A majority of pregnant women with discordant syphilis serology were isolated chemiluminescence assay (CIA) reactive; >50% became CIA non-reactive after retesting. Syphilis serology in pregnancy is often falsely positive. Reflexive testing of discordant specimens with a second treponemal test is recommended.
Keywords: treponemal immunoassay, prenatal syphilis screening, congenital syphilis, discordant treponemal serology, prenatal syphilis treatment
Abstract
Background. The reverse sequence algorithm is often used for prenatal syphilis screening by high-volume laboratories, beginning with a treponemal test such as the chemiluminescence immunoassay (CIA), followed by testing of CIA-positive (CIA+) specimens with the rapid plasma reagin test (RPR). The clinical significance of discordant serology (CIA+/RPR−) for maternal and neonatal outcomes is unknown.
Methods. From August 2007 to August 2010, all pregnant women at Kaiser Permanente Northern California with discordant treponemal serology underwent reflexive testing with Treponema pallidum particle agglutination assay (TP-PA) and were categorized as “TP-PA confirmed” (CIA+/RPR−/TP-PA+) or “isolated CIA positive” (CIA+/RPR−/TP-PA−). Demographic variables and clinical data were abstracted from the medical record and compared by TP-PA status.
Results. Of 194 pregnant women, 156 (80%) were CIA+/RPR−/TP-PA− and 38 (20%) were CIA+/RPR−/TP-PA+. Among the 77 (49%) CIA+/RPR−/TP-PA− women who were retested, 53% became CIA−. CIA+/RPR−/TP-PA+ (n = 38) women were more likely to be older, have a prior history of sexually transmitted infections, and receive treatment for syphilis during pregnancy than women who were CIA+/RPR−/TP-PA− (all P < .005). Most pregnancies (189/194 [97.5%]) resulted in a live birth; there was no difference in birth outcomes according to TP-PA status and no stillbirths attributable to syphilis.
Conclusions. Most pregnant women with discordant serology were CIA+/RPR−/TP-PA−; more than half who were retested became CIA−. CIA+/RPR−/TP-PA− serology in pregnancy is likely to be falsely positive. Reflexive testing of discordant specimens with TP-PA is important to stratify risk given the likelihood of false-positive results in this population.
Currently in the United States, screening for syphilis is recommended during the first prenatal visit for all pregnant women and again in the third trimester in high-risk populations [1, 2]. Traditionally, prenatal screening for syphilis is performed with a nontreponemal test such as the rapid plasma reagin (RPR) or the Venereal Disease Research Laboratory (VDRL) test. Recently, the availability of automated treponemal enzyme and chemiluminescence immunoassay (EIA/CIA) has led some high-volume laboratories to adopt a reverse screening algorithm for prenatal screening in which a treponemal EIA/CIA is performed first; reactive sera are then tested reflexively with an RPR/VDRL test [2]. Although use of the EIA/CIA-based algorithm allows for high-throughput testing, it also results in identification of patients with discordant serologic results (CIA+/RPR−) that were previously unidentified with the standard algorithm. The objective of this analysis was to describe outcomes among pregnant women who were CIA+/RPR−, including treatment and subsequent follow-up serology. Additionally, we describe birth/neonatal outcomes including clinical evaluation and treatment for congenital syphilis among infants born to women with discordant serology.
METHODS
Study Setting/Inclusion Criteria
We conducted a retrospective analysis of pregnant women tested with the reverse sequence algorithm between 1 August 2007 and 31 August 2010 from Kaiser Permanente Northern California (KPNC). During the study period, 106 100 live births occurred [3] (E. Walsh, personal communication). In our analysis we included only pregnant patients aged ≥18 years with discordant serology during pregnancy with a known pregnancy outcome (eg, therapeutic abortion, miscarriage, stillbirth, live birth). Women who were RPR+ at the first visit but had discordant serology later in pregnancy were excluded. Women <18 years old and/or with no known pregnancy outcome were excluded. The electronic health records of the infants born to mothers treated for syphilis antepartum were retrospectively reviewed for documentation of screening and/or treatment of congenital syphilis infection.
Laboratory Methods
In August 2007, the KPNC Regional Laboratory replaced the RPR test (Becton, Dickinson and Company, Franklin Lakes, New Jersey) with a treponemal CIA (LIAISON, DiaSorin Inc, Stillwater, Minnesota) as the initial test for syphilis screening and diagnostic testing. Patients noted to be CIA+ were subsequently reflexively tested with the RPR. Patients with discordant serology (CIA+/RPR−) were reflexively tested with the Treponema pallidum particle agglutination assay (TP-PA; Fujirebio Inc, Malvern, Pennsylvania). All serologic testing was performed on the same specimen, and the results of all 3 tests were reported simultaneously to providers.
