In a randomized controlled trial, rapid multiplex polymerase chain reaction (rmPCR) detection of organisms from positive blood culture bottles reduced treatment of contaminants and broad-spectrum antimicrobial use. Combined with audit and feedback by an antimicrobial stewardship team, rmPCR enhanced antimicrobial de-escalation.
Keywords: antimicrobial stewardship, blood culture, diagnostic, PCR
Abstract
Background. The value of rapid, panel-based molecular diagnostics for positive blood culture bottles (BCBs) has not been rigorously assessed. We performed a prospective randomized controlled trial evaluating outcomes associated with rapid multiplex PCR (rmPCR) detection of bacteria, fungi, and resistance genes directly from positive BCBs.
Methods. A total of 617 patients with positive BCBs underwent stratified randomization into 3 arms: standard BCB processing (control, n = 207), rmPCR reported with templated comments (rmPCR, n = 198), or rmPCR reported with templated comments and real-time audit and feedback of antimicrobial orders by an antimicrobial stewardship team (rmPCR/AS, n = 212). The primary outcome was antimicrobial therapy duration. Secondary outcomes were time to antimicrobial de-escalation or escalation, length of stay (LOS), mortality, and cost.
Results. Time from BCB Gram stain to microorganism identification was shorter in the intervention group (1.3 hours) vs control (22.3 hours) (P < .001). Compared to the control group, both intervention groups had decreased broad-spectrum piperacillin-tazobactam (control 56 hours, rmPCR 44 hours, rmPCR/AS 45 hours; P = .01) and increased narrow-spectrum β-lactam (control 42 hours, rmPCR 71 hours, rmPCR/AS 85 hours; P = .04) use, and less treatment of contaminants (control 25%, rmPCR 11%, rmPCR/AS 8%; P = .015). Time from Gram stain to appropriate antimicrobial de-escalation or escalation was shortest in the rmPCR/AS group (de-escalation: rmPCR/AS 21 hours, control 34 hours, rmPCR 38 hours, P < .001; escalation: rmPCR/AS 5 hours, control 24 hours, rmPCR 6 hours, P = .04). Groups did not differ in mortality, LOS, or cost.
Conclusions. rmPCR reported with templated comments reduced treatment of contaminants and use of broad-spectrum antimicrobials. Addition of antimicrobial stewardship enhanced antimicrobial de-escalation.
Clinical Trials Registration. NCT01898208.
(See the Editorial Commentary by Caliendo on pages 1081–3.)
Conventional methods for identification and susceptibility testing of microorganisms from blood cultures takes ≥2 days, during which time patients may be receiving ineffective or unnecessarily broad-spectrum antibiotics [1, 2]. Panel-based molecular diagnostic assays are now available for direct testing of positive blood culture bottles (BCBs), providing timelier results than conventional subculture and phenotypic susceptibility testing. Faster identification and resistance characterization of pathogens may lead to earlier administration of directed antimicrobial therapy, promote earlier de-escalation of broad-spectrum agents, and potentially result in better outcomes, fewer antibiotic-associated adverse effects (eg, Clostridium difficile infection), and less emergence of antimicrobial-resistant organisms [3]. However, rapid blood culture diagnostics are “add-on” tests performed in addition to conventional testing, and therefore increase complexity of laboratory testing and cost of patient care. The Infectious Diseases Society of America has recently called for research to evaluate how novel diagnostics impact patients and healthcare systems and to identify methods to integrate novel diagnostic testing into clinical practice [4].
Studies evaluating the clinical impact of rapid blood culture diagnostics have been limited by observational study designs and use of historical controls [5–11]. Whereas studies suggest that antimicrobial use, length of stay (LOS), mortality, and/or cost may be reduced through use of rapid blood culture diagnostic tests [5, 6, 9, 11–14], practice changes other than implementation of the novel tests may have affected outcomes. Additionally, prior studies have evaluated bundled interventions in which rapid tests were implemented with antimicrobial stewardship initiatives, making it impossible to determine whether the rapid test or the stewardship intervention alone might have been effective [5, 6, 8, 11, 15, 16]. Furthermore, real-time antimicrobial stewardship is not feasible in hospitals without stewardship programs. Ideally, a randomized controlled trial (RCT) is needed to evaluate outcomes associated with use of rapid diagnostics for bacterial identification and susceptibility testing directly from positive BCBs, but to date, none has been performed.
We conducted a prospective RCT comparing antimicrobial utilization and outcomes among patients with positive blood cultures who received standard culture and antimicrobial susceptibility testing alone, or with a rapid multiplex polymerase chain reaction (PCR) panel that identifies bacteria and Candida species and select antimicrobial resistance genes in approximately 1 hour [17, 18]. To determine the optimal method of communicating results, PCR test results were delivered in 2 ways, with templated comments to guide antimicrobial prescribing, or with templated comments in conjunction with real-time antimicrobial stewardship team recommendations to prescribers.
METHODS
Study Design, Randomization, and Masking
The study was a prospective RCT conducted at the Mayo Clinic, Rochester, Minnesota. Eligible patients were adults and children who had positive blood cultures processed in the clinical microbiology laboratory using the Becton Dickinson BACTEC FX system between August 2013 and March 2014. Patients were randomly assigned to either standard BCB processing (control), rapid multiplex PCR with templated comments (rmPCR), or rmPCR with templated comments and real-time antimicrobial stewardship (rmPCR/AS). Patients were excluded if they had a positive blood culture in the prior week, had not provided the Minnesota state research authorization (Minnesota Statute 144.335), were previously enrolled in the study, died or were transitioned to comfort care within 24 hours of enrollment, or had a negative BCB Gram stain. This study was approved by the Mayo Clinic Institutional Review Board with a waiver of informed consent.
