Abstract
Cytokines mediate the interaction of immune cells. Discovery of novel potential cytokines is of great value for both basic research and clinical application. In this study, we identified a novel immune-related molecule, transmembrane protein 98 (TMEM98), through a high-throughput screening platform for novel potential cytokines at a genome-wide level using the strategy of immunogenomics. So far, there is no characteristic and immune-related functional report about it. In this study, we demonstrate that TMEM98 exists as a type II transmembrane protein both in the ectopically and endogenously expressed systems. Interestingly, TMEM98 could also be secreted through exosomes. Moreover, the native secreted form of TMEM98 could be detected in the supernatants of activated human peripheral blood mononuclear cells and mouse CD4+ T cells. Further expression profile analysis showed TMEM98 was upregulated during the activation and differentiation of T helper (Th) 1 cells. Function analysis showed that eukaryotic recombinant TMEM98 (rTMEM98) promoted the differentiation of Th1 cells under both antigen-nonspecific and antigen-specific Th1-skewing conditions. These findings were further confirmed in vivo as prokaryotic rTMEM98 administration significantly increased antigen-specific IFN-γ production and serum antigen-specific IgG2a in the methylated bovine serum albumin-induced delayed-type hypersensitivity model. Overall, these observations emphasize the characteristics and essential roles of TMEM98 for the first time and will be helpful in further understanding the development of Th1 cells.
Introduction
Cytokines are secreted proteins that mediate immune and inflammatory reactions by binding cell surface receptors. They play essential roles in many physiological and pathological processes. Cytokines are mainly produced by macrophages, dendritic cells, and natural killer cells in innate immune responses and CD4+ T cells in adaptive immune responses (Lichtman and Abbas 2009). Except the canonical endoplasmic reticulum (ER)/Golgi-dependent secretory pathway, which can be blocked by brefeldin A (BFA) (Miller and others 1992), they can also be secreted through noncanonical secretory mechanisms (Duitman and others 2011). In addition to determining the differentiation and modulating the activation of CD4+ T cells, cytokines are also major effector molecules of CD4+ T cells.
CD4+ T cells play critical roles in the adaptive immune responses. According to the cytokine-producing pattern and function, they can be classified into T helper (Th) 1, Th2, Th17, Treg, etc. (Zhu and others 2010). Th1 cells, characterized by the expression of a key transcription factor, T-bet (Szabo and others 2000, 2003), are involved in clearing intracellular pathogens as well as participating in antiviral and antitumor immunity. They predominantly produce IFN-γ and are responsible for cell-mediated immune responses, such as delayed-type hypersensitivity (DTH) (Weaver and others 2007).
Until now, many cytokines have been identified to participate in the development of Th1 cells. For example, IL-12 plays a critical role in Th1 cell differentiation, and other cytokines, such as IL-18 (Zhu and others 2010), IL-21 (Suto and others 2006), and IL-27 (Owaki and others 2005), can also influence Th1 cell differentiation or activation through a different pathway. Therefore, identifying novel potential cytokines will provide new insights into understanding of the immune system and the immune responses.
Transmembrane protein 98 (TMEM98) was isolated through a previously reported data mining platform for novel potential cytokines based on the whole human genome using the strategy of immunogenomics (Guo and others 2012; Pan and others 2014; Wang and others 2014). Bioinformatic analysis indicates that it is a type I transmembrane protein and might have modulatory effects on T cells. However, there is no characteristic and immune-related functional report about it.
In this study, we demonstrate that TMEM98 is a type II transmembrane protein and can also be secreted through exosomes. It is upregulated during the activation and differentiation of Th1 cells. Recombinant TMEM98 (rTMEM98) promotes Th1 cell differentiation both in vitro and in vivo. Together, our findings demonstrate the characteristics and a pivotal role of TMEM98 in the development of Th1 cells, which may be useful in further understanding Th1 cell differentiation and development.
Materials and Methods
Databases and gene expression data
TMHMM Server v. 2.0 (www.cbs.dtu.dk/services/TMHMM/) and SignalP (www.cbs.dtu.dk/services/SignalP/) were used for transmembrane helix and signal peptide prediction analysis, respectively. Peptide Atlas (www.peptideatlas.org/) and GeneCards (www.genecards.org/) were used for the expression analysis. Protein Atlas (www.proteinatlas.org) was used for the localization analysis. The Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo/) was used to analyze the expression of TMEM98.
Reagents
The pYD11 vector was purchased from the National Research Council of Canada. pcDNA3.1-myc-his (−) B (pcDB) was purchased from Invitrogen. The pcDB-TMEM98, pEGFP-N1-TMEM98, pYD11-TMEM98-Fc, and pcDB-Tmem98 plasmids were constructed in our laboratory. Three peptides of TMEM98 and the synthetic OVA323–339 (ISQAVHAAHAEINEAGR) peptide corresponding to the immunodominant I-Ab-restricted CD4+ T-cell epitope from OVA were synthesized by the Chinese Peptide Company. DNA transfection was performed using VigoFect (Vigorous) according to the manufacturer's instructions. Protease inhibitor cocktail tablets were obtained from Roche Applied Science. Aldehyde/Sulfate latex (4% w/v, 4 μm) was obtained from Life technologies. Ni-Sepharose™ 6 Fast Flow column and protein G were obtained from GE Healthcare.
Methylated bovine serum albumin (mBSA), PMA, ionomycin, BFA, and monoclonal antibodies against β-actin and myc were purchased from Sigma-Aldrich. Cytokines were purchased from R&D Systems. The Biotin ELISA kit for mouse IFN-γ was purchased from eBiocience. Anti-mouse IgG2a was purchased from Biolegend. Anti-hamster IgG was purchased from ROCKLAND. LY294002, antibodies for Western blot, HRP-conjugated secondary antibodies against mouse, and rabbit IgG were purchased from Cell Signaling Technology (Boston). FITC-conjugated anti-rabbit or anti-mouse immunoglobulin were obtained from Beijing Zhong Shan-Golden Bridge Biological Technology CO., Ltd. Anti-mouse or anti-human CD3 and CD28 antibodies and the fluorescence-labeled antibodies (CD4, CD44, CD62L, CD25,IFN-γ, IL-4) were obtained from BD Biosciences.
Polyclonal antibody preparation
Three peptides from human TMEM98 were synthesized, and polyclonal antibodies were generated by immunizing rabbits 4 times with all 3 peptides together for 100 μg per peptide. Rabbit serum was collected before immunization as a negative control. An ELISA assay showed that serum titers against TMEM98 were as high as 1:106 and could specifically react with the mixed polypeptides.
For advanced studies, the 3 antibodies were purified by immunoaffinity chromatography, respectively. Briefly, the 3 peptides for the rabbit immunization were coupled with CNBr-activated Sepharose 4B (GE Healthcare), respectively, according to the manufacturer's instructions. Phosphate-buffered saline (PBS) was used to wash the beads and dilute the antiserum. After rolling overnight at 4°C, the beads were washed with PBS again, the antibodies were eluted with 0.1 M glycine (pH 2.4), then immediately neutralized with 1 M Tris, pH 9.0. Finally, the eluted antibodies were dialyzed into PBS.
Protein expression and purification
The eukaryotic expression and purification of the TMEM98-myc-DDK (DDK is the same epitope as FLAG from Sigma) protein were commissioned to be produced by OriGene Technologies, Inc. Purity was >90% as determined by SDS-PAGE and Coomassie blue staining, and the endotoxin concentration was <0.1 EU/μg protein by LAL analysis. The prokaryotic expression and purification of TMEM98 (27AA–226AA) were commissioned to be produced by Crown Bioscience, Inc.; the GST tag was removed using the thrombin. Protein purity was >90% as determined by SDS-PAGE and Coomassie blue staining, and the average endotoxin concentration was 40.9 EU/mg protein.
Mice
Female C57BL/6 mice (6–8 weeks old) were purchased from Vital River Laboratory Animal Technology Co., Ltd., and Beijing HFK Bioscience Co., Ltd. OT-II (OVA323–339 peptide-specific CD4 TCR transgenic) mice on a C57BL/6 background were a kind gift from Yong Zhao (State Key Laboratory of Biomembrane and Membrane Biotechnology, Institute of Zoology, Chinese Academy of Sciences). Animals were bred in the animal breeding facilities at Peking University Health Science Center (Beijing, China) under specific pathogen-free conditions. Animal experimental procedures were approved by the ethics committee of Peking University Health Science Center.
Cell preparation and culture
Human embryonic kidney (HEK) 293T (a kind gift from T. Matsuda, Japan) cells and human cervical carcinoma (HeLa) cells were maintained in Dulbecco's modified Eagle's medium (Life Technologies) supplemented with 10% fetal bovine serum (FBS; Hyclone) and L-glutamine (4 mM). Human peripheral blood mononuclear cells (PBMCs) were isolated from the peripheral blood of healthy donors (Beijing Blood Center) using Ficoll/Hypaque density gradient centrifugation as previously described (Li and others 2006).
CD4+ T cells were purified by negative selection from female C57BL/6 mice lymph nodes (LNs) following the manufacturer's instructions (Invitrogen) and stimulated in 48-well plates coated with anti-mouse CD3e (2C11; 10 μg/mL) and soluble anti-mouse CD28 (37.51; 2 μg/mL). Naïve CD4+ T cells (CD4+ CD62LhighCD25-CD44low) were sorted from the LNs of female C57BL/6 mice using the FACS Aria (BD Biosciences) cell sorter and stimulated in 48-well plates coated with rabbit anti-hamster primary antibody (pAb; 10 μg/mL, 2 h at 37°C), followed by secondary antibodies (30 min at 37°C) of hamster anti-mouse CD3e (2C11; 0.1 μg/mL) and soluble anti-mouse CD28 (37.51; 2 μg/mL) (de Souza and others 2008). The immune cells were maintained in RPMI 1640 supplemented with 10% heat-inactivated FBS, 4 mM L-glutamine, 100 IU/mL penicillin, 0.1 mg/mL streptomycin, and 200 IU/mL gentamicin for the FACS-sorted cells.
