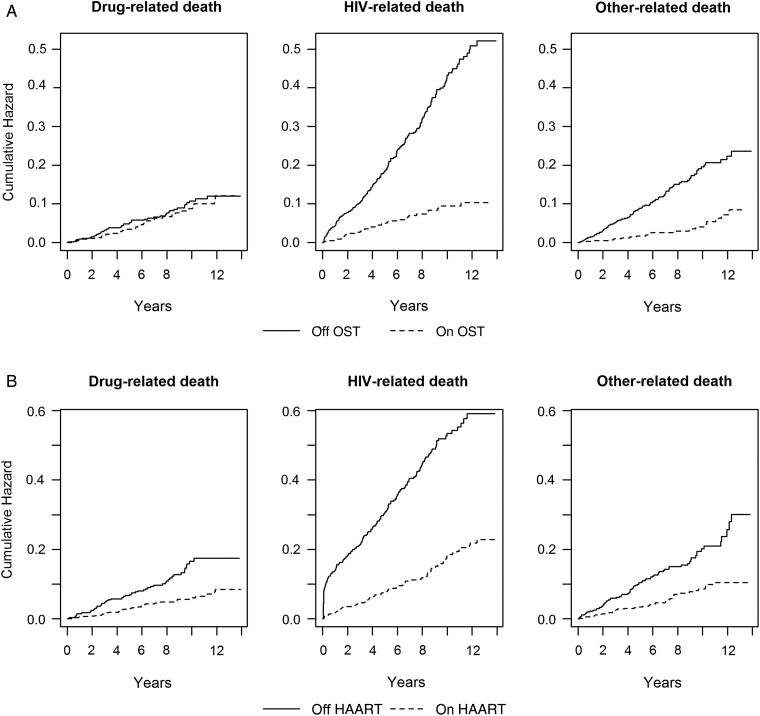
In this retrospective cohort study, we find that while opioid substitution treatment and highly active antiretroviral therapy each independently protected against human immunodeficiency virus and drug-related mortality, joint administration provided the lowest risk of death.
Keywords: opioid substitution treatment, injection drug users, highly active antiretroviral therapy, HIV/AIDS, mortality
Abstract
Background. Prior studies indicated opioid substitution treatment (OST) reduces mortality risk and improves the odds of accessing highly active antiretroviral therapy (HAART); however, the relative effects of these treatments for human immunodeficiency virus (HIV)–positive people who inject drugs (PWID) are unclear. We determine the independent and joint effects of OST and HAART on mortality, by cause, within a population of HIV-positive PWID initiating HAART.
Methods. Using a linked population-level database for British Columbia, Canada, we used time-to-event analytic methods, including competing risks models, proportional hazards models with time-varying covariates, and marginal structural models, to identify the independent and joint effects of OST and HAART on all-cause as well as drug- and HIV-related mortality, controlling for covariates.
Results. Among 1727 HIV-positive PWID, 493 (28.5%) died during a median 5.1 years (interquartile range, 2.1–9.1) of follow-up: 18.7% due to drug-related causes, 55.8% due to HIV-related causes, and 25.6% due to other causes. Standardized mortality ratios were 12.2 (95% confidence interval [CI], 9.8, 15.0) during OST and 30.0 (27.1, 33.1) during periods out of OST. Both OST (adjusted hazard, 0.34; 95% CI, .23, .49) and HAART (0.39 [0.31, 0.48]) decreased the hazard of all-cause mortality; however, individuals were at lowest risk of death when these medications were used jointly (0.16 [0.10, 0.26]). Both OST and HAART independently protected against HIV-related death, drug-related death and death due to other causes.
Conclusions. While both OST and HAART are life-saving treatments, joint administration is urgently needed to protect against both drug- and HIV-related mortality.
(See the Editorial Commentary by Hickman et al on pages 1166–8.)
Human immunodeficiency virus (HIV) and opioid use disorder carry a substantial public health burden and a high risk of mortality. A systematic review on mortality among individuals with opioid use disorders reported a standardized mortality ratio (SMR) of 14.66 (95% confidence interval [CI], 12.82, 16.50) [1], with SMRs of HIV-positive opioid users nearly 3 times higher than those of HIV-negative users (relative risk, 2.86; 95% CI, 2.18, 3.74). Comparably, the Antiretroviral Cohort Collaboration reported SMRs for people who inject drugs (PWID) that were 2–4 times higher than noninjection drug users captured within the cohort [2].
Both highly active antiretroviral therapy (HAART) and opioid substitution treatment (OST) have been proven to decrease mortality risk substantially within these populations. HAART stops viral replication, allowing for CD4 cell reconstitution and delay in the onset of AIDS and the otherwise fatal course of HIV/AIDS [3, 4]. HAART protects against mortality, with a hazard ratio of 0.48 (95% CI, .41, .57) for HAART initiators vs noninitiators [5]. Otherwise, adequately dosed methadone or buprenorphine blocks opioid receptors, satisfies opioid craving, and eliminates withdrawal symptoms while providing a blockade against subsequent opioid administration. The relative risk of mortality out of OST is nearly 2.5 times greater than in treatment (2.38; 95% CI, 1.79, 3.17) [1].
