Abstract
Rats learn to prefer a flavor mixed into a fructose–saccharin solution over a different flavor mixed into a saccharin-only solution which is considered to be a form of flavor–flavor conditioning. Fructose-conditioned flavor preferences are impaired by systemic dopamine D1 and to a lesser degree, D2 receptor antagonism as well as by NMDA, but not opioid, receptor antagonism. Given the emerging role of the endocannabinoid system in mediating hedonically-driven food intake, the present study examined whether systemic administration of the inverse CB-1 receptor agonist, AM-251 would alter fructose-conditioned flavor preferences. In Experiment 1, food-restricted rats were trained over 10 sessions (30 min/day) to drink a fructose–saccharin solution mixed with one flavor (CS+/Fs) and a less-preferred saccharin-only solution mixed with another flavor (CS−/s). Subsequent two-bottle tests with the two flavors in saccharin (CS+/s, CS−/s) occurred 15 min following counterbalanced pairs of AM-251 doses of 0, 0.1,1 or 3 mg/kg. Preference for CS+/s over CS−/s following vehicle treatment (74%) was significantly reduced by the 0.1 (67%) and 1 (65%) AM-251 doses, whereas CS+/s, but not CS−/s intake was significantly reduced by the 1 and 3 mg/kg AM-251 doses. In Experiment 2, rats received systemic injections of AM-251 (1 mg/kg) or vehicle prior to the 10 CS+/Fs and CS−/s training sessions. In subsequent two-bottle tests (drug-free) the AM-251 and control groups displayed similar preferences for the CS+ flavor (66% vs. 69%). Experiment 3 demonstrated that AM-251 significantly decreased chow intake (24 h), and 1-h intakes of fructose–saccharin and saccharin-only solutions in ad libitum-fed rats. These data indicate that functional CB-1 receptor antagonism significantly reduces the expression, but not the acquisition of fructose-conditioned flavor–flavor preferences. The endogenous endocannabinoid system is therefore implicated in the maintenance of this form of learned flavor preferences.
Keywords: AM-251, Conditioned flavor preferences, Fructose, Saccharin
1. Introduction
Learning, together with innate taste biases, plays a major role in food preferences (see review, Sclafani, 1995). The conditioned flavor preference paradigm has proved useful in studying the acquisition and expression of learned food preferences in animals. Our laboratory has used various procedures in which an arbitrary flavor (the conditioned stimulus or CS+) is paired with a nutritive source (the unconditioned stimulus or US; e.g., sugar solution), and a second flavor (the CS−) is paired with a less-preferred nonnutritive source (e.g., saccharin solution) during a series of one-bottle training sessions. Preference learning is then assessed in a two-bottle choice test with the two flavors presented in saccharin solutions to insure that any differential intake can be attributed to a learned response to the flavor cues. Learned flavor preferences may result from an association between the CS+ flavor and thepalatable flavor of the sugar (sweet taste) which is referred to as flavor–flavor conditioning, and/or between the CS+ flavor and the postingestive actions of sugar which is referred to as flavor-nutrient conditioning. We have studied flavor–nutrient learning by pairing the intakes of CS+ and CS− flavored saccharin solutions with intragastric (IG) infusions of sugar (sucrose, glucose) and water, respectively (Sclafani et al., 1993, 1999). Flavor–flavor conditioning is studied by testing either sham-feeding animals with an open gastric fistula consuming preferred CS+ flavored sucrose and less-preferred CS− flavored saccharin solutions (Yu et al., 1999) or real-feeding animals consuming preferred CS+ flavored fructose and less-preferred CS− flavored saccharin solutions (Baker et al., 2003, 2004) because fructose does not condition flavor–nutrient preferences in short training sessions (Sclafani et al., 1993, 1999).
These various flavor conditioning procedures have been combined with pharmacological treatments to investigate the neurochemical basis of food preference learning. Drug effects on the acquisition of flavor preferences are evaluated by administrating the drug just before daily one-bottle training trials, whereas drug effects on the expression of a previously learned flavor preference are evaluated by administering the drug just prior to two-bottle choice tests. Recent findings indicate that systemic pretreatment with D1 (SCH23390) and D2 (raclopride) dopamine receptor antagonists significantly reduces both the acquisition and expression of sucrose-conditioned flavor–flavor preferences in sham-feeding rats (Yu et al., 2000a,b) and of fructose-conditioned flavor–flavor preferences in real-feeding rats (Baker et al., 2003). Reductions in the expression, but not the acquisition of fructose-conditioned flavor–flavor preferences have also been observed following direct administration of D1 and D2 antagonists into the nucleus accumbens and amygdala (Bernal et al., 2006, 2007; Dostova et al., 2006). In contrast, systemic D1, but not D2 dopamine receptor antagonism blocks the acquisition, but not the expression of sucrose-conditioned flavor–nutrient preferences produced by IG sucrose infusions (Azzara et al., 2001). Direct administration of a D1 antagonist into the nucleus accumbens or amygdala also blocks acquisition of glucose-conditioned flavor–nutrient preferences following IG infusions (Touzani et al., 2006, 2007). The NMDA glutamate–glycine receptor has been implicated in flavor–flavor preferences in that the acquisition, but not the expression, of fructose-conditioned flavor preferences was respectively reduced by systemic administration of the non-competitive NMDA antagonist, MK-801, and enhanced by systemic administration of the glycine agonist, D-cycloserine (Golden and Houpt, 2007). Although the general opioid antagonist, naltrexone, is capable of reducing intakes of sucrose and saccharin solutions (see review, Bodnar, 2004), systemic administration of naltrexone failed to alter the acquisition or expression of sucrose-conditioned flavor–flavor preferences in sham-feeding rats (Yu et al., 1999), of fructose-conditioned flavor–flavor preferences in real-feeding rats (Baker et al., 2004), or of sucrose-conditioned flavor–nutrient preferences following IG infusions (Azzara et al., 2000).