Western blot analysis
Cells were washed twice with prechilled PBS, pelleted by centrifugation, and lysed in lysis buffer (10 mM Hepes, pH 7.4, 0.15 M NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 0.5% NP-40, with proteinase inhibitor cocktail). After incubation for 30 min on ice, lysates were centrifuged (12,000 g, 10 min, 4°C). The supernatants were collected, and the protein concentration was measured using a BCA protein assay reagent (Pierce), with bovine serum albumin as the standard.
Total protein (20 μg) was separated on 12.5% SDS-PAGE, and then transferred to nitrocellulose membranes (Hybond™, ECLTM; Amersham Pharmacia). After being blocked in 5% fat-free milk in Tris-buffered saline containing 0.05% Tween 20 (TBS-T) for 2 h at room temperature, the membranes were incubated with the appropriate pAbs (1 μg/mL; anti-myc, 0.5 μg/mL) overnight at 4°C, washed thrice with TBS-T, and then incubated with appropriate HRP-conjugated secondary antibody (1:2,000) with gentle agitation for 1 h at room temperature. Following 3 additional washes with TBS-T, the membranes were incubated with Super ECL Plus (APPLYGEN), drained of excess developing solution (do not let dry), wrapped in plastic wrap, and then exposed to x-ray film.
Indirect immunofluorescence staining
Cells were harvested and washed twice with PBS and blocked for 30 min in a blocking buffer (10% BSA in PBS), then stained with either pAb1 (10 μg/mL) or the anti-myc antibody (10 μg/mL), followed by the respective secondary antibodies (FITC-conjugated anti-rabbit or anti-mouse immunoglobulin).
For intracellular staining, the washed cells were fixed in 4% paraformaldehyde (PFA) and perforated with 0.1% Triton X-100/1% BSA before staining. The samples were analyzed by an FACSCalibur flow cytometer (BD Biosciences) (10,000 cells were collected) and FlowJo software (BD). For confocal analysis, cells grown on coverslips in 24-well plates were stained as described above. After staining with antibodies, cells were stained with Hoechst 33342 (1:10,000; Beyotime Institute of Biotechnology) for 5 min at 37°C. After washing, samples were observed with an Olympus laser-scanning confocal microscope (FluoView FV300; Olympus).
BFA and LY294002 inhibition assay
HEK293T cells were transfected with pcDB-TMEM98 or pcDB plasmids and incubated in HEK293 serum-free medium (SAFC Biosciences). After 24 h, 10 μg/mL of BFA, 50 μM LY294002, or ethanol/DMSO (used as a negative control) were added to the cell culture supernatants for another 24 h. Finally, the cell lysates and supernatants (50 μL) were harvested for Western blotting.
Sucrose density gradient centrifugation
Vesicles were isolated from the conditioned medium by sequential centrifugation. Briefly, the serum-free culture medium of transfected HEK293T cells was collected at 48 h after transfection and centrifuged (800 rpm for 10 min at 4°C, 2,000 g for 10 min at 4°C, and then 10,000 g for 30 min at 4°C) to remove cells and cell debris. Then, they were suspended in 1 mL of 2.5 M sucrose, 20 mM Hepes, pH 7.4, and floated into an overlaid linear sucrose density gradient (2.0–0.5 M sucrose, 20 mM Hepes, pH 7.4) at 100,000 g for 16 h at 4°C in a Beckman SW40 rotor as previously described (Li and others 2010). Fractions (400 μL) were collected from the top of the tube and the density was determined. Each fraction was sedimented by ultracentrifugation at 100,000 g for 70 min at 4°C in an SW40 rotor. After washing, the vesicles were analyzed by SDS-PAGE and Western blotting.
Immunoprecipitation
Human PBMCs (2×106/mL) were rested or stimulated using plate-bound anti-CD3 (1 μg/mL) and anti-CD28 (2 μg/mL) for 72 h. Subsequently, the supernatants (40 mL) were collected and concentrated using a filter tube (3 kD; Millipore) to 1 mL. The supernatants (6 mL) from the mouse activated CD4+ T cells were collected at different time points. Then, those supernatants were precipitated with pAb1 (2 μg) overnight at 4°C. Next, protein G beads (30 μL) were added, and the cultures were further incubated (overnight at 4°C). After washing, the pellet was resuspended with the loading buffer and microcentrifuged. The supernatants were collected as samples, and pAb2 was used for Western blot analysis.
In vitro expansion of mouse Th cells
CD4+ T cells or naïve CD4+ T cells (1×106/mL) from wild-type C57BL/6 mice were stimulated in 48-well plates as described above. For nonpolarizing (Th0) conditions, naïve CD4+ T cells were cultured with IL-2 (4 ng/mL), anti-IL-4 (10 μg/mL; 11B11), anti-IFN-γ (XMG1.2; 10 μg/mL), and anti-IL-12 (C17.8; 10 μg/mL); for Th1 conditions, cells were cultured with IL-2 (4 ng/mL), IL-12 (10 ng/mL), and anti-IL-4 (10 μg/mL; 11B11); and for Th2 conditions, cells were cultured with IL-2 (4 ng/mL), IL-4 (50 ng/mL), and anti-IFN-γ (XMG1.2; 10 μg/mL). Cells were split on day (d) 3 and supplemented with fresh cytokines and rested for an additional 2 days.
When OT-II TCR transgenic mice were used, anti-CD3e and anti-CD28 were replaced with OVA peptide (amino acids 323–339; 1 μg/mL) and DC. Briefly, CD11c+ DC cells isolated by an FACSAria (BD Biosciences) cell sorter from the spleen were pulsed overnight with OVA peptide (OVA323–339). Sorted, naïve OT-II TCR transgenic CD4+ T cells (1×106/mL) were cocultured with irradiated (3,000 rad) DCOVA (1×105/mL) in the Th1 condition as described above.
Semiquantitative reverse transcription–polymerase chain reaction and real-time polymerase chain reaction
Total RNAs of immune cells were isolated using TRIzol (Invitrogen) according to the manufacturer's instructions. Oligo (dT) primers were used for cDNA synthesis. Reverse transcription was performed on equal amounts of RNA for each sample using a RevertAid™ First Strand cDNA Synthesis Kit (Fermentas). Mouse Tmem98 PCR amplification was performed using the following nested primers: external forward primer, 5′-TGCAATTGAGCTTCCACCTG-3′, and reverse primer, 5′-GGCCGACAGAGCTCTAGAGAAC-3′; and internal forward primer, 5′-CCGGAATTCCACCATGGAGACTGTGGTGATCGTC-3′, and reverse primer, 5′-CGGGGTACCTTAAATGGCCGACTGTTCCTGC-3′.
DNA was denatured at 94°C for 5 min, followed by 30 cycles at 56°C and extension for 1 min at 72°C. The products of the first amplification were diluted 50-fold as the templates of the second amplification, in which 28 cycles were carried out at 58°C. The positive control Ifnγ was amplified for 28 cycles, while Il-4 was amplified for 32 cycles. The primers used in this study are shown in Table 1. Gapdh was amplified as before (Zhong and others 2006) by 22 cycles. Five microliters of the polymerase chain reaction (PCR) product was analyzed on a 1.0% agarose gel.
Table 1.
Primers Used for Semiquantitative Reverse Transcription–Polymerase Chain Reaction and Real-Time Polymerase Chain Reaction
| Target genes | Detection method | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|---|
| Ifnγ (Mus musculus) | SqRT-PCR | TTCTCCTCCTGCGGCCTAGCT | AAAATTCAAATAGTGCTGGCAGAAT |
| Il-4 (Mus musculus) | SqRT-PCR | ACCACAGAGAGTGAGCTCGTCT | GCATGGTGGCTCAGTACTACGAGT |
| Gapdh (Mus musculus) | SqRT-PCR | TGAAGGTCGGAGTCAACGGATTTGGT | CATGTGGGCCATGAGGTCCACCAC |
| Tmem98 (Mus musculus) | Real-time PCR (UPL) | CCAAGCTCCTGGATGCAC | CCAAGCTCCTGGATGCAC |
| Ifnγ (Mus musculus) | Real-time PCR (SYBR) | CAGCAACAGCAAGGCGAAA | CTGGACCTGTGGGTTGTTGAC |
| Tbx21 (Mus musculus) | Real-time PCR (UPL) | CAACCAGCACCAGACAGAGA | ACAAACATCCTGTAATGGCTTG |
| Actb (Mus musculus) | Real-time PCR (UPL) | CTAAGGCCAACCGTGAAAAG | ACCAGAGGCATACAGGGACA |
PCR, polymerase chain reaction; SqRT-PCR, semiquantitative reverse transcription–polymerase chain reaction; UPL, Universal Probe Library.
Real-time PCR was performed for quantitative analyses in an ABI Prism 7000 Sequence Detection System (Applied Biosystems) (Zhong and others 2006). Amplifications were carried out using the SYBR Green PCR Master Mix Kit (Applied Biosystems) or Universal Probe Library (UPL) probes. The quantification data were analyzed with ABI Prism 7000 SDS software. The expression levels of the target genes were measured as fluorescence signal intensities and normalized to the internal standard gene beta-actin (Actb). The samples with unspecific amplification were regarded as zero. The primers used in this study are shown in Table 1.
