Abstract
Mood disorders are characterized by impaired emotion regulation abilities, reflected in alterations in frontolimbic brain functioning during regulation. However, little is known about differences in brain function when comparing regulatory strategies. Reappraisal and emotional acceptance are effective in downregulating negative affect, and are components of effective depression psychotherapies. Investigating neural mechanisms of reappraisal vs emotional acceptance in remitted major depressive disorder (rMDD) may yield novel mechanistic insights into depression risk and prevention. Thirty-seven individuals (18 rMDD, 19 controls) were assessed during a functional magnetic resonance imaging task requiring reappraisal, emotional acceptance or no explicit regulation while viewing sad images. Lower negative affect was reported following reappraisal than acceptance, and was lower following acceptance than no explicit regulation. In controls, the acceptance > reappraisal contrast revealed greater activation in left insular cortex and right prefrontal gyrus, and less activation in several other prefrontal regions. Compared with controls, the rMDD group had greater paracingulate and right midfrontal gyrus (BA 8) activation during reappraisal relative to acceptance. Compared with reappraisal, acceptance is associated with activation in regions linked to somatic and emotion awareness, although this activation is associated with less reduction in negative affect. Additionally, a history of MDD moderated these effects.
Keywords: emotion regulation, remitted major depression, acceptance, reappraisal, mindfulness
INTRODUCTION
The ability to navigate one’s emotional landscape, and especially the ability to consciously downregulate negative affect that interferes with adaptive functioning, is a critical factor for psychological health. Functional magnetic resonance imaging (fMRI) studies of emotion regulation have focused on a top-down frontolimbic regulatory network (Ochsner et al., 2004). As we have summarized previously (Smoski et al., 2013), prefrontal cortical regions, including dorsolateral prefrontal cortex (dlPFC), ventrolateral prefrontal cortex (vlPFC) and anterior cingulate cortex (ACC), mediate the modulation of emotion-elicited activation in limbic regions (Ochsner and Gross, 2008). Within this regulatory circuit, activation in prefrontal cognitive control regions is negatively associated with changes in limbic activation while processing negative stimuli (Siegle et al., 2006; Urry et al., 2006).
Emotion regulation is especially critical in the context of major depressive disorder (MDD), in which biological, cognitive and behavioral responses to affective stimuli are dysregulated (Davidson et al., 2002; Ressler and Mayberg, 2007). A recent review (Rive et al., 2013) concluded that during early, automatic emotion regulation processes, individuals with MDD may engage in compensatory recruitment of lateral prefrontal cortex during successful regulation. This compensatory recruitment occurs in the medial prefrontal cortex, including the ACC. However, during conscious regulation occurring later in an emotional experience, recruitment of lateral prefrontal cortex is diminished, with less activation in dlPFC and/or vlPFC in individuals with MDD compared with healthy controls (Rive et al., 2013). Differential prefrontal cortical influence on amygdala activation in MDD has been observed during regulation of both negative and positive emotions (Greening et al., 2014), although not all studies have found evidence of altered frontolimbic indicators of regulation in MDD (Dillon and Pizzagalli, 2013). Altered frontolimbic activation during emotion regulation is also observed in euthymic individuals with a history of MDD (Kanske et al., 2012), and increases in dlPFC and ventral medial prefrontal cortex (vmPFC) activation during emotion regulation are associated with symptom improvement during treatment with antidepressant medications (Heller et al., 2013), further emphasizing the importance of emotion regulation on the trajectory of changes in depressive symptoms.
To date, functional neuroimaging studies of emotion regulation in MDD have focused almost exclusively on reappraisal as a regulation strategy. During reappraisal, the meaning of an emotional stimulus is reinterpreted to change its affective tone. In contrast, acceptance of an emotion is a component of the broader construct of mindfulness that has been defined as the awareness that arises through ‘paying attention in a particular way: on purpose, in the present moment, and nonjudgmentally’ (Kabat-Zinn, 1994, p. 4). Notably, mindfulness may be used to describe a psychological trait (i.e. naturally occurring and consistent across contexts); a mode or state of awareness (i.e. brought about through intention, through eliciting aspects of the context or as a result of experimental induction or training) or a practice of cultivating mindfulness (e.g. mindfulness meditation) (Germer et al., 2005; Keng et al., 2011). There is natural variation in trait mindfulness in the population in the absence of mindfulness training (Brown and Ryan, 2003), and training in mindfulness has been demonstrated to increase trait mindfulness (Brown and Ryan, 2003; Shapiro et al., 2007; Greeson et al., 2011; Robins et al., 2012). Within the concept of mindfulness, acceptance of emotion requires attention and awareness of one’s current emotional state, while maintaining a non-judgmental stance toward that state. In individuals who do not have an extensive history of mindfulness or meditation practice or training, both trait and state mindfulness are associated with increased prefrontal and decreased limbic activation under affective challenge (Chiesa et al., 2013), with reduced activation in midline cortical structures associated with interoception, including insular cortex, ACC, and medial prefrontal cortex during mindful practice (Ives-Deliperi et al., 2011). Mindfulness is also associated with a detached ability to observe thoughts, emotions and sensations (Feldman et al., 2010), which may be associated with a concurrent reduction in self-referential processes associated with midline cortical structures of the default mode network (DMN) (Chiesa et al., 2013).
Several studies have compared the relative effectiveness of reappraisal and emotional acceptance in downregulating negative affect. Reappraisal and acceptance appear equivalent in promoting flexible physiological regulation among individuals with heightened anxiety (as indexed by heart rate variability; Cristea et al., 2012) and in reducing physiological arousal (Hofmann et al., 2009). However, acceptance may be less effective at regulating anxiety and anger than reappraisal (Hofmann et al., 2009; Szasz et al., 2011). Other studies have found equivalent decreases in subjective distress (Wolgast et al., 2011). With the exception of Cristea et al. (2012) who studied individuals who scored high on a self-report measure of social anxiety, these studies all involved non-clinical samples. In adults with mild to moderate symptoms of MDD, both reappraisal and emotional acceptance were similarly effective in downregulating sadness (Keng et al., 2013). No study to date has compared the neural correlates or subjective effectiveness of reappraisal vs emotional acceptance among individuals with remitted major depressive disorder (rMDD).
