Abstract
Humans show stronger empathy for in-group compared with out-group members’ suffering and help in-group members more than out-group members. Moreover, the in-group bias in empathy and parochial altruism tend to be more salient in collectivistic than individualistic cultures. This work tested the hypothesis that modifying self-construals, which differentiate between collectivistic and individualistic cultural orientations, affects in-group bias in empathy for perceived own-race vs other-race pain. By scanning adults using functional magnetic resonance imaging, we found stronger neural activities in the mid-cingulate, left insula and supplementary motor area (SMA) in response to racial in-group compared with out-group members’ pain after participants had been primed with interdependent self-construals. However, the racial in-group bias in neural responses to others’ pain in the left SMA, mid-cingulate cortex and insula was significantly reduced by priming independent self-construals. Our findings suggest that shifting an individual’s self-construal leads to changes of his/her racial in-group bias in neural responses to others’ suffering.
Keywords: empathy, race, in-group bias, self-construal, fMRI
Introduction
Social intergroup relationships strongly modulate human behavior and mind. For example, people may contribute to in-group’s welfare at the cost of self-interest but to aggress against out-group members (Henrich et al., 2006; Choi and Bowles, 2007). Such behavioral parochial altruism has been associated with empathy, i.e. the ability to understand and share others’ emotions. Recent neuroimaging findings indicate that people more strongly share racial in-group members’ painful feelings compared with those of out-group members (Xu et al., 2009; Avenanti et al., 2010; Mathur et al., 2010; Sheng and Han, 2012; Azevedo et al., 2013; Contreras-Huerta et al., 2013; Sheng et al., 2014). Perceived painful stimulation applied to own-race compared with other-race individuals or perceived pain expression of own-race compared with other-race individuals elicits greater activity in the anterior cingulate (Xu et al., 2009; Contreras-Huerta et al., 2013; Sheng et al., 2014), anterior insula (Azevedo et al., 2013; Contreras-Huerta et al., 2013; Sheng et al., 2014) and medial prefrontal cortex (Mathur et al., 2010), and induces stronger modulations of the sensorimotor activity (Avenanti et al., 2010), indicating racial in-group bias in neural responses to others’ suffering. Because empathic concern produces altruistic motivation (Batson, 2011), in-group bias in empathy may provide a mechanism of behavioral parochial altruism. Indeed, it has been shown that in-group bias in neural responses to perceived pain in others can predict motivations to self-sacrifice to help in-group members (Hein et al., 2010; Mathur et al., 2010).
Interestingly, recent research suggests that parochial altruism is more prevalent in collectivistic societies compared with individualistic societies (Gelfand et al., 2012). Moreover, individuals from collectivistic compared with individualistic cultures show stronger racial in-group bias in neural responses to perceived pain in others. Mathur et al. (2010) reported stronger activity in the media prefrontal cortex to perceived pain in racial in-group than out-group individuals and the racial in-group bias in empathic neural response was greater in African compared with Caucasian-American participants. Similarly, Cheon et al. (2011) found evidence for racial in-group bias in the neural activity in temporoparietal junction to perceived pain and this effect was stronger in Korean compared with Caucasian-American participants. Although these findings uncovered cultural differences in parochial altruism and in-group bias in empathy, the underlying psychological and neural mechanisms remain unknown. One possibility is that interdependent vs independent self-construals that, respectively, dominate collectivistic and individualistic cultures (Markus and Kitayama, 1991) may provide a cognitive basis of in-group bias in empathy, which in turn influences behavioral parochial altruism. According to Markus and Kitayama (2010), an independent schema of self organizes behavior primarily in reference to an individual’s own thoughts and feelings and results in a weakened sense of in-group/out-group relationship so that people can move between in-group and out-group relatively easily. In contrast, an interdependent schema of self organizes behavior immediately in reference to the thoughts and feelings of others with whom a person is in relationship and leads to significant ingroup–outgroup distinction. This in turn results in different feeling and behavior toward in-group and out-group members. On the ground of this framework, we hypothesized that interdependent self-construals may drive in-group bias in empathy, which, however, can be reduced when independent self-construals are promoted.
To test this hypothesis, we measured neural responses to racial in-group and out-group members’ suffering after participants had been primed with interdependent or independent self-construals (Gardner et al., 1999). Self-construal priming has been widely used in recent brain imaging studies that have shown that temporary shifts of self-construals influence multiple neurocognitive processes in the human brain, including pain-related sensory processing (Wang et al., 2014), visual perceptual processing (Lin et al., 2008), self-face recognition (Sui and Han, 2007; Sui et al., 2013), reflection of personality traits of a close other (Harada et al., 2010), empathy for others’ pain (Jiang et al., 2014), motor cortical output during an action observation task (Obhi et al., 2011), monetary reward (Varnum et al., 2014), and resting state activity (Wang et al., 2013). These brain imaging results have shown robust evidence for the effect of self-construal priming on human brain activity involved in cognitive/affective processes. Using similar interdependent/independent self-construal priming procedures, this study recorded neural responses to perceived pain in others using functional magnetic resonance imaging (fMRI) as fMRI blood oxygen level dependent (BOLD) signals are much less influenced by social desirability compared with self-report empathy (e.g. Xu et al., 2009). We predicted that racial in-group bias in neural responses to perceived pain in others occurs when interdependent self-construals are promoted and that racial in-group bias in neural responses to perceived pain in others is decreased when independent self-construals are encouraged. Such findings would support a causal relationship between self-construals and racial in-group bias in empathy and suggest that racial emotional prejudice can be reduced by changing self-construals.
