Abstract
Based on studies in rodents, the basolateral amygdala (BLA) is considered a key site for experience-dependent neural plasticity underlying the acquisition of conditioned fear responses. In humans, very few studies exist of subjects with selective amygdala lesions and those studies have only implicated the amygdala more broadly leaving the role of amygdala sub-regions underexplored. We tested a rare sample of subjects (N = 4) with unprecedented focal bilateral BLA lesions due to a genetic condition called Urbach–Wiethe disease. In a classical delay fear conditioning experiment, these subjects showed impaired acquisition of conditioned fear relative to a group of matched control subjects (N = 10) as measured by fear-potentiation of the defensive eye-blink startle reflex. After the experiment, the BLA-damaged cases showed normal declarative memory of the conditioned association. Our findings provide new evidence that the human BLA is essential to drive fast classically conditioned defensive reflexes.
Keywords: amygdala, basolateral, startle, fMRI, fear conditioning, lesion
INTRODUCTION
Fear conditioning serves as a successful framework to study the neurobiological substrates underlying the acquisition of fear responses (Davis and Whalen, 2001; LeDoux, 2003). Elucidating neural mechanisms of conditioned fear acquisition might contribute to understanding and treatment of anxiety disorders (Lissek et al., 2005; Mineka and Oehlberg, 2008) one of the most prevalent categories of psychiatric disorder (Kessler et al., 2005).
A vast literature of lesion, electrical stimulation and pharmacological studies performed in the rodent points to the basolateral amygdala (BLA) as playing a key role in conditioned fear acquisition (Davis and Whalen, 2001; LeDoux, 2003). These studies indicate that the BLA integrates sensory information regarding threats and their predictors and stores a fear memory through a cascade of neuroplasticity mechanisms (Fanselow and LeDoux, 1999; Johansen et al., 2011). Given that select lesions to the human amygdala are exceedingly rare, the causal role of the amygdala has mainly been investigated through studies with patients suffering from broader lesions encompassing the amygdala (LaBar et al., 1995; Weike et al., 2005). Key evidence came from the study of a single individual with a rare genetic syndrome: Urbach–Wiethe disease (UWD). Near-complete bilateral amygdala calcification due to this disease was found to be associated with a lack of fear-conditioned skin conductance responses in this subject (Bechara et al., 1995). Recent studies showed that large individual differences in conditioned fear acquisition exist within the general population (Weike et al., 2005; Baas et al., 2008; Indovina et al., 2011). Therefore, it remains an important question whether these findings in a single subject generalize to other cases with specific amygdala lesions. A second open question concerns the contribution of human amygdala sub-regions to fear conditioning. Anatomical studies demonstrated that just as in the rodent, primate amygdala sub-regions are distinct in cellular anatomy, connectivity and function (McDonald, 1998; Price, 2003), yet no lesion studies have been able to evaluate effects of more focal lesions to the primate BLA without clear damage to neighbouring subregions.
Here, we investigate the select contribution of the BLA to human fear learning by testing a unique sample of four healthy UWD cases, selected for their specific bilateral lesions to the BLA, in a classical delay fear-conditioning paradigm. Conditioned fear levels were assessed by electromyographic recordings of the startle reflex—a defensive behaviour that reliably increases in magnitude during fear states (Lang et al., 1990; Grillon and Baas, 2003; Weike et al., 2005). Moreover, we aimed to specifically assess the uninstructed, experience-driven acquisition of fear, which has been suggested to be amygdala-dependent in both animal (Davis and Whalen, 2001; Johansen et al., 2011) and human work (Coppens et al., 2009). Therefore, there were no explicit instructions concerning the contingencies between conditioned and unconditioned stimuli, nor did we ask for concurrent ratings of these contingencies during the conditioning training. While this creates more challenging learning conditions that reduce overall fear acquisition levels, this design might be optimal for detecting experience-dependent fear acquisition impairments (Weike et al., 2005; Lissek et al., 2006; Coppens et al., 2009).
MATERIALS AND METHODS
This study was approved by the Health Sciences Faculty Human Research Ethics Committee of the University of Cape Town. All participants gave written informed consent.
Subjects
The four female UWD cases described here are part of an earlier described cohort (Thornton et al., 2008). UWD is a rare genetic syndrome that has been traced to mutations in the extracellular matrix protein 1 (ECM1) gene on chromosome 1 (1q21) and is inherited in an autosomal recessive manner. Three of the four subjects described here (identified as UWD 1-3) were previously reported on in papers focussing on working memory performance (Morgan et al., 2012), acute fear vigilance (Terburg et al., 2012) and social-economic descision making (van Honk et al., 2013). A fifth UWD case (previously described as UWD4) was also tested for the current study. This case was excluded from the final analyses because discernible startle responses were recorded in less than 30% of trials. Skin abnormalities associated with UWD, markedly present in hyperkeratotic form in UWD 4, may have disrupted the recordings (Buchanan et al., 2004; Thornton et al., 2008). Ten female subjects selected from the same geographical region in the Northern Cape of South Africa served as a control group for the analyses on fear-potentiated startle (FPS). Drawn from the same population, these subjects were carefully matched to the UWD cases in terms of age, IQ, ethnic origin (mixed Western European and Indigenous Nama/Khoesan) as well as other demographic characteristics (Morgan et al., 2012); descriptives in Table 1.
Table 1.
