Abstract
Background: Using the Surveillance, Epidemiology, and End Results—Medicare database, a substantial increase was found in the use of positron emission tomography (PET) scans after 2004 in differentiated thyroid cancer (DTC) patients. The reason for the increased utilization of the PET scan was not clear based on available the data. Therefore, the indications for and outcomes of PET scans performed at an academic institution were evaluated.
Methods: A retrospective cohort study was performed of DTC patients who underwent surgery at the University of Michigan Health System from 2006 to 2011. After identifying patients who underwent a PET scan, indications, rate of positive PET scans, and impact on management were evaluated. For positive scans, the location of disease was characterized, and presence of disease on other imaging was determined.
Results: Of the 585 patients in the cohort, 111 (19%) patients had 200 PET scans performed for evaluation of DTC. Indications for PET scan included: elevated thyroglobulin and negative radioiodine scan in 52 scans (26.0%), thyroglobulin antibodies in 13 scans (6.5%), rising thyroglobulin in 18 scans (9.0%), evaluation of abnormality on other imaging in 22 scans (11.0%), evaluation of extent of disease in 33 scans (16.5%), follow-up of previous scan in 57 scans (28.5%), other indications in two scans (1.0%), and unclear indications in three scans (1.5%). The PET scan was positive in 124 studies (62.0%); positivity was identified in the thyroid bed on 25 scans, cervical or mediastinal lymph nodes on 105 scans, lung on 28 scans, bone on four scans, and other areas on 14 scans. Therapy following PET scan was surgery in 66 cases (33.0%), chemotherapy or radiation in 23 cases (11.5%), observation in 110 cases (55.0%), and palliative care in one case (0.5%). Disease was identifiable on other imaging in 66% of cases. PET scan results changed management in 59 cases (29.5%).
Conclusions: In this academic medical center, the PET scan was utilized in 19% of patients. Indications for the PET scan included conventional indications, such as elevated thyroglobulin with noniodine avid disease, and more controversial uses, such as evaluation of extent of disease or abnormalities on other imaging tests. PET scan results changed management in about 30% of cases.
Introduction
The incidence of differentiated thyroid cancer (DTC) has been increasing progressively over the past two decades, with the largest increase in small, low-risk tumors (1,2). Conventional therapies have included total thyroidectomy and radioactive iodine treatment to reduce mortality and risk of recurrence (3). While the risk of death due to thyroid cancer is low in DTC patients, the risk of recurrence is a significant concern to the providers that follow these patients long term (4). Therefore, a variety of imaging techniques have been employed to detect persistent or recurrent disease in the postoperative setting. Several frequently utilized imaging modalities are neck ultrasound, radioactive iodine scan, computed tomography (CT) scan, and positron emission tomography (PET) scan (5,6).
The PET scan is the newest imaging modality used in the postoperative surveillance of DTC patients. As such, there remains significant controversy and uncertainty with regards to the optimal way to integrate it into practice (7–10). Previous studies have shown the ability of the PET scan to localize disease already thought to be present because of detectable thyroglobulin levels (11,12). This has been the most generally accepted indication for performing a PET scan. However, others have argued that PET scanning adds little beyond neck ultrasound and CT scan of the neck and chest in this scenario (13). Though less generally accepted, PET scanning has also been utilized in other situations, such as in patients with elevated antithyroglobulin antibodies (14,15).
The authors previously showed an increase of up to 33.4-fold in the use of PET scans in the postoperative surveillance of DTC patients after 2004 using the Surveillance, Epidemiology, and End Results (SEER)—Medicare database (16). However, this large population-based database lacked significant details to determine the indications for the PET scans being performed or the results of the test, and how they are affecting management. Therefore, a single-institution, retrospective cohort study was performed of DTC patients seen at the University of Michigan to determine the rate of utilization of and the indications for PET scans, as well as and eventual changes in management based on PET scan results.
Methods
Data source and study population
The University of Michigan Thyroid Cancer Database, a project of the Michigan Endocrine Oncology Repository (HUM00024461), includes 668 patients who underwent surgery at the University of Michigan and had a pathologically confirmed diagnosis of thyroid cancer between 2006 and 2011. It includes demographic, surgical, pathologic, radiographic, and thyroid cancer recurrence data entered into a REDCap database (17). Using this database, first only those individuals with DTC were identified, defined as International Classification of Disease for Oncology: papillary, follicular, or Hürthle cell cancer (18). In total, 585 patients with DTC were included in the analysis. Then those patients who underwent a PET scan at the authors' institution for evaluation of thyroid cancer were identified. Additionally, patients who had a PET scan at an outside institution submitted to the authors' radiology department for review were included. All PET scans were obtained based on the clinicians' opinion that a PET scan would assist with the management of their patient. Any patient who had an initial PET scan for an indication other than thyroid cancer was excluded from the analysis, as this first PET scan was felt to influence the decision to obtain additional PET scans. Approval for the study was granted by the University of Michigan Institutional Review Board.