Stratified randomization (based on age <65 or ≥65 years, intensive care unit admission, and admission to solid organ or bone marrow transplant services) was done once a BCB signaled positive. Laboratory technologists and investigators were not blinded to study arm assignment. For all groups, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) for pathogen identification of colonies isolated from positive BCBs, and rapid testing for methicillin resistance on Staphylococcus aureus colonies with the Alere PBP2a test (Alere, Waltham, Massachusetts) were used. Baseline institutional antimicrobial stewardship interventions were in place for all study groups including requiring infectious diseases approval for restricted antimicrobials, and Monday–Friday daytime prospective audit and feedback of select inpatient antimicrobial orders. A computer-based monitoring system that integrates pharmacy, laboratory, and microbiology databases was used to identify opportunities for audit and feedback [19] (see Supplementary Methods for details).
Intervention
The rmPCR panel used in both intervention arms was the FilmArray Blood Culture ID Panel (BioFire Diagnostics/bioMérieux, Salt Lake City, Utah), which was performed as soon as a BCB signaled positive, 24 hours a day, 7 days a week. This assay detects Staphylococcus species, S. aureus, Streptococcus species, Streptococcus agalactiae, Streptococcus pyogenes, Streptococcus pneumoniae, Enterococcus species, Listeria monocytogenes, Klebsiella oxytoca, Klebsiella pneumoniae, Serratia species, Proteus species, Acinetobacter baumannii, Haemophilus influenzae, Neisseria meningitidis, Pseudomonas aeruginosa, Enterobacteriaceae, Escherichia coli, Enterobacter cloacae complex, Candida albicans, Candida glabrata, Candida krusei, Candida parapsilosis, and Candida tropicalis, and 3 antimicrobial resistance genes, mecA, vanA/B, and blaKPC. Results of the rmPCR test and templated comments regarding optimal antimicrobial therapy (Supplementary Table 1) were communicated to the service by telephone by a laboratory technologist and entered into the electronic medical record in real time. Gram stain–positive blood cultures that had negative rmPCR results were reported with Gram stain result only.
In the rmPCR/AS group, the rmPCR test was performed and reported as above, and an infectious diseases clinician or pharmacist (R. B. or C. B. T.) was paged with the result, 24 hours a day, 7 days a week. The subject's rmPCR result and medical record were reviewed and the primary service (or consulting infectious diseases physician, if applicable) contacted immediately over the 3 days following enrollment if a modification to antimicrobial therapy was deemed appropriate. Discordant results between rmPCR and conventional identification and susceptibility testing were reviewed in real time by the bacteriology laboratory director (R. P.).
Outcome Measures
Subjects were followed for 30 days after enrollment. The primary outcome was duration of antimicrobial therapy (in hours) in the 4 days after enrollment. Duration of antimicrobial therapy was calculated as the difference between the date and time of the antibiotic start order (or Gram stain–positive blood culture, if antibiotics were started prior to the positive culture result) and the date and time of the antibiotic stop order. Secondary outcomes included time from positive Gram stain result to first active antibiotic; time to first appropriate antibiotic escalation (initiation of 1 or more antibiotics or switch from a narrow- to a broad-spectrum antibiotic) or de-escalation (discontinuation of 1 or more antibiotics and/or switch from a broad- to a narrow-spectrum antibiotic); proportion of contaminants not treated; time to pathogen identification and blood culture clearance; LOS; mortality; antibiotic-associated toxicities; infectious disease consultation; and costs per patient [20, 21] (see Supplementary Methods for details).
Statistical Analysis
We anticipated that two-thirds of patients (130/200 per arm) would receive vancomycin or an antipseudomonal antibiotic and that the standard deviation (SD) for antibiotic duration would be about 24 hours. Thus, we would be able to detect a difference in either vancomycin or antipseudomonal antibiotic duration of at least 0.403 SD (approximately 10 hours) with 80% power at an α level of .017 (to account for 3 comparisons) using a 2-sample t test. Comparisons among 3 groups were performed using the Kruskal–Wallis test for continuous variables and Fisher exact test or χ2 test for categorical variables. When the overall test was significant, pairwise comparisons were made using Wilcoxon rank-sum, Fisher exact, or χ2 test, as appropriate. Analysis was performed using SAS software, version 9.3 (SAS Inc, Cary, North Carolina).
RESULTS
Patients
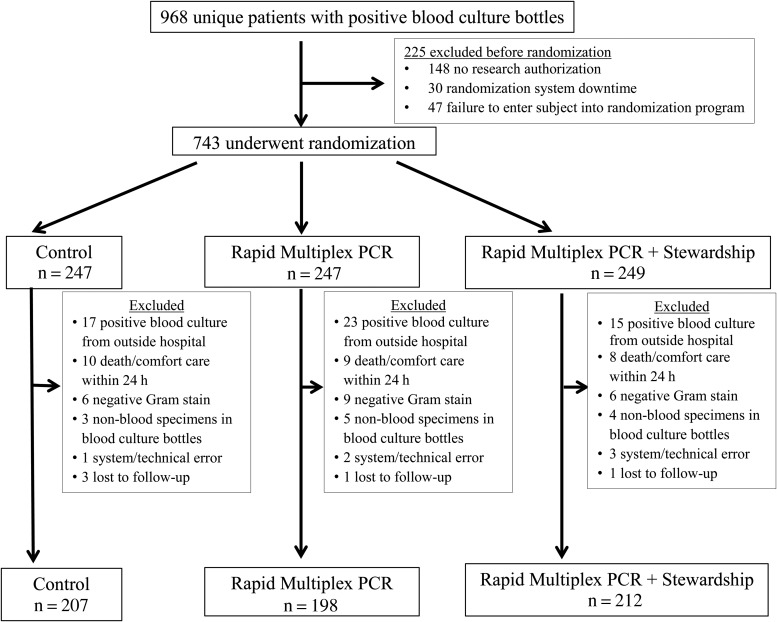
A total of 2646 positive blood cultures from 968 unique patients were identified, 743 patients underwent randomization, and 126 (17%) were excluded, leaving 617 patients included in the study (Figure 1). Compared to nonrandomized subjects, randomized patients were slightly older (61.3 vs 56.7 years, P = .01), but not different by sex. Clinical and demographic characteristics were similar between the groups (Table 1).