Mean age, intelligencea scores and raw startle amplitudes (s.d.) for the UWD and control sample
| UWD | Control | |
|---|---|---|
| Sample size | 4 | 10 |
| Age (years) | 32.2 (4.3) | 31.1 (7.0) |
| Verbal IQ | 89.3 (6.9) | 87.8 (4.8) |
| Performance IQ | 89.5 (6.4) | 86.0 (2.7) |
| Full scale IQ | 88.0 (6.9) | 85.2 (2.6) |
| Mean startle amplitude during habituation (µV) | 129.1 (102.0) | 53.5 (33.9) |
| Mean startle amplitude to aversive scream (µV) | 34.4 (34.4) | 55.5 (49.8) |
aIQ scores are derived from the Wechsler Abbreviated Scale of Intelligence (WASI). Scores of UWD and control subjects are within the normal range [for details see Morgan et al. (2012)].
Structural and functional demarcation of lesions
A high-quality, T2-weighted, whole brain anatomical scan from a Siemens Magnetom Allegra 3-Tesla head-only scanner was used to identify the lesions (1 mm isotropic resolution, TR = 3500 ms and TE = 354 ms). To allow the creation of a lesion overlap image, anatomical scans were then transformed into a common metric space using the unified normalization procedure implemented in SPM5 (http://www.fil.ion.ucl.ac.uk/spm; RRID:nif-0000-00343), which was found to operate robustly in the presence of lesions (Mineka and Ohman, 2002). Subsequently, lesion extent was quantified using the 3D volume of interest tool featured in MRIcroN (http://www.cabiatl.com/mricro/mricron). A detailed analysis of the damage relative to anatomically defined medial temporal lobe (MTL) regions was performed using the normalized cytoarchitectonic probability maps available in the SPM anatomy toolbox (Eickhoff et al., 2007). Specifically, we extracted mean cytoarchitectonic probability of the lesion voxels in each anatomical MTL region. The probability values fluctuate between 0 and 1 for a given voxel, therefore high mean probabilities across voxels indicate high certainty for overlap with a given structure because the lesion is primarily located in areas with high anatomical consistency across subjects. In this way, this method allows quantitative assessment of the probability that sub-regions of the MTL overlap with the lesion site.
To assess the impact of the lesions on basic functionality of amygdala sub-regions, the UWD cases were asked to perform a standard emotional face matching task (Hariri et al., 2002) during fMRI scanning (cf. Morgan et al., 2012; Terburg et al., 2012). In short, subjects were asked to match the emotional expression of two faces presented at the bottom of the screen to an example face presented on top. A sensorimotor control task was also included, consisting of matching the orientation of two geometric shapes at the bottom of the screen to a template shape on top. Neural activity during a total of four 30-s blocks of emotional face matching was contrasted with neural activity during five interleaved 30-s blocks of the sensorimotor control task. Each block contained six trials that each lasted 5 s. Functional whole brain 2D-EPI MRI scans were obtained (36 slices in interleaved-ascending order, 3.5 mm isotropic resolution, flip angle = 70°, TR = 2000 ms, TE = 27 ms and EPI factor = 64). For each participant, all functional scans were realigned to the first scan, co-registered to the structural T2-weighted scan and normalized using the segmentation parameters obtained from the T2-weigthed scan. No smoothing was applied to preserve spatial resolution. To assess whether subregions of the amygdala showed significant activation, signal change relative to the mean recorded activity was extracted using the MARSBAR toolbox (Brett et al., 2002). The amygdala subregions were defined by the cytoarchitectonic atlas by taking all voxels exceeding a 50% probability threshold for that region. The extracted mean activity (emotion matching vs control) was tested against zero to test which regions still show conserved functionality. To further explore and visualize the anatomical distribution of potential remaining activations in the amygdala, functional images were subsequently also subjected to a voxelwise random effects analysis in SPM. Given our small sample for such analyses, we present the voxelwise analyses with a liberal statistical threshold of P < 0.05 (uncorrected). This analysis should be seen as a confirmation and illustration of the analyses on the extracted data.
Conditioning stimuli
To serve as conditioned stimuli, two pictures of neutral male Caucasian faces were taken (PICS, http://pics.stir.ac.uk/), one coloured blue and one coloured yellow. During the acquisition training phase, one of the faces (the CS + ) was always followed by the unconditioned stimulus. Face identity of the CS + was counterbalanced across subjects. The unconditioned stimulus was presented 5500 ms after CS + onset and consisted of an aversive 100 dBA fearful female scream presented through headphones (Lissek et al., 2005; Massar et al., 2011). The other face (CS-face) was never followed by the unconditioned stimulus. A third stimulus served as a background control stimulus (CS-scrambled). This stimulus consisted of a black and white scrambled image of the same size as the faces and was therefore easy to discriminate from the two faces. Following each 6-s face presentation, the scrambled image was presented for variable durations (3.5–31 s). This CS-scrambled serves as a control for non-associative changes in startle amplitude over the course of the experiment due to habituation or sensitization. Startle reflexes were probed during each of the three stimuli by presenting 105 dB(A) bursts of 50-ms white noise with near instantaneous rise time. Startle probes were presented at 4000 or 5000 ms after onset of the face stimuli or at semi-random moments during the presentation of the scrambled image. Inter startle intervals were programmed to be 17–23 s with a mean of 20 s for each of the conditions (CS+, CS-face, CS-scrambled). To rule out confounding effects of the scream on subsequent startle reactions, the minimal interval to the next startle probe after a scream was also at least 17 s calculating from the scream. Pictures and startle probes were presented in a semi-random order designed to distribute the three conditions equally over time.