Measures
Patient demographics were obtained, including age, sex, and race/ethnicity. Race/ethnicity was categorized as white, Asian, black, and other. Primary tumor characteristics were also obtained from pathologic specimens, when available. These characteristics included: histology, tumor size, presence of capsular invasion, extrathyroidal extension, presence of lymph node metastasis, and American Joint Commission on Cancer stage (19).
The number of PET scans that were performed in each patient was identified. For each PET scan, the indication for the PET scan was determined, categorized as follows: elevated thyroglobulin with noniodine avid disease, positive thyroglobulin antibodies, rising thyroglobulin, evaluation of an abnormality on another imaging test, evaluation of extent of disease, follow-up of a previous PET scan, other indications, or unknown indication. If more than one indication was present, the documentation of the ordering physician was reviewed to determine their reason for ordering the test. For example, if a patient had a negative radioactive iodine scan with an elevated thyroglobulin that was rising, and the physician documented that the reason for ordering the PET was the increasing thyroglobulin, the indication for that PET scan was categorized as rising thyroglobulin. The method of stimulation was classified as recombinant thyrotropin (TSH), hypothyroidism, not stimulated, or unknown. Each PET scan was characterized as positive or negative for disease, verified by surgical pathology when available. If positive, the location of the disease was identified as thyroid bed, cervical and/or mediastinal lymph nodes, lungs, bone, or other. If other imaging was performed, it was ascertained whether the abnormality was identifiable on other imaging.
The management plan after each PET scan was determined: surgery, chemotherapy or radiation (including tyrosine kinase inhibitors and radioactive iodine therapy), observation, or palliative care. Finally, the study identified whether the PET scan changed the management of the patient. A PET scan was considered to have changed the management of the patient if the PET scan identified disease that was not previously known and resulted in an intervention, if the PET scan resulted in the decision to not pursue a specific intervention, or if the PET scan changed the extent of a planned surgery because of additional disease identified. For example, in a patient with an elevated thyroglobulin and nonradioiodine avid disease who had a positive PET scan and underwent surgical excision of lymph nodes in the neck, PET would be considered to have changed management. Additionally, if disease was already identified by another imaging modality but PET imaging showed additional disease that led to a change in the planned surgical procedure (reoperative central neck dissection vs. modified radical neck dissection), that was considered to have changed management. If a patient had an elevated thyroglobulin and would have empirically been treated with radioactive iodine, but treatment was withheld because of fluorodeoxyglucose (FDG) uptake on PET scanning, this was considered to be a change in management. If patients underwent chemotherapy (including tyrosine kinase inhibitors) or radiation therapy due to disease found on PET imaging, that was also considered a change in management. Finally, one patient was enrolled in hospice due to worsening disease on PET scanning, and that was considered a change in management.
For each PET scan performed, when available, the patient's thyroglobulin level at that time was recorded. Thyroglobulin was considered stimulated if obtained under recombinant thyroid stimulating hormone or thyroid hormone withdrawal. Otherwise, it was considered unstimulated. If thyroglobulin antibodies were present, the thyroglobulin was considered invalid and not included in the analysis.
Statistical analysis
For continuous variables, such as patient age and thyroglobulin levels, median and interquartile ranges were calculated. For categorical variables, such patient sex and race/ethnicity, the number and percent of patients and/or scans within each category was determined. For the location of disease on PET scan, more than one location may have been present on a scan. For example, if a patient had disease seen in the neck and lung, the PET scan would be considered positive in both those locations. Therefore, only the number of scans with disease in each location was determined, and the total may be more than the number of positive scans.
To determine if thyroglobulin levels could predict the presence of disease on PET scan, scans were categorized as positive or negative. Median stimulated and unstimulated thyroglobulin levels for each group were calculated, as well as the interquartile range. The two groups were compared for statistical significance using a two tailed t-test. A p-value of <0.05 was considered significant.
To examine pretest characteristics that increased the likelihood of PET imaging changing management, a chi-square analysis was performed with change in management as the outcome. The variables that were included in the analysis were type of stimulation, indication for PET scan, stimulated thyroglobulin, and unstimulated thyroglobulin. For the purposes of this analysis, thyroglobulin levels were characterized as <10 ng/nL, 10–30 ng/mL, and >30 ng/mL. A p-value of <0.05 was considered significant.