Flow cytometry analysis of vesicles
Thirty micrograms of vesicles prepared from the cell supernatant or medium (used as a negative control) were incubated with 10 μL 4-μm-diameter aldehyde/sulfate latex beads (Life technologies) in a final volume of 30–100 μL for 15 min at room temperature, followed by gentle shaking in 1 mL PBS for 2 h. The reaction was stopped by incubation in 100 mM glycine for 30 min. Vesicle-coated beads were washed thrice in the FACS wash (3% FBS in PBS) .Vesicle-coated beads were incubated for 40 min with pAb1 at 4°C, followed by incubation with an FITC-conjugated secondary antibody, and analyzed on an FACSCalibur flow cytometer (BD Biosciences).
DTH mice models
The DTH response to mBSA (Sigma) was measured by footpad swelling, as described (Kim and others 1998). Briefly, 8-week-old female C57BL/6 mice were sensitized by an intradermal injection of 50 μL of 2.5 mg/mL mBSA emulsified with complete Freund's adjuvant (CFA; Sigma-Aldrich) at 2 sites on the abdomen. Six days after immunization, mice were challenged by a 30 μL injection of 5 mg/mL mBSA in PBS into 1 rear footpad, while the other rear footpad received a comparable volume of PBS as the control. Footpad swelling was measured using a dial caliper at the indicated times after the challenge. The magnitude of the DTH response was determined as follows: footpad swelling (mm)=footpad thickness of mBSA-injected footpad (mm) – footpad thickness of PBS-injected footpad (mm).
Flow cytometry
For cytokine analysis, cells were restimulated with 50 ng/mL PMA and 500 ng/mL ionomycin in the presence of GolgiStop (BD Biosciences) for 5 h. For intracellular staining, cells were fixed and permeabilized with Cytofix/Cytoperm buffer (BD Biosciences) according to the manufacturer's instructions. Fixed and permeabilized cells were stained with antibodies or the appropriate isotype control. Labeled cells were analyzed by flow cytometry. Data were analyzed using FlowJo software (Tree Star) by gating on live cells based on their forward versus side scatter profiles.
Measuring cytokines and anti-mBSA antibody levels
All cytokines and mBSA-specific antibody levels were detected by ELISA according to the manufacturer's instructions. mBSA-specific antibody levels were detected by ELISA with standard methods. Briefly, plates were coated with 10 μg/mL of mBSA. Serum was diluted 10-fold in PBS containing 10% fetal calf serum and incubated in mBSA-coated wells, and then reacted with biotinylated IgG2a (0.5 μg/mL; RMG2a-62; Biolegend), followed by HRP-conjugated streptavidin (eBiosciences), and then developed with tetramethylbenzidine substrate. The detection limit of IFN-γ (eBiosciences) is 15 pg/mL.
Statistical analysis
Data are expressed as mean±SEM and tested for statistical significance by the 2-tailed Student's t-test using GraphPad Prism 5. Values of P<0.05 were considered statistically significant, where *P<0.05, **P<0.01, and ***P<0.001.
Results
Preparation and identification of pAbs against TMEM98
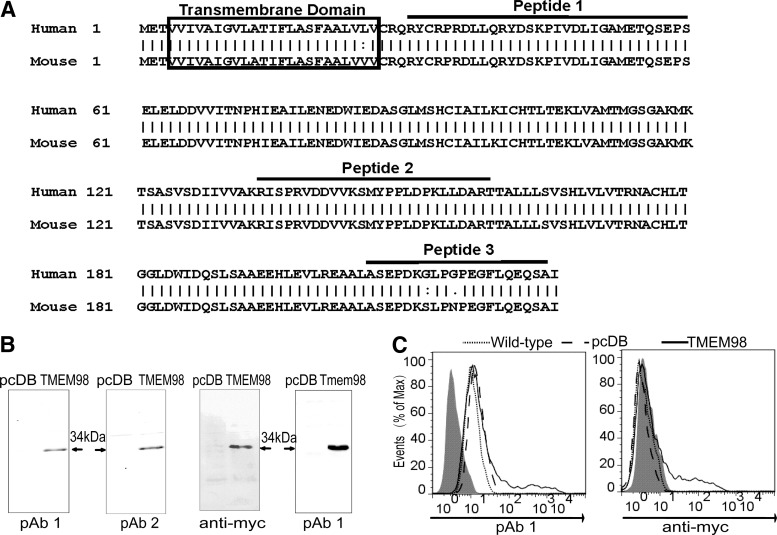
The TMHMM Server v. 2.0 indicated that TMEM98 was a type I transmembrane protein with a transmembrane helix from the 4th to the 26th amino acid (Fig. 1A). To analyze the subcellular localization of TMEM98, the polyclonal antibodies (pAbs) were first prepared using 3 synthetic peptides contained within TMEM98 (Fig. 1A) together as immunogens. After purification separately, the pAbs against TMEM98 derived from the first and the second peptide (pAb1 and pAb2) were found to have higher titers.
FIG. 1.
Preparation and identification of the pAbs against TMEM98. (A) The TMEM98 amino acid sequence was derived from GenBank (accession numbers: NM_015544.2), and human and mouse TMEM98 protein sequences were aligned. The boxes indicate the predicted transmembrane domain. The 3 peptides used to prepare polyclonal antibodies are indicated. (B) The cell lysates of HEK293T cells transiently transfected with pcDB, pcDB-TMEM98, and pcDB-Tmem98 were detected using anti-TMEM98 pAbs, respectively. The anti-myc antibody served as a positive control. pAb1 and pAb2 indicated pAbs against TMEM98 derived from the first and the second peptide, respectively. (C) pAb1 was tested for indirect immunofluorescence staining. HEK293T cells transiently transfected with pcDB-TMEM98 (TMEM98, solid line) or pcDB (pcDB, dashed line) were stained with pAb1 or anti-myc antibody after perforation. Samples were analyzed by flow cytometry. Wild-type (wild-type, dotted line) indicated the nontransfected HEK293T cells. The shading represents the staining of the isotype control. Data are representative of 3 independent experiments. HEK, human embryonic kidney; pAb, primary antibody; TMEM98, transmembrane protein 98.
Western blot analysis showed that compared with pAb2, pAb1 exhibited higher specificity and could recognize both human and mouse TMEM98 (Fig. 1B). Moreover, intracellular indirect immunofluorescence staining showed that pAb1 could also identify the overexpressed TMEM98 protein in HEK293T cells, which was consistent with the results using the anti-myc antibody (the positive control) (Fig. 1C). Therefore, pAb1 is suitable for Western blot and flow cytometry analyses.
TMEM98 is a type II transmembrane protein
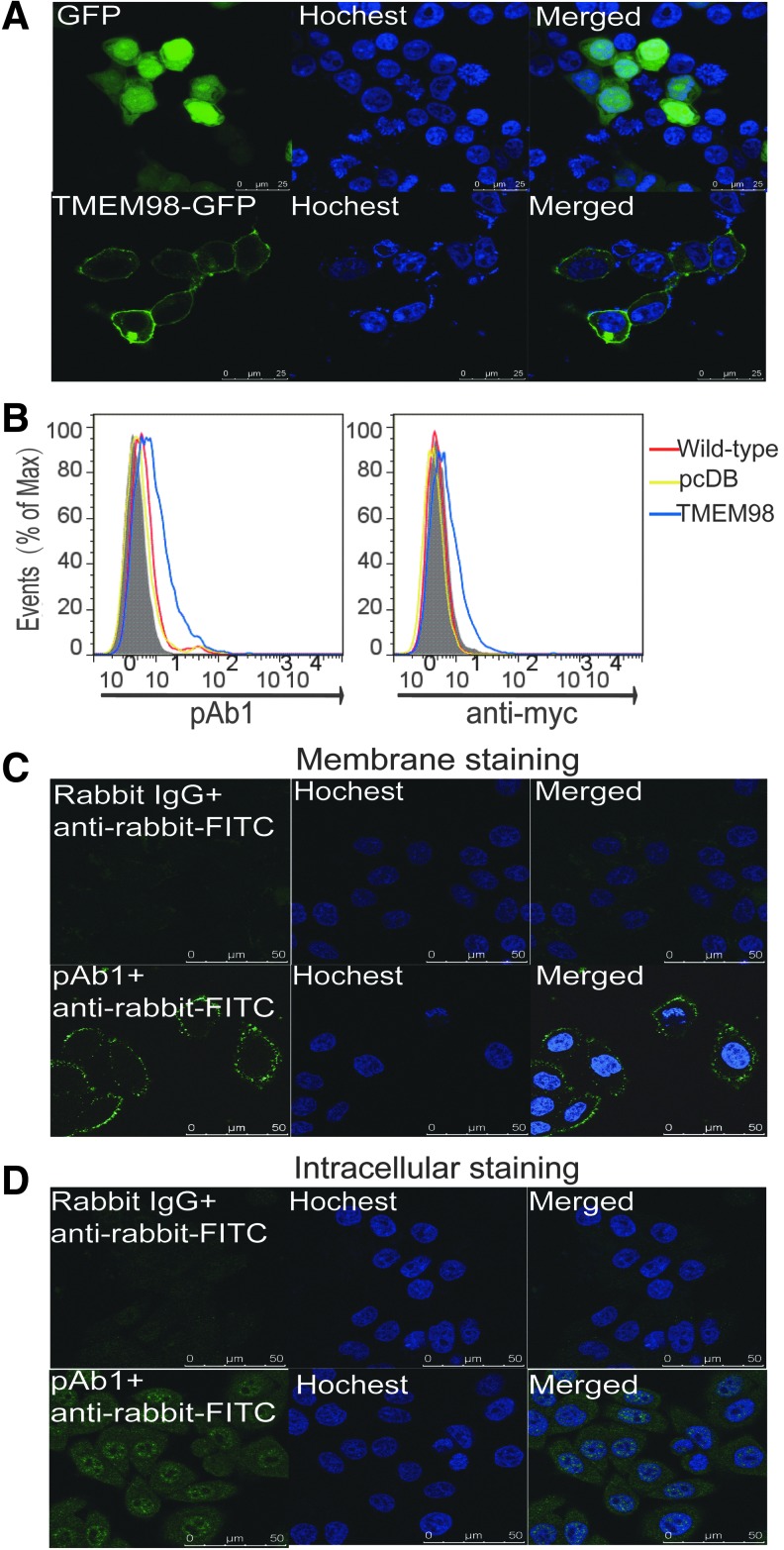
Transmembrane proteins have been classified as type I to type VI according to their transmembrane forms (Chou and Cai 2005). To verify if TMEM98 is indeed a type I transmembrane as predicted, HEK293T cells were transfected with pEGFP-N1-TMEM98 or control pEGFP-N1 plasmids. As illustrated in Fig. 2A, while EGFP signals were evenly distributed throughout the entire cell in the control group, the TMEM98-EGFP fusion proteins were clearly distributed only on the cell membrane, confirming that TMEM98 is a transmembrane protein.