Figure 1.
Participant enrollment. System/technical errors included randomization software downtime, typographical errors causing an ineligible subject to be erroneously randomized, and failure to enter an eligible subject into the randomization program. Abbreviation: PCR, polymerase chain reaction.
Table 1.
Baseline Demographic and Clinical Characteristics According to Study Group
| Characteristic | Control (n = 207) | Rapid Multiplex PCR (n = 198) | Rapid Multiplex PCR + Stewardship (n = 212) |
|---|---|---|---|
| Demographics | |||
| Male sex | 142 (69) | 116 (59) | 127 (60) |
| Age, y, mean ± SD | 61.5 ± 19.32 | 61.4 ± 21.22 | 61.2 ± 20.08 |
| Race, white | 176 (85) | 186 (93.9) | 179 (84.4) |
| Location before admission | |||
| Nursing home | 22 (10.6) | 13 (6.6) | 15 (7.1) |
| Outside hospital | 27 (13) | 34 (17.2) | 30 (14.2) |
| Patient home | 114 (55.1) | 103 (52) | 104 (49.1) |
| Outpatient clinic | 22 (10.6) | 13 (6.6) | 15 (7.1) |
| Location at enrollment | |||
| Outpatient | 14 (6.8) | 20 (10.1) | 28 (13.2) |
| General ward | 125 (60.4) | 120 (60.6) | 125 (59) |
| Intensive care unit | 68 (32.9) | 58 (29.3) | 59 (27.8) |
| Comorbidities | |||
| Charlson comorbidity score, mean ± SD | 5.3 ± 3.2 | 5.2 ± 4.7 | 4.9 ± 3.0 |
| Myocardial infarction | 17 (8.2) | 16 (8.1) | 9 (4.2) |
| Chronic heart failure | 31 (15) | 32 (16.2) | 22 (10.4) |
| Cerebrovascular accident | 24 (11.6) | 21 (10.6) | 18 (8.5) |
| Chronic obstructive pulmonary disease | 25 (12.1) | 22 (11.1) | 27 (12.7) |
| Peptic ulcer disease | 27 (13) | 15 (7.6) | 21 (9.9) |
| Lymphoma | 21 (10.1) | 21 (10.6) | 25 (11.8) |
| Leukemia | 15 (7.2) | 21 (10.6) | 21 (9.9) |
| Solid tumor | 41 (19.8) | 34 (17.2) | 31 (14.6) |
| Diabetes mellitus | 68 (32.9) | 50 (25.3) | 63 (29.7) |
| Immunosuppressant usea | 79 (38.2) | 77 (38.9) | 82 (38.7) |
| Surgery in prior 30 d | 30 (14.5) | 27 (13.6) | 45 (21.2) |
| Renal replacement therapy | 16 (7.7) | 13 (6.6) | 14 (6.6) |
| Any malignancy | 76 (36.7) | 72 (36.4) | 73 (34.4) |
| Chronic heart disease | 81 (39.1) | 87 (43.9) | 73 (34.4) |
| Chronic renal disease | 62 (30) | 49 (24.7) | 52 (24.5) |
| Chronic liver disease | 35 (16.9) | 24 (12.1) | 33 (15.6) |
| Chronic lung disease | 43 (20.8) | 44 (22.2) | 50 (23.6) |
| Central venous catheter | 95 (45.9) | 91 (46) | 94 (44.3) |
| Acute kidney injury | 53 (25.6) | 35 (17.7) | 37 (17.5) |
| Concurrent infectious syndromesb | 97 (46.9) | 87 (43.9) | 108 (50.9) |
| Neutropenic fever | 24 (11.6) | 24 (12.1) | 27 (12.7) |
| Respiratory infection | 31 (15) | 21 (10.6) | 29 (13.7) |
| Urinary tract infection | 15 (7.3) | 17 (8.6) | 19 (9) |
| Intra-abdominal infection | 22 (10.6) | 21 (10.6) | 26 (12.3) |
| Source of bacteremia | |||
| Central venous catheter | 32 (15.5) | 38 (19.2) | 37 (17.5) |
| Urinary | 31 (15) | 34 (17.2) | 33 (15.6) |
| Intra-abdominal | 38 (18.4) | 22 (11.1) | 37 (17.5) |
| Skin/soft tissue | 11 (5.3) | 13 (6.6) | 8 (3.8) |
| Respiratory | 9 (4.3) | 11 (5.6) | 8 (3.8) |
| Bone/joint | 2 (1) | 4 (2) | 12 (5.7) |
| Eyes, ears, nose, throat | 1 (0.5) | 2 (1) | 1 (0.5) |
| Cardiovascular | 2 (1) | 3 (1.5) | 2 (0.9) |
| Surgical site infection | 0 (0) | 0 (0) | 2 (0.9) |
| Unidentified | 18 (8.7) | 16 (8.1) | 9 (4.2) |
| Complicated bloodstream infectionc | 14 (6.8) | 21 (10.6) | 13 (6.1) |
| Source control within 5 dd | 44 (21.3) | 46 (23.2) | 57 (26.9) |
| Contact isolation for MRSA, VRE, or Clostridium difficile | 51 (24.6) | 40 (20.2) | 43 (20.3) |
| Possible contaminante | 63 (30.4) | 55 (27.8) | 62 (29.3) |
| Severity of illness | |||
| APACHE II score, mean ± SDf | 18.3 ± 8.2 | 17.4 ± 7.8 | 16.4 ± 7.3 |
| Requiring mechanical ventilation | 21 (10.1) | 23 (11.6) | 16 (7.5) |
| Hypotensiong | 80 (38.6) | 76 (38.4) | 63 (29.7) |
| Pitt bacteremia score, mean ± SDf | 2.0 ± 2.5 | 2.0 ± 2.3 | 1.6 ± 2.0 |
| Infectious diseases consultation within 72 h of enrollment | 103 (49.8) | 97 (49) | 96 (45.3) |
| On active antibiotic at the time of enrollmenth | 99 (69) | 102 (71) | 113 (75) |
Data are presented as No. (%) unless otherwise specified. There were no significant differences in baseline characteristics between the study groups with the exception of race (P = .005).