The purpose of this study was to compare the neural mechanisms of acceptance and reappraisal of sad images in unmedicated individuals with and without a history of MDD. As highlighted by Ochsner and Gross (2008), regulation strategies are composed of subcomponents that may differ in their attentional, linguistic and cognitive control demands, and as such may rely on differentiable neural processes. Therefore, one aim of this study was to demonstrate commonalities and differences between reappraisal and acceptance in the never-depressed control group and in the rMDD group. The second aim of this study was to test if the neural effects of acceptance are moderated by a history of MDD. Finally, we evaluated in an exploratory manner whether there were associations between neural correlates of emotion regulation and self-reported affect post-acceptance.
METHODS
Participants
Nineteen affectively healthy right-handed adult control participants (7 male, 15 Caucasian, 27.9 ± 6.3 years old, all right handed) were recruited from the lists of potential participants maintained by the UNC-Duke University Medical Center (DUMC) Brain Imaging and Analysis Center. Nineteen adults with rMDD (4 male, 13 Caucasian, 24.5 ± 5.4 years old, 17 right handed) were recruited via a participant database maintained at the Cognitive Behavioral Research and Treatment Program at DUMC. Data from one rMDD participant were excluded due to an elevated Beck Depression Inventory-II (BDI; Beck et al., 1996) score on the day of the scan (BDI = 30), resulting in a final sample of 18 rMDD participants. Exclusion criteria for both groups included age<19 or >55 years, current Axis I psychopathology assessed with the Structured Clinical Interview for DSM-IV Axis I semi-structured interview (First et al., 1996), psychiatric medication use within the past month, verbal IQ scores (estimated by the North American Adult Reading Test; Uttl, 2002) <80, BDI >8 or MRI contraindications. Inclusion in the rMDD group was contingent on a prior diagnosis of MDD. Control participants were lifetime free of MDD. None of the control participants and 2 rMDD participants were receiving psychotherapy at the time of participation. Five rMDD participants had previously used psychotropic medications. All participants consented to a protocol approved by the local Human Investigations Committees at both University of North California at Chapel Hill and Duke UMCs and were paid $35 for completing the imaging portion of the study. All participants had normal or corrected-to-normal vision and completed a mock scan session prior to imaging. Information about demographics and clinical characteristics are presented in Table 1.
Table 1.
Demographic and symptom severity information for control and rMDD participants
| Remitted subjects (n = 18) Mean (s.d.) | Control subjects (n = 19) Mean (s.d.) | P value | |
|---|---|---|---|
| Age | 24.8 (4.7) | 27.9 (6.3) | 0.10 |
| Gender: male/female | 4/14 | 7/12 | 0.33 |
| Race | 0.37 | ||
| African American | 6% | 16% | |
| Caucasian | 72% | 79% | |
| Asian | 11% | 5% | |
| American Indian | 6% | 0% | |
| Hispanic ethnicity | 22% | 6% | 0.18 |
| NAART VIQ | 110.7 (3.3) | 110.2 (5.1) | 0.70 |
| BDI | 2.9 (5.0) | 1.4 (2.4) | 0.24 |
| No. of previous depressive episodes | 1.6 (0.9) | – | – |
| No. of months since previous episode | 40.4 (46.2) | – | – |
Notes: Two-tailed P values for between-group t tests or chi-square analyses are in the final column.
BDI: Beck Depression Inventory, 2nd edition (Beck et al., 1996); NAART VIQ: North American Adult Reading Test (Uttl, 2002).
fMRI task
Each trial began with a fixation cross (6 s) followed by presentation of a sad or neutral picture (Figure 1 depicts the timing and content of each trial). After initial picture display (6–9 s, jittered) without regulation instruction, a visual regulation instruction was superimposed on the bottom of the picture, indicating the regulation strategy to use. Regulation continued for 5 s following image offset. Finally, participants rated their post-trial affect using a visual analog scale (ranging from 1 = most negative to 4 = most positive). The task included three regulation conditions. In the ‘view’ condition, used with both sad and neutral pictures, participants were instructed not to regulate their emotion response. In the ‘accept’ condition, used only with sad images, participants were asked to notice what they were thinking and feeling, and to allow those thoughts and feelings to remain, without needing to push them away. In the ‘reappraise’ condition, used with only sad images, participants were asked to reinterpret the image to reduce its negative impact. Both self-focused and situation-focused reappraisal strategies were permitted (Ochsner et al., 2004). Four runs of 12 trials each were administered (48 trials total; 4’24” per run), and there were 12 trials for each regulation condition.
Fig. 1.

The emotion regulation task. Each trial consisted of a neutral or sad image presented first without a regulation cue for 6–9 s, then the presentation of the regulation cue while the image remained presented for another 3 s, a period of 5 s after picture offset during which regulation continued and then a 5 s query for current affect. ITI were 6 s. ITI = Inter-trial intervals.
Immediately prior to the scan, participants learned and practiced the regulation strategies with an experimenter until they could correctly implement them without assistance. Instructions for the reappraise and accept conditions are shown in Table 2. Task images were drawn from two sources: (i) sad images from the International Affective Picture System based on normative sadness ratings (Mikels et al., 2005) and (ii) a normed set of sad and neutral images used in previous MDD imaging studies (Wang et al., 2005, 2008; Dichter et al., 2009, 2010).
Table 2.