Results
Of the 585 patients in the cohort, 111 (19%) patients underwent at least one PET scan. In total, 200 PET scans were identified that were performed for evaluation of DTC. The demographics and initial tumor characteristics of patients who underwent PET scan are summarized in Table 1. The median follow-up time was five years, with a minimum of three months and a maximum of 20 years. Sixty patients (54.1%) had only one PET scan, 28 patients (25.2%) underwent two PET scans, 12 patients (10.8%) underwent three PET scans, seven patients (6.3%) underwent four PET scans, three patients (2.7%) underwent five PET scans, and one patient (0.9%) had more than five PET scans.
Table 1.
Demographics of Differentiated Thyroid Cancer Patients who Underwent PET Scan
| Age, years (median, interquartile range) | 51 (37–60.5) |
| Sex | |
| Male | 42 (38%) |
| Female | 69 (62%) |
| Race/ethnicity | |
| White | 102 (91.8%) |
| Asian | 6 (5.4%) |
| African American | 2 (1.8%) |
| Other | 1 (1%) |
| Histology | |
| Papillary | 104 (92.8%) |
| Follicular | 3 (2.7%) |
| Hürthle cell | 4 (3.6%) |
| Stage at diagnosis | |
| I | 51 (45.9%) |
| II | 2 (1.8%) |
| III | 19 (17.1%) |
| IV | 31 (27.9%) |
| Unknown | 8 (7.2%) |
| Size | |
| <1.0 cm | 9 (8.1%) |
| 1.0–1.9 cm | 29 (26.1%) |
| 2.0–3.9 cm | 35 (31.5%) |
| ≥4.0 cm | 23 (20.7%) |
| Unknown | 15 (13.5%) |
| Capsular invasion | |
| Present | 61 (55.0%) |
| Absent | 22 (19.8%) |
| Unknown | 28 (25.2%) |
| Extrathyroidal extension | |
| Present | 58 (52.3%) |
| Absent | 28 (25.2%) |
| Unknown | 25 (22.5%) |
| Lymph node metastasis | |
| Present | 70 (63.1%) |
| Absent | 5 (4.5%) |
| Unknown | 36 (32.4%) |
| Number of PET scans | |
| 1 | 60 (54.1%) |
| 2 | 28 (25.2%) |
| 3 | 12 (10.8%) |
| 4 | 7 (6.3%) |
| 5 | 3 (2.7%) |
| 6+ | 1 (0.9%) |
PET, positron emission tomography.
Indications for all PET scans included: elevated thyroglobulin and negative radioactive iodine scan in 52 scans (26.0%), positive thyroglobulin antibodies in 13 scans (6.5%), rising thyroglobulin in 18 scans (9.0%), evaluation of an abnormality on other imaging test in 22 scans (11%), evaluation of extent of disease in 33 scans (16.5%), follow-up of a previous scan in 57 scans (28.4%), other indications in two scans (1.0%), and unknown indications in three scans (1.5%). In total, 97 scans (48.5%) were done using recombinant thyroid stimulating hormone, 21 scans (10.1%) were performed in a hypothyroid state, 64 scans (32%) were completed without stimulation, and 18 scans (9%) had unknown stimulation.
A total of 124 (62%) of the PET scans were positive and 76 scans (38.0%) were negative. A total of 25 scans had uptake present in the thyroid bed, 105 scans had an abnormality in the cervical and/or mediastinal lymph nodes, 28 scans showed uptake in the lung, four scans showed bone uptake, and 14 scans showed uptake in other areas. Management after PET scan was surgery in 66 cases (33.0%), chemotherapy or radiation in 23 cases (11.5%), observation in 110 cases (55.0%), and palliative care in one case (0.5%). The abnormality was identifiable on other imaging in 82 of 98 cases where other imaging was done (83%). PET changed management in 59 cases (29.5%) overall, did not change management in 138 cases (69.0%), and was unclear in three cases (1.5%). The indications and outcomes of initial PET scans and subsequent PET scans performed in the study are summarized in Table 2.
Table 2.