The cannabinoids have been implicated in the mediation of food intake. Injections of the active ingredient of marijuana, delta-9-tetrahydrocannabinol, or the putative endogenous cannabinoid neurotransmitter, anandamide, each stimulate normal and palatable food intake (Kirkham, 2005; Kirkham et al., 2002; Koch and Matthews, 2001; Williams and Kirkham, 1999, 2002a; Williams et al., 1998), and the CB-1 antagonist, SR141716 reduces ethanol and sucrose intake (Arnone et al., 1997). Although cannabinoid CB-1 and CB-2 receptors are both localized in brain sites related to feeding (e.g., striatum and nucleus accumbens: Fusco et al., 2004; Gong et al., 2006; Herkenham et al., 1991; Moldrich and Wenger, 2000), the CB-1 receptor has been more implicated in the control of food intake (see reviews, DiMarzo and Matias, 2005; Piomelli, 2003). CB-1 receptors have been localized on both GABAergic synapses and with mu-opioid receptors in striatum and accumbens (Matyas et al., 2006; Pickel et al., 2004; Schoffelmeer et al., 2006). Such neuroanatomical findings support the contention that activation of CB-1 receptors particularly stimulates intake of foods that have hedonic consequences (Arnone et al., 1997; Cooper, 2004; Higgs et al., 2003; Jarrett et al., 2005; Koch, 2001; Koch and Matthews, 2001; Mahler et al., 2007). There are proposed interactions between cannabinoid and opioid systems in mediating food intake (Cooper, 2004; Cota et al., 2006; Kirkham and Williams, 2001; Williams and Kirkham, 2002b), and between cannabinoid and dopamine systems in mediating reward (Gardner, 2005). The emerging role for cannabinoid receptor systems in palatable food intake and reward, and their interaction with dopamine and opioid neurochemical systems make them a promising candidate for a role in the acquisition and expression of the flavor–flavor conditioned preferences. The selective CB-1 receptor inverse agonist, AM-251 produces significant and sustained reductions in baseline food intake and weight in normal rats (Chambers et al., 2004, 2006), in genetically-obese Zucker rats (Vickers et al., 2003), and in mice with dietary obesity (Hildebrandt et al., 2003). AM-251 and a related CB-1 receptor inverse agonist, SR141716 each produce a synergistic reduction of food intake when paired with an opioid antagonist (Chen et al., 2004; Kirkham and Williams, 2001; Rowland et al., 2001), but AM-251 also reduces food intake in mu-opioid receptor-deficient mice (Chen et al., 2006). AM-251 also blocked the ability of delta-9-tetrahydrocannabinol to reduce quinine-induced aversions (Jarrett et al., 2007), and ventricular CB-1, but not CB-2 receptor antagonists blocked deprivation-induced intake (Werner and Koch, 2003). The present study examined the dose-dependent ability of systemic AM-251 to alter the expression and acquisition of fructose-conditioned flavor preferences as well as to replicate its inhibitory effects upon chow (24 h: Chambers et al., 2004, 2006) and sweet solution (1 h: Jarrett et al., 2007) intakes in rats.
2. Methods
2.1. Subjects
The experimental protocols were approved by the Queens College Institutional Care and Use Committee, certifying that all subjects and procedures are in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. Male albino Sprague–Dawley rats (275–300 g, Charles River laboratories, Wilmington MA, USA) were housed individually in wire mesh cages and maintained on a 12:12-h light–dark cycle with rat chow (5001, PMI Nutrition International, Brentwood, MO) and water available ad libitum. All testing took place in the rat’s home cage during the mid-light phase of the light–dark cycle in a normally illuminated animal colony room. Two weeks before testing commenced, the rats in Experiments 1 and 2 were placed on a food restriction schedule that maintained their body weights at 85–90% of their ad libitum level through the entire study.
2.2. Drugs
AM-251 (Tocris Bioscience, Ellisville, MO) was made into a stock 3 mg/ml solution by initially dissolving a 50 mg aliquot of drug in 2% DMSO (0.33 ml) by gently vortexing the solution. Subsequent additions of 1% Tween 80 (0.17 ml) and 97% normal saline (16.17 ml) occurred. This stock solution was used to prepare 0.1 and 1 mg/ml drug concentrations. The AM-251 solutions were stored at room temperature in a dark canister. Each solution was resonicated just before use. Animals were administered each particular concentration at a 1 ml/kg dose.
2.3. Test solutions
The training solutions were prepared on a weight/weight basis, and consisted of either a combined 8% fructose (Sigma, St. Louis, MO, USA) and 0.2% sodium saccharin (Sigma) solution (80 g of fructose, 2 g of saccharin, 0.5 g of Kool-Aid flavor and 917.5 g of water) or a 0.2% saccharin solution (2 g of saccharin, 0.5 g of Kool-Aid flavor and 997.5 g of water) flavored with 0.05% unsweetened grape or cherry Kool-Aid (Kraft Foods, White Plains, NY, USA). Half of the rats in Experiments 1 and 2 had the cherry flavor added to the fructose–saccharin solution and the grape flavor added to a saccharin-only solution; the flavors were reversed for the remaining rats. In the two-bottle preference tests, the cherry and grape flavors were each presented in a 0.2% saccharin solution. The flavor added to the fructose–saccharin solution is referred to as the CS+ and the flavor added to the saccharin-only solution is referred to as the CS− because 8% fructose is preferred to 0.2% saccharin (Baker et al., 2003, 2004; Sclafani and Ackroff, 1994). CS+/Fs refers to the flavored fructose–saccharin solution used in training, and CS+/s refers to the same flavor presented in saccharin-only solution during choice testing, whereas CS−/s refers to the flavored saccharin solution used in training and testing. For initial training in Experiments 1 and 2, rats were trained to drink an unflavored 0.2% saccharin solution from 100 ml bottles during daily 30-min sessions. The bottle was mounted on the front of the cage held by a taut steel spring and was positioned so that the stainless spout entered the cage about 3–6 cm above the cage floor. The training procedure was repeated daily until all rats approached the spout with short (<1 min) latency, typically within 3 days. The limited food rations were given 0.5 h after each training session.
