The deleterious effect of delaying antiretroviral therapy is stronger among patients older than 45 years at entry into care than among younger patients. These results stress the importance of improving timely linkage to human immunodeficiency virus medical care in this population.
Keywords: HIV, antiretroviral therapy, aging, epidemiologic methods
Abstract
Background. The goal of targeted antiretroviral therapy initiation is to minimize disease progression among patients with human immunodeficiency virus while minimizing the therapeutic burden on these patients. We examine whether the effect of delaying therapy initiation from 500 cells/mm3 to 350 or 200 cells/mm3 is modified by age at entry into care.
Methods. We used the parametric g-formula to compare 10-year mortality under 3 CD4 cell count thresholds for therapy initiation among 3532 patients who entered care at 1 of 8 sites in the United States between 1998 and 2013. Results are reported separately for patients 18 to 34, 35 to 44, and 45 to 65 years of age at study entry.
Results. In the observed data, 10-year mortality was 13% (165 deaths). Mortality increased from 11% under therapy initiation at 500 cells/mm3 to 12% at 350 cells/mm3 (risk difference [RD]: 0.87; 95% confidence interval [CI]: .56, 2.17) and to 14% at 200 cells/mm3 (RD: 2.71; 95% CI: 1.79, 5.38). The effect of delaying therapy became greater with age: RDs comparing the 350-cells/mm3 threshold with the 500-cells/mm3 threshold ranged from −0.03 (95% CI: −0.15, 1.76) for patients 18 to 34 years of age to 0.99 (95% CI: −.27, 1.98) for patients 35 to 44 and to 2.30 (95% CI: 1.29, 5.42) for patients 45 to 65.
Conclusions. Delaying therapy increased 10-year mortality in the full cohort. Subgroup analysis highlights that patients entering care at older ages may be more vulnerable to the consequences of delayed ART initiation than younger patients.
(See the Editorial Commentary by Walensky and Hirsch on pages 1196–8.)
Several recent large-scale observational studies have addressed when to initiate antiretroviral therapy (ART) with respect to CD4 cell count. The goal of targeted ART initiation is to minimize disease progression or mortality among people living with human immunodeficiency virus (HIV) [1–5]. These studies have reported heterogeneous optimal CD4 cell count thresholds between 300 and 500 cells/mm3 [1, 3–5] as well as thresholds over 500 cells/mm3 [2]. From a public health perspective, any unexplained heterogeneity in this evidence base is largely outweighed by the randomized evidence that early initiation of therapy reduces rates of HIV transmission [6], which has led to recent guidelines recommending treatment for all patients in care for HIV.
Nevertheless, advanced age has been a durable marker for more rapid disease progression and mortality in both the natural [7] and treated [8] histories of HIV infection. In the above-referenced observational studies, average age at study entry varied from 28 years [3] to 40 years [2]. Alterations in immune function over the life span support the hypothesis that HIV pathogenesis accelerates aging, with earlier occurrence of AIDS and non–AIDS-related events. Early therapy initiation in older patients could have a greater benefit than that reported for all ages combined. To date, there has been no report on possible age modification of the optimal CD4 cell count to initiate ART. Here, we report age-stratified 10-year mortality in the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) cohort [9] under several possible CD4 cell count treatment thresholds.
METHODS
Study Sample
The CNICS was developed to support population-based HIV research in the United States [9]. The CNICS cohort includes 26 405 HIV-positive adults engaged in clinical care from 1 January 1998 to December 31, 2013 at 8 Centers for AIDS Research sites (Case Western Reserve University; Fenway Community Health Center of Harvard University; Johns Hopkins University; University of Alabama at Birmingham; University of California, San Diego; University of California, San Francisco; University of North Carolina; and University of Washington). Patients attending 2 primary HIV medical care visits at a study site are eligible for CNICS and followed for clinical events, lab measurements, and medications while they remain in care at study sites. Institutional review boards at each site approved study protocols. Patients provided written informed consent to be included in the CNICS cohort or contributed administrative and/or clinical data with a waiver of written informed consent where approved by local institutional review boards. Details on the CNICS cohort are provide elsewhere [9].