Training instructions for (A) the accept and (B) reappraise emotion regulation strategies
| A. Accept |
| ‘To accept, your task is to notice what you are thinking and feeling, and to allow those thoughts and feelings to be there. So rather than try to push the feeling or thought away or try to feel differently, you just acknowledge it, perhaps saying, “That’s just how it is right now,” “This feeling will come and go,” or “I can accept this thought.” Note that acceptance doesn’t mean that you have to like the feeling, or that you are resigning yourself to the feeling. It is reminding yourself that it’s ok to feel what you feel without having to change it’. |
| B. Reappraise |
| ‘To reappraise something means to take a look at it in a different light or from another perspective. For the negative images in this study, that will mean reinterpreting the image in some way. For example, you could remind yourself that you don’t know the people in the picture, so no one close to you was affected by the situation. Alternatively, you could continue the story from the picture but give it a happy ending. For example, if you saw a picture of a couple arguing, you could imagine them working out their differences and being happy with one another again’. |
Notes: Following the written instructions, participants were presented with sample pictures and practiced the strategies aloud with an experimenter, who provided corrective feedback on strategy use, as necessary.
Imaging methods
Scanning was performed on a General Electric (Waukesha, WI) MR750 3.0 Tesla scanner equipped with high power, high duty cycle 50 mT/m gradients at 200 T/m/s slew rate and a 32-channel head coil for parallel imaging. A high-resolution T1-weighted image with 166 slices was acquired using a three-dimensional FSPGR pulse sequence (Repetition time (TR) = 7.484 ms, Echo time (TE) = 2.984 ms, Field of view (FOV) = 256 mm, image matrix = 256 × 256, voxel size = 1 mm3) and used for co-registration with the functional data. This structural image was aligned in a near axial plane defined by the anterior and posterior commissures. Whole-brain functional images were acquired using a spiral pulse sequence with SENSE reconstruction sensitive to blood oxygenation level-dependent contrast (TR = 1500 ms, TE = 30 ms, FOV = 256 mm, image matrix = 64 × 64, α = 60°, voxel size = 4 mm3, 32 axial slices). Functional images were aligned similarly to the T1-weighted structural image. A semi-automated high-order shimming program ensured global field homogeneity.
Imaging data analysis
Functional data were preprocessed using FSL version 4.1.8 [Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Oxford University, UK]. Preprocessing was applied in the following steps: (i) brain extraction for non-brain removal (Smith et al., 2004), (ii) motion correction using MCFLIRT (Smith, 2002), (iii) spatial smoothing using a Gaussian kernel of FWHM 5 mm, (iv) mean-based intensity normalization of all volumes by the same factor and (v) high-pass filtering (Jenkinson et al., 2002). Functional images of each participant were co-registered to structural images in native space, and structural images were normalized into a standard stereotaxic space (Montreal Neurological Institute) for intersubject comparison. This transformation included resampling voxel sizes to 2 mm3. The same transformation matrices used for structural-to-standard transformations were then used for functional-to-standard space transformations of co-registered functional images. All registrations were carried out using an intermodal registration tool (Jenkinson et al., 2002; Smith et al., 2004). Voxel-wise temporal autocorrelation was estimated and corrected using FMRIB’s Improved Linear Model (FILM; Jenkinson and Smith, 2001). Onset times of events were used to model a signal response containing a regressor for each strategy, which was convolved with a double-γ function to model the hemodynamic response. Model fitting generated whole-brain images of parameter estimates and variances representing average signal change from baseline. Group-wise activation images were calculated by a mixed-effects higher level analysis using Bayesian estimation techniques, FMRIB Local Analysis of Mixed Effects (FILM; Woolrich et al., 2001; Smith et al., 2004).
An a priori mask was created for small volume correction that included the frontal lobes and bilateral amygdala generated in FSL using the Harvard–Oxford cortical and subcortical structural probabilistic atlases. Masks were thresholded at 25%, binarized and then combined into a single mask using fslmaths. For all analyses, voxels were considered significant if they passed a statistical threshold of P < 0.005, uncorrected and were part of a 35-voxel (280 mm3) cluster of contiguous significant voxels, resulting in a cluster-corrected significance threshold of P < 0.05. This cluster size was determined by performing 1000 Monte Carlo simulations using 3dClustSim (Ward, 2000).
Only trials with sad images are analyzed here. Results from the pre-regulation phase and comparisons between the reappraise and view conditions were reported previously (Smoski et al., 2013), and here we focus on only analysis involving the accept condition during the presentation of sad images. General linear models evaluated clusters that showed significant interactions of Group (rMDD, control) with trial type (accept relative to reappraise, accept relative to view). Activation localizations were based on Harvard–Oxford cortical and subcortical structural probabilistic atlases, with Brodmann area identification via the Talairach Daemon, as implemented in FSLView version 3.1.8. Exploratory correlation analyses between brain activation magnitudes and clinical characteristics of the rMDD group were conducted by extracting contrast estimates from each participant and condition within significant clusters identified by the whole-brain general linear models described above.
RESULTS
Emotion regulation self-report
In-scanner self-reported emotion regulation was evaluated via a 2 (Group: rMDD, control) × 3 (Trial Type: view, reappraise, accept) repeated measures analysis of variance conducted on mood rating data. There was a significant main effect of Trial Type (F(2,70) = 53.78, P < 0.0001). Follow-up paired t-tests indicated less intense negative affect following accept trials (M = 2.19, s.d. = 0.34) than view trials (M = 2.05, s.d. = 0.35; t(36) = 2.24, P = 0.03), but more negative affect following accept trials than reappraise trials (M = 2.80, s.d. = 0.45; t(36) = 8.03, P < 0.001). There was no main effect of Group (F(1,35) = 0.27, P = 0.60) or Group × Trial Type interaction (F(2,70) = 1.63, P = 0.20).