2.4. Experiment 1: CB-1 expression study
Fifteen food-restricted rats were given 10 one-bottle training sessions (30 min/day) with 16 ml of the CS+/Fs solution presented on odd-numbered days, and 16 ml of the CS−/s solution presented on even-numbered days. On Days 9 and 10, the rats had access to two bottles, one containing the CS+/Fs or CS−/s solution, and the other containing water. This exposed the rats to the presence of two bottles used during the choice tests. Water intake was negligible in these training trials. The position of the CS and water bottles was counterbalanced across rats and the two days. Intakes were measured to the nearest 0.1 g by weighing the bottles prior to and at the end of the 30-min session; the food ration was presented 0.5 h later.
Following training, all rats were given 10 daily two-bottle choice test sessions (30 min/day) with the CS+/s and CS−/s solutions; intakes were unlimited (50 ml) in these tests. The positions of the two bottles were counterbalanced using a left–right–right–left pattern in half of the rats, and a right–left–left–right pattern in the remainder. All rats received vehicle injections (1 ml normal saline/kg body weight, ip) 15 min prior to the first two choice test sessions to control for the alternation of the pairs of the bottles. Two subgroups of rats, matched for their CS+ preference in the vehicle tests, received ascending (0.1, 1 and 3 mg/kg) or descending (3, 1, 0.1 mg/kg) pairs of AM-251 intraperitoneal (ip) injections 15 min prior to subsequent choice test sessions again to control for the alternation of the pairs of the bottles. Following the six days of testing the three drug doses, all rats were tested for preferences (30-min session) over two final days in the absence of injections with bottle positions alternated; the preferences observed during the first two days following vehicle were restored. The dose range employed in this and the subsequent paradigms was chosen from the range of doses used previously in feeding and food-related studies (e.g., Chambers et al., 2004, 2006; Chen et al., 2004, 2006; Hildebrandt et al., 2003; Vickers et al., 2003).
2.5. Experiment 2: CB-1 antagonist acquisition study
Twenty-four food-restricted rats were tested for unflavored saccharin solution intakes over three days, and matched into two groups on the basis of this intake. Both groups were then given 10 one-bottle training sessions (30 min/day) with 16 ml of the CS+/Fs solution presented on odd-numbered days, and 16 ml of the CS−/s solution presented on even-numbered days in a manner described above. The first group of 12 rats received AM-251 at a dose of 1 mg/kg 15 min prior to each of the ten training trials, while the second group of 12 rats received vehicle injections (1 ml normal saline/kg body weight, ip) 15 min prior to each of the ten training trials. Following training, all rats in both groups were given 6 two-bottle choice test sessions (30 min/day) with the CS+/s and CS−/s solutions; intakes were unlimited in these tests and the rats were not injected. The positions of the 2 bottles were counterbalanced using a left–right–right–left pattern in half of the rats, and a right–left–left–right pattern in the remainder. Intakes of the CS+/s and CS−/s solutions were measured to the nearest 0.1 g after 30 min during each session, and the food ration was presented 0.5 h later.
2.6. Experiment 3: CB-1 antagonist effects upon chow, fructose and saccharin intake
Baseline 24-h chowand water intakes were recorded in 12 individually housed rats over three days. Chow intake was measured by weighing the food bin before and after each 24-h session and was corrected for spillage collected on paper towels placed under the wire mesh cage. Intake was then assessed following vehicle injections (1 ml normal saline/kg body weight, ip), and used to match two subgroups (n=6 each) of rats that subsequently received ascending (0.1,1 and 3 mg/kg, ip) or descending (3, 1, 0.1 mg/kg, ip) series of AM-251 injections at 48-h intervals. Following a one-week interval, the two subgroups of rats were given an unflavored fructose (8%)–saccharin (0.2%) solution or an unflavored saccharin (0.2%) solution, respectively, in a 100 ml calibrated (0.1 ml) bottle attached to the front of the cage during daily 60-min sessions. Following stabilization, intake was assessed 15, 30, 45 and 60 min following vehicle injections (1 ml normal saline/kg body weight, ip). Animals receiving fructose–saccharin or saccharin solutions were respectively matched for vehicle intake, and subsequently received ascending (0.1, 1 and 3 mg/kg, ip) or descending (3, 1, 0.1 mg/kg, ip) series of AM-251 injections at 48-h intervals 15 min prior to presentation of the solutions. Solution intakes were measured at 15, 30, 45 and 60 min during each session.
2.7. Statistical analyses
In the expression study, training intakes were averaged over the five sessions each with the CS+/Fs and CS−/s solutions and evaluated with a t-test. Intakes during the preference tests were averaged over the two sessions at each dose and evaluated with two-way repeated-measures analysis of variance (ANOVA, CS solution×dose). Separate ANOVAs evaluated total intakes and percent of total intake consumed as CS+/s. In the acquisition study, training intakes were averaged over the five sessions each with the CS+/Fs and CS−/s solutions and were analyzed with a two-way ANOVA (CS solution×group). Intakes during the preference tests were averaged over the six sessions and analyzed with a two-way ANOVA. Changes in the percent CS+/s intakes and total intake were evaluated by t-tests comparing the vehicle and AM-251 training groups. In the ad libitum intake study, a one-way repeated-measures ANOVAwas performed to assess drug dose-induced changes in chow intake. Separate two-way repeated-measures ANOVAs were performed to assess changes in cumulative fructose–saccharin and saccharin intakes as functions of AM-251 doses and the four intake intervals. Tukey corrected comparisons (P<0.05) detected significant individual effects. Effect sizes were calculated for the preference and intake studies.