Conditioning procedure
Instructions
Before the start of the experiment, subjects were instructed that pictures and loud sounds would be presented. No instructions whatsoever were given regarding the CS-US contingencies; subjects were instructed to refrain from large movements and to keep watching the pictures on the screen.
Habituation
Subsequently, to habituate subjects to the startle probing procedure and index baseline startle levels, a series of 12 startle probes were presented while showing a fixation cross on the screen.
Preconditioning
After the habituation phase, subjects were presented with four presentations of each face stimulus. In this phase, none of the pictures was followed by the scream. For each condition (CS+, CS-face, CS-scrambled), three startle probes were delivered.
Acquisition training
After the preconditioning phase, subjects were informed that screams could be presented in the following phase. This acquisition training phase subsequently consisted of 12 presentations of each face. During this training phase, the CS+was always followed by the scream. The other pictures were never followed by the scream. In each condition, nine startle probes were delivered.
Conditioning test
Immediately following the acquisition training, the conditioning test phase began. In this phase, 12 presentations of each face, all without the scream, were presented. Again, nine startle probes were delivered in each condition to assess conditioned responding in the absence of the scream.
Awareness check
After the conditioning test phase, the subjects were asked whether they could predict the scream in any way. Following a positive response, the CS+, CS-face and CS-scrambled pictures were presented all together and subjects were asked to indicate which of the three pictures predicted presentation of the scream.
Startle reflex recording and processing
Electromyographic recording of the startle reflex was carried out using the Biosemi Active Two system (www.biosemi.nl) with Ag-AgCL electrodes positioned over the orbicularis oculi muscle below the right eye. One electrode was located below the pupil and the other ± 15 mm towards the lateral canthus of the eye. Startle data were pre-processed according to previously published guidelines (Blumenthal et al., 2005) and blind relative to participant’s identity (UWD/control). In brief, startle data were segmented, bandpass filtered (28–500 Hz, 24 dB/oct), rectified, smoothed and baseline corrected in Brainvision Analyzer (Brainproducts.com; RRID:nlx_155717). The highest peak in the resulting signal was taken as the amplitude of the response. Consistent with previous work (e.g. Klumpers et al., 2010), data were checked for artefacts such as spontaneous blinks and movement in the analysis window in a custom-built semi-automatic processing pipeline using Matlab (The Mathworks; RRID:nlx_153890). Trials with excessive activity in the 50-ms baseline period immediately preceding the response (exceeding the mean baseline activity for that subject by more than 2 standard deviations) were scored as missing values. Also trials with peak amplitude latencies outside the normal range (25–115 ms post startle probe) were set to missing. Trials that showed a less than 10% increase in standard deviation relative to a 50-ms baseline immediately preceding the response were scored as null responses. With these criteria, all subjects in the final analyses showed at least three artefact-free, non-zero responses per condition for each phase. Raw mean startle amplitudes during the habituation phase and during presentation of the scream were used to characterize unconditioned startle amplitude. Peak amplitudes from all trials in the pre-conditioning, conditioning and post-conditioning phases together were converted into T scores (T = z*10 + 50) per subject, so that individual differences in baseline startle would not confound the results (Blumenthal et al., 2005; Weike et al., 2005; Klumpers et al., 2010). In line with previous work (e.g. Weike et al., 2005; Klumpers et al., 2012a), fear responses to the CS+ are quantified by FPS and CS discrimination. Here, FPS reflects the more easy to learn contrast between CS+ and CS-scrambled, whereas CS discrimination assesses the ability to also discriminate between the two faces (CS+ and CS-face).
Statistical analysis
Apart from the voxel-wise functional MRI analyses, all statistical analyses were carried out in SPSS 21 (RRID:rid_000042). Two-tailed one-sample t-tests were used to assess amygdala differential BOLD signal changes (emotion > control) across the UWD sample against zero. Similar two-tailed one-sample t-tests were used in the conditioned fear assessment to test for significant FPS (the contrast between CS+ and CS-scrambled) and CS discrimination (the contrast between CS+ and CS-face) across all participants. As in all earlier work on these small patient samples (Morgan et al., 2012; Terburg et al., 2012; van Honk et al., 2013), two-tailed, non-parametric, independent samples Mann–Whitney U tests were conducted to provide the critical comparisons between patients and controls without assumptions about the normality of distributions. Post-hoc two-tailed one-sample t-tests were used to assess FPS and CS discrimination within each group only in case significant differences between groups in either FPS or CS discrimination were detected to limit the number of statistical comparisons. Finally, Fisher’s exact test was performed to compare the groups for differences in the classification of subjects as either aware or unaware of the contingency between CS+ and UCS as assessed by our awareness check.
