HCV+/HIV+ coinfection was associated with a 2.5-fold increased risk of death and 3-fold increase in allograft loss; however, HIV+ patients experienced outcomes comparable to HIV−/HCV− recipients. Participation in the NIH trial did not improve outcomes, suggesting that liver transplant in HIV+ and HIV+/HCV+ coinfected patients is effective in a “real world” setting.
Keywords: human immunodeficiency virus (HIV), hepatitis C virus (HCV), liver transplant, outcomes
Abstract
Background. The effectiveness of liver transplant (LT) in human immunodeficiency virus (HIV) and HIV/hepatitis C virus (HCV) coinfected recipients in the United States is unknown. We investigated (i) the effect of HIV on US patient and allograft LT outcomes, compared to HCV+ and HIV/HCV uninfected recipients and (ii) whether LT at centers that participated in the National Institutes of Health (NIH) Solid Organ Transplantation in HIV Trial, reflecting experience and a standardized approach to patient selection, impacted outcomes.
Methods. A retrospective cohort study of primary LT recipients transplanted 27 February 2002 through 31 December 2013, categorized by serostatus: HCV+ (n = 20 829), HIV+ (n = 72), HIV+/HCV+ (n = 160), and HIV−/HCV− uninfected (n = 22 926) as reference. Survival was determined using Cox regression, stratified according to center NIH trial participation.
Results. HCV (hazard ratio [HR] 1.46, 95% confidence interval [CI], 1.41–1.52) and HIV/HCV coinfection (HR 2.62, 95% CI, 2.06–3.33) were associated with mortality; HIV monoinfection was not (HR 1.37, 95% CI, .86–2.18). This was unchanged after stratification on NIH trial participation, although mortality was higher in NIH-enrolling (HIV+: HR 1.65, 95% CI, .93–2.92; HIV+/HCV+: HR 3.15, 95% CI, 2.32–4.28) than in non-enrolling centers (HIV+: HR 1.03, 95% CI, .43–2.47, HIV+/HCV+: HR 2.55, 95% CI, 1.64–3.96). Although allograft loss was higher in HIV/HCV coinfected recipients transplanted at enrolling (HR 2.64, 9%% CI, 1.91–3.64) vs nonenrolling centers (HR 2.22, 95% CI, 1.41–3.49), there was no difference in HIV and HCV monoinfected patients.
Conclusions. HIV+ LT recipient outcomes were superior to HCV+ or HIV/HCV coinfected recipients. Despite a standardized approach and plausibly more experience with HIV patients, transplantation at NIH study center did not improve outcomes.
With the widespread adoption of effective antiretroviral therapy (ART), deaths from human immunodeficiency virus (HIV) have declined [1]. Due to shared routes of transmission, many HIV patients also have viral hepatitis and end stage liver disease represents the most frequent cause of non-AIDs-related mortality in persons with HIV [2].
Until the last decade, liver transplant was contraindicated in HIV+ recipients given concerns about complications and poor survival [3, 4]. Single center experience [5] catalyzed a multicenter, prospective National Institutes of Health (NIH) study conducted in 18 US transplant centers comparing outcomes between hepatitis C virus (HCV) monoinfected liver transplant recipients and HIV/HCV coinfected patients, selected on the basis of stringent HIV virologic criteria [6]. Three-year patient and allograft survival for the coinfected cohort was lower than for HCV+ patients (60% vs 79% and 53% vs 74%, respectively). Factors linked to adverse outcomes in HIV-infected liver recipients include HCV genotype 1 coinfection [7] and recurrence [8, 9], low body mass index (BMI) [7], higher model for end stage liver disease (MELD) score at transplant, higher donor risk index (DRI) [7], use of older donors and HCV+ donors [6], and low transplant center volumes [7].
Although the NIH trial suggested that liver transplant is feasible in carefully selected HIV and HIV/ HCV coinfected patients, high rates of rejection and graft loss [5, 7], infectious [10] and vascular complications [11], and accelerated recurrent HCV [8, 9], have tempered enthusiasm for liver transplantation in this cohort. Despite this, patients with HIV or HIV/HCV are transplanted outside of a formalized study protocol on an expanding basis. Thus, although the NIH trial demonstrated the efficacy of liver transplant in HIV-infected patients as part of a study protocol, the outcomes in a real-world setting, or the effectiveness of this practice, remain unknown.
The aims of our study were 2-fold; first to determine the effect of HIV/HCV coinfection on patient and allograft outcomes in comparison to HIV monoinfected, HCV monoinfected, and HIV/HCV uninfected recipients in the entire US liver transplant population; second, to explore whether use of a standardized HIV virologic approach and careful posttransplant follow-up impacts HIV-infected liver recipient survival, by stratifying on center participation in the NIH multicenter trial.
METHODS
We performed a retrospective cohort study using data from the United Network for Organ Sharing (UNOS). The dataset included information from all liver transplants and transplant registrations in the US through 31 December 2013. The Institutional Review Board at the University of Pennsylvania approved this study (protocol 821130, exempt status).