3. Results
3.1. AM-251 and expression of fructose-conditioned flavor preference
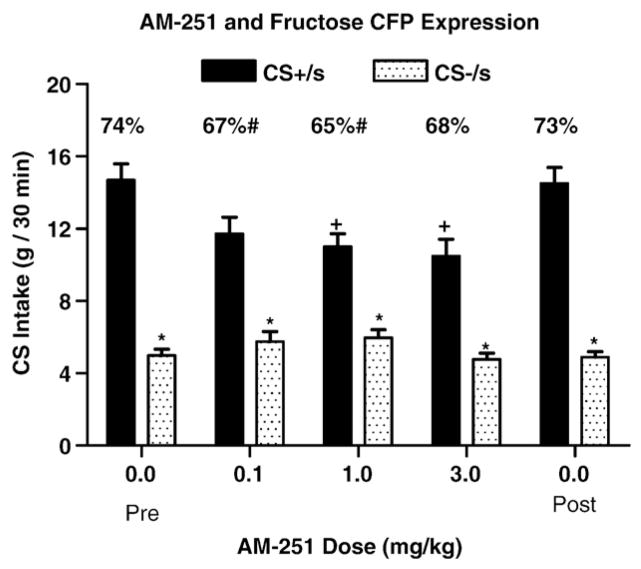
CS+/Fs training intakes (11.2+0.6 ml/session) were higher, though not significantly so (t(28)=1.65, P=0.11), than CS−/s training intakes (9.6+0.8 ml/session). Fig. 1 presents the two-bottle intakes of the CS+/s and CS−/s solutions following vehicle and AM-251 drug treatment. Significant differences in intake were observed between the CS+/s and CS−/s solutions (F(1,56)=179.70, P<0.0001; eta2=0.57), across drug doses (F(3,56)=3.53, P<0.021; eta2=0.19), and for the interaction between CS solutions and doses (F(3,56)=4.48, P<0.007; eta2=0.24). Significant differences in the percent CS+/s intake were also observed across doses (F(3,42)=5.58, P<0.003; eta2=0.62). As expected, CS+/s intake was significantly higher than CS−/s intake following vehicle treatment, resulting in a 74% CS+/s preference (Fig. 1). CS+/s intake was also significantly higher than CS−/s intake following each of the three AM-251 doses. However, CS+/s, but not CS−/s, intake was significantly and dose-dependently decreased relative to vehicle treatment following the 1 and 3, but not the 0.1, mg/kg doses of AM-251 (Fig. 1). Further, the percent CS+/s intake was significantly reduced relative to vehicle treatment following the 0.1 (67%) and 1 (65%), but not the 3 (68%) mg/kg AM-251 doses. Significant decreases in total CS solution intakes (F(3,42)=17.91, P<0.0001; eta2=0.88) relative to vehicle occurred following all three drug doses. It should be noted that animals returned to their initial pattern of CS+/s and CS−/s intakes noted during vehicle treatment after all doses of the CB-1 antagonist were administered (Fig. 1: post). These data indicate that CB-1 antagonism significantly, though marginally reduced the expression of the fructose-conditioned flavor preferences.
Fig. 1.
Expression. Intakes (g, +SEM, 0.5 h) of CS+/s and CS−/s solutions in two-bottle tests of animals receiving intraperitoneal injections (0, 0.1, 1, 3 mg/kg) of the CB-1 inverse agonist, AM-251 15 min prior to testing. Significant differences are denoted between CS+/s and CS−/s intakes within an injection condition (*) and between CS+/s intake following a drug dose relative to the vehicle treatment (+). The percentages of CS +/s intake over total intake are denoted above each pair of values with significant differences relative to vehicle treatment (#) noted.
3.2. AM-251 and acquisition of fructose-conditioned flavor preferences
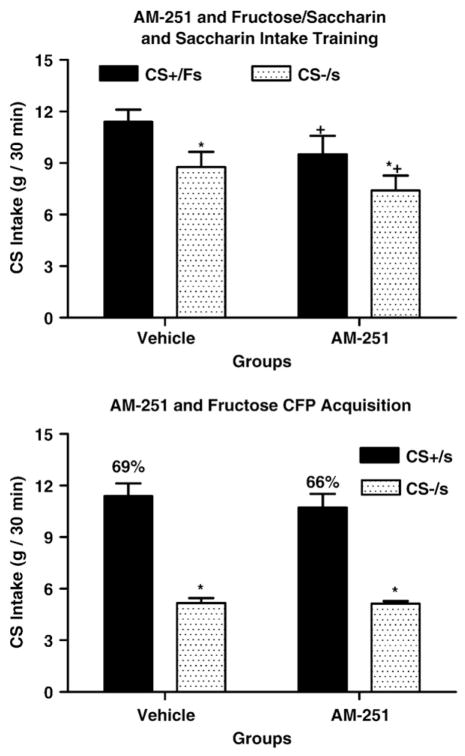
Over the 5 training sessions, significant differences in intake were observed between the CS+/Fs and CS−/s solutions (F(1,11)=87.99, P<0.0001; eta2=0.26), but not between training groups (F(1,11)=2.98, ns) or for the interaction between groups and solutions (F(1,11)=0.30, ns). Post-hoc comparisons revealed that vehicle control rats consistently consumed more of the CS+/Fs solution than the AM-251 training group, and consistently more of the CS−/s solution than the AM-251 training group.