Patients who entered HIV clinical care at a CNICS site between 1 January 1998 and 31 December 2013 and had not previously initiated combination ART, defined as treatment with 3 or more antiretroviral drugs, were eligible for inclusion in this analysis (N = 19 384). We included only patients with CD4 cell counts >500 cells/mm3 and a detectable viral load at baseline (N = 3734), as patients with undetectable viral load at baseline were likely not therapy naive. Patients were excluded if they were missing information on transmission risk factor, race, or sex (n = 202), leaving 3532 patients in the cohort for analysis.
Patients were followed from entry into care at a CNICS site until death, loss to follow-up, or administrative censoring at 10 years after study entry or 31 December 2013. Patients were considered to be lost to follow-up after 12 months without a documented clinic visit in which CD4 cell count or viral load was measured.
Mortality Ascertainment
The outcome of interest was mortality from any cause. Each CNICS site maintains a registry of deaths among patients at that site and semiannually queries the US Social Security Death Index and/or National Death Index to confirm reported deaths and record deaths not captured by the CNICS sites.
Statistical Methods
We compared the 10-year cumulative incidence of all-cause mortality under the following 3 dynamic treatment plans: initiate ART at first visit when CD4 cell count is <500 cells/mm3; initiate ART at first visit when CD4 cell count is <350 cells/mm3; and initiate antiretroviral therapy at first visit when CD4 cell count is <200 cells/mm3. We perform these comparisons separately for patients in each of the following 3 age ranges at study entry: 18 to 34 (n = 1744), 35 to 44 (n = 1132), and 45 to 65 years of age (n = 627).
The dynamic treatment regimes outlined above require patients to start therapy immediately after CD4 cell count drops below the threshold value. In practice, immediate therapy initiation may not be possible. To address possible delays in treatment, we follow existing work [4, 5] and examine the 10-year mortality under 3 dynamic treatment plans that incorporate a 6-month “grace period” between the CD4 cell count threshold and therapy initiation. These treatment plans take the form “initiate antiretroviral therapy 6 months after CD4 cell count first drops below the threshold value or the time at which the patient would have initiated therapy during the grace period with no intervention on treatment plan (ie, when the patient was expected to initiate treatment in the observe data), whichever comes first.”
We estimated the 10-year cumulative incidence of mortality under the 3 specified dynamic treatment plans with and without the grace period using the parametric g-formula, as described by Young et al [5]. Under the assumptions of no measurement error, conditional exchangeability, positivity, and no model misspecification, the parametric g-formula provides consistent estimates of the risk of mortality under each dynamic treatment plan. The g-formula accounts for time-fixed and time-varying confounders through a generalization of standardization in which we estimate the density of all possible covariate histories and sum the risk of mortality over these covariate histories [10, 11]. Briefly, to implement the parametric g-formula, we model the probability of treatment, time-varying confounders, and mortality at each time point conditional on covariates in the observed data. Then, we use these conditional probabilities to estimate the risk of mortality by time t that would have been observed if we had set exposure according to each treatment plan and observed all participants until death or the end of the study. Time-fixed covariates included in our analysis were sex, race, ethnicity, injection drug use, men who have sex with men (MSM) status, and age, year, CD4 cell count, and viral load at study entry. Time-varying covariates included CD4 cell count, viral load, and AIDS status at each clinic visit. Continuous variables were modeled flexibly using restricted quadratic splines. Full technical details are provided in the Supplementary Appendix.
Risk of mortality under each treatment plan at time t (regardless of actual treatment use at time t) was estimated using the complement of the Kaplan–Meier survival curve [12]. Mortality was compared between treatment plans using risk ratios (RRs) and risk differences (RDs), and 95% confidence intervals (CIs) were estimated using the standard deviation from 500 nonparametric bootstrap samples [13] as an approximation of the standard error. RRs, RDs, and corresponding 95% CIs were estimated separately for patients 18 to 34, 35 to 44, and 45 to 65 years of age.
