fact, during 2000–2012, almost all of the cases in our study were of papillary type, and the majority were low risk, as defined by MACIS scores less than 6. Although these cases of low-risk PTC increased from 6 per 100,000 p-y during 1990–1999 to 11.2 per 100,000 p-y during 2000–2012, the number of more extensive surgical procedures (i.e., thyroidectomies) increased three-fold and the number of more conservative procedures (unilateral lobectomies) decreased by half. Thus, despite the presence of more low-risk TC lesions, the aggressiveness of initial therapy (extent of surgical resection) has increased.
However, would these lesions, if not found incidentally, be of any threat to the patients? Are the patients better off in any way by finding these lesions? A partial answer may come from two prospective Japanese cohorts (23,24) that have followed 1,465 patients with small papillary lesions for approximately 5 years (range 1–19 years). Of these 1465 patients, only 80 (6%) experienced growth of lesions more than 3 mm and only 22 (1.5%) experienced lymph node metastasis. All patients who experienced progression received successful surgery at the time of progression, so that during follow up there were no distant metastases or deaths. A similar active surveillance prospective cohort is being conducted at Memorial Sloan-Kettering Cancer Center in New York City, with similar results to date (25). These studies indicate that many small papillary lesions are indolent and that many patients may do well without discovery and treatment of these lesions.
How to do better
Clinically silent tumors of likely trivial significance are largely responsible for the increase in thyroid cancer incidence from 2000 through 2012. One approach to curtail the detection of these lesions would be to follow clear indications for the use of certain imaging modalities (e.g., thyroid ultrasonography). These recommendations should come from society guidelines and consensus statements. For instance, the Choosing Wisely Campaign, an initiative of the American Board of Internal Medicine Foundation to reduce unnecessary tests, have, stated as part of their recommendations, “Don't routinely order a thyroid ultrasound in patients with abnormal thyroid function tests if there is no palpable abnormality of the thyroid gland” (26). Further, radiologists could also use a more careful reporting system on incidentalomas seen in computed tomographies and magnetic resonance images. Radiologists at Duke University have developed a system of reporting for these nodules that recommend work-up with ultrasound if the nodule seen in CT/MRI/PET is greater or equal than 1.5 cm. Nodules not meeting these criteria should not be mentioned in the report (27). This reporting strategy could potentially reduce the fine-needle aspiration biopsy rate for thyroid incidentalomas by 34–46% (28).
Another alternative for clinicians and patients consists in discussing the possibility of an incidental thyroid nodule before the imaging test is conducted. This includes a discussion of the likelihood of an incidental thyroid nodule and the plan for disclosing and managing any findings. Perhaps, this conversation could be facilitated with the use of decision aids (29). It is likely that some informed patients will opt not to receive information about incidental thyroid nodules.
Conclusions
We have shown the relationship between the increased incidence of thyroid cancer and the mechanism of detection using a clinically rich and population-based dataset. We found that the rapid increase in incidence of TC during 2000–2012 is attributable to the increased diagnosis of occult TCs, which are mainly found through the use of diagnostic neck imaging. The incidence of clinical TC and disease-specific TC mortality, however, remains stable since 1970, implying that the observed increased incidence is due to an increased in the detection of subclinical lesions.
Appendix Table A1.
Disease Specific Mortality Due to Thyroid Cancer in Olmsted County, Minnesota, from 1935 to 2012
| Mortality cases | Histology | Time from thyroid cancer diagnosis to death |
|---|---|---|
| 1 | Anaplastic | <1 month |
| 2 | Anaplastic | 5 months |
| 3 | Follicular | 6 years |
| 4 | Anaplastic | <1 month |
| 5 | Papillary | 9 years |
| 6 | Anaplastic | 6 months |
| 7 | Anaplastic | 1 year |
| 8 | Papillary | 5 months |
| 9 | Follicular | 9 months |
| 10 | Medullary | 9 years |
| 11 | Papillary | 6 years |
All cases were diagnosed before 2000 and presented with palpable disease.
Acknowledgments
The authors would like to thank Michael R. Gionfriddo for his valuable comments.