Fig. 2 presents the mean two-bottle intakes of CS+/s and CS−/s solutions as well as the percent of CS+/s intakes averaged over the six test sessions. Significant differences in intake were observed between the CS+/s and CS−/s solutions (F(1,11)=95.94, P<0.0001; eta2=0.78), but not between training groups (F(1,11)=0.47, ns) or for the interaction between groups and solutions (F(1,11)=0.37, ns). The groups also did not differ in their percent CS+/s intakes (vehicle: 69% vs. AM-251: 66%; t(11) =0.79, ns) or in their total intake (vehicle: 16.4 ml/session vs. AM-251: 15.8 ml/session; t(11)=0.53, ns). To determine if drug treatment during training affected CS+/s preference over the course of two-bottle training, the three counterbalanced pairs of preference tests were added as an independent variable. This analysis failed to reveal any significant interactions between groups and pairs (F(2,22)=0.24, ns), groups and solutions (F(1,11)=0.35, ns), or among groups, pairs and solutions (F (2,22)=0.15, ns). These findings indicate that treatment with the CB1 antagonist during training failed to alter the subsequent learning and acquisition of the fructose-conditioned flavor preference.
Fig. 2.
Acquisition. A. Training: Intakes (g, +SEM, 0.5 h, over 5 days of training) of CS+/Fs and CS−/s solutions in 10 one-bottle training sessions in animals receiving vehicle or AM-251 (1 mg/kg). B. Testing: Intakes (g, +SEM, 0.5 h, over 6 days) of CS+/s and CS−/s solutions in two-bottle tests in animals receiving either vehicle or AM-251 (1 mg/kg) during ten days of one-bottle CS+/Fs and CS−/s training sessions. Significant differences between CS+/Fs and CS−/s intakes during training and between CS+/s and CS−/s intakes during testing are denoted (*). Significant differences in CS+/Fs and CS−/s intakes between vehicle and AM-251 treatment during training are also denoted (+).
3.3. AM-251 and chow and fructose–saccharin intake
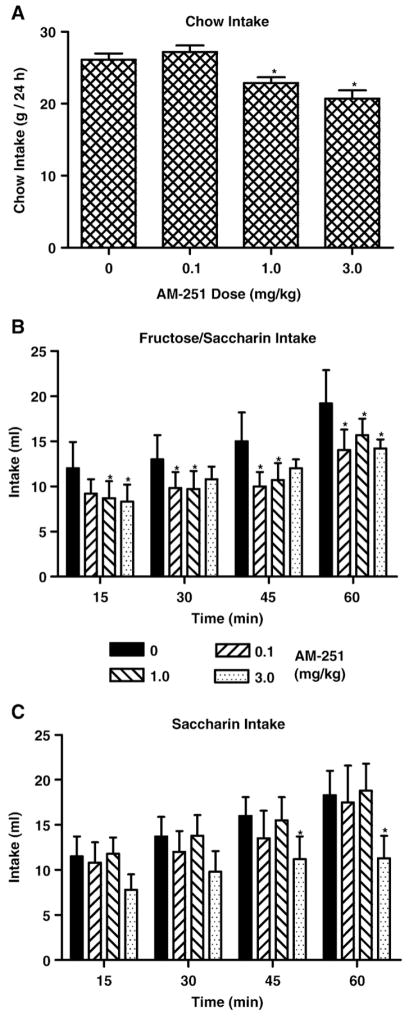
Significant reductions in chow intake over 24 h were observed (F (3,42)=15.83, P<0.0001; eta2=0.70) following the 1 and 3, but not the 0.1 mg/kg doses of AM-251 relative to vehicle treatment (Fig. 3A). Significant differences in cumulative fructose–saccharin intake were observed across times (F(3,60)=87.01, P<0.0001; eta2=0.77), approached significance for the interaction between doses and times (F(9,60)=1.85, P<0.07), but failed to differ among doses (F(3,20)=0.79, ns). AM-251 significantly reduced fructose–saccharin intake following the 0.1 (30–60 min), 1 (15–60 min) and 3 (15, 60 min) mg/kg doses (Fig. 3B). Significant differences in cumulative saccharin intake were observed across times (F(3,60)=44.69, P<0.0001; eta2=0.65), but failed to differ among doses (F(3,20)=0.90, ns) or for the interaction between doses and times (F(9,60)=1.41, ns). AM-251 significantly reduced saccharin intake only following the 3 (45–60 min) mg/kg dose (Fig. 3C).
Fig. 3.
Intake (mean +SEM) of chow (g/24 h, Panel A), combined 8% fructose and 0.2% saccharin solutions (ml/60 min, Panel B), and 0.2% saccharin solution (ml/60 min, Panel C) in animals receiving systemic injections (0, 0.1, 1, 3 mg/kg) of AM-251. Significant differences in intake following AM-251 relative to corresponding vehicle treatment are denoted (*).
4. Discussion
Systemic administration of the CB-1 receptor antagonist, AM-251, produced significant, though marginal reductions in the expression of a fructose-conditioned flavor preference; CS+ preference was reduced from 74% in the vehicle test to a low of 65% at the 1 mg/kg dose. This attenuated preference was due to selective reductions in CS+/s intake relative to vehicle without concomitant changes in CS−/s intake. Although the magnitude of reductions in the expression of fructose-conditioned preferences was significant but quite small following CB-1 antagonism, the effects were meaningful in that a subsequent pair of control tests was administered after the drug regimen revealed that the animals returned to vehicle treatment levels (74% vs. 73%). These data thereby suggest that AM-251 failed to produce any forms of rapid extinction. In contrast, the 1 mg/kg dose of AM-251 that reduced CS+ preference in the expression experiment failed to significantly alter the acquisition of the fructose-conditioned flavor preference when administered during one-bottle training. The CS+/s intakes of the control and AM-251 groups were similar over the six sessions of two-bottle testing, and the percent CS+ intakes of the two groups were also comparable (69% vs. 66%). AM-251 administration has been shown to produce a form of aversion using a gaping response (Jarrett et al., 2005, 2007), but a related novel CB-1 receptor neutral antagonist, AM4113 failed to induce signs of nausea in rats while reducing food intake and food-reinforced behavior (Sink et al., 2008a). The possibility that conditioned aversions may have had some type of effect in the present paradigm appears unlikely because the AM-251 dose of 1 mg/kg failed to alter the acquisition of a fructose-conditioned flavor preference and presumably the possibility of any conditioned aversion to the CS+/s and CS−/s flavors. Thus, AM-251 significantly reduced the expression, but not the acquisition of a fructose-conditioned flavor preference.