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; MRSA, methicillin-resistant Staphylococcus aureus; PCR, polymerase chain reaction; SD, standard deviation; VRE, vancomycin-resistant Enterococcus species.
a Received cytotoxic agents within prior 6 weeks, >15 mg prednisone for >1 week in prior 4 weeks, or any other immunosuppressant within 2 weeks of blood culture.
b A patient was classified with concurrent infection other than neutropenic fever when a site of infection was documented in the medical record (eg, pneumonia, intraabdominal infection), and culture from the concurrent infection site grew at least 1 organism that was not isolated from blood.
c Positive blood culture after 3 days of effective antimicrobial therapy, metastatic infection, or infective endocarditis.
d Catheter removal or surgical drainage procedure.
e Growth of common contaminant (eg, coagulase-negative Staphylococcus species) from a single blood culture set when ≥2 blood culture sets were collected, except among subjects suspected to have true bacteremia associated with central venous catheters or devices.
f Excludes outpatients.
g Requiring vasopressors or systolic blood pressure decrease by >20 mm Hg.
h Active antibiotic defined as an agent to which the blood culture organism was susceptible by conventional antimicrobial susceptibility testing.
Microbiology
Blood cultures grew 54.8% gram-positive bacteria, 32.6% gram-negative bacteria, 2% Candida species, and 10.5% multiple organisms. One-third of organisms isolated (29.2%) were considered contaminants. Among subjects with rmPCR testing, 81% of organisms isolated were detectable by the rmPCR panel. Study groups did not differ in terms of the distribution of microorganisms or the proportion that were contaminants or detectable by rmPCR (Supplementary Table 2).
Rapid Multiplex PCR Performance and Stewardship Interventions
Among subjects with pathogens represented on the rmPCR panel, median time from Gram stain result to organism identification was shorter in both intervention groups (both 1.3 hours) vs the control group (22 hours) (P < .0001; Figure 2). Most discrepancies between rmPCR and standard culture and susceptibility results occurred because microorganisms were not represented on the rmPCR panel (78/410 [19%]). In 13 of 410 (3.2%) cases, there were discrepancies in organism identification or susceptibility result (Table 2).
Figure 2.
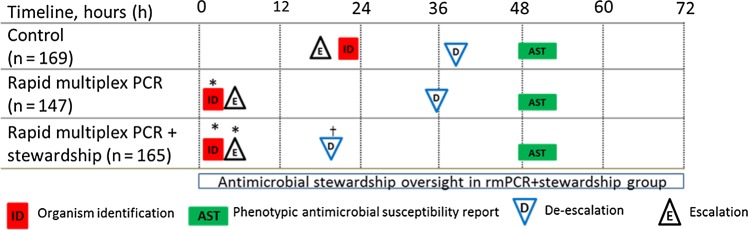
Comparison of time to organism identification, availability of phenotypic antimicrobial susceptibility results, and first appropriate modification of antimicrobial therapy for the subset of study subjects with organisms represented on the rapid multiplex polymerase chain reaction (rmPCR) panel (n = 481). Time 0 is when the positive Gram stain result was reported. Median time in hours (interquartile range [IQR]) to organism identification: control 22.3 (17–28), both rmPCR and rmPCR + stewardship 1.3 (0.9–1.6); de-escalation: control 39 (19–56), rmPCR 36 (22–61), rmPCR + stewardship 20 (6–36); escalation: control 18 (2–63), rmPCR 4 (1.5–24), rmPCR + stewardship 4 (1.8–9). *P < .05 vs control; †P < .05 vs control and rmPCR groups.
Table 2.
Discrepancies Between Rapid Multiplex Polymerase Chain Reaction Assay and Standard Culture/Phenotypic Antimicrobial Susceptibility Testing
| Rapid Multiplex PCR Panel Result | Standard Culture and Phenotypic Susceptibility Testing | No. |
|---|---|---|
| Single/few organisms detected | Multiple organisms detected | 6 |
| 1. Klebsiella pneumoniae | 1. K. pneumoniae, Staphylococcus epidermidis | |
| 2. Enterococcus species | 2. Enterococcus faecium, Staphylococcus hemolyticus, Micrococcus luteus | |
| 3. Enterobacter cloacae, Enterococcus species (vanA/B positive) | 3. Enterococcus faecalis (vancomycin susceptible), E. faecium (vancomycin resistant), E. cloacae, Streptococcus mitis, S. epidermidis, Acinetobacter species | |
| 4. (bottle a) Streptococcus agalactiae (bottle b) Escherichia colia |
4. (bottle a) S. agalactiae, E. coli (bottle b) E. coli, Proteus species |
|
| 5. Streptococcus species, Haemophilus influenzae | 5. Haemophilus sputorum, Neisseria subflava, Streptococcus salivarius, Streptococcus viridans group, Streptococcus mitis | |
| 6. Enterococcus species (vanA/B negative) | 6. E. faecium (vancomycin susceptible), S. anginosus | |
| Discrepancy in susceptibility result | 4 | |
| 1. Enterococcus species (vanA/B negative) | 1. Enterococcus casseliflavus (vancomycin MIC, 8 µg/mL) | |
| 2. Enterococcus species (vanA/B positive) | 2. E. faecium, vancomycin MIC 2 µg/mL, isolate vanA positive by PCR | |
| 3. Staphylococcus species (mecA positive) | 3. Staphylococcus capitis, oxacillin MIC 0.25 µg/mL, isolate mecA negative by PCR | |
| 4. Staphylococcus species (mecA negative) | 4. Coagulase-negative Staphylococcus species, oxacillin 1 µg/mL, isolate mecA negative by PCR | |
| Discrepancy in organism identification | 3 | |
| 1. Enterococcus species and Staphylococcus species (mecA positive)b | 1. S. epidermidis | |
| 2. Negative | 2. Enterobacter species | |
| 3. K. pneumoniae | 3. Enterobacter aerogenes | |
Abbreviations: MIC, minimum inhibitory concentration; PCR, polymerase chain reaction.