RESULTS
Of the 3532 ART-naive patients who entered care at a CNICS site with a CD4 cell count >500 cells/mm3, 82% (n = 2886) were male, 34% (n = 1194) were black, 17% (n = 581) were injection drug users, and 67% (n = 2359) were MSM. Patients entered the cohort between 1998 and 2013, and the median year of entry was 2006 (interquartile range [IQR]: 2002, 2009). At study entry, the median CD4 cell count of eligible patients was 646 cells/mm3 (IQR: 567, 780) and the median viral load was 11 722 copies/mL (IQR: 3473, 43 000).
Table 1 presents the distribution of baseline characteristics among all patients and patients in each age group. Median CD4 cell count at study entry was similar among patients entering care at 18 to 34 (median: 647 cells/mm3; IQR: 566, 782), 35 to 44 (median: 648 cells/mm3; IQR: 567, 788), and 45 to 65 years of age (median: 638 cells/mm3; IQR: 568, 782). Patients entering care during the study period at younger ages were more likely to be male, Hispanic, and MSM than older patients, while patients entering care at older ages were more likely to be injection drug users and have an AIDS diagnosis.
Table 1.
Demographics and Clinical Characteristics at Study Entry Among 3532 Patients Who Entered Care With a CD4 Cell Count >500 cells/mm3 Between 1 January 1998 and 31 December 2013 at 8 US Clinical Sites, Followed for Death for Up to 10 Years
| Characteristic | All Patients at Study Entry (n = 3532) | 18–34 Years of Age at Study Entry (n = 1744) | 35–44 Years of Age at Study Entry (n = 1132) | 45–65 Years of Age at Study Entry (n = 627) |
|---|---|---|---|---|
| n (%) | % | % | % | |
| Male | 2886 (82.1) | 83.8 | 81.3 | 78.9 |
| Black | 1194 (34.0) | 32.7 | 33.0 | 38.9 |
| Hispanic ethnicity | 329 (9.4) | 11.6 | 7.8 | 6.2 |
| Injection drug user | 581 (16.5) | 12.3 | 20.2 | 21.9 |
| Men who have sex with men | 2359 (67.1) | 73.8 | 64.2 | 54.5 |
| AIDS | 189 (5.4) | 3.6 | 6.3 | 8.6 |
| CD4 cell count at entry, cells/mm3 | ||||
| 500–600 | 1292 (36.8) | 37.2 | 35.0 | 38.6 |
| 601–750 | 1180 (33.8) | 33.4 | 34.3 | 32.5 |
| 751–1000 | 786 (22.5) | 22.3 | 23.6 | 20.4 |
| >1000 | 257 (7.31) | 7.1 | 7.2 | 8.5 |
| CD4 cell count at initiation of ART, cells/mm3 | ||||
| 0–200 | 92 (2.6) | 1.9 | 3.3 | 3.2 |
| 201–350 | 293 (8.3) | 6.2 | 10.8 | 10.0 |
| 351–500 | 374 (10.6) | 10.3 | 10.4 | 11.5 |
| >500 | 1052 (29.9) | 30.8 | 26.5 | 33.8 |
| Did not initiate ART while in the study | 1704 (48.5) | 50.1 | 49.0 | 41.5 |
| Year at study entry | ||||
| 1998–2002 | 1013 (28.8) | 25.7 | 35.8 | 25.0 |
| 2003–2007 | 1154 (32.8) | 30.7 | 36.1 | 32.5 |
| 2008–2013 | 1348 (38.3) | 43.6 | 28.1 | 42.4 |
Abbreviation: ART, antiretroviral therapy.
Patients in all age groups at study entry were most likely to initiate ART with a CD4 cell count >500 cells/mm3, though some patients in each age group initiated ART at lower CD4 cell counts. Over the follow-up period, 165 deaths occurred, including 29 among patients in the 18 to 34 age group, 65 in the 35 to 44 age group, and 66 in the 45 to 65 age group. Five deaths occurred among patients who entered care after age 65 and were excluded from subgroup analyses. The overall cumulative incidence of mortality was 5% at 5 years and 13% at 10 years.
