Abstract
We systematically reviewed the literature to estimate the incubation and latent periods of Ebola virus disease. We found limited epidemiological data from individuals with discrete 1-day exposures. Available data suggest that the incubation and latent periods may differ, and mathematical models may be improved by distinguishing between the two periods.
Keywords: Ebola, incubation period, latent period, transmission, systematic review
The current Ebola outbreak in West Africa is unprecedented in size and geographic scope. Numerous mathematical models have been developed to capture the transmission dynamics and potential impact of interventions [1–7]. However, few reliable data exist on key epidemiologic features of Ebola virus disease (EVD), and most modeling parameters come from previous models or single empirical reports [8, 9]; these include time from infection to symptom onset (incubation period), time from infection to the onset of infectiousness (latent period), and duration of infectiousness. Here we systematically reviewed the literature on Ebola outbreaks to estimate these parameters.
METHODS
Definitions
Infectious Period
Following the US Centers for Disease Control and Prevention and the World Health Organization, which report that Ebola transmission occurs primarily by direct contact with infected secretions [10, 11], we defined the EVD infectious period as the duration of any of the following wet symptoms: vomiting, diarrhea, coughing, or hemorrhage. We assumed the infectious period ends upon resolution of wet symptoms or at the time of death, and we did not consider postmortem transmission.
Incubation Period
We defined incubation period as the time from an Ebola exposure to the onset of any EVD symptoms in a probable or confirmed case. In addition to wet symptoms, we noted onset of dry symptoms including fever, myalgia, headache, oropharyngeal lesions, nausea, abdominal pain, and rash.
Latent Period
We defined the latent period as the time from an Ebola exposure to the onset of wet symptoms in a probable or confirmed EVD case. Because few sources provided a direct estimate of this period, we also considered the sum of the incubation period and the time from onset of dry to wet symptoms.
Study Selection
This systematic review conforms to PRISMA guidelines [12]. The Supplementary Material provides detail on data sources, data extraction methods, and our search strategy (Supplementary Panel 1). We reviewed all titles and abstracts identified in the specified databases excluding articles that did not report primary data on the incubation or latent periods of EVD in humans. We performed full-text reviews of the remaining articles extracting information on the incubation and latent periods and the time from onset of dry to wet symptoms. We excluded observations without a reported time of onset of any symptoms (for incubation period) or of wet symptoms (for latent period). Among the subset of articles with information on timing of wet symptoms, we also extracted the infectious period.
We considered only studies that provided a clear case definition for EVD and information about the type and timing of Ebola exposures. Because the time of a transmission event is not known from multi-day exposures, we included only data from individuals with single-day exposures.
Statistical Analysis
We performed all analyses in MATLAB (MathWorks, Natick, Massachusetts). Where appropriate, we fit a normal distribution to the point estimates extracted from the literature, weighted by the observation frequency. We calculated the latent period by summing the mean incubation period and the mean time from onset of dry to wet symptoms. We calculated the variance of this composite value as the sum of the variances.
We performed a sensitivity analysis in which we included publications that provided estimates of the incubation period based on multi-day exposures or unspecified timing and type of exposure. We also performed a sensitivity analysis of infectious period in which we considered that infectiousness ended with the resolution of plasma viremia.
RESULTS
After reviewing the titles and abstracts of 1204 citations that met our search criteria, we excluded 1113 citations that did not contain primary data regarding transmission or clinical symptoms of EVD in humans and 3 citations for which the abstracts or full texts could not be located (Supplementary Figure 1). After a full-text review of the remaining 88 citations, we excluded 70 as detailed in Supplementary Table 4. Ten publications reported an incubation period, 2 reported a latent period, and 11 reported the time from the onset of dry symptoms to the onset of wet symptoms (Table 1). We were unable to quantify heterogeneity because the majority of publications did not report the parameter variance.
Table 1.