a Gram stain results differed between 2 blood culture bottles collected on the same day for this subject. Both bottles were evaluated according to the study arm assignment of bottle a, which signaled positive first.
b Heavy growth of coagulase-negative staphylococci may lead to cross-reactivity with Enterococcus species; thus, the rapid multiplex PCR result was not reported.
In the rmPCR/AS group, investigators made 159 recommendations for the following: antibiotic de-escalation (58%), antibiotic escalation (18%), optimization of antibiotic dose or duration (15%), and infectious diseases consultation (9%); 78% of recommendations were accepted within 24 hours. In contrast, the baseline stewardship program in place for all groups identified fewer audit and feedback opportunities (50 in control, 26 in rmPCR, and 34 in rmPCR/AS) and made fewer recommendations to modify therapy (7 in control, 0 in rmPCR, and 6 in rmPCR/AS).
Antimicrobial Utilization
Within the first 4 days after enrollment, duration of vancomycin was not different between groups. However, among subjects with bloodstream infections caused by organisms not requiring vancomycin therapy (eg, monomicrobial cultures of methicillin-susceptible S. aureus, S. pyogenes, S. agalactiae, or gram-negative or fungal organisms), the median duration of vancomycin use was shorter in the rmPCR (0 hours) and rmPCR/AS (0 hours) groups compared with the control group (8.2 hours, P = .03) (Table 3). Conversely, for subjects with bloodstream infections caused by vancomycin-susceptible enterococci, vancomycin use was greater in the rmPCR (70 hours) and rmPCR/AS (82 hours) groups than the control group (20 hours, P = .037). Duration of narrow-spectrum β-lactam use (cefazolin, nafcillin, oxacillin) was greater in both the rmPCR (71 hours) and rmPCR/AS (85 hours) groups than in the control group (42 hours, P = .04). Among all subjects, the median duration of piperacillin-tazobactam was shorter in the rmPCR (44 hours) and rmPCR/AS (45 hours) groups compared with control (56 hours, P = .012). Utilization of other antibiotics was not different between groups.
Table 3.
Antibiotic Utilization Among All Study Subjects in the First 96 Hours Following Enrollment
| Outcome | Control | Rapid Multiplex PCR | Rapid Multiplex PCR + Stewardship | P Value Comparing 3 Groups |
|---|---|---|---|---|
| Duration of therapya, h | ||||
| Vancomycin | ||||
| All patients (n = 357) | 44 (22–72) | 42 (21–93) | 42 (19–90) | .92 |
| Organisms not requiring vancomycinb (n = 169) | 8.2 (0–26) | 0 (0–16) | 0 (0–3)c | .032 |
| Vancomycin-susceptible enterococci (n = 32) | 20 (1–59) | 70 (48–88)c | 82 (40–96)c | .037 |
| Methicillin-susceptible Staphylococcus aureus (n = 42) | 23 (20–53) | 11 (0–26) | 8 (0–44) | .2 |
| Nafcillin, oxacillin, or cefazolin (n = 50) | 42 (24–57) | 71 (51–79)c | 85 (42–92)c | .035 |
| Piperacillin-tazobactam (n = 214) | 56 (39–82) | 44 (27–74)c | 45 (19–78)c | .012 |
| Cefepime (n = 181) | 55 (28–96) | 71 (43–96) | 58 (32–96) | .56 |
| Antibiotic modifications | ||||
| Time to first appropriate de-escalationd (n = 344) | 34 (21–55) | 38 (22–66) | 21 (7–37)c,e | <.0001 |
| Time to first appropriate escalationf (n = 122) | 24 (3–67) | 6 (2–36) | 5 (2–22)c | .04 |
| Time to administration of active antibiotics (n = 123)g | 11 (2–51) | 6 (2–31) | 4 (2–20) | .55 |
| Contaminated blood cultures not treated or treated for <24 h, No. (%)h | 47 (75) | 49 (89)c | 57 (92)c | .015 |
Data are presented as median (IQR) unless otherwise specified.
Abbreviations: IQR, interquartile range; PCR, polymerase chain reaction.
a Duration of therapy (hours) was calculated as the difference between the date and time of the antibiotic start order (or Gram stain–positive blood culture, if antibiotics were started prior to the positive culture result) and the date and time of the antibiotic stop order, for subjects who received the specified antibiotics, according to the organisms identified and study group. Shorter duration of broad-spectrum antibiotics, longer duration of narrow-spectrum antibiotics, faster antibiotic escalation or de-escalation, and less treatment of contaminants were considered favorable outcomes.
b Organisms not requiring vancomycin included monomicrobial cultures with methicillin-susceptible Staphylococcus aureus; groups A, B, C, or G streptococci; Streptococcus anginosus species group; or gram-negative or fungal organisms.
c Statistically significant compared to control group.
d From positive Gram stain to 96 hours after enrollment. De-escalation included discontinuation of 1 or more antibiotics and/or switching from a broad- to a narrow-spectrum antibiotic.
e Statistically significant comparison between the 2 intervention groups.
f From positive Gram stain to 96 hours after enrollment. Escalation included initiation of 1 or more antibiotics and/or switching from a narrow- to a broad-spectrum antibiotic.
g From positive Gram stain to start of active antibiotic among patients not on active therapy at enrollment; excludes patients with contaminated blood cultures.
h Contaminated blood cultures were defined as growth of organisms such as coagulase-negative staphylococci from a single blood culture set when ≥2 blood culture sets were collected, except among subjects suspected to have true bacteremia associated with central venous catheters or devices.