FIG. 2.
TMEM98 is a type II transmembrane protein. (A) pEGFP-N1-TMEM98 and pEGFP-N1 were transfected in HEK293T cells, respectively. After staining nuclei with Hoechst (blue), the cells were analyzed using an Olympus laser-scanning confocal microscope. Scale bars represent 25 μm. (B) Indirect immunofluorescence was performed using the pAb1 or anti-myc antibody to examine the surface TMEM98 on nonperforated HEK293T cells transfected with pcDB-TMEM98 (TMEM98, blue) or the pcDB vector (pcDB, yellow). Samples were analyzed by flow cytometry. Wild-type (wild-type, red) indicated the nontransfected HEK293T cells. The shading represents staining with the isotype control. Representative result from 3 independent experiments is shown. The localization of endogenous TMEM98 was detected on HeLa cells without (C) or with perforation (D) with indirect immunofluorescence as described, and it was observed with a confocal microscope. Rabbit IgG or pAb1+anti-rabbit-FITC indicated that cells were first stained with either rabbit IgG or pAb1, followed by FITC-conjugated anti-rabbit immunoglobulin. Scale bars represent 50 μm. Data are representative of 2 independent experiments.
Type I membrane proteins are single-pass transmembrane proteins with their N-terminus extracellularly exposed, while type II membrane proteins have their C-terminus exposed outside. To test the localization of TMEM98, HEK293T cells transiently transfected with pcDB-TMEM98 were surface stained with pAb1 under nonpermeabilizing condition. Similar results were obtained by surface staining for the myc protein located at the C-terminal end of the protein with an anti-myc Ab (Fig. 2B), indicating that TMEM98 is a type II membrane protein with the C-terminus outside of the cell membrane.
Moreover, the localization of endogenous TMEM98 in HeLa cells was detected using pAb1 by confocal microscopy. As shown in Fig. 2C, the native TMEM98 also localizes on the cell surface with an extracellular C-terminus, which further confirms that TMEM98 is a type II transmembrane protein. Interestingly, when the membrane was perforated, positive signals could also be detected in the cytoplasm and nucleus, but not the nucleoli (Fig. 2D), which is consistent with the result from Protein Atlas.
TMEM98 could be secreted through exosomes
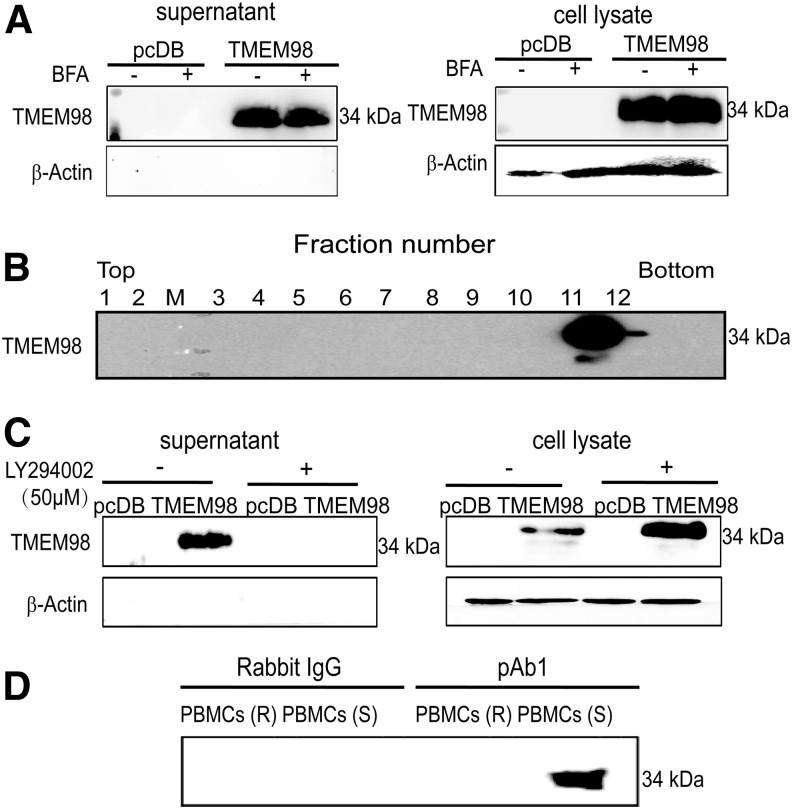
SignalP 3.0 identifies a potential signal peptide in TMEM98. Moreover, data from both the Peptide Atlas database and GeneCards show that TMEM98 is highly expressed in plasma. To verify whether TMEM98 could be secreted, we examined the supernatants from HEK293T cells transiently transfected with the pcDB-TMEM98 plasmid. Western blot analysis showed that an intense band at ∼34 kDa appeared in the supernatant (Fig. 3A), indicating that TMEM98 also exists in secreted forms.
FIG. 3.
TMEM98 can be secreted through a nonclassical pathway. (A) HEK293T cells transfected with pcDB-TMEM98 or pcDB were incubated with brefeldin A (10 μg/mL) or ethanol (control) for 24 h. The expression level of TMEM98 was detected in the supernatants and cell lysates from the transfected HEK293T cells by Western blot using pAb1. β-actin served as a cell lysate protein loading control and as the negative control for supernatant proteins. The experiment was repeated at least thrice. (B) The medium of the transfected HEK293T cells was floated into a continuous sucrose density gradient. Aliquots from each of the 12 fractions were analyzed by Western blot with pAb1 and their densities are 1, 1.04 g/mL; 2, 1.05 g/mL; 3, 1.06 g/mL; 4, 1.07 g/mL; 5, 1.09 g/mL; 6, 1.11 g/mL; 7, 1.12 g/mL; 8, 1.14 g/mL; 9, 1.16 g/mL; 10, 1.18 g/mL; 11, 1.19 g/mL; and 12, 1.15 g/mL. (C) HEK293T cells were transfected as in (A) and were incubated with 50 μM LY294002 or DMSO (control) for 24 h. The cell lysates and supernatants were harvested and analyzed as described in (A). β-actin served as a cell lysate loading control and negative control for supernatants. The pcDB vector was used as the negative control for all experiments. Data are representative of 3 independent experiments. (D) The endogenous secreted form of TMEM98 was immunoprecipitated using pAb1 or rabbit IgG from the supernatant of PBMCs, which were rested (R) or stimulated (S) using anti-CD3e and anti-CD28 for 72 h. The samples were used for Western blot analysis with pAb2. The pcDB vector was used as the negative control for all experiments. Data are representative of 2 independent experiments. PBMCs, peripheral blood mononuclear cells.
Soluble secretory proteins containing N-terminal signal peptides are usually secreted through the classical or ER/Golgi-dependent secretory pathway, which can be blocked by BFA (Miller and others 1992). Further experiments revealed that TMEM98 secretion could not be inhibited by BFA (Fig. 3A), suggesting that it is a nonclassically secreted protein. To further explicit the cleavage site of the secreted form of TMEM98, rTMEM98 was purified and subjected to N-terminal sequencing. Intriguingly, the sequence corresponded to the full-length TMEM98 without N-terminal cleavage (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/jir), supporting that TMEM98 is secreted through a nonclassical pathway.
There are 4 principal types of unconventional protein secretions that can be distinguished into nonvesicular and vesicular pathways. The nonvesicular pathways include self-sustained protein translocation across plasma membranes (type I) and ABC transporter-based secretion (type II). Vesicular pathways encompass autophagy-based secretion (type III) and proteins that bypass the Golgi complex for trafficking to the plasma membrane (type IV), such as integral membrane proteins (Rabouille and others 2012).
As TMEM98 is secreted in full length, we first determined whether TMEM98 was secreted through vesicular pathways. As shown in Fig. 3B, TMEM98 could be sedimented from the conditioned medium through sequential centrifugation, indicating that it is secreted as vesicles. Specifically, the secreted TMEM98 floated on a linear sucrose density gradient at a peak density of 1.18 g/mL (Fig. 3B). Moreover, as shown in Fig. 3C, the secreted TMEM98 could be inhibited by LY294002, a phosphatidylinositol (PI) 3′-kinase inhibitor that can inhibit internal vesicle formation within multivesicular bodies (MVBs) (Fernandez-Borja and others 1999). These data indicate that TMEM98 could be secreted through exosomes.
TMEM98 exists in plasma according to the Peptide Atlas database and GeneCards. To verify the existence of the native secreted form of TMEM98, we first analyzed the expression pattern of TMEM98 on the mRNA level in normal tissues and organs using semiquantitative reverse transcription–polymerase chain reaction (SqRT-PCR) (Supplementary Fig. S2A) and real-time PCR (Supplementary Fig. S2B). TMEM98 was widely expressed in most tissues with particularly high expression in the ovary, pancreas, and prostate.