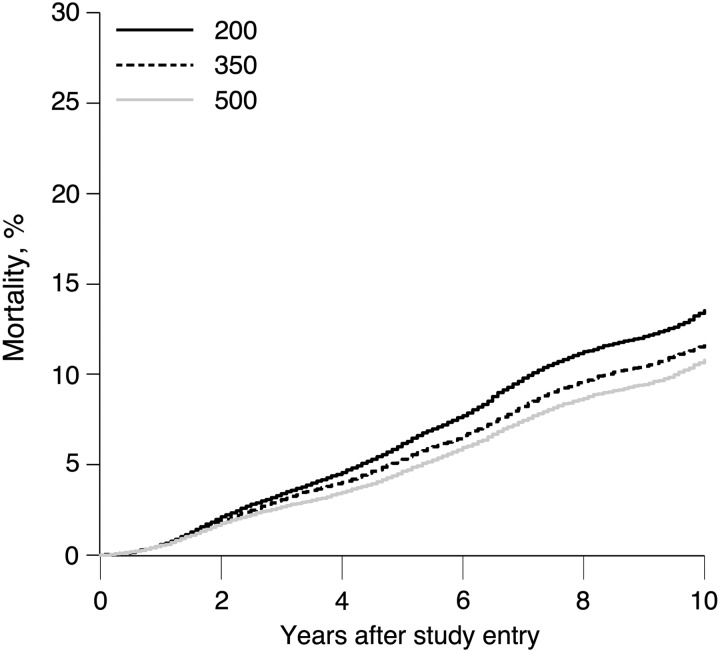
Table 2 compares the 5- and 10-year cumulative incidence of mortality for the eligible patients under no intervention and the 3 dynamic treatment plans for each age group. Overall, 10-year mortality was elevated if patients initiated ART within 6 months of CD4 cell count dropping to <350 cells/mm3 (mortality =12%) compared with if all patients initiated ART within 6 months of CD4 cell count dropping to <500 cells/mm3 (mortality = 11%), for an RR of 1.08 (95% CI: 1.00, 1.16) and an RD of 0.87% (95% CI: .07, 1.67). Ten-year mortality was further elevated to 14% if patients waited to initiate ART within 6 months of CD4 cell count dropping to <200 cells/mm3, for an RR of 1.25 (95% CI: 1.08, 1.44) and an RD of 2.71% (95% CI: .92, 4.50). Figure 1 illustrates the cumulative incidence of mortality over 10 years for each of the treatment plans.
Table 2.
Standardized 5- and 10-Year Cumulative Incidence of Mortality Under no Intervention and 3 Dynamic Treatment Plans With a 6-Month Grace Period Among 3532 Patients Who Entered Care Between 1 January 1998 and 31 December 2013 at 8 US Clinical Sites, Followed for Death for Up to 10 Years
| Treatment Plan | 5-year |
10-year |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | RR | 95% CI | RD | 95% CI | Mortality | RR | 95% CI | RD | 95% CI | |
| No intervention | 5.45 | 12.60 | ||||||||
| All ages | ||||||||||
| 500 cells/mm3 | 4.63 | 1 | 0 | 10.80 | 1 | 0 | ||||
| 350 cells/mm3 | 5.30 | 1.15 | 1.05, 1.26 | 0.67 | .23, 1.11 | 11.66 | 1.08 | 1.00, 1.16 | 0.87 | .07, 1.67 |
| 200 cells/mm3 | 6.17 | 1.33 | 1.15, 1.54 | 1.54 | .72, 2.36 | 13.51 | 1.25 | 1.08, 1.44 | 2.71 | .92, 4.50 |
| 18 to 34 years of age | ||||||||||
| 500 cells/mm3 | 2.11 | 1 | 0 | 6.11 | 1 | 0 | ||||
| 350 cells/mm3 | 2.22 | 1.05 | .87, 1.27 | 0.11 | −.33, .55 | 6.08 | 1.00 | .87, 1.15 | −0.03 | −.83, .77 |
| 200 cells/mm3 | 2.45 | 1.16 | .84, 1.60 | 0.34 | −.40, 1.08 | 6.25 | 1.02 | .78, 1.33 | 0.14 | −1.48, 1.76 |
| 35 to 44 years of age | ||||||||||
| 500 cells/mm3 | 5.09 | 1 | 0 | 11.43 | 1 | 0 | ||||
| 350 cells/mm3 | 5.51 | 1.08 | .95, 1.22 | 0.42 | −.23, 1.07 | 12.42 | 1.09 | .99, 1.20 | 0.99 | −.13, 2.11 |
| 200 cells/mm3 | 5.76 | 1.13 | .91, 1.41 | 0.67 | −.59, 1.93 | 13.59 | 1.19 | 1.98, 1.45 | 2.15 | −.39, 4.69 |
| 45 to 65 years of age | ||||||||||
| 500 cells/mm3 | 9.17 | 1 | 0 | 19.29 | 1 | 0 | ||||
| 350 cells/mm3 | 11.28 | 1.23 | 1.08, 1.40 | 2.11 | .80, 3.42 | 21.60 | 1.12 | 1.01, 1.25 | 2.30 | .23, 4.37 |
| 200 cells/mm3 | 14.50 | 1.58 | 1.31, 1.90 | 5.33 | 3.15, 7.51 | 28.07 | 1.45 | 1.21,1.71 | 8.78 | 5.89, 13.90 |
Abbreviations: CI, confidence interval; RD, risk difference; RR, risk ratio.
