Abstract
Aims: Vitamin D (VD) deficiency has become a worldwide epidemic, particularly affecting African Americans (AA). VD deficiency has been implicated in the excessive rate of complications associated with diabetes in AA. Blood levels of VD binding protein (VDBP) and glutathione (GSH) are lower in AA compared with those in Caucasians. This study tested the hypothesis that lower GSH levels are linked to VDBP and VD deficiency in AA-type 2 diabetic (AA-T2D) patients. Blood was analyzed from T2D and nondiabetic subjects (N). Experiments examining GSH deficiency and l-cysteine (LC) supplementation were performed using THP-1 monocytes. Results: Plasma levels of LC, GSH, VDBP, and VD were significantly lower in AA-T2D compared with age-matched AA-N or Caucasian-T2D. Lower levels of LC and GSH showed a significant positive correlation with lower VDBP and VD levels in AA-T2D. GSH deficiency investigated using an antisense approach depleted VDBP/vitamin D receptor (VDR); LC supplementation caused significant upregulation of GSH and of VDBP/VDR, while supplementation with VD+LC caused a significantly greater GSH and VDBP/VDR upregulation compared with that of VD alone in monocytes. Innovation and Conclusion: The reported observations suggest that VD deficiency may be linked to GSH and LC status and lead to a novel hypothesis that supplementation with LC in combination with VD will be effective in increasing VD levels and reducing health disparities in AA. Antioxid. Redox Signal. 23, 688–693.
African Americans (AA) have the highest rate of microvascular complications associated with diabetes and the highest incidence of vitamin D (VD) deficiency (1, 6). Epidemiological studies demonstrate a strong association between higher blood levels of VD and better health outcomes, including a lower risk of type 2 diabetes and cardiovascular disease (6). Blood levels of vitamin D binding protein (VDBP) and glutathione (GSH) are lower in AA compared with Caucasians (4). GSH is a physiological antioxidant, a cofactor of many enzymes, and plays an important role in a multitude of cellular processes (7). VDBP is a transporter of VD and is a key determinant of circulating VD levels under different physiological and pathological conditions (9). Our observations reported here show a positive correlation between lower blood levels of VDBP and VD with that of lower l-cysteine (LC) and increases in GSH and VDBP levels as a result of LC supplementation, leading to a novel hypothesis: is upregulation of GSH a better therapeutic target for efforts to optimize blood levels of VDBP and VD in AA-type 2 diabetic (AA-T2D) patients?
Innovation.
VD deficiency and an excessive rate of diabetes have become a worldwide epidemic, particularly affecting AA. A significant positive correlation was observed between lower VDBP and VD and LC and GSH blood levels in AA diabetic patients; raising GSH status via LC supplementation upregulated both VDBP and VDR in monocytes. VDBP is a key determinant of VD blood levels. This novel finding will impact the clinical practice of VD supplementation and the development of new therapies based on the potential of LC coupled with lower VD doses to improve the efficacy and levels of VD and health outcomes in AA.