RESULTS
Structural and functional lesion demarcation
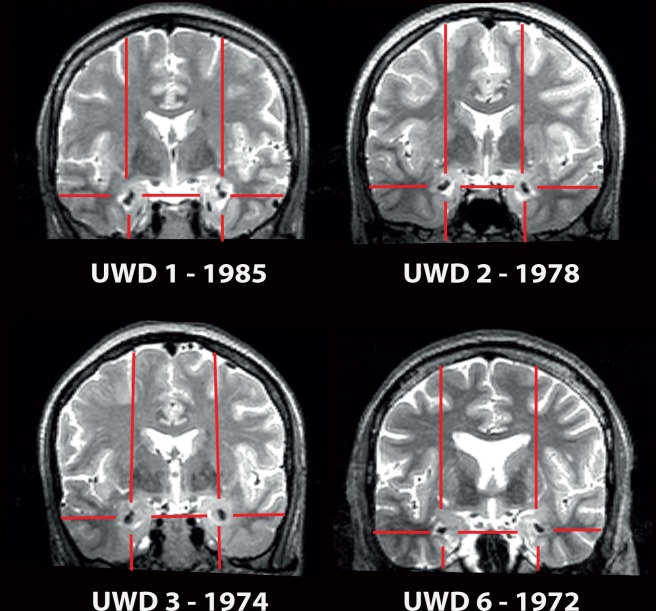
The anatomical MR images showed that the typical calcifications associated with the genetic mutation are in these four UWD cases restricted to the BLA region (see Figure 1 for raw images). In the absence of clearly visible anatomical borders between amygdala sub-regions on MRI images, we mapped each individual’s lesion onto probability maps of cytoarchitectonic MTL sub-regions identified by histological analysis of post-mortem brains (Amunts et al., 2005; Eickhoff et al., 2007). This demonstrated that the lesions in these four UWD cases were all bilaterally centred in the BLA and showed minimal overlap with other regions (Figure 2a).
Fig. 1.
Lesion location in each of the UWD cases in coronal views of a T2-weighted MR scan. For consistency, the cases are identified as in previous publications (UWD1-3, UWD6 and year of birth). Crosshairs indicate calcified brain tissue due to the genetic mutation.
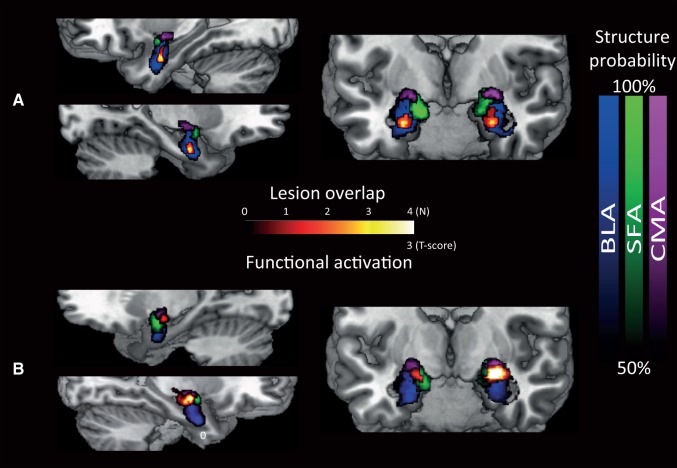
Fig. 2.
Lesion location (a) and spared functional activity (b) relative to cytoarchitectonically defined sub-regions of the amygdala. The images show voxels with a higher than 50% probability of belonging to the BLA (blue) or centromedial (violet) and cortical (green) nuclei projected on an anatomical template image. Colour coding (red to yellow) indicates the overlap in lesions across the four UWD cases in (a) and functional activation in (b). Lesions are for all subjects bilaterally centred in the BLA (a). Functional sparing is evident in dorsal amygdala nuclei (b) as confirmed by significant elevations in mean fMRI BOLD signal change for the regions outside the lesion (see text). To obtain a frank view of any remaining activity in this small sample of four UWD cases, fMRI results are shown here using a liberal statistical threshold of P < 0.05 (uncorrected) for illustrative purposes.
A quantitative probability analysis further confirmed focal BLA damage in all cases. On average, the cytoarchitectonic probability of lesion voxels in the BLA was 83% across participants and hemispheres, meaning that the lesions were located in areas with a high probability to be judged as BLA based on the probability distribution derived from the histological analysis. For all other sub-regions, mean values were lower than 25%, indicating that probabilities of damage outside the BLA were small (Figure 3). Particularly, the CMA subregion appears unaffected in each subject (probabilities < 10%). Thus, these results indicate selective structural damage in the BLA in all subjects, which is unlikely to extend significantly into surrounding regions.
Fig. 3.
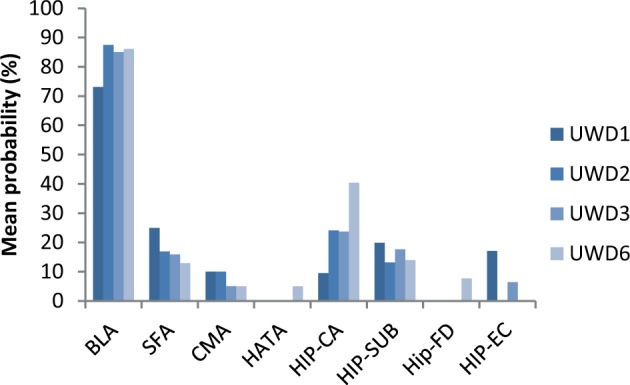
Lesion location in each UWD case quantified in terms of average probabilities for overlap with each bilateral cytoarchitectonically defined sub-region of the MTL. Although based on probability maps, these data provide strong evidence that the lesions for each UWD case were centred in the BLA and across subjects showed only minimal overlap with other MTL sub-regions if any. BL, basolateral; SF, superficial; CM, centromedial; CA, cornu ammonis; SC, subicular cortex; EC, enthorinal cortex; HA, hippocampal-amygdaloid transition area.