The proportion of subjects with any de-escalation of antimicrobial therapy after Gram stain or rmPCR result was higher in the rmPCR/AS group (24.1%) compared with the rmPCR (14.7%) and control (12.1%) groups (P = .003). Time from Gram stain result to first appropriate antimicrobial de-escalation was shorter in the rmPCR/AS group (21 hours) compared with the control (34 hours) and the rmPCR (38 hours) groups (P < .001) (Table 3). Time from Gram stain result to first appropriate antimicrobial escalation was shorter in the rmPCR/AS group (5 hours) than in the control group (24 hours, P = .04; Table 3). Results were similar among the subset of subjects with organisms on the rmPCR panel (Figure 2). In the groups with rmPCR testing, time to antimicrobial de-escalation or escalation did not change significantly over the course of the study. The proportion of contaminant blood cultures that was not treated or treated for <24 hours was higher in the rmPCR (89%) and rmPCR/AS (92%) groups vs control (75%, P = .015; Table 3). Antimicrobial utilization was not different among patients with and without infectious diseases consultation.
Other Outcomes
There were no differences in clinical or microbiologic outcomes among the groups (Table 4). Among the few patients with discrepant rmPCR and conventional culture results, no adverse consequences were observed. Both intervention groups had increased test costs but similar hospitalization costs compared with the control group. Antimicrobial costs tended to be lower for both intervention groups than for the control group, but this difference was not statistically significant. Total costs were not significantly different between intervention and control groups in sensitivity analysis of rmPCR test cost.
Table 4.
Comparison of Clinical, Microbiologic, and Cost Outcomes According to Study Group
| Outcome | Control (n = 207) | Rapid Multiplex PCR (n = 198) | Rapid Multiplex PCR + Stewardship (n = 212) | P Value Comparing 3 Groups |
|---|---|---|---|---|
| Clinical outcome | ||||
| Disposition | .12 | |||
| Home | 68 (32.9) | 62 (31.3) | 78 (36.8) | |
| Home with outpatient antimicrobial therapy | 39 (18.8) | 52 (26.3) | 38 (17.9) | |
| Nursing home/skilled nursing facility | 63 (30.4) | 42 (21.2) | 54 (25.5) | |
| Hospice/comfort care | 12 (5.8) | 8 (4) | 7 (3.3) | |
| Death | 11 (5.3) | 11 (5.6) | 8 (3.8) | |
| Length of stay (entire hospitalization), d, median (IQR) | 8 (5–15) | 8 (5–15) | 8 (5–16) | .60 |
| Length of stay (after enrollment), d, median (IQR) | 7 (4–12) | 6 (4–12) | 7 (4–12) | .61 |
| Intensive care unit admission within 14 d after enrollment | 16 (7.7) | 5 (2.5) | 10 (4.7) | .06 |
| Length of stay in intensive care unit (after enrollment), d, median (IQR) | 3 (2–4) | 2 (1–5) | 3 (2–4) | .90 |
| 30-day mortality | 22 (10.6) | 20 (10.1) | 18 (8.5) | .74 |
| 30-day attributable mortality | 7 (3.4) | 7 (3.5) | 2 (0.9) | .42 |
| 30-day readmission for infection with same organism | 6 (2.9) | 6 (3) | 8 (3.8) | .88 |
| Toxicity/adverse drug reactiona | 3 (1.4) | 3 (1.5) | 2 (0.9) | .82 |
| Microbiologic outcomes | ||||
| Blood culture clearance within 3 d after enrollment | 147 (71) | 131 (66.2) | 146 (68.9) | .79 |
| Acquisition of Clostridium difficile or multidrug-resistant organismsb within 30 days after enrollment | 15 (7.2) | 16 (8.1) | 21 (9.9) | .62 |
| Cost per hospitalized patient, mean (median) | ||||
| Overall hospitalization costs | $65 450 ($27 192) | $66 887 ($23 935) | $68 729 ($29 064) | .78 |
| Test costs | $5377 ($2082) | $5680 ($2585)c | $5743 ($2774)c | <.001 |
| Antimicrobial costs | $2194 ($990) | $1932 ($866) | $1741 ($890) | .65 |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: IQR, interquartile range; PCR, polymerase chain reaction.
a Toxicities include seizures, Clostridium difficile infection, hepatitis, myelosuppression, renal insufficiency, prolonged QTc interval, and rash that occurred within 2 weeks following enrollment and were documented in the medical record.
b Multidrug-resistant organisms including vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, extended-spectrum cephalosporin-resistant Enterobacteriaceae, and Pseudomonas aeruginosa and Acinetobacter species resistant to ≥3 antibiotic classes.
c Statistically significant compared to control group.
DISCUSSION
We report the first prospective RCT to demonstrate benefit of an rmPCR-based blood culture diagnostic test. We found that use of the rmPCR test led to more judicious antibiotic use. Additionally, we delivered rapid test results along with templated comments guiding interpretation and antimicrobial prescribing, and separately evaluated this approach with and without real-time antimicrobial stewardship. Both rapid test reporting strategies reduced unnecessary antibiotic use, although rmPCR testing combined with antimicrobial stewardship resulted in the most rapid antibiotic de-escalation.
In earlier observational studies, rapid pathogen identification methods have been associated with decreased mortality, LOS, and cost [5, 6, 9, 11–14]. However, these studies were limited by their retrospective designs, use of historical controls [5, 6, 10, 22], lack of randomization, and failure to match subjects by severity of illness [7]. In contrast, in this trial we did not observe differences in clinical or cost outcomes between patients in intervention and control arms. This may be due to the use of different study designs, the fact that this trial was not powered to detect differences in LOS, mortality, or cost, and/or the fact that 70% of our study subjects were receiving at least 1 active agent at time of enrollment and were generally being overtreated, rather than undertreated. In addition, our institution's baseline antimicrobial stewardship program, low resistance rates, high rate of infectious diseases consultation (48%), and baseline use of advanced technologies including MALDI-TOF MS and rapid PBP2a testing, even in the control arm, may have reduced differences between the control and intervention arms.