Ebola Virus Disease Incubation Period, Latent Period, and Time to Onset of Wet Symptoms
| Author | Incubation Period |
||||||
|---|---|---|---|---|---|---|---|
| Reference | Outbreak Location | Year | Species | Exposure Type | Value ± SD or (Range), Days | N | |
| Incubation period, percutaneous transmission | |||||||
| Breman et al; International Commission | [9, 13] | Zaire | 1976 | Zaire ebolavirus | Contaminated needle injection | 6.3 ± N/A (range 1–15) | 57 |
| Emond et al | [14] | Porton Down, England | 1976 | Not specified (Zaire ebolavirus or Sudan ebolavirus inferred) | Needlestick | 6 ± N/A | 1 |
| Heymann et al | [15] | Tandala, Zaire | 1977–1978 | Zaire ebolavirus | Laceration | 12 ± N/A | 1 |
| Khan et al | [16] | Kikwit, DRC | 1995 | Zaire ebolavirus | Contaminated needle injection | 3 ± N/A (range 1–6) | 11 |
| Mean incubation period (percutaneous route)a | 5.86 ± 1.42 | 70 | |||||
| Incubation period, person-to-person transmission | |||||||
| Breman et al; International Commission | [9, 13] | Zaire | 1976 | Zaire ebolavirus | Single contact | 2 ± N/A | 1 |
| Bwaka et al | [17] | Kikwit, DRC | 1995 | Zaire ebolavirus | Direct HCW contact | 6.2 ± N/A (range 5–8) | 5 |
| Bitekyerezo et al | [18] | Mbarara, Uganda | 2000 | Sudan ebolavirus | Crowded accommodation contact | 7 ± N/A | 1 |
| Leroy et al | [19] | Ndongo, DRC | 2007 | Zaire ebolavirus | Washed corpse | 8 ± N/A | 1 |
| Incubation period, animal-to-person transmission | |||||||
| Baize et al | [20] | Mayibout, Gabon | 1996 | Zaire ebolavirus | Consumed chimpanzee (decedents) | 7.8 ± 0.9 | 12 |
| Baize et al | [20] | Mayibout, Gabon | 1996 | Zaire ebolavirus | Consumed chimpanzee (survivors) | 8.4 ± 1.3 | 5 |
| Mean incubation period (non-percutaneous route)a | 7.34 ± 1.35 | 25 | |||||
| Incubation period, unknown route of transmission | |||||||
| Richards et al | [21] | Gabon/Johannesburg, RSA | 1996 | Zaire ebolavirus (inferred) | Assisted in CVC placement | 3 ± N/A | 1 |
| Mean incubation period (all routes of transmission)a | 6.22 ± 1.57 | 96 | |||||
| Latent Period |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Outbreak Location | Year | Species | Exposure Type | Initial Symptom(s) | Initial wet Symptom(s) | Value ± SD, days | N | |
| Emond et al | [14] | Porton Down, England | 1976 | Not specified (Zaire ebolavirus or Sudan ebolavirus inferred) | Needlestick | Fever, central abdominal pain, nausea | Diarrhea, vomiting | 9.5 ± N/A | 1 |
| Richards et al | [21] | Gabon/Johannesburg, RSA | 1996 | Zaire ebolavirus (inferred) | Assisted in CVC placement | Fever | Hematemesis, melena | 14 ± N/A | 1 |
| Mean latent perioda | 11.75 | 2 | |||||||
| Time to Onset of Dry to Wet Symptoms |
||||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Outbreak Location | Year | Species | Initial Symptom(s) | Initial wet Symptom(s) | Value ± SD, days | N | |
| International Commission; Isaacson et al; Sureau et al | [13, 22, 23] | Zaire | 1976 | Zaire ebolavirus | Fever, headache | Hematemesis | 6 ± N/A | 1 |
| Sureau et al | [23] | Zaire | 1976 | Zaire ebolavirus (inferred) | Fever, generalized pain, vomiting | Vomiting | 0 ± N/A | 1 |
| Sureau et al | [23] | Zaire | 1976 | Zaire ebolavirus (inferred) | Fever, lumbar pain, vomiting | Vomiting | 0 ± N/A | 1 |
| Emond et al | [14] | Porton Down, England | 1976 | Not specified (Zaire ebolavirus or Sudan ebolavirus inferred) | Fever, central abdominal pain, nausea | Diarrhea, vomiting | 3.5 ± N/A | 1 |
| WHO/International Study Team | [24] | Southern Sudan | 1976 | Not specified | Fever, headache, chest pain | Epistaxis, bloody diarrhea | 4 ± N/A | 1 |
| Richards et al | [21] | Gabon/Johannesburg, RSA | 1996 | Zaire ebolavirus (inferred) | Fever | Hematemesis, melena | 11 ± N/A | 1 |
| Shoemaker et al | [25] | Uganda | 2011 | Sudan ebolavirus | Mild headache | Vomiting | 4 ± N/A | 1 |
| Kreuels et al | [26] | Sierra Leone | 2014 | Zaire ebolavirus | Malaise, headache, myalgia, arthralgias | Vomiting, nonbloody diarrhea | 6 ± N/A | 1 |
| Lyon et al | [27] | Monrovia, Liberia | 2014 | Zaire ebolavirus | Fever, fatigue, nausea | Diarrhea, melena | 5 ± N/A | 1 |
| Lyon et al | [27] | Monrovia, Liberia | 2014 | Zaire ebolavirus | Fever, fatigue, malaise | Diarrhea | 8 ± N/A | 1 |
| Wolf et al | [28] | Sierra Leone | 2014 | Zaire ebolavirus | Fever, diarrhea | Diarrhea | 0 ± N/A | 1 |
| Lopaz et al | [29] | Spain | 2014 | Zaire ebolavirus | Fever, malaise | Vomiting, diarrhea | 7 ± N/A | 1 |
| Mean time to onset of dry to wet symptomsa | 6.05 ± 2.38 | 12 | ||||||
Abbreviations: CVC, central venous catheter; DRC, Democratic Republic of the Congo; HCW, healthcare worker; N/A, not applicable; RSA, Republic of South Africa; SD, standard deviation; WHO, World Health Organization.