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under award number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Davies L, Welch H. 2014. CUrrent thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 140:317–322 [DOI] [PubMed] [Google Scholar]
- 2.Aschebrook-Kilfoy B, Schechter RB, Shih YC, Kaplan EL, Chiu BC, Angelos P, Grogan RH. 2013. The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiol Biomarkers Prev 22:1252–1259 [DOI] [PubMed] [Google Scholar]
- 3.Lubitz CC, Kong CY, McMahon PM, Daniels GH, Chen Y, Economopoulos KP, Gazelle GS, Weinstein MC. 2014. Annual financial impact of well-differentiated thyroid cancer care in the United States. Cancer 120:1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsey S, Blough D, Kirchhoff A, Kreizenbeck K, Fedorenko C, Snell K, Newcomb P, Hollingworth W, Overstreet K. 2013. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 32:1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, Tomoda C, Takamura Y, Kobayashi K, Miya A. 2010. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 34:28–35 [DOI] [PubMed] [Google Scholar]
- 6.Brito JP, Morris JC, Montori VM. 2013. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ 347:f4706. [DOI] [PubMed] [Google Scholar]
- 7.Bahl M, Sosa JA, Nelson RC, Esclamado RM, Choudhury KR, Hoang JK. 2014. Trends in incidentally identified thyroid cancers over a decade: a retrospective analysis of 2,090 surgical patients. World J Surg 38:1312–1317 [DOI] [PubMed] [Google Scholar]
- 8.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Pankratz JJ, Brue SM, Rocca WA. 2012. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 41:1614–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke JP, Hay ID, Dignan F, Goellner JR, Achenbach SJ, Oberg AL, Melton LJ, 3rd 2005. Long-term trends in thyroid carcinoma: a population-based study in Olmsted County, Minnesota, 1935–1999. Mayo Clin Proc 80:753–758 [DOI] [PubMed] [Google Scholar]
- 10.Hay ID, Freeman SL, Bergstralh EJ, Goellner JR, Offord K, Kurland LT. 1987. Trends in the epidemiology of thyroid cancer through five decades [abstract]. Clin Res 35:347A [Google Scholar]
- 11.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ, 3rd 2012. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 87:1202–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. 2011. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol 173:1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies L, Ouellette M, Hunter M, Welch HG. 2010. The increasing incidence of small thyroid cancers: where are the cases coming from? Laryngoscope 120:2446–2451 [DOI] [PubMed] [Google Scholar]
- 14.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. 1993. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114:1050–1057; discussion 1057–1058. [PubMed] [Google Scholar]
- 15.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. 2012. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 87:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. 2007. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22:465–475 [DOI] [PubMed] [Google Scholar]
- 17.Melton LJ, 3rd, Kearns AE, Atkinson EJ, Bolander ME, Achenbach SJ, Huddleston JM, Therneau TM, Leibson CL. 2009. Secular trends in hip fracture incidence and recurrence. Osteoporos Int 20:687–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harach HR, Franssila KO, Wasenius VM. 1985. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer 56:531–538 [DOI] [PubMed] [Google Scholar]
- 19.Morris LG, Sikora AG, Tosteson TD, Davies L. 2013. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid 23:885–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malone MK, Zagzag J, Ogilvie JB, Patel KN, Heller KS. 2014. Thyroid cancers detected by imaging are not necessarily small or early stage. Thyroid 24:314–316 [DOI] [PubMed] [Google Scholar]
- 21.Grodski S, Brown T, Sidhu S, Gill A, Robinson B, Learoyd D, Sywak M, Reeve T, Delbridge L. 2008. Increasing incidence of thyroid cancer is due to increased pathologic detection. Surgery 144:1038–1043; discussion 1043. [DOI] [PubMed] [Google Scholar]
- 22.Sosa JA, Hanna JW, Robinson KA, Lanman RB. 2013. Increases in thyroid nodule fine-needle aspirations, operations, and diagnoses of thyroid cancer in the United States. Surgery 154:1420–1426; discussion 1426–1427. [DOI] [PubMed] [Google Scholar]
- 23.Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. 2010. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg 34:1222–1231 [DOI] [PubMed] [Google Scholar]
- 24.Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. 2014. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 24:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pace MD FJ, Bach A, Boucai L, Minkowitz G, Morris LG, Wong RJ, Tuttle RM. 2013. Properly selected patients with papillary thyroid cancer readily accept active Surveillance when offered as a standard of care alternative to immediate surgery. Abstracts of the 83rd Annual Meeting of the American Thyroid Association Meeting October16–20, 2013 San Juan, Puerto Rico Thyroid 23:A13–A121 [Google Scholar]
- 26.Choosing Wisely. Available at www.choosingwisely.org (accessed June2014)
- 27.Hoang JK, Raduazo P, Yousem DM, Eastwood JD. 2012. What to do with incidental thyroid nodules on imaging? An approach for the radiologist. Semin Ultrasound CT MR 33:150–157 [DOI] [PubMed] [Google Scholar]
- 28.Hobbs HA, Bahl M, Nelson RC, Kranz PG, Esclamado RM, Wnuk NM, Hoang JK. 2014. Journal Club: incidental thyroid nodules detected at imaging: can diagnostic workup be reduced by use of the Society of Radiologists in Ultrasound recommendations and the three-tiered system? AJR Am J Roentgenol 202:18–24 [DOI] [PubMed] [Google Scholar]
- 29.Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M, Llewellyn-Thomas H, Lyddiatt A, Legare F, Thomson R. 2011. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 1:CD001431. [DOI] [PubMed] [Google Scholar]