Characteristics and Outcomes of PET Scans Performed
| Initial PET scan (n=111) | Follow-up PET scan (n=89) | |
|---|---|---|
| Indication for PET scan | ||
| +TG with negative 131I scan | 44 (39.6%) | 8 (8.9%) |
| TG antibodies | 9 (8.1%) | 4 (4.5%) |
| Rising TG | 5 (4.5%) | 13 (14.6%) |
| Abnormality on other imaging test | 19 (17.2%) | 3 (3.4%) |
| Evaluation of extent of disease | 30 (27.0%) | 3 (3.4%) |
| Follow-up of previous PET | N/A | 57 (64.0%) |
| Other | 1 (0.9%) | 1 (1.1%) |
| Unknown | 3 (2.7%) | 0 (0.0%) |
| Method of stimulation | ||
| rhTSH | 46 (41.4%) | 51 (57.3%) |
| Hypothyroid | 17 (15.3%) | 4 (4.5%) |
| Unstimulated | 35 (31.5%) | 64 (32.5%) |
| Unknown | 13 (11.7%) | 5 (5.6%) |
| PET positive vs. negative | ||
| Negative | 40 (36.0%) | 36 (40.5%) |
| Positive | 71 (64.0%) | 53 (59.5%) |
| Location of abnormality* | ||
| Thyroid bed | 18 | 7 |
| Neck | 59 | 46 |
| Lung | 11 | 17 |
| Bone | 2 | 2 |
| Other | 8 | 6 |
| Abnormality present on other imaging | ||
| Yes | 53 (74.6%) | 29 (54.7%) |
| No | 8 (11.3%) | 8 (15.1%) |
| Unknown | 10 (14.1%) | 16 (30.2%) |
| Management following PET | ||
| Surgery | 50 (45.0%) | 16 (18.0%) |
| Chemo or radiation (includes 131I) | 9 (8.1%) | 14 (15.7%) |
| Observation | 52 (46.8%) | 58 (65.2%) |
| Palliation | 0 (0%) | 1 (1.1%) |
| PET changed management** | ||
| Yes | 34 (30.6%) | 25 (28.1%) |
| No | 76 (68.5%) | 138 (69.6%) |
| Unclear | 1 (0.9%) | 2 (2.2%) |
Patient may have disease in more than one location.
PET was considered to have changed management if it identified disease that was not previously identified and resulted in an intervention, led to cancelation of a previously planned intervention, or changed the extent of a previously planned surgery.
TG, thyroglobulin; rhTSH, recombinant human thyrotropin.
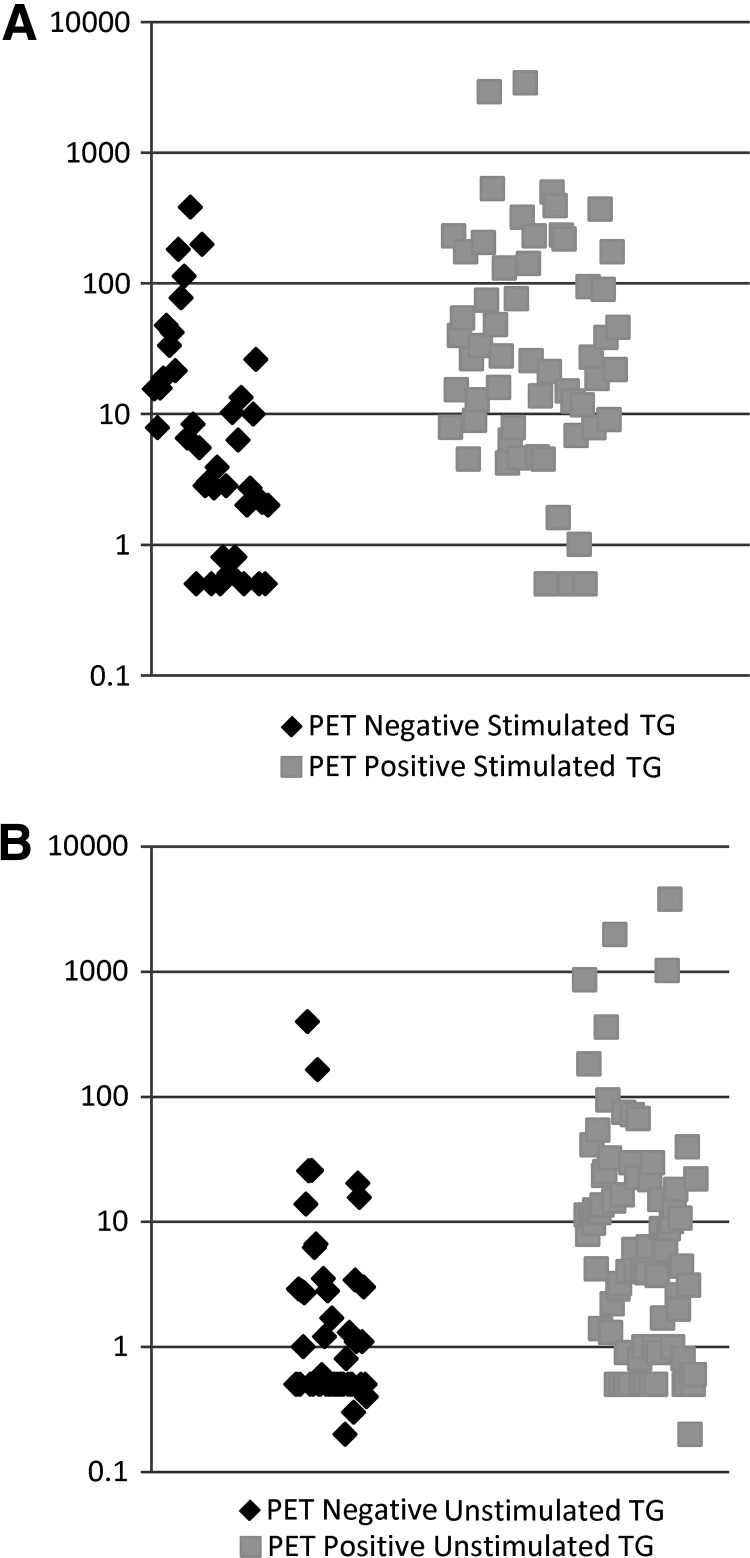
The median stimulated thyroglobulin was 10.95 ng/mL (interquartile range 2.7–47.55 ng/mL). The stimulated thyroglobulin was <10 ng/mL prior to 53 PET scans and undetectable prior to 15 PET scans. The median unstimulated thyroglobulin was 1.85 ng/mL (interquartile range 0.5–11.675 ng/mL). Thyroglobulin values were missing or antibodies were present prior to 48 scans (24.0%). The median stimulated thyroglobulin in patients with a positive PET scan was 26.4 ng/mL (interquartile range 7.9–141 ng/mL) and was 3.45 ng/mL (interquartile range 0.75–15.55 ng/mL) in patients with a negative PET scan (p=0.036). While these groups were statistically different, there was no thyroglobulin level that predicted a positive scan. Scatter plots of the stimulated (1a) and unstimulated (1b) thyroglobulin values are shown in Figure 1.