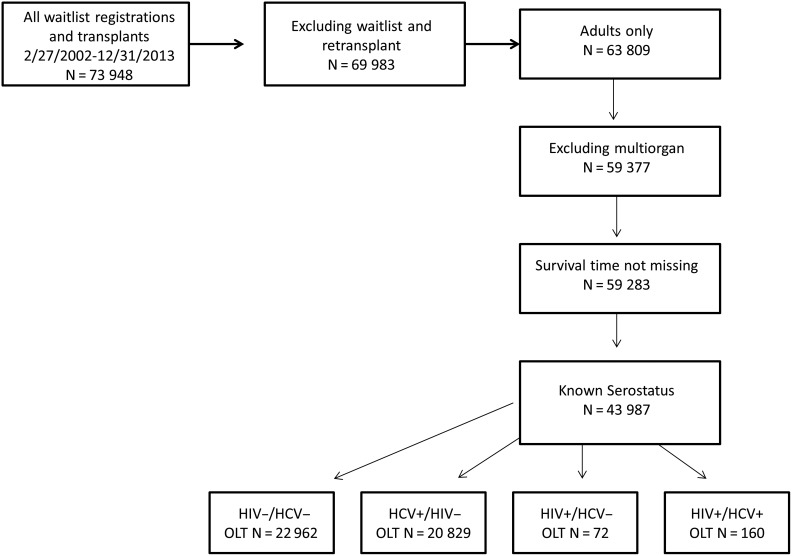
We assembled a cohort of all adult liver transplant recipients, transplanted between 27 February 2002 and 31 December 2013 (Figure 1); 27 February 2002 was chosen to reflect the clinical application of MELD-based allocation [12]. Only the first liver transplant after that date was included; subsequent episodes were censored. Only recipients with a known serostatus were included in the analysis.
Figure 1.
Creation of the patient cohort. Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; OLT, orthotopic liver transplantation.
Recipient HIV serostatus was provided in a separate file that was linked to the dataset to identify a unique patient and transplant episode. HCV serostatus was reported in the database in a variable which did not differentiate active HCV viremia from antibody positivity alone. Serostatus was supplemented with HCV and/or HIV specific information provided by liver failure diagnosis codes (Supplementary Table 1) and free text fields. Patients were divided into 4 exposure groups based upon serostatus: HIV/HCV uninfected (the reference group), HCV monoinfected, HIV monoinfected, and HIV/HCV coinfected.
The UNOS dataset does not provide information regarding HCV genotype or viral load; therefore, the HCV and HIV/HCV serostatus groups may include patients with and without active infection. There are no data regarding CD4 counts, HIV viral loads, ART or AIDS-defining illnesses for patients with HIV.
We calculated the DRI [13] and MELD score [14, 15], based on laboratory values (“calculated MELD score” and “final allocation MELD score”) and inclusive of exception points, using variables in the dataset. MELD was examined as both a continuous and dichotomous variable, using cut-points at 20 and 25, based on published data [6, 8]. Transplant center identifiers were linked to the dataset to determine whether a transplant was performed at a center that participated in the NIH trial [6].
Study Design
Kruskal–Wallis testing was used for comparison of nonnormally distributed continuous variables. Categorical variables were compared using χ2 or Fisher exact testing. Kaplan–Meier curves were compared using log rank [16]. Hazard ratios (HRs) with 95% confidence intervals (CIs) for the study outcomes, time to death, and time to all-cause allograft loss were estimated using Cox proportional hazards regression [17]. Any variable known to be a risk factor for or associated with the exposure and outcome of interest were included in the analysis. Confounders were retained in adjusted models if inclusion changed the unadjusted HR of the outcome of interest by >10% or were proposed a priori (BMI < 21, MELD score >20 at transplant, hemodialysis at transplant, older donor or transplantation at a NIH trial center) [6, 8, 18].
Model checking procedures, inclusive of examining the proportional hazards assumption of the primary exposure, and visualization of log-log plots were conducted. Variables not meeting the proportional hazards assumption by Schoenfeld residuals or inspection of log-log plots, were included in models stratified by that variable. Variables that violated the proportional hazards assumption but had a parallel appearance on the log-log plot were retained in the model without adjustment as this was attributed to the large size of the dataset [19].
All variables explored in multivariable models were <5% missing with the exception of functional status (23.3%), cold ischemic time (6.2%), Centers for Disease Control and Prevention (CDC) high-risk donor (20%), and acute rejection (25.5%). Functional status and acute rejection were tested in the multivariable models using a complete case analysis followed by single imputation to determine if there were changes in the association of interest; no significant changes were noted.
Statistical analyses were performed using Stata 12.1 (Statacorp LP, College Station, Texas). All P-values reported are 2-sided, and a P < .05 was considered statistically significant.