The longitudinal pattern and immediacy with which these treatment regimens impact the respective disease courses of HIV and opioid use disorder differ substantially. In the short term, OST decreases the risk of death through reductions in illicit drug use and resulting overdose [6]. Decreased illicit drug use may indirectly result in reduced mortality due to infectious diseases such as HIV and hepatitis C virus and also protect against death due to cardiovascular disease [7–10], accidental death, trauma, and suicide [11, 12]. In contrast, HAART suppresses virus levels in the bloodstream within 6 months, with immediate benefits perhaps only experienced by those reinitiating treatment at exceptionally low CD4 cell counts (ie, <50 cells/mm3).
Furthermore, the treatment–mortality relationship is complicated by several factors related to the pharmacodynamics of methadone and patients' initial treatment responses. The risk of death during OST is highest within 2 weeks of initiation, while risk of death out of treatment is highest in the first 2 weeks following discontinuation [12], which may be in part due to the slow elimination of methadone through the kidneys and feces [13, 14]. Ongoing drug use during these transition periods, that is, during initiation and immediately after treatment exit, is one factor that may account for this high risk of death. To mitigate these risks, clinical guidelines recommend an initial dose of 40 mg/day, with a gradual dose titration to at least 60 mg/day [15, 16], though low initial dosing may also precipitate other opioid use and mortality. Otherwise, while OST may substantially improve access and adherence to HAART [17, 18], the physiological effect of opioids on HIV disease progression is not well understood.
As a result of these complexities and the relatively coarse data with which prior studies have been executed, the protective benefits of OST have likely been underestimated, particularly among people living with HIV. Thus, the objective of this study is to determine the independent and joint effects of OST and HAART on mortality, by cause, within a population of HIV-positive PWID following HAART initiation. To fulfill our objectives, we used a linked population-level database combining pharmacy dispensation data with vital statistics records as well as inpatient and outpatient care data.
METHODS
Patient Population
This study was based on a province-level linkage of 7 health administrative databases and disease registries in British Columbia (BC), Canada. Data included antiretroviral dispensation, virology and HIV testing registries (antiretroviral dispensations, plasma viral load, CD4 tests, nominal HIV diagnoses), physician billing records (the Medical Services Plan database), hospitalizations (the discharge abstract database), and nonantiretroviral drug dispensations captured in the BC PharmaNet database and the BC Vital statistics database. Further details regarding the construction and composition of the HIV-positive cohort and available databases are provided elsewhere [19, 20].
We selected all individuals identified as HIV positive and either having a history of OST at initial HAART receipt, as indicated by methadone or buprenorphine dispensation records in the BC PharmaNet database from 1 January 1996 to 31 March 2010, or having an indication of injection drug use before HIV infection, as indicated in the HIV testing database. Buprenorphine is not commonly used in British Columbia, with the medication only gaining approval as second-line treatment in 2010. OST dispensations were identified by unique drug identification numbers. These numbers were specific to indications for opioid use disorder (as opposed to pain). We used previously defined methods to clean and process PharmaNet data for accurate estimation of OST episodes [21].
For each individual, we considered follow-up data from the point of initial HAART receipt (consisting of 2 nucleosides plus a protease-inhibitor or a nonnucleoside reverse transcriptase inhibitor). Individuals were followed until death, administrative loss to follow-up, or censorship (ongoing care as of 31 March 2010). Administrative loss was defined as no records in any of the linked databases for a period of ≥18 months.
The study cohort was followed in a unique environment where medical care (HIV and non-HIV related), antiretroviral treatment, and laboratory monitoring are fully subsidized by the British Columbia provincial government. Antiretroviral drugs are centrally distributed according to provincial treatment guidelines, which have remained consistent with those put forward by the International AIDS Society–USA [22, 23]. Antiretroviral medication and viral load data captured in the BC Centre for Excellence in HIV/AIDS registries are complete for the provincial population of people living with HIV. The CD4 cell count data captured for this population have previously been estimated at 80% [24]. This study received approval from the University of British Columbia/Providence Health Care Research Ethics Board.
Measures
Our dependent variable was mortality, stratified by cause as follows: drug related, HIV-related, and other cause of death [12]. Key time-variable exposures included OST receipt and HAART receipt. As in previous analyses [21], we defined OST episodes as continuous periods of dispensed medication with no more than a 30-day gap. We used this same threshold for HAART episodes. Alternately, in analyses featuring monthly timescales, individuals were classified as being on HAART or on OST if they had 95% adherence (29 of 30 days) within the calendar month.
We also considered a selection of other baseline and time-varying covariates hypothesized to be associated with the dependent and primary independent variable. Baseline covariates included age, gender, aboriginal ethnicity, health authority (HA; geographic healthcare delivery regions) of residence, CD4+ cell count, calendar year of HAART initiation, time from HIV diagnosis, and AIDS status. The Charlson comorbidity index (CCI) score [25] (≥1 vs 0) and OST history (number of prior OST episodes) at the time of HAART initiation were also incorporated, as well as time-varying measures of CD4+ cell count, HA, AIDS status, and CCI score.
Statistical Analyses
We first summarized mortality by cause and across key periods surrounding treatment initiation and discontinuation. The crude mortality rate (CMR) and indirect SMRs were calculated and compared with the British Columbia population with 95% CIs using the Poisson exact method. We then estimated stratified SMRs across several covariates pertaining to patient demographics and points of initiation and discontinuation of OST and HAART.