Imaging data: within groups
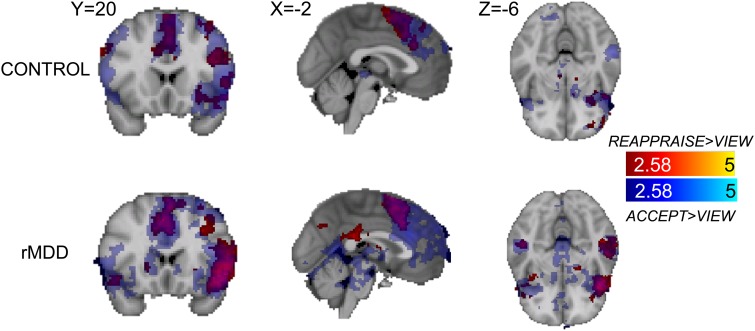
Within the control group, the accept > view contrast revealed activation in a large dorsal medial PFC with peak activation in anterior midcingulate, extending to left lateral PFC. Activation was also observed in several clusters within the frontal pole, left orbitofrontal cortex (OFC) and right dlPFC (Figure 2 and Table 3). There was one frontal pole cluster with greater activation during view than accept. The accept > reappraise contrast revealed activation in left insular cortex and left precentral gyrus (Table 4). There was greater activation during reappraisal than acceptance in several regions of the right PFC, including frontal pole, medial and inferior frontal gyrus, as well as left frontal operculum (Table 5).
Fig. 2.
Activation to accept > view (blue) and reappraise > view (red) and their overlap (green) in the control group (top) and the rMDD group (bottom).
Table 3.
Clusters showing within and between-group activation differences to the acceptance of sad images > viewing sad images contrast
| Side | BA | Size (mm3) | Z max | MNI co-ordinates |
|||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Control | |||||||
| Frontal pole | L | 9 | 472 | 3.17 | −14 | 58 | 36 |
| Frontal pole | L | 47 | 2152 | 3.86 | −44 | 44 | −16 |
| Frontal pole | L | 9 | 680 | 3.14 | −26 | 44 | 30 |
| Frontal pole | R | 8 | 1400 | 3.22 | 36 | 44 | 38 |
| Frontal pole | R | 47 | 336 | 6.25 | 32 | 24 | −14 |
| Anterior cingulate gyrus | R | 32 | 11 088 | 4.33 | 4 | 28 | 30 |
| Frontal orbital cortex | L | 13 | 784 | 4.94 | −34 | 26 | −6 |
| Precentral gyrus | R | 9 | 536 | 3.05 | 60 | 18 | 30 |
| rMDD | |||||||
| Frontal orbital cortex | L | 13 | 25 632 | 8.15 | −38 | 24 | −2 |
| Frontal orbital cortex | R | 47 | 352 | 3.15 | 50 | 30 | −6 |
| Frontal pole | R | 10 | 912 | 3.39 | 34 | 54 | 6 |
| Frontal pole | L | 9 | 456 | 3.14 | −14 | 66 | 24 |
| Paracingulate gyrus | L | 6 | 9896 | 5.05 | 4 | 18 | 44 |
| rMDD < control | |||||||
| Frontal pole | R | 9 | 280 | 3.22 | 44 | 38 | 30 |
L: left; R: right.
Table 4.
Clusters showing within- and between-group activation differences to the acceptance of sad images > reappraising sad images contrast
| Side | BA | Size (mm3) | Z max | MNI co-ordinates |
|||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Control | |||||||
| Insular cortex | L | 13 | 784 | 5.18 | −42 | −6 | 8 |
| Precentral gyrus | L | 4 | 360 | 3.32 | −40 | −14 | 52 |
| rMDD < control | |||||||
| Paracingulate gyrus | R | 24 | 360 | 3.14 | 6 | 40 | −6 |
| Middle frontal gyrus | R | 8 | 296 | 3.00 | 26 | 28 | 38 |
L: left; R: right.
Table 5.
Clusters showing within-group activation differences to the reappraisal of sad images > accepting sad images contrast
| Side | BA | Size (mm3) | Z max | MNI co-ordinates |
|||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Control | |||||||
| Frontal pole | R | 8 | 304 | 3.24 | 14 | 56 | 42 |
| Medial frontal cortex | R | 11 | 464 | 3.31 | 2 | 44 | −22 |
| rMDD | |||||||
| Inferior frontal gyrus, pars triangularis | R | 45 | 1088 | 3.49 | 52 | 28 | −6 |
| Inferior frontal gyrus, pars opercularis | R | 44 | 744 | 3.31 | 52 | 18 | 12 |
| Frontal operculum | L | 13 | 1776 | 5.25 | −40 | 12 | 2 |
| Paracingulate | R | 32 | 29 752 | 4.94 | 8 | 44 | 12 |
| Superior frontal gyrus | L | 8 | 600 | 3.39 | −24 | 24 | 40 |
| Inferior Frontal Gyrus, pars opercularis | R | 44 | 4176 | 4.67 | 46 | 18 | 16 |
L: left; R: right.
Within the rMDD group, the accept > view contrast revealed activation in a large left lateral PFC cluster with peak activation in the left OFC. Activation was also observed in the right OFC, bilateral frontal pole and anterior midcingulate (Table 3). There were no clusters with significant activation during the view > accept or accept > reappraise contrasts. The reappraise > accept contrast revealed activation in a large vmPFC cluster with peak activation in the paracingulate. Additional activations were observed in the left superior frontal gyrus and right inferior frontal gyrus.
Note that within-group analyses of the reappraisal > view contrast were reported previously (Smoski et al., 2013).