FIG. 1.
(A) Stimulated thyroglobulin levels in positive versus negative positron emission tomography (PET) scans. (B) Unstimulated thyroglobulin levels in positive versus negative PET scans.
The type of stimulation used in the PET scans did not significantly affect the likelihood of PET imaging to change management (p=0.246). Progressively higher unstimulated and stimulated thyroglobulin—stratified into <10 ng/dL, 10–30 ng/mL, and >30 ng/mL—increased the likelihood that PET scanning would change management (p=0.005 and p=0.004, respectively). Additionally, it was found that PET scans had the greatest utility when evaluating a rising thyroglobulin, where 67% of the scans changed management, compared with 15% of scans changing management if they are performed for follow-up of a previous study (p=0.046). However, the number was small in certain subgroups of the indication analysis (<5). Hence, the results should be interpreted with caution. The corresponding results are shown in Table 3.
Table 3.
Correlates of PET Scan Resulting in Change of Management
| Did PET change management? | ||||
|---|---|---|---|---|
| Yes | No | Unclear | p-Value* | |
| Method of stimulation | ||||
| rhTSH | 34 (35.4%) | 60 (62.5%) | 2 (2.0%) | 0.246 |
| Hypothyroid | 5 (23.8%) | 16 (76.2%) | 0 (0.0%) | |
| Not stimulated | 14 (19.4%) | 57 (79.2%) | 1 (1.3%) | |
| Unknown | 8 (42.1%) | 11 (57.9%) | 0 (0%) | |
| Indication for PET scan | ||||
| +TG with negative 131I scan | 17 (32.7%) | 34 (65.4%) | 1 (1.9%) | 0.046 |
| TG antibodies | 5 (38.5%) | 8 (61.5%) | 0 (0.0%) | |
| Rising TG | 12 (66.7%) | 6 (33.3%) | 0 (0.0%) | |
| Abnormality on other imaging test | 8 (34.8%) | 15 (65.2%) | 0 (0.0%) | |
| Evaluation of extent of disease | 7 (19.4%) | 29 (80.6%) | 0 (0.0%) | |
| Follow-up of previous PET | 9 (15.5%) | 47 (81.0%) | 2 (3.4%) | |
| Other | 3 (50.0%) | 3 (50.0%) | 0 (0.0%) | |
| Unknown | 1 (33.3%) | 2 (66.7%) | 0 (0.0%) | |
| Stimulated TG | ||||
| <10 ng/mL | 7 (14.0%) | 43 (86.0%) | 0.005 | |
| 10–30 ng/mL | 9 (42.9%) | 12 (57.1%) | 0 (0.0%) | |
| >30 ng/mL | 18 (52.9%) | 15 (44.1%) | 1 (2.9%) | |
| Not checked or positive antibodies | 19 (33.3%) | 36 (63.2%) | 2 (3.5%) | |
| Unstimulated TG | ||||
| <10 ng/mL | 20 (21.7%) | 72 (78.3%) | 0 (0.0%) | 0.004 |
| 10–30 ng/mL | 13 (56.5%) | 10 (43.5%) | 0 (0.0%) | |
| >30 ng/mL | 8 (50.0%) | 7 (43.8%) | 1 (6.2%) | |
| Not checked or positive antibodies | 19 (33.3%) | 36 (63.2%) | 2 (3.5%) | |
p for χ2 test.