Next, Nelson–Aalen estimators of the cumulative cause-specific hazards of death were plotted for both OST and HAART exposure and nonexposure [26, 27]. The time-variable exposures to OST and HAART were accounted for using the multistate model in the mvna package for R software [28].
Next, we implemented a series of analyses to estimate the independent effects of OST and HAART on mortality, by cause, accounting for the 2 critical features affecting our inference on these relationships: the potentially competing risks of drug-related, HIV-related, and “other” deaths and the time-variable course of OST receipt, HAART receipt, and HIV disease progression. As the development methods to account for these issues simultaneously are in development, we proceeded with several complementary models to characterize the key relationships in question.
We first compared the results of cause-specific and subdistribution proportional hazards (PH) models [29] in a counting process format to account for time-varying exposure to OST and HAART. We followed a technique proposed by Beyersmann et al [28] to obtain subdistribution hazards.
To control for the potential of time-varying confounding, we estimated marginal structural models [30, 31] using a monthly timescale. Marginal structural models are designed to handle cases in which time-dependent variables are simultaneously confounders of the effect of interest and are predicted by previous treatment. Past HAART adherence and HIV disease progression can be considered time-dependent confounders for the effect of OST on mortality, since they may be hypothesized to predict future mortality as well as subsequent initiation of OST. As the same may also be true for the effect of HAART on mortality, we estimated separate models to determine the respective independent effects of HAART on mortality, controlling for OST and the other treatment exposure (as well as CD4 progression) within the time-updated inverse probability of treatment weights. To determine the impact of the inverse probability of treatment weights, we estimated simplified pooled logit models on the same monthly timescale, including only baseline and time-varying covariates for both OST and HAART.
Finally, we reformulated our time-varying PH and marginal structural models to conform to periods of exposure to both OST and HAART, exposure to only OST, exposure to only HAART, and no treatment, according to previously defined episodic thresholds. This modification allowed us to estimate the joint effects of OST and HAART, as well as their respective individual impacts when not exposed to the other treatment.
RESULTS
Sample Selection and Description
We selected 1727 individuals with indication of injection drug use from 12 349 HIV-positive individuals in the population under study. Overall, the median follow-up time was 5.1 years (interquartile range, 2.1–9.1), during which time 493 (29%) all-cause deaths were observed. HIV-related death was most common (N = 275, 56% of all deaths); however, drug-related death was also prevalent (N = 92, 19%). Buprenorphine was observed in only 3 patients with <1 patient-month of follow-up within the study sample. At baseline, the median age was 36 years (25th–75th percentiles, 30–42), and 33% were female. A total of 26% of participants were aboriginal, 52% resided in the Vancouver coastal HA, and 12% had ≥500 CD4 cells/mm3. At baseline, 56% had no history of OST, 33% had CCI scores >0%, and 64% started HAART after 1999. Complete summary statistics are presented in Table 1.
Table 1.
Patient Characteristics at Initial Highly Active Antiretroviral Therapy Receipt
| Variable | N (%) |
|---|---|
| Injection drug using people living with HIV/AIDS | 1727 |
| Age, y | |
| <30 | 283 (16.4) |
| 30–39 | 666 (38.6) |
| 40–49 | 573 (33.2) |
| 50+ | 205 (11.9) |
| Female gender | 575 (33.3) |
| Aboriginal | 455 (26.3) |
| Health authority of residence | |
| Interior | 109 (6.3) |
| Fraser | 391 (22.6) |
| Vancouver Coastal | 900 (52.1) |
| Vancouver Island | 244 (14.1) |
| Northern | 83 (4.8) |
| On OST | 612 (35.4) |
| CD4, cells/mm3 | |
| <200 | 792 (46) |
| 200–499 | 719 (41.8) |
| ≥500 | 210 (12.2) |
| Prior OST episodes | |
| 0 | 969 (56.1) |
| 1 | 410 (23.7) |
| ≥2 | 348 (20.2) |
| Charlson comorbidity index (>0) | 575 (33.3) |
| AIDS status | 231 (13.4) |
| Calendar year | |
| 1996–1999 | 626 (36.2) |
| 2000–2003 | 378 (21.9) |
| 2004–2010 | 723 (41.9) |
| Possible endpoints: censored | 1234 (71.5) |
| Drug-related death | 92 (5.3) |
| Human immunodeficiency virus–related death | 275 (15.9) |
| Other-related death | 126 (7.3) |
Abbreviations: HIV, human immunodeficiency virus; OST, opioid substitution treatment.
Mortality in the Study Sample
Table 2 provides CMRs and SMRs across subject strata. The risk of mortality was substantially higher out of OST compared with periods in which individuals were engaged in OST (out of OST, 30.0; 95% CI, 27.1, 33.1 vs on OST, 12.2; 95% CI, 9.8, 15.0). Further, the risk of mortality was starkly elevated in the 2 weeks following OST discontinuation (SMR, 716.4 [549.2, 918.3]), with continued high risk of mortality in weeks 3–4 after OST discontinuation (203.0 [118.2, 325.0]). While a similar pattern was evident after OST initiation, the SMR was 51.7 (14.1, 132.3) ≤2 weeks after OST initiation vs 12.2 (9.8, 15.0) overall. In contrast, SMRs during the initial 4 weeks of HAART were only slightly elevated (and not statistically significantly so) from the overall on-HAART SMR of 13.4 (11.5, 15.4). Similar to OST, however, SMRs were elevated in subsequent HAART episodes and markedly higher when off HAART.