The rmPCR test enabled clinicians to quickly initiate “pathogen-directed” therapy and appropriately scale up or scale down antibiotic therapy, as needed. Specifically, in both intervention groups, we observed increased narrow-spectrum antibiotic use, less unnecessary vancomycin use, decreased treatment of blood culture contaminants, and more timely antibiotic escalation compared with the control group. Because timely initiation of effective therapy is a critical step in the management of patients with sepsis [1, 23–25], reducing time to appropriate antibiotic escalation by 14 hours, as observed in both intervention arms (Figure 2), is clinically significant. Antibiotic de-escalation occurred nearly 1 day (19 hours) faster in the rmPCR/AS group compared with control (Figure 2), which is almost a 25% reduction in broad-spectrum antibiotic days of therapy, as median duration of piperacillin-tazobactam or meropenem therapy in the control group was 4 days. This is likely to be significant at a population level; models estimate that a 5% reduction in broad-spectrum antibiotic use among hospitalized patients would result in a 26% decrease in C. difficile infection rates [26]. Among subjects with vancomycin-susceptible enterococci, vancomycin use increased in both intervention groups because of prompt de-escalation from daptomycin to vancomycin. In contrast, cefepime use did not decrease in the intervention groups, likely because this drug is commonly used for management of suspected infection in neutropenic hosts, in whom antibiotic de-escalation is often not indicated, despite negative blood cultures.
We observed that provider response differed according to how rapid test results were delivered. Others have noted that despite the availability of a rapid test result (eg, presence or absence of mecA), providers may fail to modify antibiotic therapy without pharmacist intervention [8, 27]. Observational studies have found favorable outcomes when rapid testing was implemented together with antimicrobial stewardship interventions [5, 6, 11–16]. To our knowledge, no prior studies have implemented a rapid blood culture diagnostic test using electronic decision support in the form of templated comments to guide prescribing, as done in this study. In the group that received the rmPCR test results delivered with templated comments communicated verbally and in the medical record, we observed more rapid antibiotic escalation, more narrow-spectrum antibiotic use, and less treatment of contaminants compared with the control group. Templated comments are an inexpensive and effective means of communicating straightforward results to providers, such as when coagulase-negative staphylococci or Micrococcus species represent contaminants, or when a Staphylococcus species is methicillin susceptible or resistant.
When the rmPCR test result was delivered with guidance from the antimicrobial stewardship team, there was also more frequent and timelier antibiotic de-escalation. We speculate that this is in part because notification from a stewardship team member might prompt a busy clinician to act on a test result more so than a telephone call from a laboratory technologist. Additionally, in complex clinical scenarios when critically ill or immunocompromised patients or patients with polymicrobial infections are on multiple broad-spectrum antibiotics, providers may prefer discussion with infectious disease specialists prior to modifying antimicrobial management. Although in this study the stewardship team provided feedback to providers 24 hours a day, most antibiotic de-escalation occurred during the day because housestaff preferred not to contact supervising providers at night regarding nonurgent de-escalation questions. Thus, around-the-clock antimicrobial stewardship team oversight, which is costly, may not be necessary. Rather, we estimate that at our institution, an additional 1–2 hours of stewardship effort (during the daytime) would be sufficient for daily review of positive blood cultures.
Unlike other rapid PCR-based platforms that target a limited number of organisms, the rmPCR test detected multiple targets, characterizing >80% of positive blood cultures and providing accurate results nearly a day faster than standard techniques. Additional advantages of the rmPCR test studied are that it is a closed system, can be used whether blood cultures contain gram-positive or gram-negative bacteria or yeast, and does not require significant technologist training or time. Known limitations of the test include its lack of sensitivity in detecting all organisms in polymicrobial cultures (Table 2), and the limited susceptibility information provided for gram-negative bacteria. The rmPCR test studied is “add-on” testing that does not replace conventional BCB subculture workup methods.
This study has limitations, including that it was performed at a single center and may not be generalizable to other institutions with different patient populations, prescribing and stewardship practices, and antimicrobial resistance rates. The study was not powered for subgroup analyses or to detect differences in secondary outcomes including LOS and mortality. Investigators were not blinded to study arm, and infectious diseases “curbside” consultations were not captured. We did not include a control group without rmPCR testing but with stewardship of all positive blood cultures. We were unable to account for contamination between study arms, although rarely were multiple patients in the intervention arms cared for by the same clinical service at the same time. Despite these limitations, this is the first prospective RCT to evaluate the value of a rapid diagnostic test for blood culture pathogen identification and to compare strategies to communicate rapid test results to clinicians.
In conclusion, rapid pathogen and susceptibility detection directly from blood cultures implemented with templated comments or antimicrobial stewardship oversight can optimize antibiotic prescribing for bloodstream infections. To influence clinical decision making, rapid results should be delivered with real-time decision support (using automated systems or antimicrobial stewardship programs) that assists clinicians to interpret and act on results.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are very grateful for the assistance of the outstanding staff of the Mayo Clinic Bacteriology Laboratory; the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery at Mayo Clinic; and Randy Wendt, Mayo Clinic.
Disclaimer. The contents of this work are the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). BioFire Diagnostics had no input in study design, data collection, analysis, interpretation, or manuscript preparation.