Discussion
In the present study, a high rate of PET utilization was observed at the authors' institution, with almost 20% of patients with DTC undergoing at least one PET scan. The most common indication for the initial PET scan was an elevated thyroglobulin with noniodine avid disease. However, a large proportion of patients was also seen having an initial PET scan for indications such as evaluation of extent of disease or evaluation of an abnormality that was seen on another imaging study. Overall, the most common indication for any PET scan was follow-up of another PET scan. Additionally, it was observed that most disease identified was localized to the cervical or mediastinal lymph nodes and was often visible on other imaging modalities, such as neck ultrasound or CT. It was found that the PET scan changed management in approximately 30% of cases.
Much of the existing literature surrounding PET scan use for the surveillance of DTC occurs in the setting of an elevated thyroglobulin with noniodine avid disease. For this indication, the PET scan has been shown to be capable of identifying disease with a sensitivity of about 90% and specificity of about 80% (11,12,20). Razfar et al. evaluated the likelihood that a PET scan would change management in this scenario, and found that a PET scan changed the treatment plan in 28.2% of cases, which is similar to the present findings (21). There is a smaller body of literature describing the use of the PET scan for initial staging in intermediate- to high-risk patients with conflicting conclusions regarding its utility (10,22). Others have described the use of the PET scan in the evaluation of patients with persistently elevated thyroglobulin antibodies, and found about half of patients have disease identified on a PET scan (14,15). In the present study, it was found that evaluation of elevated thyroglobulin antibodies with a PET scan was as likely to change management as evaluating elevated thyroglobulin the setting of noniodine avid disease.
While it has been shown to have utility in certain clinical scenarios, PET scans are expensive and expose the patient to radiation (23,24). Therefore, the clinician must use their judgment to determine when they should be utilized. The lesions identified on PET scans could also be found on other imaging modalities, including ultrasound or CT, in 82 of 98 patients. The present study found that the majority of disease that was detected on a PET scan was in the cervical and mediastinal lymph nodes. In the preoperative setting, ultrasound has a higher sensitivity to detect abnormal cervical lymph nodes than PET/CT (25). Given the increased cost and radiation exposure of PET/CT compared with ultrasound, its use should be reserved for indications for which there is strong evidence supporting its utility.
Previous studies have examined the correlation between PET positivity with stimulated serum thyroglobulin level. Collectively, these previous studies suggest that PET/CT may be most helpful in patients with stimulated thyroglobulin levels >20–30 ng/mL (15,26,27). The present study also shows a correlation between increasing stimulated thyroglobulin levels and PET positivity, but there was no thyroglobulin level that predicted presence or absence of disease. The present results do suggest that PET scans done in patients with low thyroglobulin levels are unlikely to change management and should be avoided.
The use of stimulation for PET scanning has been evaluated with somewhat mixed results. Petrich et al. found that the number of positive scans, as well as the number of lesions and standardized uptake values (SUV), increased with the use of rhTSH stimulation (28). In contrast, Leboulleux et al. found that the number of positive scans did not increase, but the number of identified lesions did increase after stimulation with rhTSH; the increased number of lesions changed management in about 6% of cases (29). Despite studies showing that sensitivity of PET imaging improves under TSH stimulation, a high proportion of PET scans being done without stimulation was observed in the present study. However, in this study, a significant effect of stimulation on the likelihood of PET imaging to change management was not seen.
The present study does have several important limitations that should be noted. It is a single center study, and therefore it may not capture imaging that was done at other centers. In order to minimize this limitation, imaging that was done at other centers but read by our radiology department was included. Second, as an academic referral center, we may see a higher proportion of high risk patients compared with those practicing in the community. Therefore, the applicability to smaller community practices may be limited. This is a retrospective study, which inherently has the issue of missing data. However, in the present study, missing data were low for the variables of interest, such as PET indications. Additionally, the categorization of indications for PET scan can be subjective, especially when distinguishing between subtle differences in indication, such as elevated thyroglobulin with negative radioactive iodine scan versus rising thyroglobulin. Nonetheless, it was clear when a PET scan was being ordered for an indication such as evaluation of extent of disease or an abnormality on another imaging test. Finally, with regards to the presence of disease on other imaging studies, providers may be more likely to identify disease with other imaging modalities if they know results of the patient's PET scan.
Despite these limitations, there are several strengths that make this study unique and contribute to the knowledge regarding PET scan use. First, this study includes a large population of patients compared with other studies utilizing PET scans. A real-world experience with PET scan is also reported. Much of the prior work has been done in a population of patients with elevated thyroglobulin and noniodine avid disease, with limited information on the use of PET scans in other scenarios. In addition, the present data suggest that a significant number of PET scans are being done for indications other than noniodine avid disease. Finally, ability to identify disease on other imaging was evaluated, which is often omitted from other studies. When it has been evaluated, results were similar to the present study (13,15).