Relationship of LC and GSH Status on VDBP and VD Levels in AA-T2D Patients
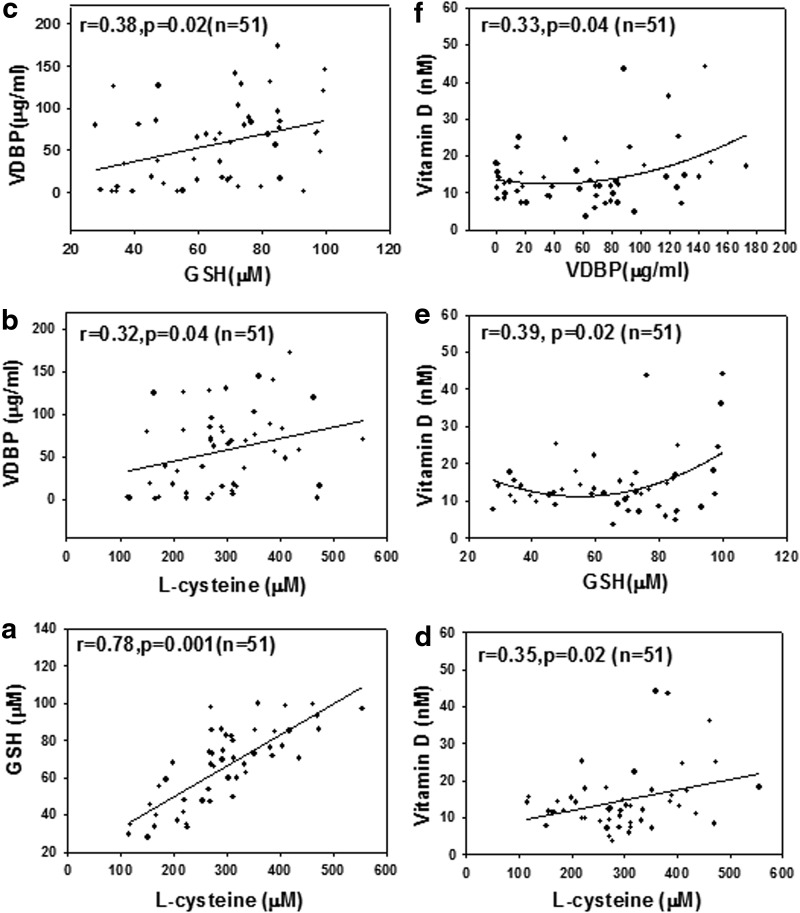
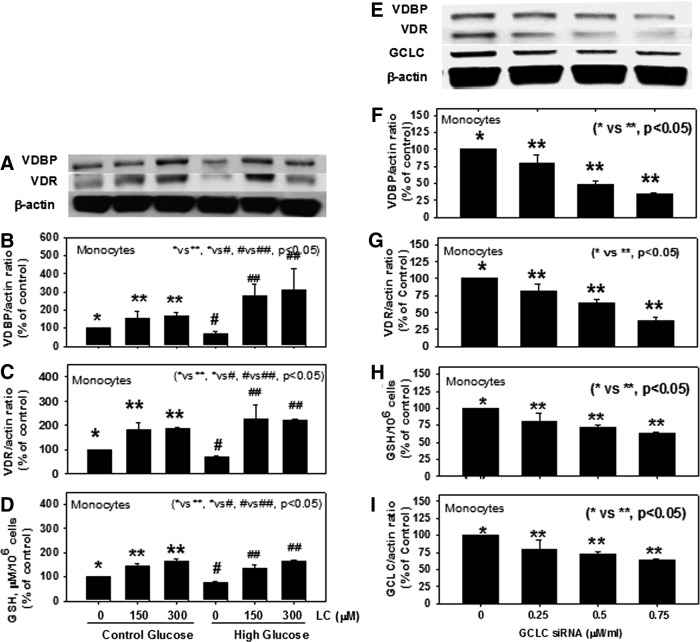
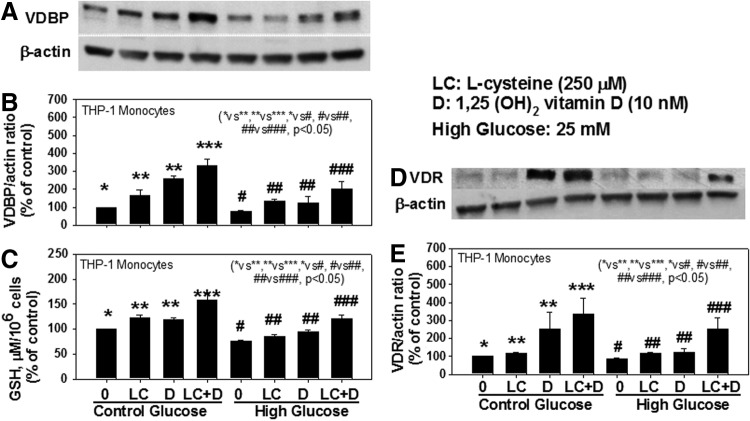
Blood levels of LC, GSH, VDBP, and VD are significantly lower in AA-T2D compared to those in age-matched AA healthy subjects (AA-N) or age-matched Caucasian-T2D (Table 1). Regression analyses showed a significant positive correlation between the blood levels of LC and VDBP (Fig. 1b), and LC and VD (Fig. 1d), and of GSH with VDBP (Fig. 1c), GSH and VD (Fig. 1e), and VDBP and VD (Fig. 1f) in AA-T2D patients. The first-order relationships were for GSH versus VD (r=0.27, p=0.07; Fig. 1e) and VDBP versus VD (r=0.29, p=0.05; Fig. 1f). The associations were stronger when a second-order relationship was calculated for GSH versus VD (r=0.39, p=0.02; Fig. 1e) and VDBP versus VD (r=0.33, p=0.04; Fig. 1f). The significant relationship between blood levels of VDBP, VD, and those of GSH and LC is a novel finding. Treatment with high glucose caused a significant decrease in VDBP and vitamin D receptor (VDR) protein expression and GSH levels in THP-1 human monocytes (Fig. 2). Supplementation with LC, a precursor of GSH, resulted in a significant upregulation of VDBP and VDR in monocytes exposed to both control and high-glucose concentrations (Fig. 2B, C). GSH is formed from LC by the enzymatic action of glutamate cysteine ligase catalytic unit (GCLC) (2). To determine whether GSH has a direct effect on VDBP and VDR expression, GSH deficiency was induced by knocking down GCLC using antisense-mRNA. Increasing the dose of GCLC-antisense caused increased levels of glutamate cysteine ligase knock-down (GCLC-KD) (Fig. 2I) and GSH deficiency (Fig. 2H), as well as a decrease in the expression of VDBP (Fig. 2F) and VDR (Fig. 2G), suggesting that GSH deficiency impairs the VDBP and VDR protein expression