Figure 1.
Standardized cumulative incidence of mortality under 3 dynamic treatment plans with a 6-month grace period among 3532 patients who entered care between 1 January 1998 and 31 December 2013 at 8 US clinical sites with a CD4 cell count >500 cells/mm3, followed for death for up to 10 years.
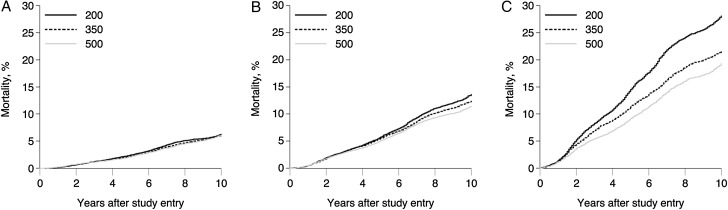
Table 2 also compares the estimated mortality under each treatment plan by age group. Patients who entered care at 18 to 34 years of age had the lowest estimated 10-year mortality for each treatment plan. In these patients, mortality was similar regardless of the CD4 cell count threshold used as a trigger for ART initiation.
The 10-year mortality for patients entering care at 35 to 44 years of age was 11% under ART initiation within 6 months of CD4 cell count dropping to <500 cells/mm3. Mortality increased slightly under the 350-cells/mm3 threshold (RR: 1.09; 95% CI: .99, 1.20; RD: 0.99%; 95% CI: −.13, 2.11) and the 200-cells/mm3 threshold (RR: 1.19; 95% CI: 1.98, 1.45; RD: 2.15%; 95% CI: −.39, 4.69).
As expected, patients who entered care at 45 to 65 years of age experienced the highest 10-year risk of mortality under all treatment plans. Under the 500-cells/mm3 threshold for ART initiation, the 10-year mortality was 19%. The 10-year risk of mortality increased to 22% under the 350-cells/mm3 threshold for an RR of 1.12 (95% CI: 1.01, 1.25) or an RD of 2.30% (95% CI: .23, 4.37). If patients in this group waited to initiate ART until CD4 cell count was <200 cells/mm3, 10-year mortality was 28%, yielding an RR of 1.45 (95% CI: 1.21,1.71) and an RD of 8.78% (95% CI: 5.89, 13.90). Figure 2 depicts the 10-year mortality under the 3 dynamic treatment plans stratified by age, demonstrating more dramatic effects of delayed ART initiation among older patients. The P value for trend in the RDs by age group was .0498 comparing the 350 and 500 thresholds and 0.2230 comparing the 200 and 500 thresholds.
Figure 2.
Standardized cumulative incidence of mortality under 3 dynamic treatment plans with a 6-month grace period among 1744 patients who entered care at 18 to 34 years of age (A), 1132 patients who entered care at 35 to 45 years of age (B), and 627 patients who entered care at 45 to 65 years of age (C) between 1 January 1998 and 31 December 2013 at 8 US clinical sites, followed for death for up to 10 years.