The rising incidence of low-risk thyroid cancer is changing treatment paradigms (30). Increasingly, management is being driven by risk assessment, and a “one size fits all” approach to thyroid cancer treatment is no longer recommended (31). The same should be true for post-treatment surveillance. The choice of imaging modality should be tailored toward the patient's risk, the clinical suspicion for recurrent or persistent disease, and the serum thyroglobulin. The present study suggests that the PET scan is most helpful in patients with higher thyroglobulin levels in the setting of a negative radioiodine scan or when evaluating a rising thyroglobulin and that routine follow-up of a previous scan is least likely to change management. In addition, attention should be paid to cost-effectiveness and radiation exposure. Future research needs to investigate whether use of an imaging modality can affect patient morbidity and mortality, rather than simply identifying recurrent or persistent thyroid cancer.
Acknowledgments
Dr. Haymart is supported by NIH 1K07CA154595-02. Dr. Papaleontiou is supported by the NIH Institutional National Research Service Award (5T32DK007245-37). Support was provided by the Punya Thyroid Cancer Research Foundation, Michigan Institute for Clinical & Health Research grant support (CTSA: UL1TR000433), and Michigan Endocrine Oncology Repository (HUM00024461). Brittany Gay set up the REDCap database and helped with data entry.
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Davies L, Welch HG. 2006. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. 2014. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 140:317–322 [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferri EL, Jhiang SM. 1994. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 97:418–428 [DOI] [PubMed] [Google Scholar]
- 4.Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, Brokhin M, Omry G, Fagin JA, Shaha A. 2010. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 20:1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacini F, Castagna MG, Brilli L, Pentheroudakis G, Group EGW. 2012. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23:vii110–119 [DOI] [PubMed] [Google Scholar]
- 6.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 7.Smallridge RC, Diehl N, Bernet V. 2014. Practice trends in patients with persistent detectable thyroglobulin and negative diagnostic radioiodine whole body scans: a survey of American Thyroid Association members. Thyroid 24:1501–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palaniswamy SS, Subramanyam P. 2013. Diagnostic utility of PETCT in thyroid malignancies: an update. Ann Nucl Med 27:681–693 [DOI] [PubMed] [Google Scholar]
- 9.Pomerri F, Cervino AR, Al Bunni F, Evangelista L, Muzzio PC. 2014. Therapeutic impact of (18)F-FDG PET/CT in recurrent differentiated thyroid carcinoma. Radiol Med 119:97–102 [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum-Krumme SJ, Gorges R, Bockisch A, Binse I. 2012. (1)(8)F-FDG PET/CT changes therapy management in high-risk DTC after first radioiodine therapy. Eur J Nucl Med Mol Imaging 39:1373–1380 [DOI] [PubMed] [Google Scholar]
- 11.Vera P, Kuhn-Lansoy C, Edet-Sanson A, Hapdey S, Modzelewski R, Hitzel A, d'Anjou J, Basuyau JP. 2010. Does recombinant human thyrotropin-stimulated positron emission tomography with [18F]fluoro-2-deoxy-D-glucose improve detection of recurrence of well-differentiated thyroid carcinoma in patients with low serum thyroglobulin? Thyroid 20:15–23 [DOI] [PubMed] [Google Scholar]
- 12.Caetano R, Bastos CR, de Oliveira IA, da Silva RM, Fortes CP, Pepe VL, Reis LG, Braga JU. 2014. Accuracy of PET and PET-CT in the detection of differentiated thyroid cancer recurrence with negative I whole body scan results: a meta-analysis. Head Neck 2014. September 23 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Lal G, Fairchild T, Howe JR, Weigel RJ, Sugg SL, Menda Y. 2010. PET-CT scans in recurrent or persistent differentiated thyroid cancer: is there added utility beyond conventional imaging? Surgery 148:1082–1089; discussion 1089–1090 [DOI] [PubMed] [Google Scholar]
- 14.Asa S, Aksoy SY, Vatankulu B, Aliyev A, Uslu L, Ozhan M, Sager S, Halac M, Sonmezoglu K. 2014. The role of FDG-PET/CT in differentiated thyroid cancer patients with negative iodine-131 whole-body scan and elevated anti-Tg level. Ann Nucl Med 28:970–979 [DOI] [PubMed] [Google Scholar]