Next we assessed remaining functionality of the amygdala sub-regions by testing mean activity per sub-region, while the UWD cases were matching the emotional expression of faces in the MR scanner. Matching emotional faces produced significant increases in mean BOLD signal across the centromedial (CMA) and superficial amygdala (SFA) regions (M = 0.38, t(3) = 4.5, P = 0.02). There was also a significant signal increase in the bilateral BLA (M = 0.26, t(3) = 11.8, P = 0.001) although as expected not in the lesioned region (defined as all voxels where at least one patient had damage) (M = 0.17, t(3) < 1). Confirming these results, an exploratory voxel-wise analysis revealed that amygdala activation was restricted to the dorsal amygdala including the CMA/SFA and potentially the most dorsal parts of the BLA but not the lesioned region (Figure 2b). Thus, we provided evidence for BOLD activity in non-calcified amygdala sub-region tissues surrounding the lesion. In sum, as previously shown for a sub-sample of three cases (Morgan et al., 2012; Terburg et al., 2012), these UWD cases exhibit selective lesions to the BLA while showing spared functionality of neighbouring amygdala sub-regions.
Fear conditioning
Startle results
Subsequently, we assessed whether these focal BLA lesions affected the experience-dependent acquisition of fear through Pavlovian conditioning. Groups did not differ in average startle reflex amplitudes during habituation to the brief bursts of loud noise (Mann–Whitney test U = 28, P = 0.30; Table 1). In the subsequent preconditioning phase, before pairing the CS+ with the scream, the amplitude of startle responses measured during the designated CS+ did not differ from responses recorded during CS-face or CS-scrambled trials, independent of the presence of amygdala lesions (P values ≥ 0.10).
During the acquisition training, groups did not differ in average unconditioned startle reactions to the scream (U = 16, P = 0.57; Table 1). Likely because fear acquisition was somewhat slow to develop, there was no difference in mean startle amplitudes during the overall acquisition training (Figure 4). During the conditioning test phase immediately after acquisition training, there was no significant conditioned startle discrimination (CS+ vs CS-face) across participants, without differences between groups (U = 12, P = 0.30) indicating that neither group consistently learned to discriminate between the face stimuli (Figure 5). However, a potentiation of startle amplitudes measured during CS+relative to CS-scrambled trials (FPS) was apparent when testing across control and UWD participants (t(13) = 2.0, P = 0.07). Crucially, FPS was significantly smaller in subjects with BLA lesions (BLA damage vs Controls: U = 5, P = 0.03; Figure 5). Post-hoc tests demonstrated that BLA-lesioned subjects showed no FPS (t(3) < 1), whereas controls showed the expected potentiation of the defensive startle response during CS+ trials relative to CS-scrambled trials (t(9) = 3.7, P = 0.03) (Figure 5). Further tests of the specificity indicated that, when contrasting the groups directly on reactions to the three stimuli, the amygdala-lesioned cases showed specifically reduced startle reactions to the CS+ compared with the controls (U = 5, P = 0.03) without differences between groups in responses to the CS-face or CS-scrambled trials (U = 12, P = 0.30 and U = 17, P = 0.67 respectively). Exploring the time course of conditioned startle potentiation revealed that this result was not due to a potential difference in extinction learning, but due to a slowly rising conditioned response level in the healthy controls that was absent in the UWD cases (Supplementary Figure S1). In sum, our fear conditioning procedure produced significant potentiation of the startle reflex in the control group during the CS+, albeit only for the contrast with the scrambled background stimulus. Compared with the matched controls, the UWD cases showed a selective reduction in startle response during the CS+ stimulus following fear conditioning.
Fig. 4.
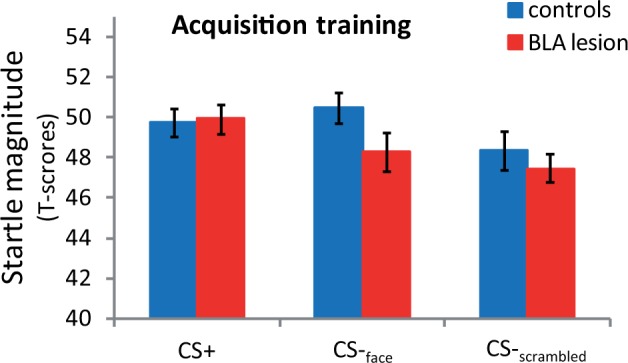
Mean startle amplitudes for BLA-damaged subjects (BLA; N = 4) and healthy controls (HC; N = 10) during the acquisition training phase. Concatenating across all conditioning training trials, there was no significant difference between startle amplitudes measured during CS+ compared with CS-scrambled or CS-face trials (t-test P values > 0.12). There was also no reliable difference between UWDs and controls in potentiation to the CS+ during the training phase (Mann–Whitney U P values ≥ 0.10). Error bars represent standard error of the mean (SEM).
Fig. 5.
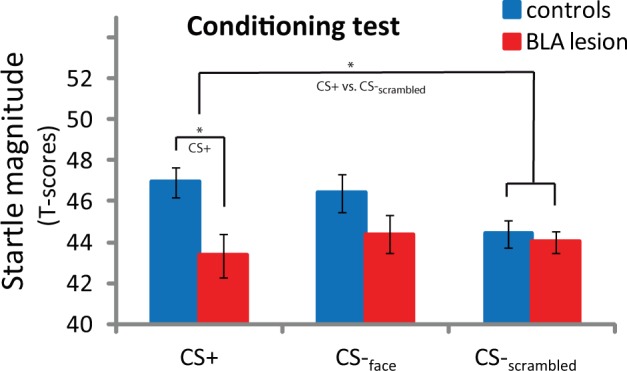
Mean startle amplitudes for BLA-damaged subjects (BLA; N = 4) and healthy controls (N = 10) during the conditioning test. Significant differences in startle potentiation (CS+ vs CS-scrambled) emerged after the acquisition training, driven by significantly higher startle amplitude during presentation of the CS+ in controls. Error bars represent SEM. *P < 0.05.