Materials and Methods
Participants
Thirty-two Chinese university students were recruited as paid volunteers. Two participants were excluded from data analysis due to excessive head movement during scanning. Thus, 30 participants (16 males, 14 females; 18–27 years, mean ± s.d. = 22.6 ± 2.4 years) were included in data analyses. This sample size was determined using a statistic estimation (VanVoorhis and Morgan, 2007) before data collection based on the effect size (ES) of self-construal priming (i.e. 0.51, Oyserman and Lee, 2008), a significance criterion of 0.05 and a power of 0.85. All were right handed, had normal or corrected-to-normal vision, and reported no abnormal neurological history. Informed consent was obtained before scanning. This study was approved by a local ethics committee.
Stimuli and procedure
The priming materials, similar to those used in our previous research (e.g. Wang et al., 2013), were four Chinese essays about tours. Two essays contained singular pronouns (‘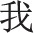 ’, Chinese character of ‘I’ or ‘me’) as target words to prime independent self-construal and two essays contained plural pronouns (‘
’, Chinese character of ‘I’ or ‘me’) as target words to prime independent self-construal and two essays contained plural pronouns (‘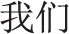 ’, Chinese characters of ‘we’ or ‘us’) as target words to prime interdependent self-construal. Each essay consisted of 18 sentences with target words in 12 sentences. The stimuli for induction of neural responses to perceived pain in others were adopted from Xu et al. (2009), consisting of 48 video clips showing faces of six Asian (three males) and six Caucasian models (three males) with neutral expressions who received painful (needle penetration) or non-painful (Q-tip touch) stimulation applied to the left or right cheeks. Each clip lasted for 3 s and subtended a visual angle of 21° × 17° (width × height) at a viewing distance of 80 cm.
’, Chinese characters of ‘we’ or ‘us’) as target words to prime interdependent self-construal. Each essay consisted of 18 sentences with target words in 12 sentences. The stimuli for induction of neural responses to perceived pain in others were adopted from Xu et al. (2009), consisting of 48 video clips showing faces of six Asian (three males) and six Caucasian models (three males) with neutral expressions who received painful (needle penetration) or non-painful (Q-tip touch) stimulation applied to the left or right cheeks. Each clip lasted for 3 s and subtended a visual angle of 21° × 17° (width × height) at a viewing distance of 80 cm.
An event-related design was used in four functional scans. Before each scan, participants underwent a priming session that lasted for 108 s. Participants read one essay sentence by sentence during the priming procedure. Each sentence was displayed for 4 s followed by a fixation of 2 s. Participants were asked to judge whether a target pronoun was present in each sentence. There were two scans following the independent self-construal priming and two scans following the interdependent self-construal priming. The order of independent or interdependent self-construal priming was counterbalanced across participants. Each scan consisted of 48 trials and lasted for 288 s. On each trial, a 3 s video clip was presented and followed by a fixation of 1, 3 or 5 s. Participants were instructed to judge whether the model in a video clip was feeling pain. Participants responded to priming sentences and video clips by pressing a button using the right index or middle finger. The video clips of Asian and Caucasian models receiving painful and non-painful stimuli were presented in a pseudo-random order during scanning. A 4.5 min structural scan was conducted after the first two functional scans.
After the scanning procedure, participants were shown the video clips again and instructed to rate pain feelings of each model (‘How painful do you think the model feels?’) and unpleasantness felt by the onlooker (‘How unpleasant do you feel when observing the video clip?’) using a 7-point Likert-type scale (1 = not painful or unpleasant at all, 7 = extremely painful or unpleasant). Individuals’ attitudes of ethnic identity were assessed using the Multigroup Ethnic Identity Measure (Phinney, 1992; 1 = strongly disagree, 4 = strongly agree). The degree of endorsement of independent and interdependent self-construals was estimated using the 24-item Self-Construal Scale (Singelis, 1994; 1 = strongly disagree, 7 = strongly agree). Individual differences in empathy traits were measured using the 28-item Interpersonal Reactivity Index (IRI; Davis, 1983; 0 = does not describes me well, 4 = describes very well), which consisted of four subscales: perspective taking, fantasy, empathic concern and personal distress.
Imaging parameters and data analysis
Image acquisition was conducted on a GE 3.0 T MR scanner (HDx, Signa MR 750 System; GE Healthcare, Milwaukee, WI) with a standard head coil. Functional images were acquired by using T2-weighted, gradient-echo, echo-planar imaging sequences sensitive to BOLD contrast [64 × 64 matrix, 32 slices, 3.75 × 3.75 × 5.00 mm voxel; repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, field of view (FOV) =24 × 24 cm, flip angle (FA) = 90°]. A high-resolution anatomical T1-weighted image was acquired for each participant (512 × 512 matrix, 180 slices, 0.47 × 0.47 × 1.00 mm voxel; TR = 8.204 ms, TE = 3.22 ms, FOV = 24 × 24 cm, FA = 12°).