Table 2.
Crude Mortality Rates and Standardized Mortality Ratios, by Key Strata
| Variable | No. Deaths | Person-Years of Follow-up | Crude Mortality Rate, per 1000 Person-Years (95% CI) | Standardized Mortality Ratio (95% CI) |
|---|---|---|---|---|
| Overall | 493 | 9913 | 49.7 (45.4, 54.3) | 23.8 (21.7, 25.9) |
| Gender | ||||
| Female | 152 | 3359 | 45.3 (38.3, 53.0) | 44.6 (37.8, 52.3) |
| Male | 341 | 6554 | 52.0 (46.7, 57.9) | 19.7 (17.6, 21.9) |
| Age, y | ||||
| 0–29 | 24 | 803 | 29.9 (19.2, 44.5) | 48.8 (31.3, 72.6) |
| 30–39 | 140 | 3273 | 42.8 (36.0, 50.5) | 43.6 (36.6, 51.4) |
| 40–49 | 192 | 4097 | 46.9 (40.5, 54.0) | 23.8 (20.5, 27.4) |
| ≥50 | 137 | 1741 | 78.7 (66.1, 93.0) | 15.3 (12.8, 18.0) |
| Calendar year | ||||
| 1996–1999 | 39 | 1139 | 34.2 (24.3, 46.8) | 19.2 (13.7, 26.3) |
| 2000–2004 | 194 | 3581 | 54.2 (46.8, 62.4) | 29.3 (25.3, 33.7) |
| 2005–2010 | 260 | 5193 | 50.1 (44.2, 56.5) | 21.5 (18.9, 24.3) |
| Aboriginal | ||||
| No | 366 | 7284 | 50.2 (45.2, 55.7) | 22.3 (20.0, 24.7) |
| Yes | 127 | 2629 | 48.3 (40.3, 57.5) | 29.5 (24.6, 35.1) |
| OST episode no. | ||||
| 0 | 219 | 4603 | 47.6 (41.5, 54.3) | 19.2 (16.8, 22.0) |
| 1 | 81 | 1892 | 42.8 (34.0, 53.2) | 22.4 (17.8, 27.8) |
| 2 | 70 | 1278 | 54.8 (42.7, 69.2) | 28.6 (22.3, 36.1) |
| ≥3 | 123 | 2140 | 57.5 (47.8, 68.6) | 37.1 (30.9, 44.3) |
| Off OST | ||||
| Overall | 404 | 5934 | 68.1 (61.6, 75.1) | 30.0 (27.1, 33.1) |
| Never | 198 | 4352 | 45.5 (39.4, 52.3) | 18.1 (15.7, 20.8) |
| ≤2 wk | 62 | 57 | 1094.9 (839.4, 1403.6) | 716.4 (549.2, 918.3) |
| 3–4 wk | 17 | 56 | 305.9 (178.2, 489.7) | 203.0 (118.2, 325.0) |
| >4 wk | 127 | 1469 | 86.4 (72.1, 102.9) | 53.9 (44.9, 64.1) |
| On OST | ||||
| Overall | 89 | 3979 | 22.4 (18.0, 27.5) | 12.2 (9.8, 15.0) |
| ≤2 wk | 4 | 52 | 76.9 (21.0, 196.9) | 51.7 (14.1, 132.3) |
| 3–4 wk | 0 | 48 | 0.0 (0.0, 77.6) | 0.0 (0.0, 52.0) |
| >4 wk | 85 | 3880 | 21.9 (17.5, 27.1) | 11.9 (9.5, 14.7) |
| HAART episode no. | ||||
| 1 | 126 | 3315 | 38 (31.7, 45.3) | 19.1 (15.9, 22.7) |
| 2 | 103 | 2093 | 49.2 (40.2, 59.7) | 24.1 (19.7, 29.3) |
| ≥3 | 264 | 4505 | 58.6 (51.7, 66.1) | 26.7 (23.6, 30.1) |
| Off HAART | ||||
| Overall | 306 | 3855 | 79.4 (70.7, 88.8) | 45.2 (40.3, 50.6) |
| ≤2 wk | 56 | 208 | 269.1 (203.3, 349.4) | 142.2 (107.4, 184.6) |
| 3–4 wk | 24 | 206 | 116.8 (74.8, 173.8) | 61.8 (39.6, 92.0) |
| >4 wk | 226 | 3441 | 65.7 (57.4, 74.8) | 37.7 (33.0, 43.0) |
| On HAART | ||||
| Overall | 187 | 6058 | 30.9 (26.6, 35.6) | 13.4 (11.5, 15.4) |
| ≤2 wk | 11 | 250 | 44.0 (22.0, 78.8) | 23.1 (11.6, 41.4) |
| 3–4 wk | 10 | 245 | 40.8 (19.5, 75.0) | 21.4 (10.3, 39.4) |
| >4 wk | 166 | 5563 | 29.8 (25.5, 34.7) | 12.7 (10.9, 14.8) |
| OST and HAART | ||||
| Off OST and HAART | 258 | 2455 | 105.1 (92.6, 118.7) | 58.4 (51.5, 65.9) |
| On OST only | 48 | 1400 | 34.3 (25.3, 45.5) | 20.4 (15.1, 27.1) |
| On HAART only | 146 | 3478 | 42.0 (35.4, 49.4) | 16.1 (13.6, 19.0) |
| On OST and HAART | 41 | 2580 | 15.9 (11.4, 21.6) | 8.3 (6.0, 11.3) |
Abbreviations: CI, confidence interval; HAART, highly active antiretroviral therapy; OST, opioid substitution treatment.