Study Variables
For all pregnant women with discordant serology, we obtained demographic, clinical, behavioral, and follow-up syphilis testing data from the KPNC electronic health record using a standardized abstraction protocol. Data elements collected included but were not limited to date of birth, race (black/African American, white/Caucasian, Asian/Pacific Islander, Hispanic/Latino, and other), medical history, human immunodeficiency virus (HIV) status, history of sexually transmitted infections (STIs) other than syphilis (gonorrhea, chlamydia, and genital herpes), current and past pregnancies, pregnancy-related morbidity and medical conditions, and prior history of treated syphilis. Stage of syphilis (if diagnosed), treatment, and subsequent clinical management were also recorded. All women were tested as part of standard prenatal care and serial tests were performed based on their index serology during gestation. Finally, we queried the KPNC laboratory database and the California Department of Public Health state syphilis case report registry to collect historical RPR titers, as well as any follow-up syphilis serology results (repeat CIA, RPR, and/or TP-PA) in the 12 months following the initial CIA testing.
The following criteria were used to determine whether patients had a prior history of treated syphilis: (1) documentation in the health record; (2) patient self-report (documented by provider in the clinical encounter note); or (3) prior positive RPR test with TP-PA or fluorescent treponemal antibody-absorption test (FTA-ABS) documented in the KPNC laboratory database prior to August 2007 with subsequent clinical follow-up at KPNC. For patients for whom none of these were documented, the state syphilis case registry was searched for prior positive syphilis serology. Women with no documentation in the KPNC health record and no record in the state syphilis case registry were considered to have no prior history of syphilis.
Data Management and Statistical Analysis
All abstracted medical record and laboratory data were entered into a Microsoft Access database. Demographic data were analyzed using descriptive statistics. The χ2 or Fisher's exact test was used to compare proportions, and Student t test was used for continuous variables. Mantel–Haenszel χ2 test was used to test for trends. Medians for nonnormally distributed variables (eg, gestational age, birth weight) were compared using the Wilcoxon rank-sum test. A P value <.05 was considered to be statistically significant. Analyses were performed using Stata version 12 (StataCorp, College Station, Texas) and SAS version 9.2 (SAS Institute, Cary, North Carolina). This study was approved by the institutional review boards of the University of California, San Francisco, the California Department of Public Health, and the Kaiser Foundation Research Institute, with a waiver of informed consent for study participants.
RESULTS
During the study period of August 2007–August 2010, 224 women had discordant serology at some point during pregnancy. Excluding 30 women who were CIA+/RPR+ at their initial visit, 194 of 224 (87%) were included in the final analysis. Table 1 describes a comparison of demographic and clinical characteristics of the 194 women overall and according to TP-PA status. The majority (156/194 [80%]) of pregnant women with discordant serology were CIA+/RPR−/TP-PA−. The remaining pregnant women with discordant serology who were CIA+/RPR−/TP-PA+ (n = 38) were significantly more likely to be older and to have a higher number of prior pregnancies and living children than those who were CIA+/RPR−/TP-PA− (all P ≤ .005). CIA+/RPR−/TP-PA+ women were significantly more likely to have had an STI other than syphilis in the prior 24 months and more likely to have a prior history of syphilis compared with women who were CIA+/RPR−/TP-PA−.
Table 1.
Characteristics of Pregnant Women With Discordant Syphilis Serologya According to Treponema pallidum Particle Agglutination Assay Status (N = 194)
| Characteristic | CIA+RPR−TP-PA+ (n = 38) | CIA+RPR−TP-PA– (n = 156) | P Value |
|---|---|---|---|
| Age at delivery, y, mean (SD) | 35 (5.1) | 30 (5.9) | <.0001 |
| Prior pregnancies, median | 4 | 2 | <.0001 |
| Living children, median | 2 | 1 | .001 |
| HIV status, No. (%) | |||
| Positive | 1 (3) | 0 | |
| Negative | 37 (97) | 156 (100) | |
| STI (not syphilis) in past 24 mo, No. (%) | 6 (16) | 4 (3) | .005 |
| Prior history of syphilis, No. (%) | 26 (70) | 3 (2) | <.0001 |
Abbreviations: CIA, chemiluminescence immunoassay; HIV, human immunodeficiency virus; RPR, rapid plasma reagin test; SD, standard deviation; STI, sexually transmitted infection (includes chlamydia, gonorrhea, and genital herpes); TP-PA, Treponema pallidum particle agglutination assay.
a CIA reactive, RPR nonreactive.