RESULTS
Cohort Assembly
In total, 73 948 transplant and wait list registrations were recorded in the dataset from 27 February 2002 through 31 December 2013. After excluding waitlisted patients, retransplants, and pediatric patients, we retained 63 809 adult first liver transplant recipients. Multi-organ recipients and patients lacking a discretely recorded survival time were excluded, leaving 43 987 recipients with complete serostatus information; these patients comprised the cohort for analysis. Categorization according to serostatus yielded 22 926 uninfected, 20 829 HCV monoinfected, 72 HIV monoinfected, and 160 HIV/HCV coinfected patients (Figure 1).
Patient and Transplant Characteristics
The groups differed significantly with respect to demographic and clinical characteristics (Table 1 and Supplementary Table 2). HIV-infected patients were younger than the HCV monoinfected or reference patients (P < .001). The majority of patients across all groups were white, but there were higher percentages of blacks in the HIV and HIV/HCV coinfected groups (P <.001). Male sex was predominant among recipients regardless of serostatus, but the highest proportion of males was in the HIV and HIV/HCV coinfected groups.
Table 1.
Clinical and Demographic Characteristics of the Patient Cohort
| HIV−/HCV− n = 22 926 | HCV+/HIV− n = 20 829 | HIV+/HCV− n = 72 | HIV+/HCV+ n = 160 | P Valuea | |
|---|---|---|---|---|---|
| Age (median, years) | 55 (46–62) | 55 (51–59) | 48 (44–55) | 52 (46–56) | .001 |
| Male | 13 770 (60.1) | 15 593 (74.9) | 58 (80.6) | 129 (80.6) | <.001 |
| Race | <.001 | ||||
| White | 16 958 (74.0) | 14 719 (70.7) | 42 (58.3) | 100 (62.5) | |
| Black | 1786 (7.8) | 2262 (10.9) | 21 (29.2) | 36 (22.5) | |
| Latino | 2712 (11.8) | 3024 (14.5) | 6 (8.3) | 22 (13.8) | |
| Asian | 1204 (5.2) | 588 (2.8) | 3 (4.2) | 2 (1.2) | |
| Other | 266 (1.2) | 236 (1.1) | 0 | 0 | |
| Cause of Liver Disease | <.001 | ||||
| Hepatitis C | 0 | 15 725 (75.5) | 0 | 124 (77.6) | |
| Alcohol | 5394 (23.5) | 444 (2.1) | 1 (1.4) | 1 (0.6) | |
| Hepatitis B | 909 (4.0) | 60 (0.3) | 25 (34.7) | 1 (0.6) | |
| NASH/cryptogenic | 5362 (23.4) | 158 (0.8) | 12 (16.7) | 1 (0.6) | |
| Cholestatic disease | 3433 (15.0) | 90 (0.4) | 2 (2.8) | 0 | |
| Autoimmune | 1102 (4.8) | 27 (0.1) | 0 | 0 | |
| Otherb | 6726 (29.3) | 4325 (20.8) | 32 (44.4) | 33 (20.6) | |
| Blood group | .777 | ||||
| A | 8519 (37.2) | 7636 (36.7) | 26 (36.1) | 52 (32.5) | |
| AB | 1129 (4.9) | 1009 (4.8) | 2 (2.8) | 9 (5.6) | |
| B | 3048 (13.3) | 2724 (13.1) | 8 (11.1) | 21 (13.2) | |
| O | 10 230 (44.6) | 9460 (45.4) | 36 (50.0) | 78 (48.7) | |
| Final Allocation MELD at transplant | 25 (20–32) | 24 (22–29) | 27 (22–35) | 25 (22–31) | <.001 |
| Laboratory MELD at transplant | 21 (15–30) | 17 (12–25) | 22 (12–32) | 18 (13–27) | <.001 |
| HCC exception points | 3242 (14.1) | 7264 (34.9) | 20 (27.8) | 50 (31.2) | <.001 |
| Status-1 | 1390 (6.1) | 46 (0.2) | 7 (9.7) | 1 (0.6) | <.001 |
| BMI | 27.5 (23.8–32.0) | 27.8 (24.7–31.6) | 24.8 (21.8–27.7) | 25.4 (23.1–28.6) | <.001 |
| Pretransplant diabetes | 5741 (25.3) | 4195 (20.4) | 13 (18.1) | 24 (15.0) | <.001 |
| Dialysis at transplant | 1815 (7.9) | 1113 (5.3) | 5 (6.9) | 5 (3.1) | <.001 |
| HBV core + | 2593 (11.9) | 6538 (33.6) | 41 (59.4) | 99 (66.0) | <.001 |
| HBV surface antigen + | 1653 (7.3) | 573 (2.8) | 30 (41.7) | 12 (7.9) | <.001 |
| Functional status | <.001 | ||||
| No impairment | 5345 (23.3) | 5416 (26.0) | 16 (22.2) | 38 (23.7) | |
| Mild | 7881 (34.4) | 7890 (37.9) | 22 (30.5) | 66 (41.2) | |
| Moderate-severe | 6645 (29.0) | 4528 (21.7) | 25 (34.7) | 37 (23.1) | |
| Median days on wait list | 66 (13–237) | 105 (27–305) | 60 (6–265) | 92 (20–225) | <.001 |
Abbreviations: BMI, body mass index; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MELD, model for end stage liver disease; NASH, nonalcoholic steatohepatitis.
a P value represents the overall comparison of all 4 groups.
b Other group includes liver failure attributed to drugs, hepatocellular carcinoma or unknown etiology.