Functional images were preprocessed using SPM8 (the Wellcome Trust Centre for Neuroimaging, London, UK). The functional data were first time corrected to compensate for delays associated with acquisition time differences between slices during the sequential imaging. The functional images were then realigned to the first scan to correct for head motion within and between scans. The anatomical image was coregistered with the mean realigned functional image and then was normalized to a 1 × 1 × 1 mm3 Montreal Neurological Institute (MNI) template. The functional images were normalized to a 3 × 3 × 3 mm3 MNI space and then spatially smoothed using an isotropic of 8 mm full-width half-maximum Gaussian kernel.
A general linear model was applied to the fMRI data. Parameter estimations were conducted by convolving the images in a design matrix with a hemodynamic response function. Fixed effect analyses were first performed to estimate effects at each voxel and to compare regionally specific effects in individual participants using linear contrasts. To assess neural responses to perceived pain in others, contrast values of painful vs non-painful stimuli were calculated by collapsing fMRI data in all conditions. Random effect analyses were then conducted across all participants based on statistical parameter maps from each individual participant to allow population inference.
To test our hypothesis, we first conducted region of interest (ROI) analyses to examine priming effects on racial in-group bias in neural responses to perceived pain in others. We defined ROIs based on a meta-analysis of 50 fMRI studies of empathy for pain (Fan et al., 2011) because the brain regions involved in empathy for pain identified in the meta-analysis are, to a certain degree, independent of stimuli, tasks and participants. Five ROIs that show neural responses to perceived pain in others were defined as spheres with a radius of 5 mm centered at MNI coordinates −6/20/46 (mid-cingulate cortex, MCC),1 −42/18/0 (left insula), 38/24/−2 (right insula), −4/14/54 (left supplementary motor area, SMA) and 6/8/60 (right SMA). Contrast values of painful vs non-painful stimuli were extracted from each ROI using the Toolbox of MarsBar, which were then subjected to a repeated measure analysis of variance (ANOVA) with Priming (independent vs interdependent) and Race (Asian vs Caucasian models) as within-subjects independent variables. Furthermore, original ES and standardized ES (Cumming, 2014) were estimated. As we assumed a racial in-group bias in empathy for others’ suffering, original ES of racial bias was defined as the difference in contrast values of painful vs non-painful stimuli applied to Asian vs Caucasian models. Standardized ES was defined as Cohen’s d:
where MAS was the mean of contrast values of painful vs non-painful stimuli applied to Asian models, MCA was the mean of contrast values of painful vs non-painful stimuli applied to Caucasian models, and SCA was the standard deviation of contrast values of painful vs non-painful stimuli applied to Caucasian models. Confidence interval (CI) reported along with the ES referred to 95% CI. We also conducted whole-brain analyses using a 2 × 2 factorial design with Priming and Race as independent variables to assess the priming effects on racial in-group bias in empathic neural activity (i.e. the contrast of painful vs non-painful stimuli) in other brain regions.
Results
Behavioral results
The response accuracies during the identification of painful and non-painful stimuli during scanning were high (interdependent priming: Chinese faces: 95.5 ± 7.8%, Caucasians faces: 95.0 ± 8.9%; independent priming: Chinese faces: 97.2 ± 5.1%, Caucasians faces: 95.4 ± 9.5%). Response accuracies were subjected to ANOVAs with Priming (interdependent vs independent priming) and Race (Asian vs Caucasian models). Neither the main effect of Priming or Race nor their interaction reached significance [F(1,29) = 0.784–2.100, Ps > 0.1].
Rating scores of pain intensity and self-unpleasantness after scanning were subjected to ANOVAs with Pain (painful vs non-painful stimuli) and Race (Asian vs Caucasian models) as within-subjects independent variables. This revealed a significant main effect of Pain on ratings of pain intensity and self-unpleasantness [F(1,29) = 207.6 and 195.6, d = 5.63 and 5.26, both Ps < 0.001], suggesting stronger feelings of others’ pain and one’s own unpleasantness when perceiving painful compared with non-painful stimuli. However, the interaction of Pain and Race did not reach significant [F(1,29) < 1 and F(1,29) = 1.461, both Ps > 0.2; Table 1], suggesting similar subjective feelings of Asian and Caucasian models’ pain. Thus, self-report of one’s subjective feeling did not show racial in-group bias.
Table 1.