GEO profile analysis (GDS2164/223170_at/TMEM98) showed that TMEM98 was increased in simian immunodeficiency virus Nef protein-expressing CD4+ T cells. Therefore, we further detected the protein expression of TMEM98 in the supernatants of the anti-CD3e and anti-CD28-activated PBMCs. As the pAb1could enrich the secreted form of TMEM98 in the supernatants from transiently transfected HEK293T cells (Supplementary Fig. S3), the native secreted TMEM98 was immunoprecipitated using pAb1 from the supernatants of resting and activated PBMCs and subjected to Western blotting with pAb2 as the detection Ab. The intense band suggested the existence of secreted TMEM98 in the supernatants of the activated, but not the rested, PBMCs (Fig. 3D), which also indicates that TMEM98 might be involved in the activation of T cells.
TMEM98 is upregulated during the activation and differentiation of Th1 cells
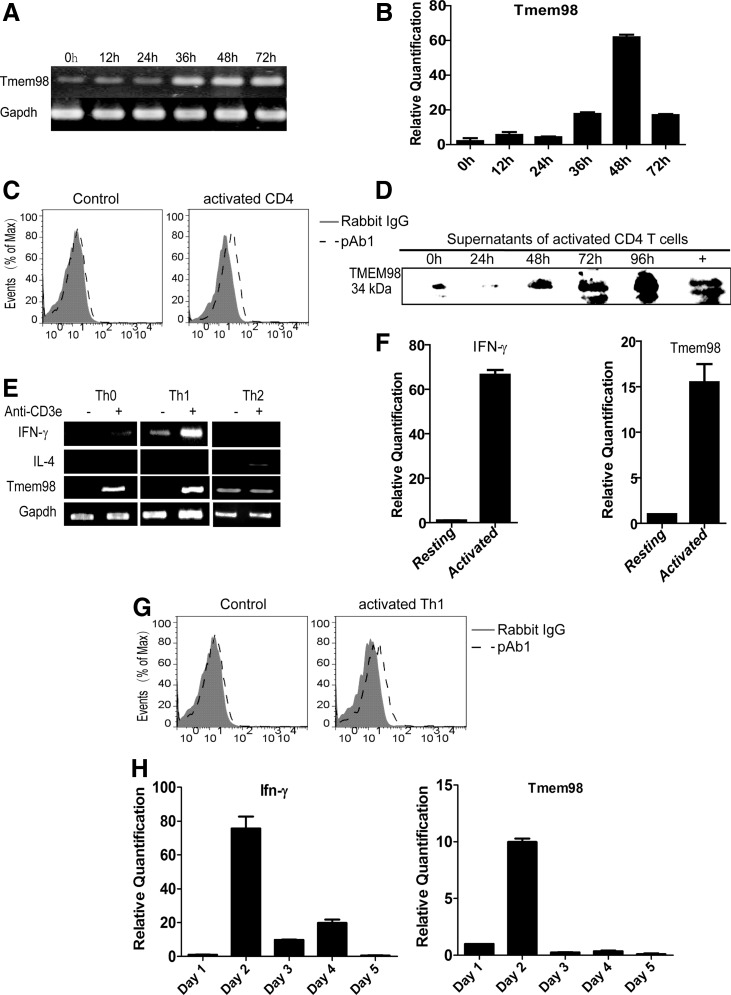
TMEM98 is highly conserved during evolution, with humans and mice sharing 98.7% identity on the whole amino acid sequence. To explore the expression and function of TMEM98 deeply, we detected the kinetic expression of Tmem98 on the naïve CD4+ T cells harvested from wild-type C57BL/6 mice and primed with anti-CD3e and anti-CD28. As shown in Fig. 4A, B, Tmem98 was significantly increased on the stimulated CD4+ T cells compared with resting cells, peaking at 48 h after activation.
FIG. 4.
TMEM98 is upregulated during the activation and differentiation of Th1 cells. Sorted naïve CD4+ T cells were stimulated with anti-CD3e/CD28 and harvested at various time points. Tmem98 transcriptional expression was analyzed by SqRT-PCR (A) and real-time PCR (B). Gapdh and Actb were used as the internal control. The expression level of Tmem98 at 0 h was treated as the baseline. The experiment was repeated at least twice. (C, D) The protein expression of TMEM98 during the activation of CD4+ T cells through immunofluorescence analysis (C) and Western blot (D). After being coated on latex beads and labeled by pAb1 (pAb1, dashed line) indirectly, vesicles prepared from the supernatants of activated mouse CD4+ T cells (C) and Th1 (G) cells by sequential centrifugation were detected by flow cytometry. Gray histograms were obtained with a matching isotype (Rabbit IgG, solid line and filled with gray). Vesicles prepared from the medium were used as the negative control. Data are representative of 2 independent experiments. The secreted form of TMEM98 was immunoprecipitated using pAb1 from the supernatant of activated CD4+ T cells at different time points and the samples were used for Western blot analysis with pAb2.+means supplementing 1.5 μg prokaryotic protein into the medium before immunoprecipitation. Naïve CD4+ T cells were stimulated in vitro with anti-CD3e/CD28 antibodies and cultured under the Th0 and Th1 conditions separately as described in the Materials and Methods section. On day 5, cells were rested or restimulated with plate-bound anti-CD3e antibody for 24 h, and the expression of Tmem98 was detected by SqRT-PCR (E) and real-time PCR (F). The expression level of Tmem98 on the resting Th1 cells was treated as the baseline. Gapdh and Actb were used as the internal control, and Ifnγ and Il-4 were used as the positive control. The experiment was repeated at least thrice. (H) Naïve CD4+ T cells were stimulated in vitro with anti-CD3e/CD28 antibodies and cultured on the Th1 conditions separately as described in the Materials and Methods section. Cells were collected at various time points during the differentiation process, and RNA was isolated for expression profile analysis by real-time PCR. The expression level of Tmem98 on day 1 was set as 1. Actb was used as the internal control, and Ifnγ was used as the positive control. Data are representative of 2 independent experiments. SqRT-PCR, semiquantitative reverse transcription–polymerase chain reaction; Th1, T helper 1.
The vesicles can be accurately quantified by regular flow cytometry after adsorption onto the latex beads (Valadi and others 2007). Using this method, we found that the protein expression of TMEM98 in the supernatant of stimulated CD4+ T cells was also increased (Fig. 4C), which was further confirmed through Western blot analysis (Fig. 4D). These results demonstrate that the expression of TMEM98 is increased both at the mRNA and at the protein level during the activation of CD4+ T cells.
GEO profile analysis (GDS892/10383/TMEM98) suggested that ectopic expression of mutant GATA3 in 293T cells resulted in the upregulation of TMEM98. Therefore, we investigated the expression of Tmem98 on resting and activated Th0, Th1, and Th2 cells further by SqRT-PCR and real-time PCR. SqRT-PCR results showed that Tmem98 was rapidly induced upon activation on Th0 and Th1 cells (Fig. 4E), and real-time PCR confirmed that Th1 cells prominently expressed Tmem98 (Fig. 4F). However, although they existed on the resting Th2 cells, the expression of Tmem98 had no obvious changes upon activation (Fig. 4E). Using the above demonstrated vesicle quantification analysis method, we found that the secreted TMEM98 was also increased in the supernatant of stimulated Th1 cells (Fig. 4G).
Furthermore, we detected the mRNA expression of Tmem98 during the differentiation of mouse Th1 cells. As shown in Fig. 4H, Tmem98 was elevated significantly during Th1 cell differentiation and peaked at day 2, which was similar to the expression kinetics of Ifnγ. These results indicate that TMEM98 is probably involved in the activation and differentiation of Th1 cells.
TMEM98 promotes the differentiation of Th1 cells in vitro
Based on the expression profile analysis, it is reasonable to evaluate the effects of TMEM98 on Th1 cells. To perform a functional study, we first expressed and purified the eukaryotic rTMEM98 with high quality (Supplementary Fig. S4A). Then, its effects on the differentiation and activation of Th1 cells were investigated.
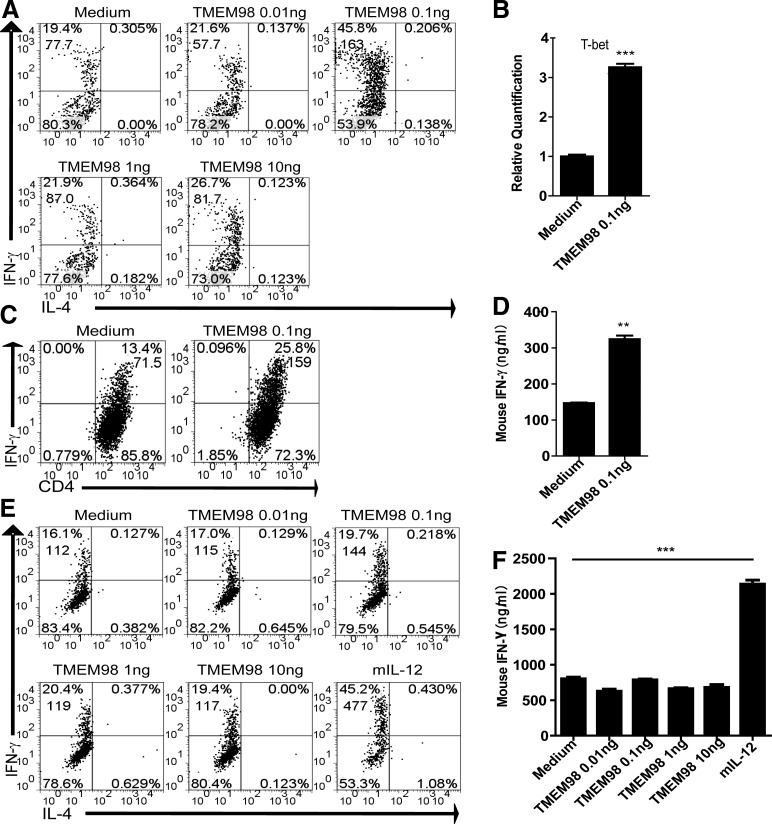
Various concentrations of eukaryotic rTMEM98 (0.01, 0. 1, 1, 10 ng/mL) were supplemented to the culture medium during the Th1 cell differentiation process. As shown in Fig. 5A, flow cytometry analysis showed that eukaryotic rTMEM98 not only increased the percentage of IFN-γ+ cells but also upregulated intracellular IFN-γ levels (represented by mean fluorescence intensity, MFI). These data suggest that eukaryotic rTMEM98 could promote increased Th1 cell generation during in vitro Th1 differentiation. Furthermore, we also analyzed the expression level of Tbx21, the key Th1 transcription factor, at the optimal concentration of eukaryotic rTMEM98 (0.1 ng/mL). As shown in Fig. 5B, Tbx21 was significantly increased at 72 h, verifying that TMEM98 was able to further promote Th1 differentiation.