a Combined parameter values were fitted to the normal distribution and weighted by observation frequency.
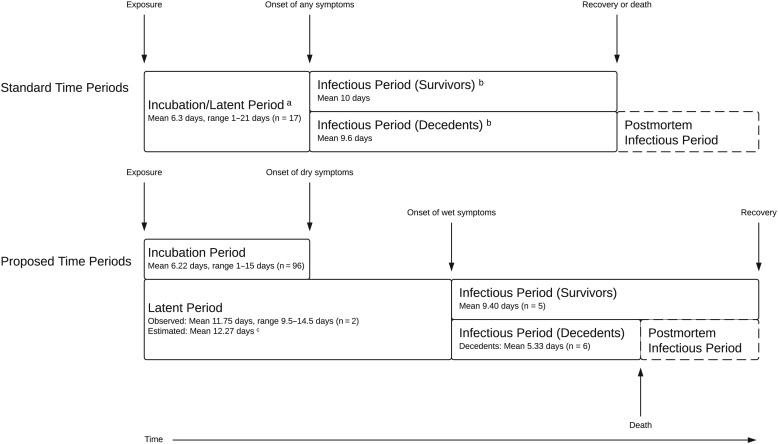
The mean incubation period was 6.22 ± 1.57 days for all routes of transmission (n = 96, Table 1). The 5 publications on the incubation period following percutaneous transmission yielded a mean of 5.86 ± 1.42 days (n = 70) and the 6 following person-to-person transmission or contact with infected animals yielded a mean of 7.34 ± 1.35 days (n = 25). The mean of the latent period in individual patients was 11.75 (Table 1). The mean time from onset of dry to wet symptoms was 6.05 ± 2.38 days (n = 12). When we summed this mean with the time from exposure to onset of dry symptoms, we estimated a mean latent period of 12.27 ± 2.85 days. Stratified by exposure type, we calculated a mean latent period of 11.91 ± 2.77 days after percutaneous exposure, and 13.39 ± 2.74 days after non-percutaneous exposure. From 9 publications that reported the duration of wet symptoms, we calculated a mean infectious period of 9.40 ± 5.50 days for EVD survivors (n = 5) and a mean time from onset of wet symptoms to death of 5.33 ± 4.03 days (n = 6, Supplementary Table 3). Figure 1 summarizes these findings.
Figure 1.
Standard vs proposed time periods for Ebola virus disease. aSource: [9];
bSource: [8]; cThe mean latent period was estimated by summing the mean time from exposure to onset of dry symptoms with the mean time from onset of dry to wet symptoms.
The sensitivity analysis of incubation period included 6 publications covering an additional 1189 patients (Supplementary Table 4) previously excluded due to multi-day exposures (n = 2), unspecified exposure type (n = 3), or unreported timing (n = 1). The mean incubation period for all routes of transmission was 10.07 ± 1.95 days (n = 1274), and the mean non-percutaneous incubation period was 10.32 ± 1.67 (n = 1203). The sensitivity analysis of infectious period including 5 publications reporting time from onset of wet symptoms to resolution of plasma viremia (Supplementary Table 3) yielded a mean infectious period of 14.00 ± 5.55 days among EVD survivors (n = 6).