Calculated laboratory and final allocation MELD scores were significantly higher in the HIV and HIV/HCV coinfected groups (P < .001). A higher proportion of patients received hepatocellular carcinoma HCC exception points in the HCV (34.9%) and HIV/HCV (31.2%) groups (P < .001). HCV and HIV/HCV coinfected patients were more likely to require hemodialysis at transplant. Calculated DRI differed significantly with the highest DRI livers (DRI of 1.61; P < .001) allocated to the HIV monoinfected group. Tacrolimus based therapy was the most common immunosuppression regimen at discharge from the index hospitalization.
Transplant Center Characteristics
We compared the characteristics of patients transplanted at the 18 centers that participated in the NIH trial to those transplanted at centers that did not enroll (Table 2). These centers were represented in all transplant regions except for the 14 states comprising regions 4, 6, and 8 (Oklahoma, Texas; Alaska, Hawaii, Idaho, Montana, Oregon, Washington; Colorado, Iowa, Kansas, Missouri, Nebraska, Wyoming). Overall, the patients transplanted at these centers were similar. NIH centers transplanted older (56, IQR 49–61) and more black (12.7% vs 8.4%, P < .001) patients than did nonenrolling centers. There was no significant difference in the final allocation MELD score, but the livers transplanted at NIH centers had a higher median DRI (1.56; IQR, 1.27–1.88). Choice of maintenance immunosuppression and acute rejection rates were similar.
Table 2.
A Comparison of the Clinical and Demographic Characteristics of Patients Transplanted at a National Institutes of Health (NIH) Center Compared to Those Transplanted at a Non-NIH Center
| NIH Center n = 18 | Non-NIH Center n = 136 | P Value | |
|---|---|---|---|
| Patients transplanted | 9148 | 34 839 | <.001 |
| Serostatus group | <.001 | ||
| HIV−/HCV− | 4700 (51.4) | 18 226 (52.3) | |
| HCV+ | 4304 (47.1) | 16 525 (47.4) | |
| HIV+ | 42 (0.5) | 30 (0.1) | |
| HIV+/HCV+ | 102 (1.1) | 58 (0.2) | |
| Age at transplant | 56 (49–61) | 55 (49–60) | <.001 |
| Male | 6203 (67.8) | 23 347 (67) | .150 |
| Race | <.001 | ||
| White | 6257 (68.4) | 25 562 (73.4) | |
| Black | 1163 (12.7) | 2942 (8.4) | |
| Latino | 1208 (13.2) | 4556 (13.1) | |
| Asian | 473 (5.2) | 1324 (3.8) | |
| Other | 47 (0.5) | 455 (1.3) | |
| Final allocation MELD at transplant | 24 (21–30) | 24 (21–30) | .421 |
| Calculated MELD at transplant | 18 (12–27) | 19 (13–28) | <.001 |
| HCC exception points | 2426 (26.5) | 8150 (23.4) | <.001 |
| Status 1 | 301 (3.3) | 1143 (3.3) | .964 |
| Functional status | .003 | ||
| No impairment | 2422 (26.5) | 8398 (24.1) | |
| Mild | 3350 (36.6) | 12 509 (35.9) | |
| Moderate-severe | 2308 (25.2) | 8927 (25.6) | |
| BMI | 27.4 (24–31) | 27.7 (24–32) | .001 |
| Pretransplant diabetes | 2226 (24.7) | 7747 (22.5) | <.001 |
| Dialysis at transplant | 562 (6.2) | 2376 (6.8) | .020 |
| Donor risk index | 1.56 (1.27–1.88) | 1.45 (1.18–1.77) | <.001 |
| Immunosuppression | .001 | ||
| Tacrolimus | 8113 (93.8) | 31 066 (93.6) | |
| Cyclosporine | 435 (5) | 1544 (4.6) | |
| Rapamycin | 106 (1.2) | 589 (1.8) | |
| Acute rejection (in the 1st yr) | 970 (13.8) | 3576 (13.6) | .581 |
Abbreviations: BMI, body mass index; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MELD, model for end stage liver disease; NIH, National Institutes of Health.