Rating scores of pain intensity and self-unpleasantness (mean ± s.d.)
| Video types | Chinese face |
Caucasian face |
||
|---|---|---|---|---|
| Needle | Q-tip | Needle | Q-tip | |
| Pain intensity | 5.66 ± 1.51 | 1.60 ± 0.72 | 5.64 ± 1.52 | 1.62 ± 0.86 |
| Self-unpleasantness | 5.31 ± 1.26 | 1.72 ± 0.78 | 5.11 ± 1.38 | 1.67 ± 0.77 |
The mean ethnic identity score was 2.91 ± 0.34, suggesting similar ethnic identity in our participants and Asian samples reported in the previous work (Phinney, 1992). Self-report revealed slightly more endorsement of interdependent than independent self-construals [4.98 ± 0.70 vs 4.75 ± 0.66, t(29) = 1.908, P = 0.066]. IRI rating scores of the subscales were 2.31 ± 0.51 (perspective takings), 2.39 ± 0.68 (fantasy), 1.89 ± 0.59 (empathic concern) and 1.96 ± 0.47 (personal distress).
fMRI results
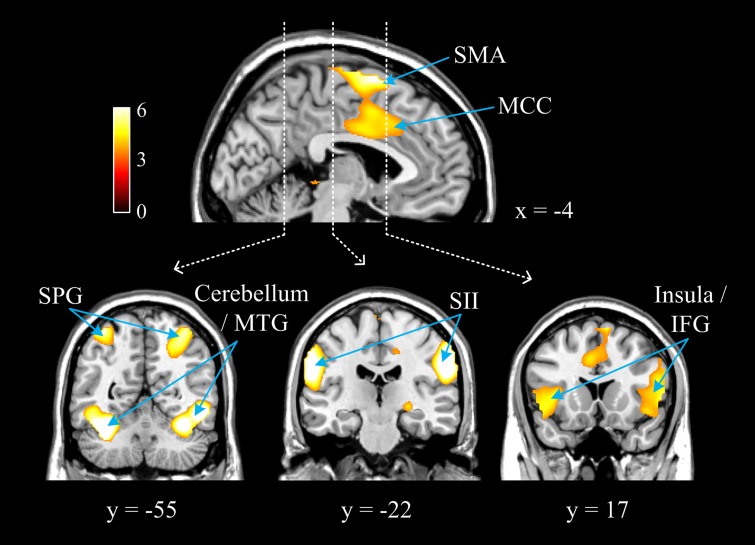
A whole-brain analysis of fMRI data was conducted to identify neural responses to others’ suffering. The contrast of painful vs non-painful stimuli across all conditions revealed significant activations at a threshold of P < 0.05 (cluster-level corrected) in the MCC, bilateral SMA, bilateral insula/inferior frontal gyrus (IFG), bilateral second somatosensory cortex (SII), bilateral superior parietal gyrus (SPG), bilateral middle temporal gyrus (MTG) and bilateral posterior cerebellum (Figure 1 and Table 2).2 These results replicate the previous findings that perceiving others’ pain activates parts of the typical pain matrix (Fan et al., 2011; Lamm et al., 2011).
Fig. 1.
Illustration of enhanced neural responses to painful compared with non-painful stimuli across all conditions.
Table 2.
Neural activations shown in the contrast of painful vs non-painful stimuli
| Brain region | k (voxels) | t-value | Peak coordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| Right SMA | 879 | 7.06 | 9 | 2 | 67 |
| Left SMA | 6.46 | −3 | 8 | 70 | |
| MCC | 4.65 | 0 | −1 | 34 | |
| Left insula/IFG | 2174 | 9.24 | −57 | 5 | 1 |
| 7.89 | −42 | −4 | 13 | ||
| Right insula/ IFG | 1264 | 7.37 | 60 | 11 | 7 |
| 7.20 | 57 | 11 | 22 | ||
| Right MTG | 2302 | 8.61 | 51 | −55 | −11 |
| Right SII/SPC | 8.13 | 57 | −22 | 34 | |
| Right posterior cerebellum | 8.10 | 36 | −52 | −23 | |
| Left posterior cerebellum | 1172 | 9.57 | −33 | −61 | −26 |
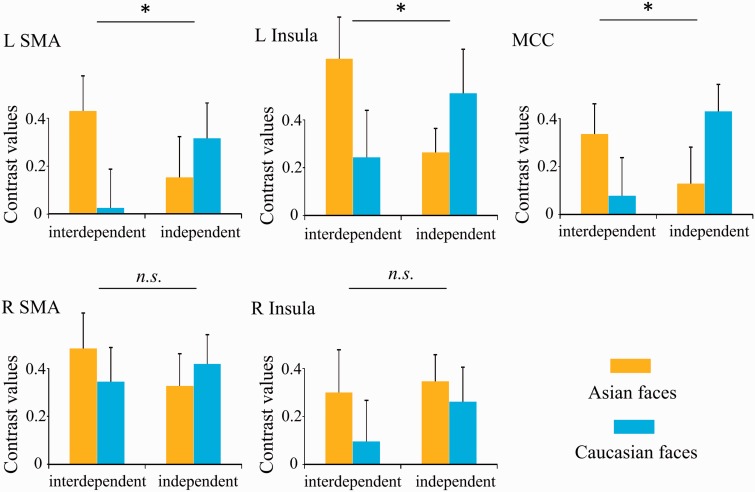
ROI analyses were conducted to test the hypothesis that priming independence reduces the racial in-group bias in neural responses to perceived pain in others. ANOVAs of contrast values of painful vs non-painful stimuli did not show significant main effects of Priming (F < 1, Ps > 0.5) or Race [F(1,29) = 0.033–1.328, Ps > 0.25] in any of the five ROIs. However, ANOVAs of the contrast values revealed significant interactions of Priming × Race in the left SMA [F(1,29) = 4.882, P = 0.035], MCC [F(1,29) = 5.144, P = 0.031] and left insula [F(1,29) = 4.643, P = 0.040], indicating distinct patterns of neural responses to perceived pain in Asian and Caucasian models in the two priming conditions, i.e. stronger neural responses to perceived pain in others to Asian compared with Caucasian models after the interdependent self-construal priming but a reverse pattern after the independent self-construal priming (Figure 2). Post hoc analyses revealed significantly stronger activity in the left SMA and marginally significantly stronger activity in the left insula to perceived pain in racial in-group compared with out-group individuals in the interdependent self-construal priming condition (t = 2.144 and 1.782, P = 0.041 and 0.085) but not in the independent self-construal priming condition (t = −0.967 and −1.251, P = 0.342 and 0.221). In contrast, there was significantly stronger activity in the MCC to perceived pain in racial out-group compared with in-group individuals in the independent self-construal priming condition (t = −2.095, P = 0.045) but not in the interdependent self-construal priming condition (t = 1.335, P = 0.192). Pairwise comparisons across priming conditions were also conducted, which identified stronger MCC activation to out-group faces under independent vs interdependent priming (t = 2.121, P = 0.043).