Post-experiment contingency awareness
After the experiment, 5 out of 10 control subjects (50%) correctly selected the CS+ out of the three possible stimuli after the experiment. In line with the inconsistent startle discrimination between face stimuli in both groups, all incorrect subjects indicated that the CS-face predicted the scream except for one control subject who indicated the CS-scrambled. Interestingly, BLA-damaged subjects appeared unimpaired in contingency knowledge. Of the BLA-damaged subjects, three out of four subjects correctly identified the CS+ (75%—only UWD3 indicated CS-face), which was not statistically different from controls (Fisher’s exact test, P = 0.58). Thus, together with the startle data, these data confirm that while almost all subjects learned that the faces predicted the scream, they found it difficult to indicate which face. More interesting, these data indicate that the BLA-damaged cases exhibited an intact ability to obtain explicit knowledge on the association between conditioned and unconditioned stimuli relative to the matched control sample.
DISCUSSION
We studied the neural mechanisms underpinning the acquisition of human fear through classical conditioning, an important pathway to the development of fear reactions in everyday life and disease. Although numerous rodent studies have shown that conditioned fear acquisition is dependent on the BLA, it remained unclear whether this crucial insight translates to the human species. We addressed this question in a rare selected group of UWD cases with focal selective lesions of the BLA. Four UWD cases with, to our knowledge, the most selective human BLA lesions ever described showed a specific reduction in fear-potentiation of the startle response to a fear-conditioned stimulus compared with a group of matched control subjects. These results provide new support for theories supposing a causal role for the human BLA in conditioned fear acquisition (Fanselow and LeDoux, 1999; Davis and Whalen, 2001; Maren and Quirk, 2004).
We used T2-weighted MRI scans to map the lesions in the UWD cases to amygdala subregions. Previous studies investigating fear conditioning in amygdala-damaged patients relied on qualitative, sometimes unreported criteria to define amygdala damage (LaBar et al., 1995; Bechara, 2004; Weike et al., 2005; Coppens et al., 2009), with lesions displayed on selected MR images. Our study aimed to provide a quantification of the anatomical specificity of lesions with respect to anatomical subregions of the MTL through objective criteria and a replicable approach. As with any lesion, qualification procedure based on MR images, and due to a lack of clearly visible anatomical boundaries, the accuracy of our procedure is limited by (i) the resolution of the MR scans and (ii) inter-individual differences in anatomy. Given the resulting uncertainty in defining the amygdala subregions, we calculated the average certainty for all lesion voxels to belong to a particular subregion, reflecting the centrality of the lesion relative to that area. To this end, we utilized the estimates of the inter-individual anatomical variance as reported in a cytoarchitectonic atlas (Amunts et al., 2005; Eickhoff et al., 2007). With this objective method, we show that the likelihood for the lesions being located in the BLA is consistently high in all subjects, whereas across subjects the likelihood is low to very low for other regions. Although these probability estimates cannot provide absolute certainty that only the BLA is affected, they provide strong and objective evidence of highly specific lesions to the BLA.
Remaining responsiveness of the non-lesioned amygdala subregions was assessed using functional MRI. For these analyses, we extracted mean signal for each anatomical region defined as all voxels showing more than 50% probability to belong to the region. The lesioned region showed no indications of functionality, as expected from calcified tissue; however, we observed evidence for spared activity in dorsal amygdala regions as was confirmed in a voxelwise analysis. Because the functional scans are of considerably lower resolution than the anatomical scans (3.5mm3 vs 1mm3), it is difficult to discern which regions were spared exactly. Nevertheless, the remaining amygdala tissue, particularly the CMA which is considered the output region of the amygdala (Davis and Whalen, 2001; Kalin et al., 2004; Jimenez and Maren, 2009), appears to retain basic functionality in spite of the lesions in the BLA.
Considering our fear conditioning results, a limitation to this study is that there was no significant startle discrimination as assessed relative to the second face (CS-face), which provides a more specific measure of conditioning. In the current sample, with our uninstructed fear conditioning paradigm without concurrent ratings, we did not observe such discrimination in either group and therefore could not assess whether the BLA-damaged group was impaired in this learning. However, we did observe significant potentiation of the startle reflex during the CS+ compared with the scrambled stimulus in the control group. The scrambled stimulus provided an additional control to assess whether any observed differences between groups might be due to non-associative, stimulus-unspecific changes in startle amplitude. This stimulus was not the most conservative control given that it differed from the CS+ both in appearance and duration. Importantly, however, our main finding of fear-conditioned startle impairment in the BLA-damaged group was driven specifically by reduced reactions to the CS+ trials in BLA-damaged cases. There were no differences in reactions to the other two stimuli and this impairment appeared only after the CS+ had been paired with the aversive UCS. With significant differences between the groups in FPS, specifically caused by reduced startle reactions to the CS+ in the BLA-damaged group following conditioning, these data provide unique first evidence from a human lesion study indicating a causal role for the human BLA in Pavlovian fear acquisition.