Patient and Allograft Outcomes
In sum, 1- and 3-year survival were as follows: 89% and 76% in the uninfected group, 87% and 66% in the HCV, 86% and 67% in the HIV, and 75% and 47% in the HIV/HCV coinfected group (Figure 2A, P < .001). Increased mortality was observed in the first 6 months in the HIV and HIV/HCV coinfected groups; by 1 year the slope for the HIV monoinfected plateaued (Supplementary Figures 1 and 2). Infections caused early deaths in 20% of the HIV and 29.6% of the HIV/HCV coinfected patients. Among patients with a reported cause of death, cancer (8.8%–16.7%), cardiovascular disease (4.4%–12.8%), and infections (11.1%–19.1%) were common to all, but approximately 20% of the HIV or HIV/HCV coinfected patients' deaths were attributed to allograft failure. Death or allograft failure was attributed to “recurrent disease” in 6% of the reference group, 24.4% of the HCV, 11.1% of the HIV, and 26.5% of the HIV/HCV coinfected groups. Vascular complications were implicated as a cause of death or allograft loss in 11.1% of the reference group, 6.2% of the HCV, 38.9% of the HIV, and 8.8% of the HIV/HCV coinfected groups. All cause graft loss (Figure 2B) was lowest in the reference group (23.6%) compared to the HCV (30.6%), HIV (31.4%), and HIV/HCV coinfected patients (44.8%; P < .001). Acute rejection in the first year was highest in the HIV (16.7%) and HIV/HCV coinfected (11.2%) patients.
Figure 2.
Kaplan–Meier curves illustrating (A) patient and (B) allograft survival in the liver transplant cohort, stratified by infectious status. Log rank test P < .001 for patient survival and P < .001 for allograft loss. Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Multivariable Models
Cox regression models were fit for mortality and graft loss (Tables 3 and 4, Supplementary Table 3). In univariable models, HCV infection (HR 1.46, 95% CI, 1.41–1.52) and HIV/HCV coinfection (HR 2.62, 95% CI, 2.06–3.33) were associated with an increased risk of death, whereas HIV infection was not (HR 1.37, 95% CI, .86–2.18). Age, male sex, race, BMI < 21, diabetes, hemodialysis at transplant, MELD score, impaired functional status, use of a HCV+ donor, and increasing DRI were associated with an increased risk of mortality. In a multivariable model for death stratified by NIH center, there was again no significant difference in HIV monoinfected patient survival compared to the reference group. The risk of death was higher in HIV patients transplanted at an NIH study center (NIH center HR 1.65, 95% CI, .93–2.92 vs non-NIH center HR death 1.03, 95% CI, .43–2.47) but did not meet statistical significance. An increased hazard for death was also observed for HIV/HCV coinfected patients transplanted at NIH study centers (NIH center HR 3.15, 95% CI, 2.32–4.28 vs non-NIH center 2.55, 95% CI, 1.64–3.96) but not for HCV infected patients; this difference did not meet statistical significance. Functional status was tested in the model but did not change estimates significantly; it was excluded from the final model due to a high degree of missingness. HCV+ donor was tested in model but did not have a significant effect on model estimates.
Table 3.
Multivariable Model for Death Stratified by Transplant Center Participation in the National Institutes of Health Trial
| Variable | NIH Study Centers |
Non-NIH Study Centers |
||||
|---|---|---|---|---|---|---|
| HR | P Value | 95% CI | HR | P Value | 95% CI | |
| HIV−/HCV− | Ref | Ref | ||||
| HCV+ | 1.56 | <.001 | 1.43–1.71 | 1.52 | <.001 | 1.45–1.60 |
| HIV+ | 1.65 | .088 | .93–2.92 | 1.03 | .951 | .43–2.47 |
| HIV+/HCV+ | 3.15 | <.001 | 2.32–4.28 | 2.55 | <.001 | 1.64–3.96 |
| Age (continuous) | 1.02 | <.001 | .92–1.10 | 1.02 | <.001 | 1.01–1.02 |
| Male recipient | 1.01 | .110 | .99–1.08 | 1.05 | .064 | 1.00–1.10 |
| Race | ||||||
| White | Ref | Ref | ||||
| Black | 1.13 | .049 | 1.00–1.28 | 1.35 | <.001 | 1.26–1.46 |
| Hispanic | 0.88 | .053 | .77–1.00 | 0.94 | .072 | .87–1.01 |
| Asian | 0.78 | .030 | .62–.98 | 0.82 | .005 | .72–.94 |
| Other | 1.19 | .496 | .72–1.99 | 0.89 | .282 | .71–1.10 |
| Final allocation MELD score (continuous) | 1.01 | <.001 | 1.01–1.02 | 1.01 | <.001 | 1.01–1.01 |
| Dialysis at Transplant | 1.59 | <.001 | 1.48–1.78 | 1.46 | <.001 | 1.32–1.61 |
| Donor risk index | 1.62 | <.001 | 1.48–1.78 | 1.56 | <.001 | 1.48–1.65 |
Abbreviations: CI, confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HR, hazard ratio; MELD, model for end stage liver disease; NIH, National Institutes of Health; Ref, reference.
Table 4.