Fig. 2.
Contrast values to painful vs non-painful stimuli extracted from pre-defined ROIs. Error bars denote standard errors. *Significant interactions of Priming × Race in the brain regions (P < 0.05).
Similarly, the racial in-group bias in neural responses to perceived pain in others in the right SMA and insula tended to be reduced in the independent compared with interdependent priming conditions, though the effects did not reach significance [right SMA: F(1,29) = 1.092, P = 0.305; right insula: F(1,29) = 0.271, P = 0.607; Figure 2]. To further estimate the difference of racial in-group bias in neural responses to perceived pain in others, we calculated the original and standardized ESs of racial in-group bias in neural responses to perceived pain in others in each condition. As can be seen in Table 3, the mean ESs of racial in-group bias in the all ROIs were decreased in the independent relative to interdependent self-construal priming conditions.
Table 3.
ES of racial in-group bias for each ROI
| L SMA | R SMA | MCC | L insula | R insula | |
|---|---|---|---|---|---|
| Interdependent | |||||
| Original ES | 0.35 | 0.11 | 0.21 | 0.33 | 0.17 |
| 95% CI | [0.03, 0.67] | [−0.11, 0.34] | [−0.10, 0.52] | [−0.03, 0.70] | [−0.15, 0.48] |
| Cohen’s d | 0.49 | 0.18 | 0.30 | 0.38 | 0.22 |
| Independent | |||||
| Original ES | −0.11 | −0.05 | −0.24 | −0.18 | 0.07 |
| 95% CI | [−0.45, 0.22] | [−0.35, 0.24] | [−0.52, 0.05] | [−0.53, 0.16] | [−0.23, 0.37] |
| Cohen’s d | −0.18 | −0.10 | −0.47 | −0.23 | 0.11 |
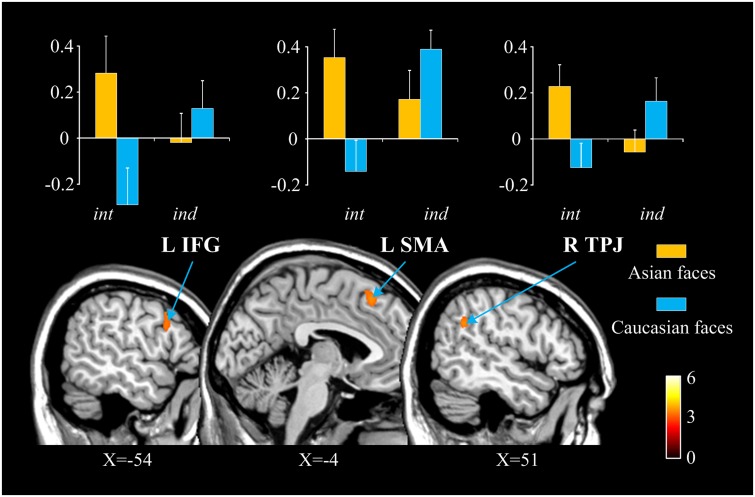
We also conducted a whole-brain Priming × Race interaction analysis of the contrast of painful vs non-painful stimuli to examine the modulations of racial in-group bias in neural responses to perceived pain in others by self-construal priming. This analysis did not show any activation at a threshold of P < 0.05 (FDR cluster-level corrected). However, using a voxel-level threshold of P < 0.001 and an extent threshold k > 20 voxels (Lieberman and Cunningham, 2009), this analysis revealed significant activations in the left SMA (−3/26/52, k = 21), left IFG (−50/17/31, k = 23) and right temporo-parietal junction (51/−52/31, k = 26). As can be seen in Figure 3, the pattern of modulations of racial in-group bias in neural responses to perceived pain in others in these brain areas by self-construal priming was similar to those observed in the ROI analyses. Post hoc analyses revealed significantly stronger activity in all the three brain regions to perceived pain in racial in-group compared with out-group individuals in the interdependent self-construal priming condition (t = 2.578–2.787, P = 0.009–0.015) but not in the independent self-construal priming condition (t = −1.181 and −0.893, P = 0.081 and 0.379).