Results were similar when the dynamic treatment plans did not include a 6-month grace period, when patients were censored after a 6- or 18-month (rather than 12-month) gap in care, and after excluding patients with AIDS diagnosis or mono or dual-therapy prior to study entry (Supplementary Tables 2–5).
DISCUSSION
The effect of delaying ART initiation on 10-year mortality was not homogenous among age groups. For patients who entered care at a CNICS site at 18 to 34 years of age, delaying ART until CD4 cell count dropped to <200 cells/mm3 had little effect. The effect of delaying ART was slightly more pronounced among patients who entered care at 35 to 44 years of age. However, among patients who entered care at 45 to 65 years of age, delaying ART had a profoundly deleterious effect; delaying ART until CD4 cell count dropped to <200 cells/mm3 increased 10-year mortality from 19% (had patients initiated ART when CD4 cell count first dropped to <500 cells/mm3) to 28%.
The overall results agree with existing work on “when to start” ART. As in recent work [1–5], delaying ART initiation until the CD4 cell count is <200 cells/mm3 resulted in higher mortality during the 10-year study period. In particular, the 5-year RR comparing the dynamic treatment plan “initiate ART within 6 months of CD4 cell count dropping below 200 cells/mm3” to “initiate ART within 6 months of CD4 cell count dropping below 500 cells/mm3” (RR = 1.33) was similar to RRs presented by Cain [4] (RR = 1.19) and Young [5] (RR = 1.38).
Our approach provides consistent (ie, unbiased) estimates of the cumulative incidence of mortality under several assumptions. First, we assume that all variables (eg, CD4 cell count, date of treatment initiation, and date of death, among others) were measured without error. We also assume that patients initiating treatment at different CD4 cell counts are exchangeable within levels of measured covariates or that there is no unmeasured confounding [14]. For example, in the observed data, only a small proportion of eligible patients in each age group initiated ART at CD4 cell counts <200 cells/mm3, and this proportion decreased over calendar time; if these patients were systematically different from patients initiating ART at higher CD4 cell counts, conditional on observed covariates, the estimated cumulative incidence of mortality <200 cells/mm3 treatment threshold could be biased.
Similarly, we assume that right censoring is ignorable conditional on measured covariates [15]. Though loss to follow-up was high (over the 10-year study period, 56% of patients were censored due to a 1-year or greater gap in lab measurements), few measured variables predicted loss to follow-up and the proportion lost to follow-up was similar across the age groups, though patients in the oldest age group were somewhat less likely to be lost (Supplementary Table 8). In addition, previous work has shown that loss to follow-up in the CNICS cohort does not predict mortality [16], indicating that selection bias resulting from the high proportion of patients lost to follow-up is likely to be modest.
We used parametric models for treatment, time-varying covariates, and mortality, and the validity of our results depends on correct specification of these models. Though it is not possible to check the performance of the models under the intervention scenarios, we note agreement between the observed time to mortality and values of the covariates and the predicted values from our models under no intervention (Supplementary Figure 1).
We excluded patients who entered care in CNICS with a CD4 cell count <500 cells/mm3 so that we could compare the 10-year mortality had we implemented treatment plans to initiate ART at 500, 350, and 200 cells/mm3 in the same group of patients and so that we could compare our results to those from existing studies. Because these patients compose only roughly 20% of the therapy-naive population of CNICS and unmeasured variables such as predictors of health-seeking behaviors likely predict both early entry into care and mortality, our results may not be generalizable to the entire CNICS population [17]. In addition, including only patients entering with CD4 cell counts >500 cells/mm3 likely preferentially captures a slow progression population, as “slow progressors” have more time to enter care before CD4 cell count drops below this threshold.
We did not have information on cause of death or comorbidities. It is possible that the heterogeneity in the estimated effect of delaying ART initiation by age could be explained, in part, by differences in the distribution of comorbidities and, therefore, causes of death for patients entering CNICS in each age group. For example, we know that a higher proportion of patients in the older age groups reported injection drug use (21% in the 45–65 group and 20% in the 34–45 group) than patients aged 18 to 34 years (12%), meaning that older patients may have been more likely to be infected with other blood-borne diseases, such as hepatitis C.