Multivariable Model for Allograft Loss Stratified by Transplant Center Participation in the National Institutes of Health Trial
| Variable | NIH Study Centers |
Non-NIH Study Centers |
||||
|---|---|---|---|---|---|---|
| HR | P Value | 95% CI | HR | P Value | 95% CI | |
| HIV−/HCV− | Ref | Ref | ||||
| HCV+ | 1.46 | <.001 | 1.33–1.59 | 1.50 | <.001 | 1.44–1.57 |
| HIV+ | 1.24 | .500 | .66–2.32 | 1.10 | .811 | .49–2.45 |
| HIV+/HCV+ | 2.64 | <.001 | 1.91–3.64 | 2.22 | .001 | 1.41–3.49 |
| Age (continuous) | 1.01 | <.001 | 1.00–1.01 | 1.01 | <.001 | 1.00–1.01 |
| Male Recipient | 1.06 | .192 | .96–1.16 | 1.07 | .005 | 1.02–1.12 |
| Race | ||||||
| White | Ref | Ref | ||||
| Black | 1.15 | .029 | 1.01–1.29 | 1.35 | <.001 | 1.25–1.45 |
| Hispanic | 0.93 | .243 | .82–1.05 | 0.96 | .258 | .89–1.02 |
| Asian | 0.72 | .004 | .57–.90 | 0.79 | .001 | .70–.91 |
| Other | 1.01 | .985 | .59–1.70 | 0.94 | .574 | .76–1.16 |
| Final allocation MELD Score (continuous) | 1.01 | <.001 | 1.00–1.02 | 1.01 | <.001 | 1.01–1.02 |
| Donor risk index | 1.69 | <.001 | 1.54–1.85 | 1.70 | <.001 | 1.61–1.79 |
| BMI <21 | 1.14 | .094 | .98–1.33 | 1.20 | <.001 | 1.11–1.31 |
| Immunosuppression | ||||||
| Tacrolimus | Ref | Ref | ||||
| Cyclosporine | 1.09 | .320 | .92–1.29 | 1.11 | .041 | 1.00–1.22 |
| Rapamycin | 1.33 | .078 | .97–1.83 | 1.44 | <.001 | 1.26–1.65 |
Abbreviations: BMI, body mass index; CI, confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HR, hazard ratio; MELD, model for end stage liver disease; NIH, National Institutes of Health; Ref, reference.
In the univariable model for all-cause allograft loss, HCV (HR 1.37, 95% CI, 1.32–1.42), HIV (HR 1.52, 95% CI, 1.01–2.28), and HIV/HCV coinfection (HR 2.41, 95% CI, 1.92–3.04) were associated with increased risk. Older recipient age, male sex, race, BMI < 21, hemodialysis at transplant, functional impairment, increasing DRI, diabetic donor, hepatitis B surface antigen + donor, HCV+ donor, acute rejection, and cyclosporine or rapamycin use were associated with an increased risk of allograft loss. In the multivariable model stratified by NIH transplant center, HIV infection was not associated with an increased risk of graft loss (NIH center HR 1.24, 95% CI, .66–2.32; non-NIH center HR 1.10, 95% CI, .49–2.45), whereas HCV infection and HIV/HCV coinfection were. An increased hazard for allograft loss was observed in HIV/HCV coinfected patients transplanted at NIH centers (NIH center HR 2.64, 9%% CI, 1.91–3.64; non-NIH center HR 2.22, 95% CI, 1.41–3.49) but not for HCV+ patients. The inclusion of HCV+ donor in the model did not change estimates significantly. Acute rejection was an effect modifier, increasing the hazard for allograft loss for HCV (NIH center HR 1.73, 95% CI, 1.55–1.92; non-NIH center HR 1.73, 95% CI, 1.63–1.83) and HIV/HCV coinfected patients (NIH center HR 2.66, 95%CI, 1.74–4.06; non-NIH center HR 2.92, 95% CI, 1.69–5.05) but attenuating the risk observed in HIV monoinfected patients (NIH center HR 0.35, 95% CI, .10–1.40; non-NIH center HR 1.21, 95% CI, .45–3.22).
DISCUSSION
In our US primary liver transplant population-based study, we found that HIV/HCV coinfection was associated with a 2.5-fold increased risk of mortality and an almost 3-fold increase in allograft loss, consistent with prior studies [6, 7]. However, HIV monoinfected patients experienced outcomes comparable to HIV/HCV uninfected recipients. This suggests that liver disease etiology is of great importance to patient and allograft outcomes in HIV patients assumed to be on a stable ART regimen, with an appropriately high CD4 count, and undetectable HIV RNA at time of transplant, as per the protocol set forth in the NIH multicenter trial [6]. The HCV cohort was found to have rates of allograft loss and mortality comparable to other studies [20].