Fig. 3.
Illustration of activated brain regions in the whole-brain interaction analysis. Contrast values to painful vs non-painful stimuli in each brain region are shown in the upper panel. int, interdependent self-construal priming; ind, independent self-construal priming.
Finally, we calculated correlations between neural responses in all the ROIs related to empathy for pain, racial in-group bias, and priming effects on racial in-group bias and subjective ratings of self-construals, empathy traits and ethnic identity. But these analyses did not show any significant results (Ps > 0.05).
Discussion
Our neuroimaging results first revealed reliable neural responses to others’ suffering, i.e. viewing others in pain significantly activated the neural circuit consisting of the MCC, SMA, insula/IFG and SII. This replicates the previous fMRI findings (Singer et al., 2004; Jackson et al., 2005; Gu and Han, 2007; Han et al., 2009). More interestingly, our results uncovered that the racial in-group bias in neural responses to perceived pain in others was significantly modulated by self-construal priming. Specifically, we found that viewing racial in-group compared with out-group members in pain elicited stronger responses in the MCC, left SMA and insula when interdependent self-construals were primed. In contrast, the racial in-group bias in neural responses to perceived pain in others was eliminated when independent self-construals were primed. The modulations of racial in-group bias in neural responses to perceived pain in others were confirmed in the ROI, whole-brain and ES analyses, though self-report did not show any racial in-group bias possibly due to the influences of social desirability. Our fMRI results in the condition of interdependent self-construal priming replicated the previous findings of racial in-group bias in empathic neural responses (Xu et al., 2009; Azevedo et al., 2013; Contreras-Huerta et al., 2013; Sheng et al., 2014) and the fMRI results in the independent self-construal priming provide the first evidence that the independent self-construal functions to reduce the in-group bias in empathy.
Our findings are consistent with a social-cognitive model of the link between self-construals and intergroup relationship (Markus and Kitayama, 2010) that suggests that interdependent self-construals induce a strong boundary between in-group and out-group whereas independent self-construals define a strong boundary between the self and any others and lead to a weakened boundary between in-group and out-group. The causal relationship between independent self-construals and decreased racial in-group bias in empathy, as indicated by our fMRI results, suggests a possible mechanism of weakened racial in-group bias in empathic neural responses in individualistic cultures that are characterized with independent self-construals compared with collectivistic cultures that are dominated by interdependent self-construals (Mathur et al., 2010; Cheon et al., 2011; Zuo and Han, 2013). The MCC and left anterior insula (AI) activities are associated with both affective and cognitive processes of empathy (Fan et al., 2011). The in-group bias in the left AI activity also predicts individual differences in helping in-group members (Hein et al., 2010). The right temporoparietal junction (TPJ) and left SMA are, respectively, associated with mental state inference (Cheon et al., 2011; Sheng et al., 2014) and motor motivation (Fan et al., 2011) during empathy. Given the functional roles of these brain regions in empathy, our findings further suggested that self-construals may influence parochial altruism in collectivistic and individualistic societies (Gelfand et al., 2012) by modulating in-group bias in empathy.
The neural activity in all the ROIs showed a similar pattern that, relative to interdependent self-construal priming, independent self-construal priming tended to reduce the neural activity to racial in-group members’ pain but to increase the neural activity to racial out-group members’ pain, though the cross-priming comparison only confirmed a significant effect on the MCC activity to racial out-group members’ pain. Therefore, priming independent vs interdependent self-construals may produce opposite effects on neural responses to racial in-group and out-group members’ suffering. One possible account is that independent self-construal priming shifted our participants’ cultural identity toward Western cultures and Caucasian models were thus treated as in-group members, which in turn enhanced neural responses to their suffering. Alternatively, priming independent self-construals may weaken race-based group affiliation between Chinese participants and Chinese models shown in video clips. In such a context, participants became more sensitive to other-race individuals’ pain that may imply a danger signal but less sensitive to own-race individuals’ pain that implicates requirement for help. Although these possible accounts should to be verified in future research, the findings of differential neural responses to racial in-group and out-group individuals raise an interesting issue, i.e. does priming independent compared with interdependent self-construals undermine behavioral parochial altruism?
Chronic self-construals measured from participants in this study did not correlate with patterns of their racial in-group bias in neural activity. One possible account is that the chronic interdependent self-construal style did not vary significantly across our participants who were all educated in China. Another possibility is that empathic neural responses were measured in the current experiment after participants had been primed with independent or interdependent self-construals. The priming procedure may reduce the effect of chronic self-construals.