Syphilis Diagnosis and Treatment
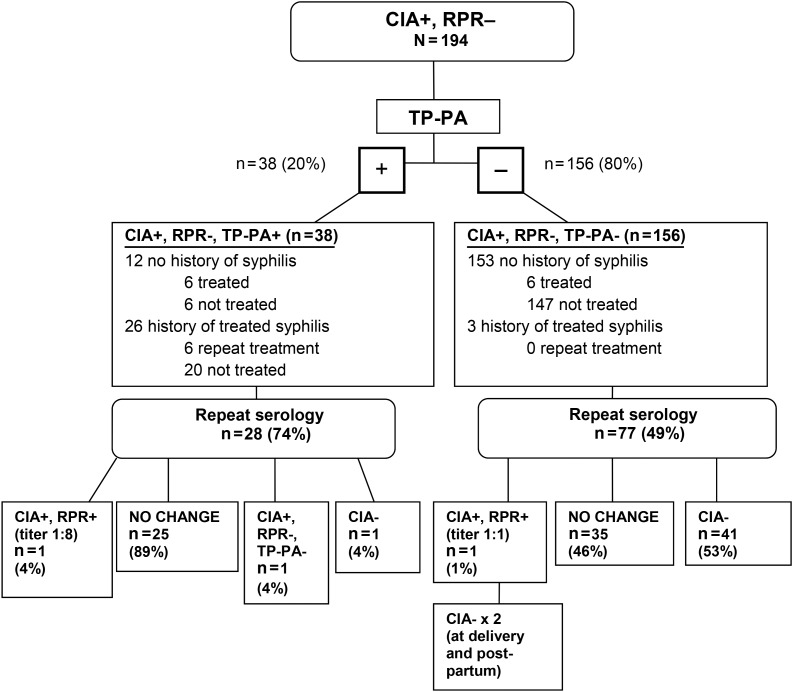
Few women (18/194 [9%]) were treated for syphilis based on their discordant serology results (Figure 1). Staging among the 18 women treated for syphilis included early latent (3 cases), late latent (14 cases), and 1 case of latent syphilis where stage was not specified; there were no cases of primary or secondary syphilis. Syphilis staging was determined by review of the medical chart, not based on traditional surveillance case definitions that require a positive RPR. All women received benzathine penicillin for treatment. Treatment was given more frequently for women who were CIA+/RPR−/TP-PA+ than those who were CIA+/RPR−/TP-PA− (32% vs 4%; P < .0001).Treatment status of patients according to prior syphilis history is illustrated in Figure 1.
Figure 1.
Serology results and clinical management after prenatal screening with the treponemal chemiluminescence immunoassay (CIA). Abbreviations: RPR, rapid plasma reagin test; TP-PA, Treponema pallidum particle agglutination assay.
Follow-up Syphilis Testing
Reversion to CIA nonreactive was more likely among younger pregnant women and significantly declined with increasing age (Ptrend = .001). Follow-up serology results according to initial TP-PA status are described in Figure 1. The majority (89%) of women who were initially CIA+/RPR−/TP-PA+ had no change in serology after repeat testing, whereas just over half (53%) of women who were initially CIA+/RPR−/TP-PA− became CIA–.
Among the 2 women who seroconverted to CIA+/RPR+, 1 woman was initially CIA+/RPR−/TP-PA+ and became CIA+/RPR+ (titer 1:8). The other was initially CIA+/RPR−/TP-PA− and became CIA+/RPR+ (titer 1:1), and then became CIA– during the third trimester and remained CIA− at delivery. Her infant was also RPR nonreactive; this patient may have had both a false-positive CIA and RPR. Eleven women seroconverted from CIA− in the first 20 weeks of pregnancy to CIA+/RPR−/TP-PA− in the second half of pregnancy and were not treated for syphilis; later 4 of these seroreverted back to CIA−; the remaining 7 women were not retested again before delivery.
Among 6 women who were CIA+/RPR−/TP-PA+ with no history of syphilis and no documented treatment during pregnancy, 1 became CIA− and another had no change in repeat serology. The remaining women were not retested during pregnancy.
Birth Outcomes
The majority of pregnancies in the cohort (189/194 [97.5%]) resulted in a live birth; 3 stillbirths (>20 weeks) and miscarriages (<20 weeks) were observed. There was no difference in the distribution of birth outcomes or preterm delivery by TP-PA status (P = .26 and P = .69, respectively), and no stillbirths were attributed to syphilis infection in the medical records. Two infants received empiric antibiotic therapy, and 1 infant also received a lumbar puncture; however, none were RPR reactive or diagnosed with congenital syphilis.