in monocytes. There was no change in cell viability as a result of any of the treatments. This suggests that LC supplementation upregulates the VDBP and VDR protein expression mediated by the GSH in monocytes. Treatment of VD along with LC resulted in a significantly greater upregulation of both VDBP (Fig. 3B) and VDR (Fig. 3E) expression compared to treatment with VD alone in THP-1 monocytes. GSH levels were also significantly higher in VD- and LC-treated cells compared to cells treated with VD or LC alone (Fig. 3C). The increase in VDBP and VDR expression caused by VD was lower under high-glucose conditions, which suggests that uncontrolled glycemia in diabetes may cause impairment in the metabolic actions of VD. A promising hypothesis thus presents itself and raises the question of whether supplementation with VD in combination with LC can correct the impaired status of VD and its action in patients with type 2 diabetes.
Table 1.
Body Weight, Glucose, Vitamin D Binding Protein, Vitamin D, and l-Cysteine Levels in the Fasting Blood of Type 2 Diabetic Patients and Age- and Race-Matched Healthy Nondiabetic Subjects
| AA-T2D | AA-N | Caucasians-T2D | Caucasians-N | |
|---|---|---|---|---|
| N | 51 | 32 | 27 | 20 |
| Age (years) | 47.6±1.3 | 47±2.1 | 50.3±1.6 | 46.8±3.1 |
| Body weight (kg) | 104.2±3.7a | 77.2±6.5b | 102.3±8.6c | 84.1±4.3d |
| Glucose (mg/dl) | 139±8a | 108±5b | 126±8c | 87±4d |
| HbA1C (%) | 7.99±0.3 | ND | 7.0±0.2 | ND |
| GSH (μM) | 66.1±2.9a | 80.4±6.8b | 79.6±6.1c,e | 102±5.3d |
| l-Cysteine (μM) | 296±14a | 363±26b | 342±26e | 398±18 |
| VDBP (μg/ml) | 63.2±7.2a | 156±26b | 126±27e | 177±12 |
| Vitamin D (nM) | 14.3±1.1a | 18.9±2.1b | 18.7±2.2e | 21.8±2.7 |
Values are mean±SE. Values marked “a” versus “b,” between “b” versus “d,” “c” versus “d,” and “a” versus “e” are significant (p<0.05). Normals are age- and race-matched healthy nondiabetic subjects.