Financial support. This work was supported in part by the National Institute of Allergy and Infectious Diseases of the NIH (award number UM1AI104681) and the National Center for Advancing Translational Sciences (grant number KL2 TR000136). Supplies and instrumentation were provided by BioFire Diagnostics (bioMérieux, Salt Lake City, Utah).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ibrahim E, Sherman G, Ward S, Fraser V, Kollef M. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000; 118:46–55. [DOI] [PubMed] [Google Scholar]
- 2.Garnacho-Montero J, Gutierrez-Pizarraya A, Escoresca-Ortega A et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med 2014; 40:32–40. [DOI] [PubMed] [Google Scholar]
- 3.Dellit T, Owens R, McGowan J et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an an institutional program for enhancing antimicrobial stewardship. Clin Infect Dis 2007; 44:159–77. [DOI] [PubMed] [Google Scholar]
- 4.Caliendo A, Gilbert D, Ginocchio C et al. Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis 2013; 57:S139–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez K, Olsen R, Musick W et al. Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Arch Pathol Lab Med 2012; 137:1247–54. [DOI] [PubMed] [Google Scholar]
- 6.Perez KK, Olsen RJ, Musick WL et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant gram-negative bacteremia. J Infection 2014; 69:216–25. [DOI] [PubMed] [Google Scholar]
- 7.Barenfanger J, Drake C, Kacich G. Clinical and financial benefits of rapid bacterial identification and antimicrobial susceptibility testing. J Clin Microbiol 1999; 37:1415–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carver P, Lin S-W, DePestel D, Newton D. Impact of mecA gene testing and intervention by infectious disease clinical pharmacists on time to optimal antimicrobial therapy for Staphylococcus aureus bacteremia at a university hospital. J Clin Microbiol 2008; 46:2381–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clerc O, Prod'hom G, Vogne C, Bizzini A, Calandra T, Greub G. Impact of matrix-assisted laser desorption ionization time of flight mass spectrometry on the clinical management of patients with gram-negative bacteremia: a prospective observational study. Clin Infect Dis 2013; 56:1101–7. [DOI] [PubMed] [Google Scholar]
- 10.Galar A, Leiva J, Espinosa M, Guillen-Grima F, Hernaez S, Yuste J. Clinical and economic evaluation of the impact of rapid microbiological diagnostic testing. J Infection 2012; 65:302–9. [DOI] [PubMed] [Google Scholar]
- 11.Huang A, Newton D, Kunapuli A et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis 2013; 57:1237–45. [DOI] [PubMed] [Google Scholar]
- 12.Forrest GN, Mankes K, Jabra-Rizk M et al. Peptide nucleic acid fluorescence in situ hybridization-based identification of Candida albicans and its impact on mortality and antifungal therapy costs. J Clin Microbiol 2006; 44:3381–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forrest GN, Roghmann M-C, Toombs L et al. Peptide nucleic acid fluorescent in situ hybridization for hospital-acquired enterococcal bacteremia: delivering earlier effective antimicrobial therapy. Antimicrob Agents Chemother 2008; 52:3558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen D, Yeh E, Perry S et al. Real-time PCR testing for mecA reduces vancomycin usage and length of hospitalization for patients infected with methicillin-sensitive staphylococci. J Clin Microbiol 2010; 48:785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer K, West J, Balada-Llasat J-M, Pancholi P, Stevenson K, Goff D. An antimicrobial stewardship program's impact with rapid polymerase chain reaction methicillin-resistant Staphylococcus aureus/S. aureus blood culture test in patients with S. aureus bacteremia. Clin Infect Dis 2010; 51:1074–80. [DOI] [PubMed] [Google Scholar]
- 16.Forrest G, Mehta S, Weekes E, Lincalis D, Johnson J, Venezia R. Impact of rapid in situ hybridization testing on coagulase-negative staphylococci positive blood cultures. J Antimicrob Chemother 2006; 58:154–8. [DOI] [PubMed] [Google Scholar]
- 17.Blaschke A, Heyrend C, Byington C et al. Rapid identification of pathogens from positive blood cultures by multiplex polymerase chain reaction using the FilmArray system. Diagn Microbiol Infect Dis 2012; 74:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altun O, Almuhayawi M, Ullberg M, Ozenci V. Clinical evaluation of FilmArray(R) Blood Culture ID Panel in identification of bacteria and yeast from positive blood culture bottles. J Clin Microbiol 2013; 51:4130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson J, Oyen L, Ou N et al. Hospital rules-based system: the next generation of medical informatics for patients safety. Am J Health Syst Pharm 2005; 62:499–505. [DOI] [PubMed] [Google Scholar]
- 20.Wagner J, Alberts S, Sloan J, Cha S, Killian J, O'Connell M. Incremental costs of enrolling cancer patients in clinical trials: a population-based study. J Natl Cancer Inst 1999; 91:847–53. [DOI] [PubMed] [Google Scholar]
- 21.Van Grevenhof P, Wagner J, Naessens J, Darby D, Killian J, Helgeson E. Development of a multi-year population-based data warehouse of standardized medical costs: methods and results. Proc AMIA Symp 1998:1091. [Google Scholar]
- 22.Parta M, Goebel M, Thomas J, Matloobi M, Stager C, Musher D. Impact of an assay that enables rapid determination of Staphylococcus species and their drug susceptibility on the treatment of patients with positive blood culture results. Infect Cont Hosp Epidemiol 2010; 31:1043–8. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Roberts D, Wood K et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–96. [DOI] [PubMed] [Google Scholar]
- 24.Kang C, Kim S, Kim H et al. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin Infect Dis 2003; 37:745–51. [DOI] [PubMed] [Google Scholar]
- 25.Ferrer R, Martin-Loeches I, Phillips G et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med 2014; 42:1749–55. [DOI] [PubMed] [Google Scholar]
- 26.Fridkin S, Baggs J, Fagan R et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014; 63:194–200. [PMC free article] [PubMed] [Google Scholar]
- 27.Frye A, Baker C, Rustvold D et al. Clinical impact of a real-time PCR assay for rapid identification of staphylococcal bacteremia. J Clin Microbiol 2012; 50:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.