Nelson–Aalen estimates of the cumulative hazard of mortality due to OST exposure, not adjusting for HAART exposure, demonstrated a strong protective effect of OST on the hazard of HIV and other causes of death, but not drug-related death (Figure 1A). In contrast, HAART had a similarly strong effect on the hazard of HIV-related death and comparable, significant effects on deaths due to drugs and other causes (Figure 1B).
Figure 1.
Nelson–Aalen estimates of the cumulative hazard of death, by cause, for opioid substitution treatment (OST)– and highly active antiretroviral therapy (HAART)–exposed and nonexposed individuals. A, Nelson–Aalen estimates of the cumulative hazard of mortality due to OST exposure, not adjusting for HAART exposure, demonstrated a strong protective effect of OST on the hazard of human immunodeficiency virus (HIV) and other causes of death, but not drug-related death. B, In contrast, HAART had a similarly strong effect on the hazard of HIV-related death and comparable, significant effects on deaths due to drugs and other causes.
Multiple Regression Analysis
Table 3 provides results of the multiple regression models used to estimate the independent effects of OST and HAART on all-cause and cause-specific mortality. The results of all model formulations were qualitatively similar. Compared with cause-specific Cox PH models, subdistribution PH models provided similar but relatively weaker-magnitude associations for all competing causes of death, while pooled logistic regression model results were attenuated toward the null in all cases, compared with marginal structural model results (see Supplementary Table A4 for full results).
Table 3.
Results of Multiple Regression Analyses on the Effect of Opioid Substitution Treatment and Highly Active Antiretroviral Therapy on All-Cause and Cause-Specific Mortality
| Model Type | Covariate | Categories | All-Cause Mortality | COD: Drug-Related | COD: Human Immunodeficiency Virus–Related | COD: Other |
|---|---|---|---|---|---|---|
| AHR (95% CI) | AHR (95% CI) | AHR (95% CI) | AHR (95% CI) | |||
| Subdistribution PH model 1a | OST | Yes vs no | 0.20 (.15, .26) | 0.65 (.39, 1.09) | 0.20 (.14, .30) | 0.26 (.15, .45) |
| HAART | Yes vs no | 0.29 (.24, .35) | 0.54 (.35, .84) | 0.31 (.24, .40) | 0.50 (.34, .74) | |
| Subdistribution PH model 2a | OST and HAART | Off OST and HAART | Ref | Ref | Ref | Ref |
| On OST only | 0.18 (.13, .25) | 0.60 (.32, 1.12) | 0.21 (.13, .35) | 0.30 (.15, .59) | ||
| On HAART only | 0.29 (.23, .36) | 0.49 (.28, .87) | 0.31 (.23, .42) | 0.54 (.35, .81) | ||
| On OST and HAART | 0.07 (.05, .09) | 0.37 (.20, .70) | 0.06 (.04, .11) | 0.11 (.05, .25) | ||
| Marginal Structural Model 1b,c | OST | Yes vs no | 0.34 (.23, .49) | 0.68 (.42, 1.12) | 0.33 (.23, .48) | 0.19 (.12, .32) |
| Marginal Structural Model 2b,d | HAART | Yes vs no | 0.39 (.31, .48) | 0.49 (.32, .75) | 0.34 (.26, .44) | 0.42 (.29, .60) |
| Marginal Structural Model 3b | OST and HAART | Off OST and HAART | Ref | Ref | Ref | Ref |
| On OST only | 0.34 (.22, .52) | 0.45 (.22, .92) | 0.35 (.21, .56) | 0.23 (.10, .52) | ||
| On HAART only | 0.46 (.34, .62) | 0.37 (.21, .64) | 0.35 (.26, .46) | 0.64 (.46, .90) | ||
| On OST and HAART | 0.16 (.10, .26) | 0.40 (.22, .73) | 0.14 (.08, .23) | 0.08 (.04, .17) |
Abbreviations: AHR, adjusted hazard ratio; CI, confidence interval; COD, cause of death; HAART, highly active antiretroviral therapy; OST, opioid substitution treatment; PH, proportional hazards; Ref, reference.
a Data are organized into durations on and off OST and HAART.
b Data are organized in monthly intervals; OST and HAART receipt in at least 95% of days in a given month; estimated parameters are cause-specific hazard ratios.
c HAART is included as a time-dependent confounder in the model.
d OST is included as a time-dependent confounder in the model.