Of the 18 infants born to the women treated for syphilis infection antepartum, 10 had normal clinical evaluations up to 12 months of age; 6 charts were unavailable (eg, patient no longer a KPNC member); 2 neonates were admitted to the neonatal intensive care unit (NICU) after birth. One of the children admitted to the NICU was evaluated for jaundice and was RPR nonreactive at birth and at 2 months of life; the other received empiric antibiotic therapy for syphilis but was RPR nonreactive at birth and at 2 months of life.
DISCUSSION
Based on our observational analysis, women with discordant serology and their infants were not at increased risk for adverse outcomes, regardless of subsequent TP-PA results. Management of pregnant women with discordant serology results presents a dilemma for providers who weigh the risk of congenital syphilis vs the risks of overtreating for a false-positive CIA result and associated detriment to the patient's well-being and costs incurred for neonatal/maternal management. Costs may be significant if the pregnant patient is allergic to penicillin (guidelines recommend desensitization and treatment, typically as an inpatient). Most pregnant women in our study were CIA+/RPR−/TP-PA−, suggesting false-positive serology; a prior study of reverse-sequence screening also observed that pregnant women were more likely to be isolated CIA reactive compared with nonpregnant women [4].
Current guidelines recommend retesting low-risk patients with CIA+/RPR−/TP-PA− results rather than immediate treatment [2, 5]. In our study, the vast majority of pregnant women with CIA+/RPR−/TP-PA− results were indeed managed with repeat testing. Reflexive testing of discordant results with a second treponemal test such as TP-PA is an important tool to distinguish true syphilis vs a false-positive CIA before initiating treatment. Among low-risk women, treatment can be reserved for those with reactive nontreponemal serology (eg, CIA reactive, RPR reactive) or with 2 reactive treponemal tests [2, 5].
Our study is the first to our knowledge to describe fluctuations of CIA reactivity and reversion of isolated CIA-reactive results to nonreactive status during pregnancy. The reasons for false-positive treponemal CIA results in pregnancy are unknown. Pregnancy is a well-recognized cause of biologic false-positive (BFP) nontreponemal tests (eg, RPR or VDRL) [6, 7]; however, data are limited around BFP treponemal tests in pregnancy [6, 7]. A study of pregnant women by Tinajeros et al found that 0.91% of all pregnant women had BFP RPR and 1.5% had an isolated reactive treponemal rapid test; it is unknown whether these were false-positive results or reflective of prior infection [8]. Seroreversion of treponemal tests is uncommon but has been described for older treponemal tests (eg, FTA-ABS) among patients with HIV (38% seroreversion) and among 13%–24% of patients treated for early syphilis [9]. However, in our study there were few patients with a prior history of syphilis and only 1 patient with HIV. We noted one patient who likely had both a false-positive RPR and CIA during her pregnancy. This was the only observation that occurred in >100 000 births at KPNC during the study period, and therefore is likely an uncommon occurrence.
Current guidelines recommend repeated syphilis screening in the third trimester only for high-risk patients or those living in areas of high syphilis prevalence [5, 10]. When using the reverse sequence algorithm, our data suggest that pregnant women identified as CIA+/RPR−/TP-PA− should also receive routine repeat screening later in pregnancy to assess whether reversion to CIA– status occurs. Repeat testing would only be indicated for asymptomatic pregnant women at low risk for syphilis. Those with symptoms or at high risk for syphilis (eg, sexual contacts to syphilis) should continue to be staged and treated with benzathine penicillin [5].
Our data did not indicate a difference in birth outcomes between the groups of pregnant women according to TP-PA status, with nearly all women having a full-term live birth. There were no cases of probable or confirmed congenital syphilis, even among mothers who became RPR+ during pregnancy. However, congenital syphilis is a rare outcome and our study was likely not large enough to detect differences in this outcome by initial TP-PA status. A large analysis of congenital syphilis case reports by Peterman et al found no confirmed congenital syphilis among mothers with persistently negative nontreponemal serology [11]. Yet, most of the women were tested with a traditional RPR-based algorithm rather than the reverse sequence algorithm, so generalizability to our data is limited.