The failure of AM-251 to alter acquisition of a fructose-conditioned flavor preference is in agreement with a preliminary report (Mendez-Diaz and Propero-Garcia, 2007) demonstrating that AM-251 at the same 1 mg/kg dose failed to alter the acquisition of conditioned place preference reinforced by a palatable food (Rice Krispies cereal). Yet the drug reduced the intake of the palatable food reward. Many other studies report that AM-251, as well as the related compound, SR141716, reduces baseline food intake and body weight in ad libitum-fed (Chambers et al., 2004, 2006) and food-deprived (Werner and Koch, 2003) normal rats, in genetically-obese Zucker rats (Vickers et al., 2003), and in mice with dietary obesity (Hildebrandt et al., 2003), as well as decreases ethanol or sucrose intake (Arnone et al., 1997).
The present study confirmed and extended these latter observations. In Experiment 3, AM-251 significantly reduced 24-h chow intake as well as short-term (1 h) intake of a fructose–saccharin and saccharin solutions. Interestingly, AM-251 appeared to produce more potent inhibitory effects at lower doses and at shorter latencies on the intake of the fructose–saccharin solution compared to the saccharin-only solution. Intakes of the two solutions were similar following vehicle treatment yet the fructose–saccharin solution is preferred to the saccharin-only solution (unpublished observations). The similar baseline intakes and greater drug effects on the fructose–saccharin solution may be related to the postingestive satiating effect of the fructose in the mixed solution. This may have limited the intake of the solution so that the rats consumed similar amounts of the fructose–saccharin and saccharin solutions in the vehicle test and also made the mixed solution more susceptible to drug suppression. This interpretation could be tested by comparing the effects of AM-251 on the sham-feeding response to both solutions. In any case, the ability of a CB-1 inverse agonist to reduce intake of the sweet solutions is consistent with the stimulation of palatable food and fluid intakes by activation of CB1 receptors (Cooper, 2004; Higgs et al., 2003; Jarrett et al., 2005; Koch, 2001; Koch and Matthews, 2001; Mahler et al., 2007). Given that AM-251 reduces intake of fructose–saccharin as well as saccharin solutions per se in one-bottle tests, and given that AM-251 reduces the expression of a fructose-conditioned flavor preference, it would be of interest to investigate in future studies whether AM-251 would similarly reduce the strong unconditioned preference for the CS+/Fs solution relative to the CS−/s solution.
The endocannabinoid system appears to interact with dopamine systems in mediating reward (Gardner, 2005). This together with the ability of full and inverse CB-1 agonists to stimulate and reduce food intake, respectively, was a major rationale for studying AM-251 effects on fructose-conditioned flavor preferences. D1, and to a lesser degree, D2 dopamine receptor antagonists reduced the expression of fructose-conditioned flavor preferences in real-feeding rats (Baker et al., 2003) and strongly reduced the expression of sucrose-conditioned flavor preferences in sham-feeding rats (Yu et al., 2000a,b). In contrast, AM-251 produced at best only a partial, but significant reduction in CS+ preference. Together, these findings indicate that conditioned flavor preferences are more dependent upon dopamine receptor activity than CB-1 receptor activity. Among the sites at which CB1 receptors may exert such effects is the accumbens (Pickel et al., 2004; Schoffelmeer et al., 2006). Interestingly, D1 receptor antagonism in the accumbens reduced CS+/s preference to a degree comparable to that obtained with systemic AM-251 treatment in the present study (Dostova et al., 2006). Finally, a recent finding (Sink et al., 2008b) that came to the same conclusion of greater dopamine than endocannabinoid antagonist effects demonstrated differences between D1 and D2 receptor antagonists on the one hand and CB-1 functional antagonism on the other hand in their ability to decrease an operant task involving response allocation and effort-related choice in food-seeking behavior.
Further, in contrast to the failure of AM-251 treatment to alter the acquisition of fructose-conditioned flavor preferences, D1, and to a lesser degree, D2 dopamine receptor antagonists reduced the acquisition of fructose-conditioned flavor preferences (Baker et al., 2003). Another neurotransmitter system implicated in flavor conditioning is the glutamatergic system. The non-competitive NMDA receptor antagonist, MK-801 was observed to reduce the acquisition, but not expression of fructose-conditioned flavor preferences, whereas the glycine agonist, D-cycloserine enhanced this response (Golden and Houpt, 2007). It may be that dopamine interacts with different neurotransmitter systems in mediating different aspects (e.g., acquisition, expression) of flavor–flavor preference learning.
CB-1 receptors have been co-localized with mu-opioid receptors in striatum and accumbens (Matyas et al., 2006; Pickel et al., 2004; Schoffelmeer et al., 2006), and cannabinoid and opioid interactions have been observed in the mediation of food intake (Cooper, 2004; Cota et al., 2006; Kirkham and Williams, 2001; Williams and Kirkham, 2002b). Further, AM-251 and SR141716 each produce a synergistic reduction of food intake when paired with an opioid antagonist (Chen et al., 2004; Kirkham and Williams, 2001; Rowland et al., 2001), and AM-251 is capable of also reducing food intake in mu-opioid receptor-deficient mice (Chen et al., 2006). However, in contrast to the ability of systemic AM-251 to reduce the expression of a fructose-conditioned flavor preference, systemic naltrexone failed to reduce fructose-conditioned flavor preferences in real-feeding rats (Baker et al., 2004) or sucrose-conditioned flavor preferences in sham-feeding rats (Yu et al., 1999).