The rather selective fear-conditioned startle deficit we observed in these BLA-damaged subjects suggests a specific contribution of the BLA to the distributed neurobiology underlying emotional responses. In line with a sparing of hippocampal regions (Bechara et al., 1995; Clark and Squire, 1998; Weike et al., 2005), BLA-damaged cases exhibited an intact ability to obtain explicit knowledge on the association between conditioned and unconditioned stimuli. After the experiment, the UWD cases performed similarly to matched controls when asked to indicate the threat-predicting cue. Thus, the human BLA does not appear to be required for the experience-dependent acquisition of declarative knowledge concerning contingent threats but appears to be essential for the coupling of fear memories to fast defensive reflex physiology.
Second, we observed no significant alterations in unconditioned defensive responses. General startle reactions to the auditory startle probe and to the aversive scream were highly variable between subjects but not significantly altered after BLA damage, and as in rodents likely depend on the CMA and brainstem (Davis et al., 1993; Davis and Whalen, 2001). This dissociation suggests that, just as in non-human primates (Antoniadis et al., 2007, 2009), the human amygdala, and particularly the BLA, plays a crucial role in developing fear responses to conditioned threats without being indispensable for the expression of unconditioned fear (Feinstein et al., 2013).
This dissociation, between impaired conditioned startle potentiation on the one hand and unimpaired unconditioned defensive reactions and associative knowledge acquisition on the other hand, provides support for multi-level accounts of fear conditioning (Weike et al., 2005; Adolphs, 2013). Our results suggest that fear is represented in multiple anatomical substrates that to some extent independently support unconditioned defensive reactions, cognitive awareness of threats and fast conditioned fear reactions.
Consistent with earlier work, large inter-individual differences were also observed in control subjects’ ability to acquire conditioned startle potentiation. This individual variation might reflect diversity in attentional processes (Mackintosh, 1975) and amygdala reactivity (Indovina et al., 2011) both potentially originating in genetic variance (Lonsdorf et al., 2009; Klumpers et al., 2012b). Similarly, a substantial proportion of control subjects (50%) did not correctly indicate the threat-predicting stimulus at the end of the study. Evidently, our choice to not have any explicit prior indications signalling the importance of the contingencies to subjects established relatively challenging learning conditions. We cannot rule out that more favourable training conditions might have resulted in normalized startle potentiation in the presence of BLA lesions. Indeed, when provided with additional training trials, BLA-lesioned rodents acquire normal defensive responses through the involvement of slower learning systems in the brain (Maren, 1999; Poulos et al., 2009). However, three out of four BLA-lesioned subjects were cognitively aware of the cue-threat association in our study. Such cognitive awareness is typically slower to develop than startle potentiation (Hamm and Vaitl, 1996; Weike et al., 2005; Baas et al., 2008). Tentatively, this suggests that even slower learning systems might not be sufficient to prime fast defensive reflexes when BLA function is compromised. Regardless, our findings suggest a critical role for the human BLA in helping to rapidly acquire defensive reflexes under challenging learning conditions. On a final note, it deserves mentioning that these BLA-damaged subjects were previously also shown to exhibit hypervigilance for innate, unconditioned threat stimuli (fearful facial expressions) and impaired instrumental social-economic behaviours (Terburg et al., 2012; van Honk et al., 2013). Taken together with the current findings, these data translate the findings in rodents showing that the BLA might be essential in the instrumental learning of fear and socio-emotional behaviour (Davis and Whalen, 2001; LeDoux, 2003; Wolff et al., 2014) but inhibits impulsive social behaviours and unconditioned acute fear responses (Macedo et al., 2006; Martinez et al., 2007; Tye et al., 2011; Felix-Ortiz and Tye, 2014).
In conclusion, selective focal lesions of the BLA in four females with a rare genetic mutation were shown to be associated with an absence of normal FPS development when undergoing a classical fear conditioning procedure. These data provide new evidence that the human BLA is indispensable for the experience-driven pairing of fast somatic fear responses to fear-conditioned stimuli.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The work in this paper was supported by grants from Utrecht University, the Netherlands Society of Scientific Research (Brain and Cognition: no. 056-24-010), the South African MRC/DST Professional Development Program and the University of Cape Town (Brain Behavior Initiative).
The authors thank Dr. Johanna Baas and Prof. Leon Kenemans for commenting on earlier manuscript drafts.