DISCUSSION
We found limited data from individuals with discrete 1-day exposures with which to estimate the incubation and latent periods for EVD. A 1976 report on the first known Ebola outbreak gave an incubation period of 1 to 21 days, and this range has been used in many modeling studies [9]. In contrast, the studies that met our inclusion criteria provided support for a mean incubation period of 6.2 days with a range of 1 to 15 days. It is important to note that the standard errors we report are measures of uncertainty in the point estimate of each population mean and are not measures of the degree of individual-level variation in the population. The standard errors should not be used to infer the optimal length of quarantine during an Ebola outbreak.
Many modeling studies assume that the EVD latent period is equal to its incubation period [1, 5, 6]. The studies reviewed here suggest that dry symptoms precede wet ones by a mean of 6.1 days. This means that the incubation period likely underestimates the time to infectiousness, and that models using the incubation period as the latent period may capture a slower dynamic. More important, however, are our findings on the infectious period of EVD. The infectious periods used in many models have come from limited empirical data [2–6, 8], fitted estimates from prior models [1], or relied on models that estimated serial intervals rather than infectious periods [7]. If these estimates were based on empirical data on the time from onset of any symptoms until either death or clinical recovery, then the infectious periods may have been systematically overestimated.
Our stringent inclusion criteria represent a potential source of selection bias. The mean incubation period estimated in our sensitivity analysis was longer than that estimated in our primary analysis, consistent with our expectation that reports on multi-day exposures may overestimate the incubation period. Recall bias may introduce misclassification of the timing of exposure to EVD and the incubation period in either direction, as patients may recall exposures to known EVD patients more than with unrecognized infectious sources.
The assumption that Ebola is only transmitted from patients with wet symptoms may underestimate the infectious period if Ebola can be transmitted by fomites, droplets, or aerosols that hosts may generate when they have dry symptoms. Postmortem Ebola transmission is also well recognized, and therefore the duration of the infectious period is likely underestimated for decedents. When we considered the end of infectiousness to be the resolution of plasma viremia, this sensitivity analysis yielded a mean infectious period of 14.0 days in contrast to the 9.4 days until resolution of wet symptoms.
In summary, we demonstrate that limited epidemiological data from individuals with discrete 1-day exposures underpins estimates for the EVD incubation period used in the current modeling literature, and we summarized empirical data suggesting that the time to symptoms underestimates the time to infectiousness for EVD. These findings may have important implications for models of EVD intervention strategies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute on Drug Abuse, the National Institute of General Medical Sciences, or the National Institutes of Health (NIH).
Contributors. All authors conceived the study. G. E. V., O. A., E. J. L., E. Q. M., and I. D. conducted the literature review. All authors contributed to the writing of the manuscript.
Financial support. This work was supported by the NIAID [T32 AI007433 to G. E. V.]; the National Institute on Drug Abuse [T32 DA013911 to O. A.]; and the National Institute of General Medical Sciences [U54 GM088558 to E. Q. M.] at the NIH.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rivers CM, Lofgren ET, Marathe M, Eubank S, Lewis BL. Modeling the impact of interventions on an epidemic of Ebola in Sierra Leone and Liberia. PLoS Curr 2014; 6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewnard JA, Ndeffo Mbah ML, Alfaro-Murillo JA et al. . Dynamics and control of Ebola virus transmission in Montserrado, Liberia: a mathematical modelling analysis. Lancet Infect Dis 2014; 14:1189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandey A, Atkins KE, Medlock J et al. . Strategies for containing Ebola in West Africa. Science 2014; 346:991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med 2014; 371:1481–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meltzer MI, Atkins CY, Santibanez S et al. . Estimating the future number of cases in the Ebola epidemic—Liberia and Sierra Leone, 2014–2015. MMWR Surveill Summ 2014; 63(suppl 3):1–14. [PubMed] [Google Scholar]
- 6.Fasina FO, Shittu A, Lazarus D et al. . Transmission dynamics and control of Ebola virus disease outbreak in Nigeria, July to September 2014. Euro Surveill 2014; 19:20920. [DOI] [PubMed] [Google Scholar]
- 7.Fisman D, Khoo E, Tuite A. Early epidemic dynamics of the West African 2014 Ebola outbreak: estimates derived with a simple two-parameter model. PLoS Curr 2014; 6:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Legrand J, Grais RF, Boelle PY, Valleron AJ, Flahault A. Understanding the dynamics of Ebola epidemics. Epidemiol Infect 2007; 135:610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breman JG, Piot P, Johnson KM et al. . The epidemiology of Ebola hemorrhagic fever in Zaire, 1976. In: Pattyn SR, ed. Ebola virus haemorrhagic fever. Amsterdam, NY, USA: Elsevier/North-Holland Biomedical Press, 1978:103–24. [Google Scholar]
- 10.Review of Human-to-Human Transmission of Ebola Virus. U.S. Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/vhf/ebola/transmission/human-transmission.html. Accessed 26 February 2015.