- 15.Ozkan E, Aras G, Kucuk NO. 2013. Correlation of 18F-FDG PET/CT findings with histopathological results in differentiated thyroid cancer patients who have increased thyroglobulin or antithyroglobulin antibody levels and negative 131I whole-body scan results. Clin Nucl Med 38:326–331 [DOI] [PubMed] [Google Scholar]
- 16.Wiebel JL, Banerjee M, Muenz DG, Worden FP, Haymart MR. 2015. Trends in imaging after diagnosis of thyroid cancer. Cancer 121:1387–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritz A, Percy C, Jack A, Shanmurgarathnam K, Sobin L, Parkin DM, Whelan S. (Eds) 2000. International Classification of Diseases for Oncology. Third edition. World Health Organization, Geneva, Switzerland [Google Scholar]
- 19.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M. (Eds) 2002. AJCC Cancer Staging Manual. Sixth edition. Springer, New York, NY [Google Scholar]
- 20.Weber T, Ohlhauser D, Hillenbrand A, Henne-Bruns D, Reske SN, Luster M. 2012. Impact of FDG-PET computed tomography for surgery of recurrent or persistent differentiated thyroid carcinoma. Horm Metab Res 44:904–908 [DOI] [PubMed] [Google Scholar]
- 21.Razfar A, Branstetter BFt, Christopoulos A, Lebeau SO, Hodak SP, Heron DE, Escott EJ, Ferris RL. 2010. Clinical usefulness of positron emission tomography-computed tomography in recurrent thyroid carcinoma. Arch Otolaryngol 136:120–125 [DOI] [PubMed] [Google Scholar]
- 22.Kim MH, O JH, Ko SH, Bae JS, Lim DJ, Kim SH, Baek KH, Lee JM, Kang MI, Cha BY, Lee KW. 2012. Role of [(18)F]-fluorodeoxy-D-glucose positron emission tomography and computed tomography in the early detection of persistent/recurrent thyroid carcinoma in intermediate-to-high risk patients following initial radioactive iodine ablation therapy. Thyroid 22:157–164 [DOI] [PubMed] [Google Scholar]
- 23.Look Hong NJ, Petrella T, Chan K. 2014. Cost-effectiveness analysis of staging strategies in patients with regionally metastatic melanoma. J Surg Oncol 111:423–430 [DOI] [PubMed] [Google Scholar]
- 24.Guttikonda R, Herts BR, Dong F, Baker ME, Fenner KB, Pohlman B. 2014. Estimated radiation exposure and cancer risk from CT and PET/CT scans in patients with lymphoma. Eur J Radiol 83:1011–1015 [DOI] [PubMed] [Google Scholar]
- 25.Jeong HS, Baek CH, Son YI, Choi JY, Kim HJ, Ko YH, Chung JH, Baek HJ. 2006. Integrated 18F-FDG PET/CT for the initial evaluation of cervical node level of patients with papillary thyroid carcinoma: comparison with ultrasound and contrast-enhanced CT. Clin Endocrinol 65:402–407 [DOI] [PubMed] [Google Scholar]
- 26.Ozdemir E, Yildirim Poyraz N, Polat SB, Turkolmez S, Ersoy R, Cakir B. 2014. Diagnostic value of 18F-FDG PET/CT in patients with TENIS syndrome: correlation with thyroglobulin levels. Ann Nucl Med 28:241–247 [DOI] [PubMed] [Google Scholar]
- 27.Vural GU, Akkas BE, Ercakmak N, Basu S, Alavi A. 2012. Prognostic significance of FDG PET/CT on the follow-up of patients of differentiated thyroid carcinoma with negative 131I whole-body scan and elevated thyroglobulin levels: correlation with clinical and histopathologic characteristics and long-term follow-up data. Clin Nucl Med 37:953–959 [DOI] [PubMed] [Google Scholar]
- 28.Petrich T, Borner AR, Otto D, Hofmann M, Knapp WH. 2002. Influence of rhTSH on [(18)F]fluorodeoxyglucose uptake by differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging 29:641–647 [DOI] [PubMed] [Google Scholar]
- 29.Leboulleux S, Schroeder PR, Busaidy NL, Auperin A, Corone C, Jacene HA, Ewertz ME, Bournaud C, Wahl RL, Sherman SI, Ladenson PW, Schlumberger M. 2009. Assessment of the incremental value of recombinant thyrotropin stimulation before 2-[18F]-Fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography imaging to localize residual differentiated thyroid cancer. J Clin Endocrinol Metab 94:1310–1316 [DOI] [PubMed] [Google Scholar]
- 30.Durante C, Costante G, Filetti S. 2013. Differentiated thyroid carcinoma: defining new paradigms for postoperative management. Endocr Relat Cancer 20:R141–154 [DOI] [PubMed] [Google Scholar]
- 31.Nixon IJ, Ganly I, Patel SG, Palmer FL, Di Lorenzo MM, Grewal RK, Larson SM, Tuttle RM, Shaha A, Shah JP. 2013. The results of selective use of radioactive iodine on survival and on recurrence in the management of papillary thyroid cancer, based on Memorial Sloan-Kettering Cancer Center risk group stratification. Thyroid 23:683–694 [DOI] [PubMed] [Google Scholar]