Imaging data: group differences
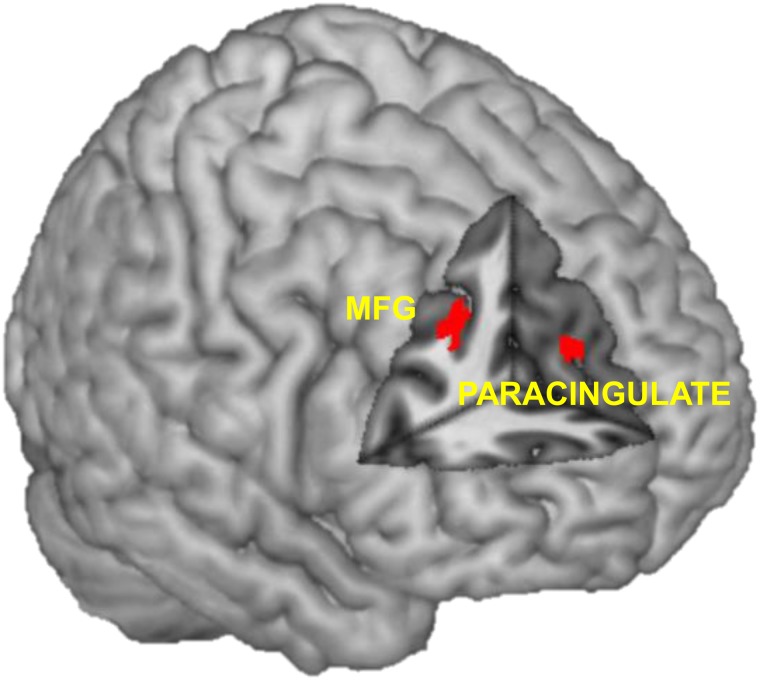
The accept > view contrast revealed less activation in the right frontal pole in the rMDD relative to the control group, and no regions with relatively greater activation in the rMDD group than control group view (Figure 3 and Table 3). The accept > reappraise contrast revealed less activation in the rMDD group relative to the control group in the right paracingulate gyrus and the right middle frontal gyrus, and no regions with relatively greater activation in the rMDD group than the control group (Table 4).
Fig. 3.
Clusters with less activation in the rMDD group than the control group in the accept > reappraise contrast were found in the right paracingulate gyrus and the right MFG. MFG = middle frontal gyrus.
Correlations between brain activation and self-reported affect post-acceptance
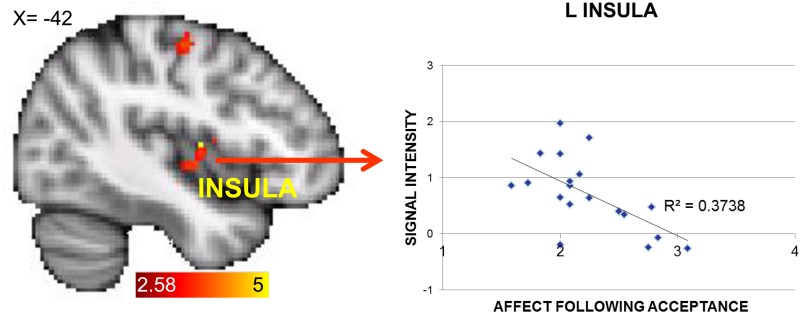
To test for relations between brain activation magnitudes and self-reported affect during acceptance, correlations between in-scanner reports of post-acceptance affect and activation magnitudes of within-groups clusters are reported in Tables 3–5. As these analyses were exploratory, they were not corrected for multiple comparisons. Within the control group, there was a significant correlation between self-reported negative affect post-acceptance and activation in left insular cortex during the accept > reappraise contrast (r(18) = −0.61, P = 0.005) (Figure 4). No other correlations were significant in the control group. Within the rMDD group, no clusters correlated significantly with self-reported affect post-acceptance.
Fig. 4.
The control group demonstrated greater activation during acceptance > reappraisal in the left precentral gyrus and the left insula, and greater signal intensity in this left insula cluster was associated with less change in negative affect following acceptance.
DISCUSSION
The purpose of this study was to examine the neural mechanisms of acceptance, compared with reappraisal, of sad images in unmedicated individuals with and without a history of MDD. Acceptance and reappraisal recruited overlapping brain circuits, including dorsal medial prefrontal cortex (dmPFC), dlPFC, vlPFC and anterior midcingulate. However, there were also key differences between these emotion regulation strategies. In both rMDD and control groups, acceptance was associated with less dlPFC and frontal pole activation than reappraisal. Within the control group, acceptance was associated with greater activation in the left precentral gyrus and insula than reappraisal. Greater activation to acceptance than reappraisal in the left insular cortex was associated with greater intensity of negative affect post-acceptance, suggesting that this activation reflected less effective emotion regulation, or alternately, reflected heightened emotional awareness rather than regulation. Acceptance was associated with less effective subjective regulation than reappraisal, which is consistent with some (Hofmann et al., 2009; Szasz et al., 2011) but not all (Keng et al., 2013) previous comparisons of these strategies.
Most previous fMRI investigations of mindfulness broadly, or mindful acceptance specifically, have focused on mindfulness as a trait (Creswell et al., 2007; Farb et al., 2011) or as an outcome of mindfulness training (Farb et al., 2007; Taylor et al., 2011). Trait mindful observing, or noticing of internal and external stimuli (a component of the acceptance instructions administered in this study), is associated with activation in dorsal medial PFC during negative emotional imagery (Frewen et al., 2010). Olsson and Ochsner (2008) have proposed that medial ACC and anterior insula support experiential awareness, with metacognitive awareness of that experience supported by dorsal and rostral PFC. By examining acceptance as state that can be brought forth with intention, the present findings broaden the understanding of the neural underpinnings of acceptance as an emotion regulation strategy. Greater activation in left insular cortex during awareness may reflect the task instructions to be aware of one’s emotional states. In individuals with little training or experience in mindful acceptance beyond brief experimental task instructions, this awareness may in itself be mildly distressing, or may interfere with other more practiced or preferred regulation strategies. In individuals with greater training and experience in mindful acceptance, emotional awareness may function differently. For example, in a study of pain perception in expert and novice meditators, increased insula activation was associated with higher ratings of pain unpleasantness in novice meditators but lower ratings in experts (Gard et al., 2012), suggesting different effects of sensory processing on pain reduction. Similarly, mindfulness training in individuals with elevated depression symptoms was associated with reduced insula reactivity to sad stimuli, while heightened insula reactivity correlated with depression symptom severity, suggesting that mindfulness training may promote reduced neural reactivity to affective stimuli (Farb et al., 2010).