Our finding that independent self-construal priming reduced the racial bias in neural responses to perceived pain in others in the MCC/SMA and insula does not necessarily indicate the absence of racial in-group bias in individuals from Western cultures. Behavioral studies have reported evidence for racial in-group favoritism in Westerners (Johnson et al., 2002; Drwecki et al., 2011). Brain imaging studies also reported evidence for racial in-group bias in neural responses to perceived pain in others in Westerners (e.g. Xu et al., 2009; Avenanti et al., 2010). The findings of this study raised an interesting question, i.e. does interdependent self-construal priming increase Westerners’ racial in-group bias in empathic neural responses to others’ suffering? To address this issue would help us to understand the opposite effects of independent/interdependent self-construal priming on racial in-group bias in empathy. Previous studies have revealed that multiple factors influence neural activity in response to others’ suffering, including attention and stimulus reality (Gu and Han, 2007; Fan and Han, 2008), ones’ expertise (Cheng et al., 2007), perceived fairness (Singer et al., 2006), emotional contexts (Han et al., 2009) and mortality salience (Luo et al., 2014), and personal experiences (Zuo and Han, 2013). Therefore, how the human brain responds to perceived pain in others depends on both social contexts/experiences and perceivers’ psychological traits, which in turn produces strong impact on human social behaviors.
In conclusion, our neuroimaging results provide new insight into the causal relationship between self-construals and racial in-group bias in empathy. Our findings that self-construals modulate cognitive/affective processes involved in empathy for in-group and out-group members’ emotional states suggest a mechanism of cultural differences in parochial altruism (Gelfand et al., 2012).
Conflict of Interest
None declared.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Project 31470986, 31421003, 91332125, 81161120539) and the Ministry of Education of China (Project 20130001110049). We thank Xiuyun Wu for helping with subject recruitment.
*C. W. and B. W. contributed equally to this study.
Footnotes
1 All ROIs were adopted from Table 1 of Fan et al. (2011), expect that MCC (−6/20/46) was adopted from Table 2 of Fan et al. (2011). This is because, according to Fan et al. (2011), the dorsal MCC (−6/20/46) is recruited more frequently in the cognitive-evaluative form of empathy, and the racial bias in in-group empathy for pain was also observed in a cognitive-evaluation task [e.g. pain vs no-pain judgments on each video clip in Xu et al. (2009)]. Thus, we choose the dorsal MCC (−6/20/46) as the ROI in our work.
References
- Avenanti A, Sirigu A, Aglioti SM. Racial bias reduces empathic sensorimotor resonance with other-race pain. Current Biology. 2010;20:1018–22. doi: 10.1016/j.cub.2010.03.071. [DOI] [PubMed] [Google Scholar]
- Azevedo RT, Macaluso E, Avenanti A, Santangelo V, Cazzato V, Aglioti SM. Their pain is not our pain: brain and autonomic correlates of empathic resonance with the pain of same and different race individuals. Human Brain Mapping. 2013;34:3168–81. doi: 10.1002/hbm.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson CD. Altruism in Humans. New York: Oxford University Press; 2011. [Google Scholar]
- Cheng Y, Lin CP, Liu HL, et al. Expertise modulates the perception of pain in others. Current Biology. 2007;17:1708–13. doi: 10.1016/j.cub.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Cheon BK, Im DM, Harada T, et al. Cultural influences on neural basis of intergroup empathy. NeuroImage. 2011;57:642–50. doi: 10.1016/j.neuroimage.2011.04.031. [DOI] [PubMed] [Google Scholar]
- Choi JK, Bowles S. The coevolution of parochial altruism and war. Science. 2007;318:636–40. doi: 10.1126/science.1144237. [DOI] [PubMed] [Google Scholar]
- Contreras-Huerta LS, Baker KS, Reynolds KJ, Batalha L, Cunnington R. Racial bias in neural empathic responses to pain. PLoS One. 2013;8(12):e84001. doi: 10.1371/journal.pone.0084001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming G. The new statistics: why and how. Psychological Science. 2014;25:7–29. doi: 10.1177/0956797613504966. [DOI] [PubMed] [Google Scholar]
- Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44:113–26. [Google Scholar]
- Drwecki BB, Moore CF, Ward SE, Prkachin KM. Reducing racial disparities in pain treatment: the role of empathy and perspective-taking. Pain. 2011;152:1001–6. doi: 10.1016/j.pain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Fan Y, Han S. Temporal dynamic of neural mechanisms involved in empathy for pain: an event-related brain potential study. Neuropsychologia. 2008;46:160–73. doi: 10.1016/j.neuropsychologia.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience & Biobehavioral Reviews. 2011;35:903–11. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Gardner WL, Gabriel S, Lee AY. “I” value freedom, but “we” value relationships: self-construal priming mirrors cultural differences in judgment. Psychological Science. 1999;10:321–6. [Google Scholar]