Fructose-conditioned flavor preferences in rats represent a useful model of flavor–flavor learning. Whereas brain dopamine and glutamatergic systems appear quite important in acquiring flavor–flavor preferences, brain dopamine and now endocannabinoid systems appear important in maintaining the expression of such preferences. Demonstrating interactions between these and other candidate neurotransmitter systems and identifying the brain site(s) of action at which such interactions occur are important steps in the complete understanding of food preferences and appetite.
Acknowledgments
This research was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK071761.
References
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, et al. Selective inhibition of sucrose and ethanol intake by SR141176, an antagonist of central cannabinoid (CB1) receptors. Psychopharm. 1997;132:104–6. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. Naltrexone fails to block the acquisition or expression of a flavor-preference conditioned by intragastric carbohydrate infusions. Pharmacol Biochem Behav. 2000;67:545–57. doi: 10.1016/s0091-3057(00)00395-6. [DOI] [PubMed] [Google Scholar]
- Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. D-1 but not D-2 dopamine receptor antagonism blocks the acquisition of a flavor-preference conditioned by intragastric carbohydrate infusions. Pharmacol Biochem Behav. 2001;68:709–20. doi: 10.1016/s0091-3057(01)00484-1. [DOI] [PubMed] [Google Scholar]
- Baker RW, Li Y, Lee MG, Sclafani A, Bodnar RJ. Naltrexone does not prevent acquisition or expression of flavor preferences conditioned by fructose in rats. Pharmacol Biochem Behav. 2004;78:239–46. doi: 10.1016/j.pbb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Baker RW, Shah MJ, Sclafani A, Bodnar RJ. Dopamine D1 and D2 antagonists reduce the acquisition and expression of flavor-preferences conditioned by fructose in rats. Pharmacol Biochem Behav. 2003;75:55–65. doi: 10.1016/s0091-3057(03)00039-x. [DOI] [PubMed] [Google Scholar]
- Bernal SY, Abayev Y, Miner P, Kandova E, Pinhas A, Touzani K, et al. Expression of fructose-conditioned flavor preferences: role of D1 and D2 dopamine receptors in the amygdala. Soc Neurosci Abstr. 2006;32 SFN Abstract Viewer. [Google Scholar]
- Bernal SY, Miner P, Abayev Y, Kandova E, Pulatov P, Touzani K, et al. Acquisition of fructose-conditioned flavor preferences in rats: role of D1 and D2 dopamine receptors in the nucleus accumbens and amygdala. Soc Neurosci Abstr. 2007;33 SFN Abstract Viewer. [Google Scholar]
- Bodnar RJ. Endogenous opioids and feeding behavior: a thirty-year historical perspective. Peptides. 2004;25:697–725. doi: 10.1016/j.peptides.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Sharkey KA, Koopmans HS. Cannabinoid (CB)1 receptor antagonist, AM 251, causes a sustained reduction of daily food intake in the rat. Physiol Behav. 2004;82:863–9. doi: 10.1016/j.physbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Koopmans HS, Pittman QJ, Sharkey KA. AM 251 produces sustained reductions in food intake and body weight that are resistant to tolerance and conditioned taste aversion. Br J Pharmacol. 2006;147:109–16. doi: 10.1038/sj.bjp.0706439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RZ, Frassetto A, Fong TM. Effects of the CB1 cannabinoid receptor inverse agonist AM251 on food intake and body weight in mice lacking mu opioid receptors. Brain Res. 2006;1108:176–8. doi: 10.1016/j.brainres.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Huang RRC, Shen CP, MacNeil DJ, Fong TM. Synergistic effects of cannabinoid inverse agonist AM251 and opioid antagonist nalmefene on food intake in mice. Brain Res. 2004;999:227–30. doi: 10.1016/j.brainres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Cooper SJ. Endocannabinoids and food consumption: comparisons with benzodiazepine and opioid-palatability-dependent appetite. Eur J Pharmacol. 2004;500:37–49. doi: 10.1016/j.ejphar.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Cota D, Tschop MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res Rev. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- DiMarzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8:585–9. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- Dostova I, Kest A, Bernal SY, Abayev Y, Kandova E, Touzani K, et al. Expression of fructose-conditioned flavor preferences: role of D1 and D2 dopamine receptors in the nucleus accumbens shell. Soc Neurosci Abstr. 2006;32 doi: 10.1016/j.bbr.2008.02.003. SFN Abstract Viewer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco FR, Martorana A, Giampa C, De March Z, Farini D, D’Angelo V, et al. Immunolocalization of CB1 receptor in rat striatal neurons: a confocal microscopy study. Synapse. 2004;53:159–67. doi: 10.1002/syn.20047. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–84. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Golden GJ, Houpt TA. NMDA receptor in conditioned flavor–taste preference learning: blockade by MK-801 and enhancement by D-cycloserine. Pharmacol Biochem Behav. 2007;86:587–96. doi: 10.1016/j.pbb.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991;547:267–74. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Higgs S, Williams CS, Kirkham TC. Cannabinoid influences on palatability: microstructural analysis of sucrose drinking after delta(9)-tetrahydrocannibinol, amandamide, 2-arachidonoyl glycerol and SR141716. Psychopharmacol. 2003;165:370–7. doi: 10.1007/s00213-002-1263-3. [DOI] [PubMed] [Google Scholar]
- Hildebrandt AL, Kelly-Sullivan DM, Black SC. Antiobesity effects of chronic cannabinoid CB1 receptor antagonist treatment in diet-induced obese mice. Eur J Pharmacol. 2003;462:125–32. doi: 10.1016/s0014-2999(03)01343-8. [DOI] [PubMed] [Google Scholar]