FIG. 5.
TMEM98 promotes Th1 cell differentiation, but does not affect polarized Th1 cells in vitro. (A) CD4+ T cells (1×106/mL) were cultured under Th1 conditions, and various concentrations of eukaryotic rTMEM98 were added. On day 5, cells were harvested and replated at a density of 1×106/mL. The cells were stimulated by PMA and ionomycin for 5 h with GolgiStop, followed by intracellular staining. Data are representative of 3 independent experiments. Live cells were gated for flow cytometry analysis. (B) Naïve CD4+ T cells (1×106/mL) were cultured under Th1 conditions, and the optimal concentration of eukaryotic rTMEM98 (0.1 ng/mL) was added. Tbx21 expression was evaluated at 72 h without restimulation using real-time PCR. The experiment was repeated twice. Values are expressed as mean±SEM. ***P<0.0001. (C, D) Naïve CD4+ T cells from OT-II TCR transgenic mice (1×106/mL) were stimulated with irradiated OVA peptide-pulsed (1 μg/mL) CD11c+ DC cells (1×105/mL) in Th1 conditions (IL-2, IL-12, anti-IL-4), and the optimal concentration of eukaryotic rTMEM98 (0.1 ng/mL) was added. On day 5, cells were collected as described above, and flow cytometry and ELISA analyses were performed. The experiment was repeated thrice. Values are expressed as mean±SEM. **P=0.0034. (E, F) Polarized Th1 cells were restimulated with plate-bound anti-CD3e antibody in the presence of eukaryotic rTMEM98 for 72 h. Intracellular staining (E) and ELISA (F) were used to detect the percentage of IFN-γ+ cells and IFN-γ concentration in the supernatant, respectively. IL-12 was used as a positive control. Live cells were gated for flow cytometry analysis. Data are representative of 3 independent experiments. Values are expressed as mean±SEM. ***P<0.0001. Top number in quadrants expresses percentage of cells in the quadrant, and the lower number expresses MFI. rTMEM98, recombinant TMEM98.
To test whether TMEM98 had a similar effect on Th1 differentiation under antigen-specific Th1-skewing condition, we stimulated OVA323–339-specific Th1 cells derived from OT-II TCR transgenic mice with the OVA323–339 peptide in the presence of eukaryotic rTMEM98 at the optimal concentration (0.1 ng/mL). Eukaryotic rTMEM98 increased both the percentage of IFN-γ+ cells (Fig. 5C) and the IFN-γ levels secreted into the supernatant (Fig. 5D), further suggesting that eukaryotic rTMEM98 can promote Th1 cell differentiation and IFN-γ secretion in vitro.
Since expression profile analysis showed that Tmem98 was elevated on the activated Th1 cells, we further evaluated whether eukaryotic rTMEM98 affected already polarized Th1 cells. We measured the percentage of IFN-γ+ cells and IFN-γ secretion 72 h after adding various concentrations of eukaryotic rTMEM98 (0.01, 0.1, 1, 10 ng/mL) to already polarized Th1 cells in the presence of anti-CD3e. In contrast to the effect of eukaryotic rTMEM98 during Th1 differentiation, no effect on IFN-γ production was observed from already polarized Th1 cells (Fig. 5E, F), while IL-12, the positive control of the system, could induce the production of IFN-γ successfully. Therefore, eukaryotic rTMEM98 could promote the differentiation, but not activation, of Th1 cells in vitro in our system.
TMEM98 promotes the differentiation of Th1 cells in vivo
The above data show that eukaryotic rTMEM98 promotes Th1 cell differentiation in vitro. According to SignalP 3.0, there was a potential signal peptide in TMEM98 from the 1st to the 26th amino acid. Moreover, TMHMM Server v. 2.0 indicated that TMEM98 had a transmembrane helix from the 4th to the 26th amino acid (Fig. 1A). Therefore, we further generated the prokaryotic rTMEM98 without the first 26 amino acids with high quality (Supplementary Fig. S4B). First, our in vitro functional analysis showed that consistent with eukaryotic rTMEM98, the prokaryotic rTMEM98 could also promote Th1 cell differentiation (Supplementary Fig. S5). Since there was not enough eukaryotic protein for in vivo functional analysis, we further used the prokaryotic rTMEM98 to extend these findings in vivo by evaluating whether TMEM98 could modulate an antigen-specific response.
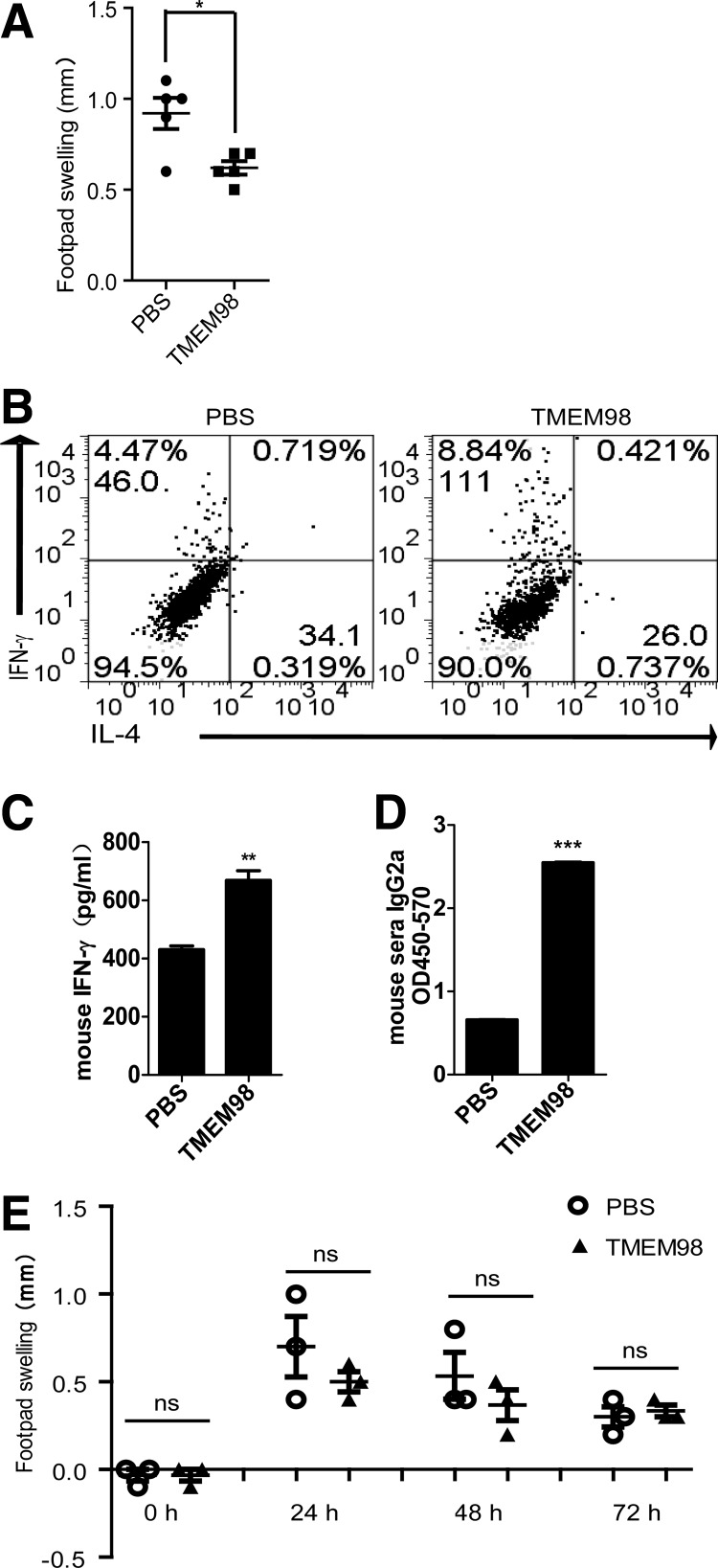
DTH, a T-cell-mediated type IV allergy, develops in 2 phases: a sensitization phase, in which T cells are sensitized and memory T cells are formed, and an elicitation phase, in which T-cell recall responses are induced upon secondary challenge with antigens (Grabbe and Schwarz 1998). After sensitization with mBSA/CFA, 400 ng/mouse of prokaryotic rTMEM98 was intraperitoneally injected every day for 6 days, and footpad swelling was measured 24 h after antigen challenge. As shown in Fig. 6A, prokaryotic rTMEM98-treated mice had significantly abrogated footpad swelling compared with control PBS-injected mice (n=5, P=0.0127).
FIG. 6.