The subdistribution PH models resulted in a strong estimate on the association of OST; the adjusted hazard of (all-cause) mortality was 0.20 (0.15, 0.26), while the association of HAART was also strong (0.29 [0.24, 0.35]). Marginal structural models demonstrated a similarly large negative effect of OST on mortality, with the effect again slightly stronger for OST (0.34 [0.23, 0.49]) compared with HAART (0.39 [0.31, 0.48]).
We found OST had relatively weaker and not statistically significant results on drug-related deaths compared with those of HIV-related deaths and deaths due to other causes from the subdistribution PH (0.65 [0.39, 1.09]) and marginal structural models (0.68 [0.42, 1.12]). On the other hand, HAART also had a strong and statistically significant negative association with drug-related mortality. Results were similar in subdistribution hazards (0.54 [0.35, 0.84]) and marginal structural models (0.49 [0.32, 0.75]).
Both OST and HAART had strong negative associations with HIV-related mortality. In separately constructed marginal structural models, OST had an adjusted cause-specific hazard of 0.33 (0.23, 0.48), while HAART had an adjusted cause-specific hazard of 0.34 (0.26, 0.44). Finally, both OST and HAART had strong protective effects against mortality due to other causes, with an adjusted hazard for OST of 0.19 (0.12, 0.32) and an adjusted hazard for HAART of 0.42 (0.29, 0.60) from marginal structural models. Once again, these estimates were similar to those derived from PH models with time-varying covariates.
Joint Effects of HAART and OST
Concurrent use of both OST and HAART resulted in the strongest negative association with all-cause mortality across model formulations, with the marginal structural model formulation producing an adjusted hazard of 0.16 (0.10, 0.26; Table 3). OST alone was also strong and significant (0.34 [0.22, 0.52]), as well as HAART alone (0.46 [0.34, 0.62]).
Cause-specific hazards for the joint effect of OST and HAART on drug-related death were also strong (marginal structural model, 0.40 [0.22, 0.73]), with a significant negative association for OST alone (0.45 [0.22, 0.92]) and HAART alone (0.37 [0.21, 0.64]). While HAART alone did have a statistically significant negative association with HIV-related mortality (0.35 [0.26, 0.46]), the joint effect of OST and HAART (0.14 [0.08, 0.23]) was substantially stronger. Finally, concurrent use of OST and HAART was most strongly negatively associated with death due to other causes (0.08 [0.04, 0.17]).
DISCUSSION
Our analyses, which were focused on HIV-positive PWID following HAART initiation, found that both forms of treatment were strongly negatively associated with all-cause mortality and HIV-related mortality. However, HAART had a stronger independent association with drug-related death, while OST better protected against causes of death other than HIV and drugs. An alternate set of analyses found that the risk of death was lowest when individuals were engaged in both forms of treatment, but individuals had a slightly lower hazard of death when only receiving OST compared with only HAART. We interpret these findings as being indicative of the stabilizing effects of OST among HIV-positive PWID and its importance in protecting against mortality.
A novel finding to emerge from our analyses is the relatively stronger association of OST on HIV-related death compared with drug-related death and the generally stronger independent effect compared with HAART. There are both biological and methodological factors that require careful consideration in interpreting these findings.
First, while there is no doubt that environmental and sociological factors associated with illicit drug use compromise HAART retention and downstream HIV-related health outcomes, the evidence on the independent effects of opioid and other illicit drug use on HIV disease progression, at the molecular level, are mixed. Epidemiological studies have difficulty specifying such independent effects given issues with drug use measurement, confounding, and sample selection, while laboratory studies on simian subjects have been underpowered to clearly distinguish a causal effect [32]. Baum et al found that crack cocaine use, but not other illicit substances such as marijuana or heroin, was associated with CD4 decline, controlling for HAART adherence [33]. Reviews by Kapadia et al [34] and Celentano and Lucas [35] suggested that while unstable patterns of opioid use and withdrawal may speed HIV progression [36], stable opioid administration (including in the context of OST) may slow disease progression; a finding consistent with those observed in our study. Taken together, these studies provide support for the impact of OST on HIV-related mortality, particularly immediately after OST discontinuation.
Second, the nonsignificant negative association of OST on drug-related death may be attributable to the known risks of death early in the treatment course [12, 37], though it may have simply been an artifact of the sample selection scheme (only HIV-positive PWID who had accessed HAART). Alternately, OST dosing dynamics (exceptionally high or low doses or rapid dose changes) may have had an influence on our findings. While dosing information was available, it could not be adequately incorporated into the episodic or monthly counting processes within the PH and marginal structural modeling frameworks we implemented to clearly define its effect; this remains a topic for future study.
Finally, while methodological development continues for statistical methods to handle competing risks data with time-varying confounders [38], our results were robust to multiple modeling techniques and model formulations, thus providing strong evidence supporting our key findings.