While there are certainly population health benefits to early ART initiation, the individual, patient-level benefits of initiating ART at 500 cells/mm3 rather than 350 cells/mm3 or 200 cells/mm3 are unclear for younger patients in terms of mortality risk, according to our analyses. Acknowledging that clinical guidelines increasingly incorporate public health considerations, particularly in light of the efficacy of treatment as prevention approaches [6], medical providers entertain treatment decisions with individual patients, one at a time. Our findings suggest that for younger patients entering HIV medical care with high CD4 counts, there may be less urgency in initiating ART from the standpoint of individual health outcomes. These results contribute a new layer of evidence by describing the variable individual-level benefits of more immediate ART initiation according to age. Ultimately, treatment decisions must weigh as many benefits and risks as possible, at an individual and population health level, and should be made by patient–provider dyads informed by the best available evidence.
As expected, this study illustrates that delaying ART increases 10-year mortality. Subgroup analysis highlights that adults entering care at 45 to 65 years of age are more vulnerable to the consequences of delayed ART initiation than younger adults. While current guidelines recommend early ART initiation for all adults to improve both individual and population-level health outcomes, the striking increase in 10-year mortality under delayed ART initiation for adults older than 45 stresses the heightened importance of early ART initiation in this group. Increasing early ART initiation among adults older than 45 will require both the clinical guidelines already in place as well as a renewed commitment to improving timely diagnosis and linkage to HIV medical care in this population.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was funded, in part, by the National Institutes of Health (NIH; R01AI100654, R24AI067039, DP2 HD084070 01, and P30AI50410).
Potential conflicts of interest. M. J. M. reports personal fees from Georgetown University/American Foundation for AIDS Research, the National Alliance of State and Territorial AIDS Directors, Bristol-Myers Squibb (BMS), and Merck, outside the submitted work. J. J. E. reports grants from NIH/National Institute of Allergy and Infectious Diseases during the conduct of the study; personal fees from BMS, GlaxoSmithKline, Janssen, and Gilead; and grants and personal fees from ViiV Healthcare, outside the submitted work. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Sterne JAC, May M, Costagliola D et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet 2009; 373:1352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitahata MM, Gange SJ, Abraham AG et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 2009; 360:1815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funk MJ, Fusco JS, Cole SR et al. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med 2011; 171:1560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cain LE, Logan R, Robins JM et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med 2011; 154:509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young JG, Cain LE, Robins JM, O'Reilly EJ, Hernán MA. Comparative effectiveness of dynamic treatment regimes: an application of the parametric g-formula. Stat Biosci 2011; 3:119–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MS, Chen YQ, McCauley M et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muñoz A, Sabin CA, Phillips AN. The incubation period of AIDS. AIDS 1997; 11(suppl A):S69–76. [PubMed] [Google Scholar]
- 8.Egger M, May M, Chêne G et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 2002; 360:119–29. [DOI] [PubMed] [Google Scholar]
- 9.Kitahata MM, Rodriguez B, Haubrich R et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol 2008; 37:948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keil A, Edwards JK, Richardson DB, Naimi AI, Cole SR. The parametric g-formula for time-to-event data intuition and a worked example. Epidemiology 2014; 25:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westreich D, Cole SR, Young JG et al. The parametric g-formula to estimate the effect of highly active antiretroviral therapy on incident AIDS or death. Stat Med 2012; 31:2000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53:457–81. [Google Scholar]
- 13.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman & Hall, 1993:436. [Google Scholar]
- 14.Greenland S, Robins JM. Identifiability, exchangeability, and epidemiological confounding. Int J Epidemiol 1986; 15:413–9. [DOI] [PubMed] [Google Scholar]
- 15.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology 2004; 15:615–25. [DOI] [PubMed] [Google Scholar]
- 16.Edwards JK, Cole SR, Westreich D et al. Loss to clinic and five-year mortality among HIV-infected antiretroviral therapy initiators. PLoS One 2014; 9:e102305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole SR, Stuart EA. Generalizing evidence from randomized clinical trials to target populations: the ACTG 320 trial. Am J Epidemiol 2010; 172:107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.