We, like others, found poor patient and allograft outcomes in HIV/HCV coinfected liver transplant recipients. Prior data [6, 7] have demonstrated that high MELD scores, low BMI candidates, and use of older or HCV+ donor organs in this population portend a poor prognosis. Despite our expectation that these studies would guide subsequent donor and recipient selection, transplant outcomes have not improved since publication of these findings. This continued posttransplant disparity, coupled with acceptable patient and allograft survival in the HIV monoinfected group, suggests that the differential outcomes in the HIV/HCV+ patients are based solely on coinfection with HCV. These data argue for treatment of HCV infection either in the pretransplant setting or immediately posttransplant. Although not previously feasible in patients intolerant of interferon based regimens, the recent approval of highly effective direct acting antiviral agents such as simeprevir, sofosbuvir, ledipasvir, and the ombitasvir/ritonavir/paritaprevir/dasabuvir combination, enables treatment of HCV/HIV coinfected patients prior to transplant [21–23]. Alternatively, in order to decrease wait times and to transplant coinfected recipients with lower MELD scores, one could consider using HCV+ organs and initiating HCV therapy immediately posttransplant; this strategy may equally benefit HCV monoinfected recipients, who also experienced longer wait times in our analysis. The ratification of the HIV Organ Policy Equity Act in 2013, which permits research into the use of HIV+ organs for transplant, may also increase organ availability for HIV-infected patients. These approaches, which require further study, will increase the potential donor pool and may result in improved outcomes for these patients.
Our study corroborates earlier findings [10] that patients with HIV, and especially those with HCV coinfection, had more infection-related deaths, especially in the early posttransplant period. Although information could not be gleaned regarding the specific infections, the initial trajectory of the survival curves for the HIV and HIV/HCV coinfected groups suggests that infection may be one basis for early mortality seen in these groups. However, once HIV monoinfected patients passed this 6-month threshold, their mortality rate leveled off while the trajectory was unchanged in the HIV/HCV coinfected group.
We observed increased rates of vascular complications and acute rejection in the HIV and HIV/HCV coinfected patients, consistent with other studies [7, 11, 24, 25]. This finding in our analysis should be interpreted with caution given the amount of missing data in the cause of graft loss and rejection variables, and the nonstandardized approach to their characterization in the dataset. When we adjusted for both acute rejection and immunosuppression regimen, there was an attenuation in the protective effect of tacrolimus-based therapy. This suggests that the association between tacrolimus and allograft failure may be on the basis of a reduction in acute rejection episodes. Given the limited data, these associations could not be fleshed out further, but highlight the effect of acute rejection and immunosuppression in early allograft survival.
In light of studies demonstrating the relationship between transplant center effect and transplant outcomes [26], we explored whether consistent patient selection criteria and careful posttransplant follow-up across centers that participated in the NIH trial impacts HIV-infected liver recipient survival. Though we found no change in outcomes in HCV monoinfected patients, there was a difference in the hazard ratio between NIH centers and nonenrolling centers for HIV and HIV/HCV coinfected patients. This may in part be due to the willingness of larger and more experienced centers to take on higher complexity cases that are associated with a greater risk of allograft loss and patient mortality. Center practice merits further exploration, as does whether center performance influences the continued acceptance of high-risk transplant recipients. We conclude on the basis of these data that liver transplant in the HIV and HIV/HCV coinfected patient is effective in the “real world” setting.
Our study has limitations. We lacked complete serologic data on all subjects within the cohort, although patients with missing data were comparable in demographics to those with complete data. The UNOS dataset does not provide information regarding HCV viral load or genotype, so we could not distinguish those patients who had been successfully treated and cleared their HCV infection. The UNOS dataset also does not provide information regarding CD4 counts or viral loads for patients with HIV infection. Additionally, the dataset had a significant amount of missing data in some variables of clinical importance (functional status and acute rejection). However, no significant change in the HRs for the outcomes of interest was noted with imputation of these variables. Lastly, the sample size in the HIV monoinfected group was small, and hence the estimates of association may be unstable in fully adjusted models.
In summary, our data demonstrate outcomes for HIV monoinfected liver transplant patients that are similar to uninfected recipients but poor allograft and patient outcomes in HIV/HCV coinfected recipients. Two distinct strategies can be considered in these patients that may improve outcomes: (1) pretransplant HCV eradication or (2) early posttransplant preemptive HCV therapy. The second option supports a strategy that would broaden the donor pool for HIV/HCV coinfected patients, as well as for HCV monoinfected patients, by making transplant with an HCV+ donor feasible. In this new era of direct acting antiviral therapy, both of these strategies can be implemented and merit further study, as does the use of HIV-positive donor organs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Author contributions. D. S. designed the study, analyzed the data and wrote the report. D. S. G. analyzed the data and revised the report. E. B., P. L. A., and R. D. B. designed the study and revised the report. K. A. F. designed the study, analyzed the data, and wrote the report.