Despite the cited advantages of our study analyses, there are several limitations that require consideration. First, since we only had cause-specific death data for those accessing HAART, our analysis excludes HIV-positive PWID who accessed OST but did not access HAART throughout follow-up. Otherwise, our patient timeline, beginning at HAART initiation, excludes pre-HAART periods when individuals may have accessed OST. The selectivity of the study population and the time frame need to be taken into account when interpreting our results and determining whether, and to what extent, they may apply to other settings. Second, as with any observational study, there is potential for unmeasured confounding factors to influence our results. In our context, information on the breadth and frequency of illicit drug use over time, including illicit opioids, stimulants, and other drugs, likely drove results, particularly immediately after OST initiation and discontinuation. Otherwise, changes in HAART regimens were only partially controlled for with indication of calendar year of HAART initiation. Nonetheless, our results are indicative of mean patterns of mortality pertaining to HAART and OST access for the population under study. Third, some individuals included in the study on the basis of injection drug use history may have been exclusive nonopioid injectors, though epidemiological studies on PWID in British Columbia suggest this is unlikely [39]. Finally, our analyses on cause-specific mortality may have been subject to a degree of outcome misclassification [40]. If this misclassification is nondifferential, it would bias results toward the null hypothesis; in this context, cause-specific hazards would be attenuated toward the all-cause hazard ratio.
In a setting with universally covered HIV-related medical care and widely available office-based OST, we had previously demonstrated engagement in OST nearly doubles the odds of HAART adherence within HIV-positive PWID [18]. This population-level study further finds that both HAART and OST are independently negatively associated with mortality in this population; however, the risk of death was lowest when individuals were engaged in both forms of treatment. These findings call for renewed efforts to engage HIV-positive PWID into life-saving OST. Health systems should strive toward integrating the delivery of these medications where possible in order to optimize the individual and public health benefits of these treatments.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We acknowledge the administrative and editorial support of Michelle Olding. We acknowledge all BC Ministry of Health (BCMoH) and Vancouver Coastal Health Decision Support Staff involved in data access and procurement, including Monika Lindegger, Clinical Prevention Services, British Columbia Centre for Disease Control; Elsie Wong, Public Health Agency of Canada; Al Cassidy, BCMoH Registries; and Joleen Wright and Karen Luers, Vancouver Coastal Health decision support.
Financial support. This work was supported by the National Institutes of Health (NIH) and National Institute for Drug Abuse (R01-DA033424) as well as the BCMoH-funded ‘Seek and treat for optimal prevention of HIV & AIDS' pilot project.
B. N. is a Michael Smith Foundation for Health Research Scholar. V. D. L. is a Michael Smith Foundation for Health Research Scholar and Canadian Institutes of Health Research (CIHR) New Investigator. E. W. is supported with grants paid to his institution from the NIH, CIHR, MAC AIDS Fund and Open Society Foundation. J. S. G. M. is supported with grants paid to his institution by the British Columbia Ministry of Health and by the US NIH (R01DA036307 and 1DP1DA026182).
Potential conflicts of interest. J. S. G. M. has received limited unrestricted funding, paid to his institution, from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. L. Liu has consulted for Celladon, Outcome Research Solutions, and Zensum. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Degenhardt L, Bucello C, Mathers B et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta‐analysis of cohort studies. Addiction 2011; 106:32–51. [DOI] [PubMed] [Google Scholar]
- 2.Antiretroviral Therapy Cohort Collaboration; Zwahlen M, Harris R, May M et al. Mortality of HIV-infected patients starting potent antiretroviral therapy: comparison with the general population in nine industrialized countries. Int J Epidemiol 2009; 38:1624–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palella FJ, Baker RK, Moorman AC et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006; 43:27–34. [DOI] [PubMed] [Google Scholar]
- 4.Mellors JM, Kingsley LA, Rinaldo CR et al. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med 1995; 122:573–9. [DOI] [PubMed] [Google Scholar]
- 5.The HIV-CAUSAL Collaboration. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS 2010; 28:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J Subst Abuse Treat 2005; 28:321–9. [DOI] [PubMed] [Google Scholar]
- 7.Frishman WH, Del Vecchio A, Sanal S, Ismail A. Cardiovascular manifestations of substance abuse: part 2: alcohol, amphetamines, heroin, cannabis, and caffeine. Heart Dis 2003; 5:253–71. [DOI] [PubMed] [Google Scholar]
- 8.Herning RI, Better WE, Tate K, Umbricht A, Preston KL, Cadet JL. Methadone treatment induces attenuation of cerebrovascular deficits associated with the prolonged abuse of cocaine and heroin. Neuropsychopharmacology 2003; 28:562–8. [DOI] [PubMed] [Google Scholar]
- 9.Reece AS, Hulse GK. Lifetime opiate exposure as an independent and interactive cardiovascular risk factor in males: a cross-sectional clinical study. Vasc Health Risk Manag 2013; 9:551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reece AS, Hulse GK. Opiate dependence as an independent and interactive risk factor for arterial stiffness and cardiovascular ageing—a longitudinal study in females. J Clin Med Res 2013; 5:356–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caplehorn JRM, Dalton MSYN, Cluff MC, Petrenas AM. Retention in methadone maintenance and heroin addicts’ risk of death. Addiction 1994; 89:203–9. [DOI] [PubMed] [Google Scholar]
- 12.Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend 2009; 105:9–15. [DOI] [PubMed] [Google Scholar]
- 13.Inturrisi CE, Colburn WA, Kaiko RF, Houde RW, Foley KM. Pharmacokinetics and pharmacodynamics of methadone in patients with chronic pain. Clin Pharmacol Ther 1987; 41:392–401. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari A, Rosario Coccia CP, Bertolini A, Stemieri E. Methadone—metabolism, pharmacokinetics and interactions. Pharmacol Res 2004; 50:551–9. [DOI] [PubMed] [Google Scholar]
- 15.College of Physicians and Surgeons of British Columbia. Methadone Maintenance Program: Clinical Practice Guideline; Available at: https://www.cpsbc.ca/files/pdf/MMP-Clinical-Practice-Guideline.pdf. Accessed 26 June 2015. [Google Scholar]
- 16.Stephenson DK, California Society of Addiction Medicine. Guideline for physicians working in California Opioid Treatment Programs. Available at: http://www.csam-asam.org/sites/default/files/CSAMOTPGuideline21Apr09.pdf. Accessed 26 June 2015.