- Jarrett MM, Limebeer CL, Parker LA. Effect of delta9-tetrahydrocannibinol on sucrose palatability as measured by the taste reactivity test. Physiol Behav. 2005;86:475–9. doi: 10.1016/j.physbeh.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Jarrett MM, Scantlebury J, Parker LA. Effect of delta9-tetrahydrocannibinol on quinine palatability and AM251 on sucrose and quinine palatability using the taste reactivity test. Physiol Behav. 2007;90:425–30. doi: 10.1016/j.physbeh.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Kirkham TC. Endocannabinoids in the regulation of appetite and body weight. Behav Pharmacol. 2005;16:297–313. doi: 10.1097/00008877-200509000-00004. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM. Synergistic effects of opioid and cannabinoid antagonists on food intake. Psychopharmacol. 2001;153:267–70. doi: 10.1007/s002130000596. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di MV. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–7. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch JE. Delta(9)-THC stimulates food intake in Lewis rats: effects on chow, high-fat and sweet high-fat diets. Pharmacol Biochem Behav. 2001;68:539–43. doi: 10.1016/s0091-3057(01)00467-1. [DOI] [PubMed] [Google Scholar]
- Koch JE, Matthews SM. Delta9-tetrahydrocannabinol stimulates palatable food intake in Lewis rats: effects of peripheral and central administration. Nutr Neurosci. 2001;4:179–87. doi: 10.1080/1028415x.2001.11747361. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacol. 2007;32:2267–78. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Matyas F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neurosci. 2006;137:337–61. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Mendez-Diaz M, Prospero-Garcia O. AM251 a CB1 antagonist prevents food intake but not food seeking. Soc Neurosci Abstr. 2007;33 SFN Abstract Viewer. [Google Scholar]
- Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21:1735–42. doi: 10.1016/s0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kash TL, Rodriguez JJ, MacKie K. Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in the rat nucleus accumbens. Neurosci. 2004;127:101–12. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signaling. Nat Rev Neuroci. 2003;4:873–84. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Mukherjee M, Robertson K. Effects of the endocannabinoid receptor antagonist SR 141716, alone and in combination with dexfenfluramine or naloxone, on food intake in rats. Psychopharmacol. 2001;159:111–6. doi: 10.1007/s002130100910. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AN, Hogenboom F, Wardeh G, De Vries TJ. Interactions between CB1 cannabinoid and mu opioid receptors mediating inhibition of neurotransmitter release in rat nucleus accumbens core. Neuropharmacol. 2006;51:773–81. doi: 10.1016/j.neuropharm.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Sclafani A. How food preferences are learned: laboratory animal models. Proc Nutrit Soc. 1995;54:419–27. doi: 10.1079/pns19950011. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Glucose- and fructose-conditioned flavor preferences in rats: taste versus post-ingestive conditioning. Physiol Behav. 1994;56:399–405. doi: 10.1016/0031-9384(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. Intragastric glucose but not fructose conditions robust flavor-preferences in rats. Am J Physiol. 1993;265:R320–5. doi: 10.1152/ajpregu.1993.265.2.R320. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusions of galactose, glucose and fructose in rats. Physiol Behav. 1999;67:227–34. doi: 10.1016/s0031-9384(99)00053-0. [DOI] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown V, Fan P, Vemuri VK, et al. The novel cannabinoid CB(1) receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea. Neuropsychopharm. 2008a;33:946–55. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Olszawska T, Makriyannis A, Salamone JD. Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharm. 2008b;196:565–74. doi: 10.1007/s00213-007-0988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Critical role of dopamine D1 receptors in nucleus accumbens shell in flavor preferences conditioned by intragastric glucose infusion. Appetite. 2006;388 [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Critical role of dopamine D1 receptors in amygdala in the acquisition of flavor preferences conditioned by intragastric glucose infusion in rats. Soc Neurosci Abstr. 2007;33 SFN Abstract Viewer. [Google Scholar]
- Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacol. 2003;167:103–11. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- Werner NA, Koch JE. Effects of the cannabinoid antagonists AM281 and AM630 on deprivation-induced intake in Lewis rats. Brain Res. 2003;967:290–2. doi: 10.1016/s0006-8993(02)04274-9. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharm. 1999;143:315–7. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Observational analysis of feeding induced by delta9-THC and anandamide. Physiol Behav. 2002a;76:241–50. doi: 10.1016/s0031-9384(02)00725-4. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Reversal of delta9-THC hyperphagia by SR141716 and naloxone but not dexfenfluramine. Pharmacol Biochem Behav. 2002b;71:333–40. doi: 10.1016/s0091-3057(01)00694-3. [DOI] [PubMed] [Google Scholar]
- Williams CM, Rogers PJ, Kirkham TC. Hyperphagia in pre-fed rats following oral delta9-THC. Physiol Behav. 1998;65:343–6. doi: 10.1016/s0031-9384(98)00170-x. [DOI] [PubMed] [Google Scholar]
- Yu WZ, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor-preference conditioning in sham-feeding rats: effects of naltrexone. Pharmacol Biochem Behav. 1999;64:573–84. doi: 10.1016/s0091-3057(99)00124-0. [DOI] [PubMed] [Google Scholar]
- Yu WZ, Silva RM, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor-preference conditioning in sham-feeding rats: effects of dopamine receptor antagonists. Pharmacol Biochem Behav. 2000a;65:635–47. doi: 10.1016/s0091-3057(99)00239-7. [DOI] [PubMed] [Google Scholar]
- Yu WZ, Sclafani A, Delamater AR, Bodnar RJ. Role of D1 and D2 dopamine receptors in the acquisition and expression of flavor-preference conditioning in sham-feeding rats. Pharmacol Biochem Behav. 2000b;67:537–44. doi: 10.1016/s0091-3057(00)00396-8. [DOI] [PubMed] [Google Scholar]