TMEM98 promotes the differentiation of Th1 cells in vivo. (A) After sensitization with methylated bovine serum albumin (mBSA)/complete Freund's adjuvant (125 μg in 50 μL), prokaryotic rTMEM98 (400 ng in 400 μL phosphate-buffered saline [PBS]) was injected every day for 6 days. On day 7, mice were challenged by injecting PBS or mBSA (150 μg in 30 μL) into the footpads. Footpad thickness was measured at 24 h after the challenge. Bars represent mean±SEM for each group. *P=0.0127 versus control by t-test. n=5 for each group. The result shown is 1 representative of 3 repeated experiments. (B, C) Six days after sensitization, spleen cells (5×106/mL) were isolated before the challenge, and then stimulated with mBSA in vitro for 72 h. IFN-γ levels in the supernatants and the percentage of IFN-γ+ cells were determined by ELISA and flow cytometry, respectively. The mean±SEM of each group is also shown. **P=0.0026 versus control. Top number in quadrants expresses the percentage of cells in the quadrant, and the lower number expresses MFI. (D) Six days after challenge, sera were collected from PBS- and TMEM98-treated mice. Serum IgG2a levels were analyzed in 1:10 diluted samples by ELISA. The mean±SEM of each group is also shown. ***P<0.0001 versus control. (E) Six days after sensitization, prokaryotic rTMEM98 (400 ng in 400 μL PBS, TMEM98, filled triangle) or PBS (400 μL, PBS, open circle) was injected before (indicated as 0 h) and after challenge every day for 3 days, and the thickness of each footpad was measured with an engineer's caliper. n=3 for each group. The result shown is 1 representative of 2 repeated experiments.
To further examine the mechanism of this effect, 6 days after mBSA sensitization, spleen cells were isolated before the challenge and cultured in the presence of mBSA for 72 h. Both the percentage of IFN-γ+ cells and the MFI of IFN-γ were increased in the prokaryotic rTMEM98 group. Although the percentage of IL-4+ cells was also upregulated, their MFI had no obvious change (Fig. 6B). Moreover, IFN-γ levels in culture supernatants from spleen cells derived from prokaryotic rTMEM98-treated mice were also increased compared with the PBS-treated mice (P=0.0026) (Fig. 6C). Consistent with our in vitro results, these data indicate that TMEM98 predominantly affects Th1 cell differentiation in an in vivo mouse DTH model.
A combination of T-cell-derived cytokines induces B cells to proliferate and differentiate into immunoglobulin-secreting plasma cells (Finkelman and others 1990). The Th1-derived cytokine, IFN-γ, is important for enhancing the Th1-related secretion of the IgG2a isotype (Finkelman and others 1988; Snapper and others 1988). To determine whether TMEM98 could induce increased serum IgG2a during the DTH response, we measured serum mBSA-specific IgG2a levels by ELISA 6 days after the mBSA challenge. We found that serum mBSA-specific IgG2a was significantly increased in the prokaryotic rTMEM98-treated group compared with the PBS-treated group (P<0.0001) (Fig. 6D), further suggesting that TMEM98 can efficiently promote Th1 immune responses.
In the elicitation phase of the DTH reaction, antigen-specific Th1 cells are already activated. To test whether TMEM98 could influence the function of these already activated cells in vivo, prokaryotic rTMEM98 was injected into mice at the same time as the challenge for 3 consecutive days. As illustrated in Fig. 6E, no obvious change in footpad swelling was observed between the 2 groups (n=3), indicating that prokaryotic rTMEM98 has no effect on the activation of committed Th1 cells in vivo, which is consistent with the results in vitro.
Discussion
Cytokines play key roles in important biological processes, such as morphogenesis, cell differentiation, and modulation of the immune response, and lead to new understandings of disease paradigms. More importantly, they could be utilized as therapeutic agents or targets and provide potential opportunities for the development of therapeutics. Therefore, identifying and characterizing novel potential cytokines and transmembrane proteins are of great value for both basic research and clinical application. TMEM98 was identified as a candidate molecule through the data mining platform for novel potential cytokines using the strategy of immunogenomics (Guo and others 2012; Pan and others 2014; Wang and others 2014).
In this study, we demonstrated the existence of the secretion of TMEM98 both in the transfected cells and the primary cells for the first time. To date, many cytokines have been verified to present in 2 forms: the transmembrane form and the secreted form, such as the TNF superfamily cytokine TNF-α, a type II transmembrane protein, which can be cleaved by an enzyme, and exert their function as secreted cytokines (Black and others 1997). Although being a type II transmembrane protein, TMEM98 is secreted in full length and not through cleavage like TNF-α.
Recently, an increasing number of cytokines devoid of signal peptides, such as IL-1β, MIF, FGF-1, and FGF-2 (Nickel 2003; Duitman and others 2011), have been shown to be exported through nonclassical secretory mechanisms that are independent of ER/Golgi trafficking. Our ultracentrifugation results (Fig. 3B) showed that TMEM98 could be secreted through vesicles. Secreted membrane vesicles can be divided into exosomes, exosome-like vesicles, membrane particles, microvesicles, and ectosomes (Thery and others 2009). Of them, exosomes are released from cells through endosomal vesicle/MVB pathways that result in fusion with the plasma membrane (Kesimer and others 2009). They are commonly purified based on the size by serial centrifugation with a buoyant floatation density of 1.13–1.19 g/mL on sucrose gradients (Thery and others 2009).
Our further study showed that secreted TMEM98 could be enriched by ultracentrifugation and floated at a peak density of 1.18 g/mL on a continuous sucrose gradient. Moreover, the secretion of TMEM98 could be blocked by LY294002, which can inhibit internal vesicle formation within MVBs and suppress the secretion of exosomes (Denzer and others 2000). These characters are consistent with the criteria for exosomes (Thery and others 2009); therefore, TMEM98 could be secreted through exosomes.
Furthermore, although the predicted molecule weight of TMEM98 is 24.6 kDa, the observed band size is about 34 kDa in our system. That may be due to some post-translational modifications, which might lead to increased protein size, including glycosylation, phosphorylation, acetylation, and methylation . The post-translational modifications may be different among different expression systems. As shown in Supplementary Fig. S4B, TMEM98 has a weight of 23 kDa because the prokaryotic expression systems do not have post-translational modifications and the loss of the first 26 amino acids.
TMEM98 was previously proposed as one of the signature genes for adenocarcinoma (Imadome and others 2010). Recently, it has been described as a novel chemoresistance-conferring gene in hepatocellular carcinoma (Ng and others 2014). However, there has been no immune-related report about it until now. Our results showed that native secreted TMEM98 existed on the activated PBMCs, CD4+ T cells, and Th1 cells. More interestingly, it was increased significantly during the differentiation of Th1 cells. In vitro studies showed that TMEM98 displays a bell-shaped activity curve and its optimal effect is about 0.1 ng/mL, which is in accordance with the characteristics of cytokines.
Our in vivo results showed that prokaryotic rTMEM98 inhibited the development of mBSA-induced DTH, in which Th1 cells play a protective role (Feuerer and others 2006; Irmler and others 2007), while Th17 cells play a pathogenic role (Nakae and others 2002; Umemura and others 2007), indicating that it may promote the Th1 response. Consistent with this finding, the percentages of antigen-specific IFN-γ+ cells, IFN-γ secretion, and mBSA-specific IgG2a antibody levels were also increased. In a word, these results demonstrate a promoting role of TMEM98 in the differentiation of Th1 cells both in vitro and in vivo.
In recent years, several large-scale functional screenings have been performed using culture supernatant containing overexpressed proteins (Lin and others 2008), purified recombinant proteins (Lin and others 2008), and genetic knockouts (Tang and others 2010). Genentech, Inc., has constructed 472 knockout mouse strains of secreted and membrane proteins and analyzed their phenotype. Tmem98 is included in this catalog.
Genetic data indicated that knocking out this gene resulted in greatly reduced viability of the homozygous mutants (−/−). The surviving homozygous mutants exhibited numerous abnormalities in most tested areas, indicating that Tmem98 plays essential roles in embryonic development. Moreover, antigen-specific immunoglobulin levels were also evaluated in the serum of these mice by ELISA; interestingly, OVA-specific IgG2a secretion is decreased both in Tmem98+/− and Tmem98−/− mice (Tang and others 2010), which is consistent with our results using recombinant protein.
Transmembrane proteins also have modulatory effects on the differentiation and activation of immune cells. For example, the interaction between B-7 and CD28 provides the essential costimulatory signal for CD4+ T cells. TMHMM Server v. 2.0 indicated that TMEM98 had a transmembrane helix from the 4th to the 26th amino acid (Fig. 1A). Moreover, the 1–26 amino acids were predicted as signal peptides by SignalP 3.0. Our results demonstrate that TMEM98 is a type II membrane protein with the C-terminus outside of the cell membrane (Fig. 2).
To test if TMEM98 functions as a membrane ligand through the region outside of the membrane, we produced the prokaryotic protein without the first 26 amino acids. First, we tested the function of the prokaryotic protein in vitro and found that it was the same as the eukaryotic protein (Supplementary Fig. S5). Because there was not enough eukaryotic protein, we further used the prokaryotic protein to test its effects in vivo, which was proved to be the same as in vitro (Fig. 6). These data reflect that the predicated transmembrane and inside regions have no function to the development of Th1 cells. That means, except the secreted form, the transmembrane form of TMEM98 could also function as a membrane ligand through the region outside of the membrane to promote Th1 cell differentiation.
Cytokines mediate immune and inflammatory reactions by binding cell surface receptors. Since rTMEM98 promotes the differentiation of Th1 cells at a very low concentration, it may function through receptor binding. Therefore, the receptor of TMEM98 needs to be further identified.