- 17.Roux P, Carrieri MP, Cohen J et al. Retention in opioid substitution treatment: a major predictor of long-term virological success for HIV-infected injection drug users receiving antiretroviral treatment. Clin Infect Dis 2009; 49:1433–40. [DOI] [PubMed] [Google Scholar]
- 18.Nosyk B, Min J, Colley G et al. The causal effect of opioid substitution treatment on highly active antiretroviral treatment adherence. Drug Alcohol Depend 2015; 146:e53–4. [Google Scholar]
- 19.Nosyk B, Colley G, Yip B et al. Application and validation of case-finding algorithms for identifying individuals with human immunodeficiency virus from administrative data in British Columbia, Canada. PLoS One 2013; 8:e54416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heath K, Samji H, Nosyk B et al. Cohort profile: seek and treat for the optimal prevention of HIV/AIDS in British Columbia (STOP HIV/AIDS BC). Int J Epidemiol 2014; 43:1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nosyk B, MacNab YC, Sun H et al. Proportional hazards frailty models for recurrent methadone maintenance treatment. Am J Epidemiol 2009; 170:783−92. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter CC, Fischl MA, Hammer SM et al. Antiretroviral therapy for HIV infection in 1996. Recommendations of an international panel. International AIDS Society–USA. JAMA 1996; 276:146–54. [PubMed] [Google Scholar]
- 23.Thompson MA, Aberg JA, Hoy JF et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society–USA panel. JAMA 2012; 308:387–402. [DOI] [PubMed] [Google Scholar]
- 24.Hogg RS, Heath K, Lima VD et al. Regional disparities in the burden of HIV/AIDS in Canada. PLoS One 2012; 7:e47260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneeweiss S, Maclure M. Use of comorbidity scores for control of confounding in studies using administrative databases. Int J Epidemiol 2000; 29:891–8. [DOI] [PubMed] [Google Scholar]
- 26.Schumacher M, Wangler M, Wolkewitz M, Beyersmann J. Attributable mortality due to nosocomial infections: a simple and useful application of multistate models. Methods Inf Med 2007; 46:595–600. [PubMed] [Google Scholar]
- 27.Allignol A, Schumacher M, Beyersmann J. Estimating summary functionals in multistate models with an application to hospital infection data. Comput Stat 2011; 26:181–97. [Google Scholar]
- 28.Beyersmann J, Allignol A, Schumacher M. Competing risks and multistate models with R. New York: Springer, 2012. [Google Scholar]
- 29.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009; 170:244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000; 11:550–60. [DOI] [PubMed] [Google Scholar]
- 31.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000; 11:561–70. [DOI] [PubMed] [Google Scholar]
- 32.Donahoe RM. Multiple ways that drug abuse might influence AIDS progression: clues from a monkey model. J Neuroimmunol 2004; 147:28–32. [DOI] [PubMed] [Google Scholar]
- 33.Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr 2009; 50:93–9. [DOI] [PubMed] [Google Scholar]
- 34.Kapadia F, Vlahov D, Donahoe RM, Friedland G. The role of substance abuse in HIV disease progression: reconciling differences from laboratory and epidemiologic investigations. Clin Infect Dis 2005; 41:1027–34. [DOI] [PubMed] [Google Scholar]
- 35.Celentano D, Lucas G. Optimizing treatment outcomes in HIV-infected patients with substance abuse issues. Clin Infect Dis 2007; 45:S318–23. [DOI] [PubMed] [Google Scholar]
- 36.Banerjee A, Strazza M, Wigdahl B, Pirrone V, Meucci O, Nonnemacher MR. Role of mu-opioids as cofactors in human immunodeficiency virus type 1 disease progression and neuropathogenesis. J Neurovirol 2011; 17:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornish R, Macloed J, Strang J, Vickerman P, Hickman M. Risk of death during and after opiate substitution treatment in primary care: prospective observational study in UK General Practice Research Database. BMJ 2010; 341:c5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekeart M, Vansteelandt S, Mertens K. Adjusting for time-varying confounding in the subdistribution analysis of a competing risk. Lifetime Data Anal 2010; 16:45–70. [DOI] [PubMed] [Google Scholar]
- 39.Kerr T, Werb D, DeBeck K et al. Drug situation in Vancouver (UHRI Report): Urban Health Research Initiative, British Columbia Centre for Excellence in HIV/AIDS, 2009. Available at: http://www.cfenet.ubc.ca/sites/default/files/uploads/news/releases/war_on_drugs_failing_to_limit_drug_use.pdf. Accessed 26 June 2015.
- 40.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One 2014; 9:e92286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.