AA, African Americans; Caucasians, European Americans; GSH, glutathione; N, healthy nondiabetic subjects; ND, not determined; T2D, type 2 diabetic patients; VDBP, vitamin D binding protein.
FIG. 1.
The relationship between plasma levels of LC and those of GSH (a), VDBP (b), and VD (d); between GSH levels and those of VDBP (c) and VD (e); and between VDBP and VD (f) in AA-T2D patients. Body weight was always used as an additional independent variable to determine regression and p-value. Data show a significant positive association between the blood levels of LC and those of GSH (a), VDBP (b), and VD (d), and those of GSH and VDBP (c), and those of VDBP and VD (f) levels in AA-T2D patients. AA, African Americans; AA-T2D, AA-type 2 diabetic; GSH, glutathione; LC, l-cysteine; VD, vitamin D; VDBP, vitamin D binding protein.
FIG. 2.
Effect of LC supplementation on VDBP and VDR protein expression (A), VDBP/actin ratio (B), VDR/actin ratio (C), and GSH levels (D) in control and high-glucose-treated THP-1 monocytes. second column: Effect of GSH deficiency on VDBP, VDR, and GCLC-protein expression (E) and VDBP/actin ratio (F), VDR/actin ratio (G), GSH (H), and GCLC knockdown level (I) in THP-1 monocytes treated with different concentrations of GCLC-siRNA. Values are mean±SE (n=4). GCLC, glutamate cysteine ligase catalytic unit; VDR, vitamin D receptor.
FIG. 3.
First column: Effect of LC and VD supplementation on VDBP expression (A), VDBP/actin ratio (B), GSH levels (C), VDR protein expression (D), and VDR/actin ratio (E) in control and high-glucose-treated THP-1 monocytes. Values are mean±SE (n=4).
VD Link to VDBP Status and Better Health
VDBP is a transporter of VD, protects against VD deficiency, and is a novel regulator of VD action and metabolism (6, 9). Both humans with a genetic mutation for VDBP and VDBP-knockdown mice demonstrate low plasma levels of VD (6, 9). Genetic variations in VDBP are known to influence VD blood levels in response to VD supplementation (6, 9). VDBP is produced by monocytes and by the liver (9). VDBP is a 52–59 kDa monomeric glycoprotein with a short half-life, 2.5–3 days compared with the 1–2 months for VD (9). Other factors such as age, sex, skin pigmentation, season, geographic latitude, food and supplemental sources of VD, and adiposity also influence the VD status in the human body (1, 6). It has been thought that dark pigmentation of the skin absorbs much of the UV B energy before it has an opportunity to produce VD, to which is attributed the higher rates of VD deficiency observed in AA (1, 6). VD deficiency has been associated with an increased risk of atherosclerosis, carotid artery thickness, and cardiovascular disease (CVD) (6). This has led to the practice of widespread VD supplementation. However, supraphysiological doses of VD are required to reach desired concentrations of circulating VD (1). High doses of VD can cause undesirable elevated blood levels of calcium, which leads to vascular and tissue calcification, with subsequent damage to the heart, blood vessels, and kidneys (6). These conflicting observations cause us to wonder whether simultaneous upregulation of VDBP will require lower doses of VD supplementation to reach desired circulating VD levels.