REFERENCES
- Adolphs R. The biology of fear. Current Biology. 2013;23:R79–93. doi: 10.1016/j.cub.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology. 2005;210:343–52. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. Role of the primate amygdala in fear-potentiated startle: effects of chronic lesions in the rhesus monkey. Journal of Neuroscience. 2007;27:7386–96. doi: 10.1523/JNEUROSCI.5643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. The nonhuman primate amygdala is necessary for the acquisition but not the retention of fear-potentiated startle. Biological Psychiatry. 2009;65:241–8. doi: 10.1016/j.biopsych.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas JM, van Ooijen L, Goudriaan A, Kenemans JL. Failure to condition to a cue is associated with sustained contextual fear. Acta Psychologica. 2008;127:581–92. doi: 10.1016/j.actpsy.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain and Cognition. 2004;55:30. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–8. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Presented at the 8th International Conference on Functional Mapping of the Human Brain. Sendai, Japan: Neuroimage; 2002. Region of interest analysis using an SPM toolbox [abstract] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Anteromedial temporal lobe damage blocks startle modulation by fear and disgust. Behavioral Neuroscience. 2004;118:429–37. doi: 10.1037/0735-7044.118.2.429. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Coppens E, Spruyt A, Vandenbulcke M, Van Paesschen W, Vansteenwegen D. Classically conditioned fear responses are preserved following unilateral temporal lobectomy in humans when concurrent US-expectancy ratings are used. Neuropsychologia. 2009;47:2496–503. doi: 10.1016/j.neuropsychologia.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behavioural Brain Research. 1993;58:175–98. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–21. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–32. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Buzza C, Hurlemann R, et al. Fear and panic in humans with bilateral amygdala damage. Nature Neuroscience. 2013;16:270–72. doi: 10.1038/nn.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. Journal of Neuroscience. 2014;34:586–95. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology. 2003;114:1557–79. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Vaitl D. Affective learning: awareness and aversion. Psychophysiology. 1996;33:698–710. doi: 10.1111/j.1469-8986.1996.tb02366.x. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Indovina I, Robbins TW, Nunez-Elizalde AO, Dunn BD, Bishop SJ. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron. 2011;69:563–71. doi: 10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez SA, Maren S. Nuclear disconnection within the amygdala reveals a direct pathway to fear. Learning and Memory. 2009;16:766–8. doi: 10.1101/lm.1607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–24. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24:5506–16. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Klumpers F, Denys D, Kenemans JL, Grillon C, van der Aart J, Baas JM. Testing the effects of Delta9-THC and D-cycloserine on extinction of conditioned fear in humans. Journal of Psychopharmacology. 2012a;26:471–8. doi: 10.1177/0269881111431624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F, Heitland I, Oosting RS, Kenemans JL, Baas JM. Genetic variation in serotonin transporter function affects human fear expression indexed by fear-potentiated startle. Biological Psychology. 2012b;89:277–82. doi: 10.1016/j.biopsycho.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Klumpers F, Raemaekers MA, Ruigrok AN, Hermans EJ, Kenemans JL, Baas JM. Prefrontal mechanisms of fear reduction after threat offset. Biological Psychiatry. 2010;68:1031–8. doi: 10.1016/j.biopsych.2010.09.006. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. Journal of Neuroscience. 1995;15:6846–55. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–95. [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23:727. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Pine DS, Grillon C. The strong situation: a potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biological Psychology. 2006;72:265–70. doi: 10.1016/j.biopsycho.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour Research and Therapy. 2005;43:1391–24. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Ohman A. Genetic gating of human fear learning and extinction: possible implications for gene-environment interaction in anxiety disorder. Psychological Science. 2009;20:198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- Macedo CE, Martinez RC, Brandao ML. Conditioned and unconditioned fear organized in the inferior colliculus are differentially sensitive to injections of muscimol into the basolateral nucleus of the amygdala. Behavioral Neuroscience. 2006;120:625–31. doi: 10.1037/0735-7044.120.3.625. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82:276. [Google Scholar]
- Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. Journal of Neuroscience. 1999;19:8696–703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Reviews. Neuroscience. 2004;5:844–52. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Martinez RC, Ribeiro de Oliveira A, Brandao ML. Serotonergic mechanisms in the basolateral amygdala differentially regulate the conditioned and unconditioned fear organized in the periaqueductal gray. European Neuropsychopharmacology. The Journal of the European College of Neuropsychopharmacology. 2007;17:717–24. doi: 10.1016/j.euroneuro.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Massar SA, Mol NM, Kenemans JL, Baas JM. Attentional bias in high- and low-anxious individuals: evidence for threat-induced effects on engagement and disengagement. Cognition and Emotion. 2011;25:805–17. doi: 10.1080/02699931.2010.515065. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in Neurobiology. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychologica. 2008;127:567–80. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Mineka S, Ohman A. Phobias and preparedness: the selective, automatic, and encapsulated nature of fear. Biological Psychiatry. 2002;52:927–37. doi: 10.1016/s0006-3223(02)01669-4. [DOI] [PubMed] [Google Scholar]
- Morgan B, Terburg D, Thornton HB, Stein DJ, van Honk J. Paradoxical facilitation of working memory after basolateral amygdala damage. PLoS One. 2012;7:e38116. doi: 10.1371/journal.pone.0038116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Li V, Sterlace SS, Tokushige F, Ponnusamy R, Fanselow MS. Persistence of fear memory across time requires the basolateral amygdala complex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11737–41. doi: 10.1073/pnas.0905257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Annals of the New York Academy of Sciences. 2003;985:50. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Terburg D, Morgan BE, Montoya ER, et al. Hypervigilance for fear after basolateral amygdala damage in humans. Translational Psychiatry. 2012;2:e115. doi: 10.1038/tp.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton HB, Nel D, Thornton D, van Honk J, Baker GA, Stein DJ. The neuropsychiatry and neuropsychology of lipoid proteinosis. Journal of Neuropsychiatry and Clinical Neuroscience. 2008;20:86–92. doi: 10.1176/jnp.2008.20.1.86. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–62. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Honk J, Eisenegger C, Terburg D, Stein DJ, Morgan B. Generous economic investments after basolateral amygdala damage. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2506–10. doi: 10.1073/pnas.1217316110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weike AI, Hamm AO, Schupp HT, Runge U, Schroeder HW, Kessler C. Fear conditioning following unilateral temporal lobectomy: dissociation of conditioned startle potentiation and autonomic learning. Journal of Neuroscience. 2005;25:11117–24. doi: 10.1523/JNEUROSCI.2032-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff SB, Grundemann J, Tovote P, Krabbe S, Jacobson GA, Muller C, Herry C, Ehrlich I, Friedrich RW, Letzkus JJ, Luthi A. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–8. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.