The rMDD and control groups did not differ in self-reported affect following acceptance or reappraisal. However, the groups did differ in neural activation when implementing the emotion regulation strategies. Compared with the control group, acceptance in rMDD was associated with relatively reduced activation in the right frontal pole, a medial prefrontal region that has been linked to self-referential processing and rumination in individuals with rMDD (Farb et al., 2011). Mindfulness training has been previously linked to reduced reactivity to sad stimuli in a cluster that included vmPFC (Farb et al., 2010). Given that one of the key aims of mindfulness-based interventions targeting depressive relapse is to reduce rumination and maladaptive self-focus (Barnhofer et al., 2009), the finding of relatively reduced medial prefrontal activation during acceptance is consistent with this therapeutic goal. Compared with controls, the rMDD group demonstrated relatively reduced activation in the right paracingulate and middle frontal gyrus when using acceptance than reappraisal. The middle frontal gyrus is recruited during tasks involving working memory, selective attention and successful emotion regulation (Ochsner et al., 2004; Ochsner and Gross, 2008). Decreased middle frontal gyrus activity has been observed in MDD during tasks involving cognitive control (Okada et al., 2009; Kikuchi et al., 2012), emotion processing (Wang et al., 2008; Dichter et al., 2009; Feeser et al., 2013) and during reappraisal in rMDD (Smoski et al., 2013). Likewise, the rostral cingulate/paracingulate is thought to function as a ‘hub’ within the DMN (Shackman et al., 2011) that mediates task switching with dorsal cognitive control networks in depression (Pizzagalli, 2011). Relatively decreased middle frontal gyrus and paracingulate activity in rMDD during acceptance may reflect decreased recruitment of neural resources to exert cognitive control during acceptance relative to reappraisal, with potential implications for the success of the strategy under more difficult task demands.
The relative effectiveness and neural underpinnings of different emotion regulation strategies in the rMDD group are relevant to informing treatments that focus on reducing vulnerability to MDD relapse. MDD is cyclical, with previous episodes serving as a powerful risk factor for future episodes (Lewinsohn et al., 1988). Emotional acceptance and reappraisal mirror key elements of mindfulness-based cognitive therapy (MBCT; Segal et al., 2002) and traditional cognitive behavioral therapy (CBT; Beck et al., 1979), respectively. CBT reduces relapse risk and the need for further treatment among patients with acute MDD (Blackburn et al., 1986; Evans et al., 1992; Shea et al., 1992), presumably through altering dysfunctional attitudes and thoughts that are vulnerability factors for MDD. MBCT reduces relapse rates among those with a history of three or more MDD episodes (Teasdale et al., 2000; Ma and Teasdale, 2004; Kuyken et al., 2008) and prolongs time to relapse (Bondolfi et al., 2010), presumably by facilitating the ability to decenter from and accept thoughts and emotions non-judgmentally. Although both interventions are effective in reducing MDD relapse, less is known regarding the neural mechanisms by which these interventions improve clinical outcomes.
One limitation in our design is the lack of ratings of familiarity with, or ease of implementation of, acceptance vs reappraisal. With its roots in Eastern contemplative practices, mindfulness and mindful acceptance have until recently been relatively unfamiliar and under-studied as a means of promoting emotional health (Baer, 2003; Keng et al., 2011), and the relatively reduced regulatory success of acceptance vs reappraisal may be in part related to the novelty of mindful acceptance. Although all participants were trained in acceptance and reappraisal before the scan and successfully articulated the use of the acceptance strategy during that training, measure of the extent to which the strategy was implemented correctly during the scan would further confirm correct implementation of the strategy. In addition, future studies that incorporate long-term clinical course will increase the translational implications for sustained MDD remission vs relapse.
Although the intent of this study was to examine the relative effectiveness and comparative neural activation of reappraisal and acceptance, it should be noted that these two strategies are not mutually exclusive. As shown in Figure 2, there is significant overlap in frontolimbic activation between the two strategies, consistent with prefrontal regions widely associated with emotion regulation (Ochsner and Gross, 2008). In practice, acceptance and reappraisal may mutually facilitate effective regulation. Cross-sectional (Jermann et al., 2009; Desrosiers et al., 2013) and intervention-based (Bormann and Carrico, 2009; M.J. Smoski, J.G. Brantley, M.M. Llabre, T.R. Lynch, E.C. Suarez, R.Q. Wolever and J.M. Greeson, submitted for publication) studies suggest that reappraisal use mediates the relationship between mindfulness and reduced depressive symptoms. Similarly, Garland et al. (2011) found a reciprocal relationship between positive reappraisal and mindfulness over the course of a mindfulness-based stress and pain reduction program, whereby increases in mindfulness predicted increases in reappraisal, and vice versa. Although reappraisal and acceptance are theoretically and empirically separable, they may be best thought of as complementary regulation strategies.
In summary, although both reappraisal and acceptance were associated with more effective subjective regulation than a no-regulation control condition, reappraisal was more effective than acceptance in regulating subjective negative affect. Both acceptance and reappraisal showed similar patterns of prefrontal cortex activation in both individuals remitted from depression as well as never-depressed controls, with a few notable exceptions. Acceptance was associated with greater activation in regions associated with somatic awareness (i.e. insula) and with less overall right-lateralized prefrontal cortex activation. Reappraisal was associated with greater regulatory success than acceptance, with no group differences in self-reported emotion regulation effectiveness. Currently, euthymic individuals with a history of MDD showed decreased right middle frontal gyrus and paracingulate activation during acceptance compared with reappraisal, and decreased medial prefrontal activation during acceptance compared with a control condition. These findings were evident despite the fact that the rMDD and control groups were matched to have equivalently low levels of current depressive symptom severity. These patterns of brain activity suggest complex implications regarding the use of acceptance as a regulation strategy in rMDD: less vmPFC activation that has been associated with rumination, a problematic process that is predictive of depressive relapse, but also less activation in cognitive control regions (i.e. paracingulate) generally associated with regulatory success. Further research is needed to determine whether these patterns of brain activation convey greater risk for future MDD episodes or are modifiable by interventions such as MBCT that target relapse prevention.