GSH, VDBP, and VD Status in Diabetes
The blood levels of VDBP, VD, and GSH are lower in diabetes (6, 7). Elevated urinary loss of VDBP and decreased reabsorption in proximal tubule by megalin/Dab2 have been suggested to contribute to the lower VDBP and VD levels in diabetes (6). The VDBP downregulation by high glucose observed in this study suggests that uncontrolled glycemia may also contribute to lower VDBP and VD levels in diabetes. This study shows that the decrease in VDBP and GSH levels associated with diabetes is greater in AA diabetic patients than that observed in Caucasian patients. This study also reports for the first time that a significant positive correlation exists between the blood levels of LC and GSH and those of VDBP and VD in AA-T2D patients. Cell culture studies demonstrate that high-glucose exposure can cause a significant decrease in the VDBP and VDR protein expression and GSH levels in monocytes; VDBP and VDR were significantly upregulated by LC, a precursor of GSH; the deficiency of GSH accomplished by GCLC knockdown resulted in a significant decrease in VDBP and VDR expression, which suggests that GSH status influences expression of VDBP and VDR in monocytes. Treatment with VD and LC together resulted in a significantly greater upregulation of GSH and VDBP and VDR in monocytes exposed to high glucose (HG) in comparison to treatment with VD alone. This finding provides evidence for a link between VDBP and VD with that of GSH status. Cellular actions influenced by circulating levels of VD are regulated by the VDR. Therefore, it seems logical that higher levels of both VD and VDR are needed to achieve the desired benefits resulting from higher VD levels that have been observed in data from various epidemiological studies (6). Is simultaneous supplementation with LC in combination with VD a better therapeutic approach in the design of VD supplemental studies?
Potential of LC Plus VD to Raise Blood Levels of VDBP and VD in AA
Hyperglycemia and diabetes are associated with increased oxidative stress (5). AA are at a higher risk for impaired GSH status due to their nearly 11% incidence of G6PD deficiency and 6%–7% incidence of sickle cell trait, a disease associated with elevated oxidative stress and reduced GSH (3). This study demonstrates a potential link between GSH status and those of VDBP and VD blood levels. GSH is a physiological antioxidant, a cofactor of many enzymes, and plays an important role in cellular processes (2, 3, 7). LC can also have a direct effect on posttranslational modification or S-glutathionylation of proteins, which can cause modification of structure and function and thereby prevention of sulfhydryl overoxidation and proteolysis. It can also boost the protection provided against the harm caused by oxidative signaling events. VDR is a potential antioxidant and VDR expression is regulated by physiological factors and hormones, including Ca and 1,25 (OH)2D (6, 8). The finding that LC upregulates VDBP and VDR suggests that LC supplementation can stimulate both circulating levels of VDBP and VD and the overall efficacy of VD. Whether supplementation with LC can improve VDBP and VD levels in AA subjects needs investigation. Can the validation of the novel link among the statuses of GSH, VDBP, and VD enable the discovery and practice of new therapeutic approaches based on the potential of LC supplementation coupled with lower VD doses to be used as an adjuvant therapy in reducing the VD deficiency and associated health hazards in the AA population?
Note
Patient enrollment
Patients included in this study were adults with type 2 diabetes. All patients who provided written informed consent according to the protocol approved by the institutional review board were invited to return to have blood drawn after fasting overnight. Nondiabetic healthy subjects were also enrolled from siblings of patients or from workers at LSUHSC. Patients were excluded if they had any history of cardiovascular disease, sickle cell disease, treatment with insulin, or metabolic disorders, including uncontrolled hypertension, hypothyroidism, or hyperthyroidism. Patients were excluded if they showed signs of significant hepatic dysfunction, defined as any underlying chronic liver disease or liver function tests greater than 1.5 times the upper limit of normal or renal dysfunction, defined as a serum creatinine value greater than 1.5 mg%. Women with a positive pregnancy test or those nursing infants were also excluded. Subjects who were taking any supplemental vitamins or herbal products were excluded. Blood was drawn after an overnight fast (8 h). Serum tubes containing blood for chemistry profiles and EDTA-blood tubes for HbA1C and CBC were promptly delivered to the LSUHSC clinical laboratories. Additional EDTA-blood was brought to the research laboratory and the resulting plasma was stored at −80°C for analyses of the biochemical parameters.