- 11.Ebola virus disease. Fact sheet No. 103. Updated September 2014. World Health Organization; Available at: http://www.who.int/mediacentre/factsheets/fs103/en/ Accessed 26 February 2015. [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ 1978; 56:271–93. [PMC free article] [PubMed] [Google Scholar]
- 14.Emond RT, Evans B, Bowen ET, Lloyd G. A case of Ebola virus infection. Br Med J 1977; 2:541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heymann DL, Weisfeld JS, Webb PA, Johnson KM, Cairns T, Berquist H. Ebola hemorrhagic fever: Tandala, Zaire, 1977–1978. J Infect Dis 1980; 142:372–6. [DOI] [PubMed] [Google Scholar]
- 16.Khan AS, Tshioko FK, Heymann DL et al. . The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidémies à Kikwit. J Infect Dis 1999; 179(suppl 1):S76–86. [DOI] [PubMed] [Google Scholar]
- 17.Bwaka MA, Bonnet MJ, Calain P et al. . Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis 1999; 179(suppl 1):S1–7. [DOI] [PubMed] [Google Scholar]
- 18.Bitekyerezo M, Kyobutungi C, Kizza R et al. . The outbreak and control of Ebola viral haemorrhagic fever in a Ugandan medical school. Trop Doct 2002; 32:10–5. [DOI] [PubMed] [Google Scholar]
- 19.Leroy EM, Epelboin A, Mondonge V et al. . Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Dis 2009; 9:723–8. [DOI] [PubMed] [Google Scholar]
- 20.Baize S, Leroy EM, Georges AJ et al. . Inflammatory responses in Ebola virus-infected patients. Clin Exp Immunol 2002; 128:163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards GA, Murphy S, Jobson R et al. . Unexpected Ebola virus in a tertiary setting: clinical and epidemiologic aspects. Crit Care Med 2000; 28:240–4. [DOI] [PubMed] [Google Scholar]
- 22.Isaacson M, Sureau P, Courteille G, Pattyn SR. Clinical aspects of Ebola virus disease at the Ngaliema hospital, Kinshasa, Zaire, 1976. In: Pattyn SR, ed. Ebola virus hemorrhagic fever. Amsterdam, NY, USA: Elsevier/North-Holland Biomedical Press, 1978:15–20. [Google Scholar]
- 23.Sureau PH. Firsthand clinical observations of hemorrhagic manifestations in Ebola hemorrhagic fever in Zaire. Rev Infect Dis 1989; 11(suppl 4):S790–3. [DOI] [PubMed] [Google Scholar]
- 24.Ebola haemorrhagic fever in Sudan, 1976. Report of a WHO/International Study Team. Bull World Health Organ 1978; 56:247–70. [PMC free article] [PubMed] [Google Scholar]
- 25.Shoemaker T, MacNeil A, Balinandi S et al. . Reemerging Sudan Ebola virus disease in Uganda, 2011. Emerg Infect Dis 2012; 18:1480–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreuels B, Wichmann D, Emmerich P et al. . A case of severe Ebola virus infection complicated by Gram-negative septicemia. N Engl J Med 2014; 371:2394–401. [DOI] [PubMed] [Google Scholar]
- 27.Lyon GM, Mehta AK, Varkey JB et al. . Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med 2014; 371:2402–9. [DOI] [PubMed] [Google Scholar]
- 28.Wolf T, Kann G, Becker S et al. . Severe Ebola virus disease with vascular leakage and multiorgan failure: treatment of a patient in intensive care. Lancet 2015; 385:1428–35. [DOI] [PubMed] [Google Scholar]
- 29.Lopaz MA, Amela C, Ordobas M et al. . First secondary case of Ebola outside Africa: epidemiological characteristics and contact monitoring, Spain, September to November 2014. Euro Surveill 2015; 20:1–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.