ACKNOWLEDGMENTS
The authors thank Josh Bizzell and Chris Petty for assistance with image analysis; Alison Rittenberg for assistance with data collection; and MRI technologists Susan Music, Natalie Goutkin and Luke Poole for assistance with data acquisition. This research was supported by grants from the NARSAD Young Investigator Program. Investigator effort was supported by National Institute of Mental Health grants K23 MH087754 to M.S., K23 MH081285 to G.D., R21 MH094781 to M.S. and G.D. and R21 MH094781-02S1 to T.M.
REFERENCES
- Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clinical Psychology: Science and Practice. 2003;10:125–43. [Google Scholar]
- Barnhofer T, Crane C, Didonna F. Clinical Handbook of Mindfulness. New York: Springer Science + Business Media; 2009. Mindfulness-Based Cognitive Therapy for Depression and Suicidality. [Google Scholar]
- Beck AT, Rush S, Shaw P, Emery N. Cognitive Therapy of Depression. New York: Guilford Press; 1979. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Blackburn IM, Eunson KM, Bishop S. A two-year naturalistic follow-up of depressed patients treated with cognitive therapy, pharmacotherapy and a combination of both. Journal of Affective Disorders. 1986;10:67–75. doi: 10.1016/0165-0327(86)90050-9. [DOI] [PubMed] [Google Scholar]
- Bondolfi G, Jermann F, der Linden MV, et al. Depression relapse prophylaxis with Mindfulness-Based Cognitive Therapy: replication and extension in the Swiss health care system. Journal of Affective Disorders. 2010;122:224–31. doi: 10.1016/j.jad.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann JE, Carrico AW. Increases in positive reappraisal coping during a group-based mantram intervention mediate sustained reductions in anger in HIV-positive persons. International Journal of Behavioral Medicine. 2009;16:74–80. doi: 10.1007/s12529-008-9007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology. 2003;84:822–48. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A, Jakobsen JC. Mindfulness: top-down or bottom-up emotion regulation strategy? Clinical Psychology Review. 2013;33:82–96. doi: 10.1016/j.cpr.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosomatic Medicine. 2007;69:560–5. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Cristea IA, Valenza G, Gentili C, Szentagotai Tatar A, Scilingo EP, David D. Cognitive reappraisal and acceptance distinctly impact heart rate variability in socially anxious individuals. International Journal of Psychophysiology. 2012;85:339. [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–74. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Desrosiers A, Vine V, Klemanski DH, Nolen-Hoeksema S. Mindfulness and emotion regulation in depression and anxiety: common and distinct mechanisms of action. Depression & Anxiety. 2013;30:654–61. doi: 10.1002/da.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Smoski MJ. Affective context interferes with cognitive control in unipolar depression: an fMRI investigation. Journal of Affective Disorders. 2009;114:131–42. doi: 10.1016/j.jad.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Smoski MJ. The effects of Brief Behavioral Activation Therapy for Depression on cognitive control in affective contexts: an fMRI investigation. Journal of Affective Disorders. 2010;126:236–44. doi: 10.1016/j.jad.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Pizzagalli DA. Evidence of successful modulation of brain activation and subjective experience during reappraisal of negative emotion in unmedicated depression. Psychiatry Research. 2013;212:99–107. doi: 10.1016/j.pscychresns.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MD, Hollon SD, DeRubeis RJ, et al. Differential relapse following cognitive therapy and pharmacotherapy for depression. Archives of General Psychiatry. 1992;49:802–8. doi: 10.1001/archpsyc.1992.01820100046009. [DOI] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Bloch RT, Segal ZV. Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biological Psychiatry. 2011;70:366–72. doi: 10.1016/j.biopsych.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Andersen AK, Mayberg H, Bean J, McKeon D, Segal ZV. Minding one's emotions: mindfulness training alters the neural expression of sadness. Emotion. 2010;10:25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2:313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeser M, Schlagenhauf F, Sterzer P, et al. Context insensitivity during positive and negative emotional expectancy in depression assessed with functional magnetic resonance imaging. Psychiatry Research. 2013;212:28–35. doi: 10.1016/j.pscychresns.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Feldman G, Greeson J, Senville J. Differential effects of mindful breathing, progressive muscle relaxation, and loving-kindness meditation on decentering and negative reactions to repetitive thoughts. Behaviour Research and Therapy. 2010;48:1002–11. doi: 10.1016/j.brat.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Clinician Version, Administration Booklet. Washington, DC: American Psychiatric Press; 1996. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) [Google Scholar]
- Frewen PA, Dozois DJA, Neufeld RWJ, et al. Individual differences in trait mindfulness predict dorsomedial prefrontal and amygdala response during emotional imagery: an fMRI study. Personality and Individual Differences. 2010;49:479–84. [Google Scholar]
- Gard T, Holzel BK, Sack AT, et al. Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb Cortex. 2012;22:2692–702. doi: 10.1093/cercor/bhr352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Gaylord SA, Fredrickson BL. Positive reappraisal mediates the stress-reductive effects of mindfulness: An upward spiral process. Mindfulness. 2011;2:59–67. [Google Scholar]
- Germer CK, Siegel RD, Fulton PR. Mindfulness and Psychotherapy. New York: Guilford Press; 2005. [Google Scholar]