LC, VD, and high-glucose treatment and GCLC KD in THP-1 monocytes
The THP-1 human monocytic cell line purchased from ATCC was cultured and maintained in the complete RPMI 1640 medium (2). Pretreatment of the cells, maintained at 1×106/ml of media, was done for 2 h with LC (0–300 μM), then 24 h with 1, 25-(OH)2-D3, followed by treatment for 24 h with HG (25 mM). Control cells were treated with mannitol, which is considered an osmolarity control. To investigate the role of GSH, siRNA specific for GCLC (the catalytic component of the enzyme) was used to knock down the enzyme and induce GSH deficiency. Complexes of GCLC siRNA (Santa Cruz Biotechnology) and Lipofectamine (Invitrogen) were allowed to form in culture flasks in serum-free media, to which cells suspended in serum-free media were added. After 24 h, the complete medium with serum was added and the cells were then treated as described in the figures.
VD, VDBP, GSH, LC, cell viability assays, and immunoblotting
Plasma levels of 25-OH-VD were determined using an ELISA kit (Eagle Biosciences) and those of VDBP using the kit from ALPCO. The kit includes polyclonal antibodies that detect total VDBP levels. Levels of GSH and LC were determined using HPLC (2). Details of immunoblotting and cell viability are similar to those given in our previous publication (2). The antibodies for GCLC (73 kDa), VDBP (52 kDa), and VDR (48 kDa) were purchased from Abcam.
All chemicals were purchased from the Sigma Chemical Co. unless otherwise mentioned. Data were analyzed using ANOVA with SigmaStat. Body weight was used as an additional independent variable to determine regression analyses and p-value using SigmaStat software. A p-value of less than 0.05 for a statistical test was considered significant.
Abbreviations Used
- AA
African Americans
- AA-T2D
AA-type 2 diabetic
- GCLC
glutamate cysteine ligase catalytic unit
- GSH
glutathione
- LC
l-cysteine
- VD
vitamin D
- VDBP
vitamin D binding protein
- VDR
vitamin D receptor
Acknowledgments
The authors are supported by grants from NIH (R01 AT007442) and the Malcolm Feist Endowed Chair in Diabetes. The authors thank Ms. Morgan for excellent editing.
References
- 1.Garrett-Mayer E, Wagner CL, Hollis BW, Kindy MS, and Gattoni-Celli S. Vitamin D3 supplementation (4000 IU/d for 1 y) eliminates differences in circulating 25-hydroxyvitamin D between African American and white men. Am J Clin Nutr 96: 332–336, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain SK, Micinski D, Huning L, Kahlon G, Bass PF, and Levine SN. Vitamin D and l-cysteine levels correlate positively with GSH and negatively with insulin resistance levels in the blood of type 2 diabetic patients. Eur J Clin Nutr 68: 1148–1153, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain SK. Effect of glucose-6-phosphate dehydrogenase deficiency on reduced and oxidized glutathione and lipid peroxide levels in the blood. Clin Chim Acta 253: 181–183, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, and Thadhani R. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 369: 1991–2000, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rains JL. and Jain SK. Oxidative stress, insulin signaling and diabetes. Free Radic Biol Med 50: 567–575, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, Murad MH, and Kovacs CS. The nonskeletal effects of vitamin D: an endocrine society scientific statement. Endocr Rev 33: 456–492, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekhar RV, McKay SV, Patel SG, et al. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 34: 162–167, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao T, Ying X, Zhao Y, Yuan A, He Q, Tong H, Ding S, Liu J, Peng X, Gao E, Pu J, and He B. Vitamin D receptor activation protects against myocardial reperfusion injury through inhibition of apoptosis and modulation of autophagy. Antioxid Redox Signal 22: 633–650, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yousefzadeh P, Shapses SA, and Wang X. Vitamin D binding protein impact on 25-hydroxyvitamin D levels under different physiologic and pathologic conditions. Int J Endocrinol 98: 1581, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]