Our study has several limitations. First, the analysis was based on observational data and therefore systematic retesting of all pregnant patients was not performed, which may have resulted in selection bias. Due to limitations with the laboratory data, we were not able to determine the exact number of pregnant women who were screened during the study period. Nevertheless, we were able to determine the exact number of live births during the study period, which serves as a proxy denominator. Sexual history data were largely incomplete in the medical records, which limited our ability to assess risk for syphilis. Given the absence of a control group, we are not able to conclude whether similar fluctuations in CIA reactivity occur in the nonpregnant state, although our prior study at KPNC did also document some reversions to CIA– among nonpregnant patients [12]. The seroprevalence of syphilis at KPNC overall is low (approximately 2%), and our findings are not generalizable to populations with high syphilis seroprevalence, where the predictive value of an isolated CIA-reactive result is higher than in our population [13].
In conclusion, routine retesting of pregnant women with CIA+/RPR−/TP-PA− serology and reflexive testing of CIA+/RPR− specimens with a second treponemal test is useful given the high likelihood of false-positive CIA results in pregnancy. Further prospective studies in pregnant populations with both high and low syphilis seroprevalence could further inform guidelines on use of the reverse sequence algorithm for prenatal syphilis screening.
Notes
Acknowledgments. The authors thank Stacey Holly of the California Department of Public Health, Raquel Fernandez of the Los Angeles Department of Public Health, and Bob Kohn, MPH, of the San Francisco Department of Public Health, for assistance with syphilis registry searches. We thank Katie Gustafson of the California Department of Public Health for assistance with programming/data analysis, and Eileen Walsh, RN, MPH, of Kaiser Permanente Northern California for providing data on volume of births during the study period.
Disclaimer. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health (NIH) or the Centers for Disease Control and Prevention (CDC).
Financial support. At the time of this study, O. M. was at the University of California, San Francisco and was supported by an NIH training grant (T32AI065388) from the National Institute of Allergy and Infectious Diseases. J. M. C. and I. U. P. were supported by the CDC (grant number H25PS001379-01).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Wolff T, Shelton E, Sessions C et al. Screening for syphilis infection in pregnant women: evidence for the U.S. Preventive Services Task Force Reaffirmation Recommendation Statement. Ann Intern Med 2009; 150:710–6. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Discordant results from reverse sequence syphilis screening—five laboratories, United States, 2006–2010. MMWR Morb Mortal Wkly Rep 2011; 60:133–7. [PubMed] [Google Scholar]
- 3.Kaiser Permanente. Fast facts about Kaiser Permanente. Available at: http://share.kaiserpermanente.org/article/fast-facts-about-kaiser-permanente/. Accessed 13 December 2013.
- 4.Henrich TJ, Yawetz S. Impact of age, gender, and pregnancy on syphilis screening using the Captia Syphilis-G assay. Sex Transm Dis 2011; 38:1126–30. [DOI] [PubMed] [Google Scholar]
- 5.Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep 2010; 59(RR-12):1–110. [PubMed] [Google Scholar]
- 6.Watson-Jones D, Gumodoka B, Weiss H et al. Syphilis in pregnancy in Tanzania. II. The effectiveness of antenatal syphilis screening and single dose benzathine penicillin treatment for the prevention of adverse pregnancy outcomes. J Infect Dis 2002; 186:948–57. [DOI] [PubMed] [Google Scholar]
- 7.Nandwani R, Evans DT. Are you sure it's syphilis? A review of false positive serology. Int J STD AIDS 1995; 6:241–8. [DOI] [PubMed] [Google Scholar]
- 8.Tinajeros F, Grossman D, Richmond K et al. Diagnostic accuracy of a point-of-care syphilis test when used among pregnant women in Bolivia. Sex Transm Infect 2006; 82:v17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas JS, Bolan G, Larsen SA et al. Sensitivity of treponemal tests for detecting prior treated syphilis during human immunodeficiency virus infection. J Infect Dis 1990; 162:862–6. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Pediatrics. Syphilis. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, eds. Red Book: 2012 report of the Committee on Infectious Diseases. 29th ed Elk Grove Village, IL: American Academy of Pediatrics; 2012:690–703. [Google Scholar]
- 11.Peterman QA, Newman DR, Davis D et al. Do women with persistently negative non-treponemal test results transmit syphilis during pregnancy? Sex Transm Dis 2013; 40:311–5. [DOI] [PubMed] [Google Scholar]
- 12.Park IU, Chow JM, Bolan G et al. Screening for syphilis with the treponemal immunoassay: analysis of discordant serology results and implications for clinical management. J Infect Dis 2011; 204:1297–304. [DOI] [PubMed] [Google Scholar]
- 13.Hunter MG, Robertson PW, Post JJ. Significance of isolated reactive treponemal chemiluminescence immunoassay results. J Infect Dis 2013; 207:1416–23. [DOI] [PubMed] [Google Scholar]