Financial support. D. S. is supported by Penn Center for AIDS Research P 30 AI 045008. K. A. F. is supported by National Institutes of Health (NIH) K23 DK090209. D. S. G. is supported by NIH K08 DK098272.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med 2012; 156:271–8. [DOI] [PubMed] [Google Scholar]
- 2.Weber R, Sabin CA, Friis-Moller N et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006; 166:1632–41. [DOI] [PubMed] [Google Scholar]
- 3.Erice A, Rhame FS, Heussner RC, Dunn DL, Balfour HH Jr. Human immunodeficiency virus infection in patients with solid organ transplants: report of five cases and review. Rev Infect Dis 1991; 13:537–47. [DOI] [PubMed] [Google Scholar]
- 4.Bouscarat F, Samuel D, Simon F, Debat P, Bismuth H, Saimot AG. An observational study of 11 French liver transplant recipients infected with human immunodeficiency virus type 1. Clin Infect Dis 1994; 19:854–9. [DOI] [PubMed] [Google Scholar]
- 5.Roland ME, Barin B, Carlson L et al. HIV-infected liver and kidney transplant recipients: 1- and 3-year outcomes. Am J Transplant 2008; 8:355–65. [DOI] [PubMed] [Google Scholar]
- 6.Terrault NA, Roland ME, Schiano T et al. Outcomes of liver transplant recipients with hepatitis C and human immunodeficiency virus coinfection. Liver Transpl 2012; 18:716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miro JM, Montejo M, Castells L et al. Outcome of HCV/HIV co-infected liver transplant recipients: a prospective and multicenter cohort study. Am J Transplant 2012; 12:1866–76. [DOI] [PubMed] [Google Scholar]
- 8.de Vera ME, Dvorchik I, Tom K et al. Survival of liver transplant patients co-infected with HIV and HCV is adversely impacted by recurrent hepatitis C. Am J Transplant 2006; 6:2983–93. [DOI] [PubMed] [Google Scholar]
- 9.Duclos-Vallee JC, Feray C, Sebagh M et al. Survival and recurrence of hepatitis c after liver transplantation in patients co-infected with human immunodeficiency virus and hepatitis C virus. Hepatology 2008; 47:407–17. [DOI] [PubMed] [Google Scholar]
- 10.Moreno A, Cervera C, Fortun J et al. Epidemiology and outcome of infections in human immunodeficiency virus/hepatitis C virus co-infected liver transplant recipients: a FIPSE/GESIDA prospective cohort study. Liver Transplant 2012; 18:70–82. [DOI] [PubMed] [Google Scholar]
- 11.Cherian PT, Alrabih W, Douiri A et al. Liver transplantation in human immunodeficiency virus infected patients: procoagulant but is antithrombotic prophylaxis required? Liver Transplant 2012; 18:83–9. [DOI] [PubMed] [Google Scholar]
- 12.Wiesner R, Edwards E, Freeman R et al. Model for end stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003; 14:91–6. [DOI] [PubMed] [Google Scholar]
- 13.Feng S, Goodrich NP, Bragg-Gresham JL et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant 2006; 6:783–90. [DOI] [PubMed] [Google Scholar]
- 14.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000; 31:864–71. [DOI] [PubMed] [Google Scholar]
- 15.Kamath PS, Wiesner RH, Malinchoc M et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001; 33:464–70. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53:457–81. [Google Scholar]
- 17.Cox D. Regression models and life tables. J R Stat Soc 1972; 34:187–220. [Google Scholar]
- 18.Rothman KJ, Greenland S. Modern Epidemiology. 2nd ed Philadelphia: Lippincott Williams and Wilkins, 1998. [Google Scholar]
- 19.Thereau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model (Statistics for Biology and Health). New York: Springer Verlag, 2001. [Google Scholar]
- 20.O'Leary JG, Randall H, Onaca N, Jennings L, Klintman GB, Davis GL. Post liver transplant survival in hepatitis C patients is improving over time. Liver Transplant 2009; 15:360–8. [DOI] [PubMed] [Google Scholar]
- 21.Kowdley KV, Gordon SC, Reddy KR et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014; 370:1879–88. [DOI] [PubMed] [Google Scholar]
- 22.Charlton M, Gane E, Manns MP et al. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis c virus infection after liver transplantation. Gastroenterology 2015; 148:108–17. [DOI] [PubMed] [Google Scholar]
- 23.Kwo PY, Mantry PS, Coakley E et al. An interferon free antiviral regimen for HCV after liver transplantation. N Engl J Med 2014; 371:2375–82. [DOI] [PubMed] [Google Scholar]
- 24.Stock PG, Roland ME, Carlson L et al. Kidney and liver transplantation in human immunodeficiency virus infected patients: a pilot safety and efficacy study. Transplantation 2003; 76:370–5. [DOI] [PubMed] [Google Scholar]
- 25.Roland ME, Stock PG. Review of solid organ transplantation in HIV infected patients. Transplantation 2003; 75:425–9. [DOI] [PubMed] [Google Scholar]
- 26.Asrani SK, Kim WR, Edwards EB et al. Impact of the center on graft failure after liver transplantation. Liver Transplant 2013; 19:957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.