IL-12-STAT4 and IFN-γ-STAT1 are 2 major feedback loops mediating the Th1 cell differentiation (Szabo and others 2003). Our results showed that although TMEM98 could activate STAT1 slightly, the effect was not so powerful (Supplementary Fig. S6). Therefore, we cannot formally rule out the possibility that additional mechanisms may contribute to the effect of TMEM98 on Th1 cell differentiation.
Ng and others (2014) have found that TMEM98 might function through activation of the AKT pathway and deactivation of p53 in conferring chemoresistance of hepatocellular carcinoma. As reported previously, Akt could upregulate IFN-γ production by CD4+ T cells from C57BL/6 mice (Arimura and others 2004). Moreover, active Akt could rescue Th1, but not Th2 cell differentiation, and T-bet, but not GATA-3 (Lee and others 2010). Therefore, TMEM98 might promote the differentiation of Th1 cells through the Akt pathway. Consequently, further study is necessary to identify the signaling molecules as well as the TMEM98 receptor to understand the underlying mechanism, which will provide novel insights into the mechanism of Th1 cell development.
In summary, we have provided the first comprehensive annotation of the characteristics of TMEM98, a novel immune-related molecule with both the transmembrane form and the secreted form. Unraveling the promoting effects of the secreted form of TMEM98 on the differentiation of Th1 cells will be helpful in understanding the characteristics of TMEM98 and the development of Th1 cells.
Supplementary Material
Acknowledgment
This work was supported by grants from the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20110001110016) and the Program for Innovation of New Drugs (2013ZX09103003-023).
Author Disclosure Statement
No competing financial interests exist.
References
- Arimura Y, Shiroki F, Kuwahara S, Kato H, Dianzani U, Uchiyama T, Yagi J. 2004. Akt is a neutral amplifier for Th cell differentiation. J Biol Chem 279(12):11408–11416 [DOI] [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. 1997. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385(6618):729–733 [DOI] [PubMed] [Google Scholar]
- Chou KC, Cai YD. 2005. Prediction of membrane protein types by incorporating amphipathic effects. J Chem Inf Model 45(2):407–413 [DOI] [PubMed] [Google Scholar]
- de Souza AJ, Oak JS, Jordanhazy R, DeKruyff RH, Fruman DA, Kane LP. 2008. T cell Ig and mucin domain-1-mediated T cell activation requires recruitment and activation of phosphoinositide 3-kinase. J Immunol 180(10):6518–6526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. 2000. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 113(Pt 19):3365–3374 [DOI] [PubMed] [Google Scholar]
- Duitman EH, Orinska Z, Bulfone-Paus S. 2011. Mechanisms of cytokine secretion: a portfolio of distinct pathways allows flexibility in cytokine activity. Eur J Cell Biol 90(6–7):476–483 [DOI] [PubMed] [Google Scholar]
- Fernandez-Borja M, Wubbolts R, Calafat J, Janssen H, Divecha N, Dusseljee S, Neefjes J. 1999. Multivesicular body morphogenesis requires phosphatidyl-inositol 3-kinase activity. Curr Biol 9(1):55–58 [DOI] [PubMed] [Google Scholar]
- Feuerer M, Eulenburg K, Loddenkemper C, Hamann A, Huehn J. 2006. Self-limitation of Th1-mediated inflammation by IFN-gamma. J Immunol 176(5):2857–2863 [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Holmes J, Katona IM, Urban JF, Jr, Beckmann MP, Park LS, Schooley KA, Coffman RL, Mosmann TR, Paul WE. 1990. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol 8:303–333 [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Katona IM, Mosmann TR, Coffman RL. 1988. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol 140(4):1022–1027 [PubMed] [Google Scholar]
- Grabbe S, Schwarz T. 1998. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol Today 19(1):37–44 [DOI] [PubMed] [Google Scholar]
- Guo X, Zhang Y, Wang P, Li T, Fu W, Mo X, Shi T, Zhang Z, Chen Y, Ma D, Han W. 2012. VSTM1-v2, a novel soluble glycoprotein, promotes the differentiation and activation of Th17 cells. Cell Immunol 278(1–2):136–142 [DOI] [PubMed] [Google Scholar]
- Imadome K, Iwakawa M, Nakawatari M, Fujita H, Kato S, Ohno T, Nakamura E, Ohkubo Y, Tamaki T, Kiyohara H, Imai T. 2010. Subtypes of cervical adenosquamous carcinomas classified by EpCAM expression related to radiosensitivity. Cancer Biol Ther 10(10):1019–1026 [DOI] [PubMed] [Google Scholar]
- Irmler IM, Gajda M, Brauer R. 2007. Exacerbation of antigen-induced arthritis in IFN-gamma-deficient mice as a result of unrestricted IL-17 response. J Immunol 179(9):6228–6236 [DOI] [PubMed] [Google Scholar]
- Kesimer M, Scull M, Brighton B, DeMaria G, Burns K, O'Neal W, Pickles RJ, Sheehan JK. 2009. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: a possible role in innate defense. FASEB J 23(6):1858–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Maslinski W, Zheng XX, Stevens AC, Li XC, Tesch GH, Kelley VR, Strom TB. 1998. Targeting the IL-15 receptor with an antagonist IL-15 mutant/Fc gamma2a protein blocks delayed-type hypersensitivity. J Immunol 160(12):5742–5748 [PMC free article] [PubMed] [Google Scholar]
- Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. 2010. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 32(6):743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Guo X, Shao L, Plate M, Mo X, Wang Y, Han W. 2010. CMTM5-v1, a four-transmembrane protein, presents a secreted form released via a vesicle-mediated secretory pathway. BMB Rep 43(3):182–187 [DOI] [PubMed] [Google Scholar]
- Li T, Zhong J, Chen Y, Qiu X, Zhang T, Ma D, Han W. 2006. Expression of chemokine-like factor 1 is upregulated during T lymphocyte activation. Life Sci 79(6):519–524 [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Abbas AK. 2009. Basic immunology: Functions and disorders of the immune system. Philadelphia, PA: Saunders/Elsevier [Google Scholar]
- Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein SK, Williams LT. 2008. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 320(5877):807–811 [DOI] [PubMed] [Google Scholar]
- Miller SG, Carnell L, Moore HH. 1992. Post-Golgi membrane traffic: brefeldin A inhibits export from distal Golgi compartments to the cell surface but not recycling. J Cell Biol 118(2):267–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17(3):375–387 [DOI] [PubMed] [Google Scholar]
- Ng KT, Lo CM, Guo DY, Qi X, Li CX, Geng W, Liu XB, Ling CC, Ma YY, Yeung WH, Shao Y, Poon RT, Fan ST, Man K. 2014. Identification of transmembrane protein 98 as a novel chemoresistance-conferring gene in hepatocellular carcinoma. Mol Cancer Ther 13(5):1285–1297 [DOI] [PubMed] [Google Scholar]
- Nickel W. 2003. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur J Biochem 270(10):2109–2119 [DOI] [PubMed] [Google Scholar]
- Owaki T, Asakawa M, Morishima N, Hata K, Fukai F, Matsui M, Mizuguchi J, Yoshimoto T. 2005. A role for IL-27 in early regulation of Th1 differentiation. J Immunol 175(4):2191–2200 [DOI] [PubMed] [Google Scholar]
- Pan W, Cheng Y, Zhang H, Liu B, Mo X, Li T, Li L, Cheng X, Zhang L, Ji J, Wang P, Han W. 2014. CSBF/C10orf99, a novel potential cytokine, inhibits colon cancer cell growth through inducing G1 arrest. Sci Rep 4:6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C, Malhotra V, Nickel W. 2012. Diversity in unconventional protein secretion. J Cell Sci 125(Pt 22):5251–5255 [DOI] [PubMed] [Google Scholar]
- Snapper CM, Peschel C, Paul WE. 1988. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J Immunol 140(7):2121–2127 [PubMed] [Google Scholar]
- Suto A, Wurster AL, Reiner SL, Grusby MJ. 2006. IL-21 inhibits IFN-gamma production in developing Th1 cells through the repression of Eomesodermin expression. J Immunol 177(6):3721–3727 [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100(6):655–669 [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. 2003. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol 21:713–758 [DOI] [PubMed] [Google Scholar]
- Tang T, Li L, Tang J, Li Y, Lin WY, Martin F, Grant D, Solloway M, Parker L, Ye W, Forrest W, Ghilardi N, Oravecz T, Platt KA, Rice DS, Hansen GM, Abuin A, Eberhart DE, Godowski P, Holt KH, Peterson A, Zambrowicz BP, de Sauvage FJ. 2010. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol 28(7):749–755 [DOI] [PubMed] [Google Scholar]
- Thery C, Ostrowski M, Segura E. 2009. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9(8):581–593 [DOI] [PubMed] [Google Scholar]
- Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. 2007. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol 178(6):3786–3796 [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9(6):654–659 [DOI] [PubMed] [Google Scholar]
- Wang W, Li T, Wang X, Yuan W, Cheng Y, Zhang H, Xu E, Zhang Y, Shi S, Ma D, Han W. 2014. FAM19A4 is a novel cytokine ligand of formyl peptide receptor 1 (FPR1) and is able to promote the migration and phagocytosis of macrophages. Cell Mol Immunol [Epub ahead of print]; DOI: 10.1038/cmi.2014.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25:821–852 [DOI] [PubMed] [Google Scholar]
- Zhong J, Wang Y, Qiu X, Mo X, Liu Y, Li T, Song Q, Ma D, Han W. 2006. Characterization and expression profile of CMTM3/CKLFSF3. J Biochem Mol Biol 39(5):537–545 [DOI] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. 2010. Differentiation of effector CD4 T cell populations. Annu Rev Immunol 28:445–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.