- Gelfand M, Shteynberg G, Lee T, et al. The cultural contagion of conflict. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2012:367, 692–703. doi: 10.1098/rstb.2011.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. NeuroImage. 2007;36:256–67. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Han S, Fan Y, Xu X, et al. Empathic neural responses to others’ pain are modulated by emotional contexts. Human Brain Mapping. 2009;30:3227–37. doi: 10.1002/hbm.20742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Li Z, Chiao JY. Differential dorsal and ventral medial prefrontal representations of the implicit self modulated by individualism and collectivism: an fMRI study. Social Neuroscience. 2010;5:257–71. doi: 10.1080/17470910903374895. [DOI] [PubMed] [Google Scholar]
- Hein G, Silani G, Preuschoff K, Batson CD, Singer T. Neural responses to ingroup and outgroup members’ suffering predict individual differences in costly helping. Neuron. 2010;68(1):149–60. doi: 10.1016/j.neuron.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Henrich J, McElreath R, Barr A, et al. Costly punishment across human societies. Science. 2006;312:1767–70. doi: 10.1126/science.1127333. [DOI] [PubMed] [Google Scholar]
- Jiang C, Varnum ME, Hou Y, Han S. Distinct effects of self-construal priming on empathic neural responses in Chinese and Westerners. Social Neuroscience. 2014;9:130–8. doi: 10.1080/17470919.2013.867899. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–9. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Simmons CH, Jordav A, et al. Rodney King and OJ revisited: the impact of race and defendant empathy induction on judicial decisions. Journal of Applied Social Psychology. 2002;32:1208–23. [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Lin Y, Han S. Self-construal priming modulates visual activity: underlying global/local perception. Biological Psychology. 2008;77:93–7. doi: 10.1016/j.biopsycho.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Luo S, Shi Z, Yang X, Wang X, Han S. Reminders of mortality decrease midcingulate activity in response to others’ suffering. Social Cognitive and Affective Neuroscience. 2014;9:477–86. doi: 10.1093/scan/nst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus HR, Kitayama S. Culture and the self: implications for cognition, emotion, and motivation. Psychological Review. 1991;98:224. [Google Scholar]
- Markus HR, Kitayama S. Cultures and selves: a cycle of mutual constitution. Perspectives on Psychological Science. 2010;5:420–30. doi: 10.1177/1745691610375557. [DOI] [PubMed] [Google Scholar]
- Mathur VA, Harada T, Lipke T, Chiao JY. Neural basis of extraordinary empathy and altruistic motivation. NeuroImage. 2010;51:1468–75. doi: 10.1016/j.neuroimage.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Obhi SS, Hogeveen J, Pascual-Leone A. Resonating with others: the effects of self-construal type on motor cortical output. Journal of Neuroscience. 2011;31:14531–5. doi: 10.1523/JNEUROSCI.3186-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyserman D, Lee SW. Does culture influence what and how we think? Effects of priming individualism and collectivism. Psychological Bulletin. 2008;134:311. doi: 10.1037/0033-2909.134.2.311. [DOI] [PubMed] [Google Scholar]
- Phinney JS. The multigroup ethnic identity measure a new scale for use with diverse groups. Journal of Adolescent Research. 1992;7:156–76. [Google Scholar]
- Sheng F, Han S. Manipulations of cognitive strategies and intergroup relationships reduce the racial bias in empathic neural responses. NeuroImage. 2012;61:786–97. doi: 10.1016/j.neuroimage.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Sheng F, Liu Q, Li H, Fang F, Han S. Task modulations of racial bias in neural responses to others' suffering. NeuroImage. 2014;88:263–70. doi: 10.1016/j.neuroimage.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Singelis TM. The measurement of independent and interdependent self-construals. Personality and Social Psychology Bulletin. 1994;20:580–91. [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–9. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Han S. Self-construal priming modulates neural substrates of self-awareness. Psychological Science. 2007;18:861–6. doi: 10.1111/j.1467-9280.2007.01992.x. [DOI] [PubMed] [Google Scholar]
- Sui J, Hong Y, Liu CH, Humphreys GW, Han S. Dynamic cultural modulation of neural responses to one’s own and friend’s faces. Social Cognitive and Affective Neuroscience. 2013;8:326–32. doi: 10.1093/scan/nss001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanVoorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutorials in Quantitative Methods for Psychology. 2007;3:43–50. [Google Scholar]
- Varnum MEW, Shi Z, Chen A, Qiu J, Han S. When “Your” reward is the same as “my” reward: self-construal priming shifts neural responses to own vs. friends’ rewards. NeuroImage. 2014;87:164–9. doi: 10.1016/j.neuroimage.2013.10.042. [DOI] [PubMed] [Google Scholar]
- Wang C, Ma Y, Han S. Self-construal priming modulates pain perception: event-related potential evidence. Cognitive Neuroscience. 2014;5:3–9. doi: 10.1080/17588928.2013.797388. [DOI] [PubMed] [Google Scholar]
- Wang C, Oyserman D, Li H, Liu Q, Han S. Accessible cultural mindset modulates default mode activity: evidence for the culturally situated brain. Social Neuroscience. 2013;8:203–16. doi: 10.1080/17470919.2013.775966. [DOI] [PubMed] [Google Scholar]
- Xu X, Zuo X, Wang X, Han S. Do you feel my pain? Racial group membership modulates empathic neural responses. Journal of Neuroscience. 2009;29:8525–9. doi: 10.1523/JNEUROSCI.2418-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X, Han S. Cultural experiences reduce racial bias in neural responses to others’ suffering. Culture and Brain. 2013;1:34–46. [Google Scholar]