- Greening SG, Osuch EA, Williamson PC, Mitchell DG. The neural correlates of regulating positive and negative emotions in medication-free major depression. Social Cognitive and Affective Neuroscience. 2014;9:628–37. doi: 10.1093/scan/nst027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeson JM, Webber DM, Smoski MJ, et al. Changes in spirituality partly explain health-related quality of life outcomes after Mindfulness-Based Stress Reduction. Journal of Behavioural Medicine. 2011;34:508–18. doi: 10.1007/s10865-011-9332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA Psychiatry. 2013;70:1181–9. doi: 10.1001/jamapsychiatry.2013.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Heering S, Sawyer AT, Asnaani A. How to handle anxiety: the effects of reappraisal, acceptance, and suppression strategies on anxious arousal. Behaviour Research and Therapy. 2009;47:389–94. doi: 10.1016/j.brat.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives-Deliperi VL, Solms M, Meintjes EM. The neural substrates of mindfulness: an fMRI investigation. Society for Neuroscience. 2011;6:231–42. doi: 10.1080/17470919.2010.513495. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jermann F, Billieux J, Laroi F, et al. Mindful Attention Awareness Scale (MAAS): psychometric properties of the French translation and exploration of its relations with emotion regulation strategies. Psychological Assessment. 2009;21:506–14. doi: 10.1037/a0017032. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Wherever you go there you are: Mindfulness meditation in everyday life. New York, NY: Hyperion; 1994. [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Wessa M. Neural correlates of emotion regulation deficits in remitted depression: the influence of regulation strategy, habitual regulation use, and emotional valence. NeuroImage. 2012;61:686–93. doi: 10.1016/j.neuroimage.2012.03.089. [DOI] [PubMed] [Google Scholar]
- Keng SL, Robins CJ, Smoski MJ, Dagenbach J, Leary MR. Reappraisal and mindfulness: a comparison of subjective effects and cognitive costs. Behaviour Research and Therapy. 2013;51:899–904. doi: 10.1016/j.brat.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keng SL, Smoski MJ, Robins CJ. Effects of mindfulness on psychological health: a review of empirical studies. Clinical Psychology Review. 2011;31:1041–56. doi: 10.1016/j.cpr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Miller JM, Schneck N, et al. Neural responses to incongruency in a blocked-trial Stroop fMRI task in major depressive disorder. Journal of Affective Disorders. 2012;143:241–7. doi: 10.1016/j.jad.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyken W, Byford S, Taylor RS, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. Journal of Consulting and Clinical Psychology. 2008;76:966–78. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Hoberman HM, Rosenbaum M. A prospective study of risk factors for unipolar depression. Journal of Abnormal Psychology. 1988;97:251–64. doi: 10.1037//0021-843x.97.3.251. [DOI] [PubMed] [Google Scholar]
- Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. Journal of Consulting and Clinical Psychology. 2004;72:31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- Mikels JA, Fredrickson BL, Larkin GR, Lindberg CM, Maglio SJ, Reuter-Lorenz PA. Emotional category data on images from the International Affective Picture System. Behavior Research Methods. 2005;37:626–30. doi: 10.3758/bf03192732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–8. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Okada G, Okamoto Y, Yamashita H, Ueda K, Takami H, Yamawaki S. Attenuated prefrontal activation during a verbal fluency task in remitted major depression. Psychiatry and Clinical Neurosciences. 2009;63:423–5. doi: 10.1111/j.1440-1819.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN. The role of social cognition in emotion. Trends in Cognitive Sciences. 2008;12:65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nature Neuroscience. 2007;10:1116–24. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2013;37:2529–53. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Robins CJ, Keng SL, Ekblad AG, Brantley JG. Effects of mindfulness-based stress reduction on emotional experience and expression: a randomized controlled trial. Journal of Clinical Psychology. 2012;68:117–31. doi: 10.1002/jclp.20857. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York: Guilford Press; 2002. [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12:154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SL, Brown KW, Biegel GM. Teaching self-care to caregivers: effects of mindfulness-based stress reduction on the mental health of therapists in training. Training and Education in Professional Psychology. 2007;1:105–15. [Google Scholar]
- Shea T, Elkin I, Imber SD, et al. Course of depressive symptoms over follow-up. Findings from the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Archives of General Psychiatry. 1992;49:782–7. doi: 10.1001/archpsyc.1992.01820100026006. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry. 2006;163:735–8. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Keng SL, Schiller CE, Minkel J, Dichter GS. Neural mechanisms of cognitive reappraisal in remitted major depressive disorder. Journal of Affecticve Disorders. 2013;151:171–7. doi: 10.1016/j.jad.2013.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szasz PL, Szentagotai A, Hofmann SG. The effect of emotion regulation strategies on anger. Behaviour Research and Therapy. 2011;49:114–9. doi: 10.1016/j.brat.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Taylor VA, Grant J, Daneault V, et al. Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. NeuroImage. 2011;57:1524–33. doi: 10.1016/j.neuroimage.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology. 2000;68:615–23. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttl B. North American Adult Reading Test: age norms, reliability, and validity. Journal of Clinical and Experimental Neuropsychology. 2002;24:1123–37. doi: 10.1076/jcen.24.8.1123.8375. [DOI] [PubMed] [Google Scholar]
- Wang L, LaBar KS, Smoski M, et al. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Research. 2008;163:143–55. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, McCarthy G, Song AW, LaBar KS. Amygdala activation to sad pictures during high-field (4 Tesla) functional magnetic resonance imaging. Emotion. 2005;5:12–22. doi: 10.1037/1528-3542.5.1.12. [DOI] [PubMed] [Google Scholar]
- Wolgast M, Lundh LG, Viborg G. Cognitive reappraisal and acceptance: an experimental comparison of two emotion regulation strategies. Behaviour Research and Therapy. 2011;49:858–66. doi: 10.1016/j.brat.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]