Abstract
The amygdala is a highly interconnected region of the brain that is critically important to emotional processing and affective networks. Previous studies have shown that the response of the amygdala to emotionally arousing stimuli can be modulated by sex hormones. Because oral contraceptive pills dramatically lower circulating sex hormone levels with potent analogs of those hormones, we performed a functional magnetic resonance imaging experiment to measure amygdala reactivity in response to emotional stimuli in women using oral contraceptives, and compared their amygdala reactivity with that of naturally cycling women. Here, we show that women who use oral contraceptive pills have significantly decreased bilateral amygdala reactivity in response to negatively valenced, emotionally arousing stimuli compared with naturally cycling women. We suggest that by modulating amygdala reactivity, oral contraceptive pills may influence behaviors that have previously been shown to be amygdala dependent—in particular, emotional memory.
Keywords: amygdala, oral contraceptive pills, emotional memory, fMRI, menstrual phase
INTRODUCTION
The amygdala is best known for its role in modulating memory for emotionally arousing stimuli (Ledoux, 1993; Cahill et al., 1995; Canli et al., 2000; Hamann, 2001; McGaugh, 2004; LaBar and Cabeza, 2006), and mounting evidence suggests that the amygdala’s response to emotional stimuli can be influenced by sex hormones (van Wingen et al., 2008, 2009, 2011; Zeidan et al., 2011). A sex difference exists in amygdala activity such that in men, the activity in the right amygdala predicts subsequent memory, but in women, the activity in the left amygdala does (Canli et al., 2002; Cahill et al., 2004).
Furthermore, and more directly tied to the action of sex hormones in the brain, amygdala activity has been shown to be influenced by menstrual phase. In healthy, naturally cycling women approaching ovulation, in whom estrogen levels are elevated, amygdala reactivity to arousing, negatively valenced stimuli is reduced (Goldstein et al., 2005). However, women in the luteal phase of the menstrual cycle show both enhanced emotional memory (Ertman et al., 2011) and an increased amygdala response to emotional stimuli (Andreano and Cahill, 2010). This pattern of reactivity across the menstrual cycle suggests an attenuating effect of estrogen on the amygdala, and an agonistic effect of progesterone in naturally cycling women.
However, the vast majority of women in the USA and millions of other women around the world do not experience normal menstrual cycles for the entirety of their reproductive years. The CDC estimates that 82% of women in the USA will use oral contraceptive (OC) pills for at least part of their reproductive years, and there are an estimated 100 million current users worldwide (Trussell, 2007). OCs suppress ovulation and interrupt the pattern of hormonal fluctuations normally observed in naturally cycling women. Combined OC pills including synthetic estrogen and progestins are much more widely used than progestin-only pills. OCs generally consist of 3 weeks of active pills, during which the hormones are ingested, followed by 1 week of inactive placebo pills. Very little research currently exists on the cognitive effects of this steroid hormone modulation, and two recent reviews have called for additional research on the topic (Gogos et al., 2014; Pletzer and Kerschbaum, 2014).
Structural and functional investigations have revealed baseline changes to the brain in women using hormonal contraceptives. In a structural brain analysis, hormonal contraceptive use was shown to significantly increase gray matter volume in the pre- and post-central gyri; parahippocampal gyrus; fusiform gyrus and the superior, middle and inferior temporal gyri (Pletzer et al., 2010). White matter differences have also been observed in the fornix, with higher mean diffusivity scores in OC users (De Bondt et al., 2013). A resting state functional connectivity investigation using independent component analysis revealed that the connectivity during the resting state of the default mode network and executive control network is decreased in OC users (Petersen et al., 2014).
Functional magnetic resonance imaging (fMRI) studies have also shown differences in the pattern of brain activity in OC users. Cortisol administration reduces hippocampal response to a learned fear stimulus in naturally cycling women, but increases the hippocampal BOLD (blood oxygen level-dependent) signal in women using OCs (Merz et al., 2012). OC users also show less activity in several prefrontal regions and fail to show the attenuation of activity in the amygdala observed in naturally cycling women (Gingnell et al., 2013).
The imaging studies summarized above suggest that OCs alter the function and structure of the brain. Furthermore, behavioral studies have shown that OCs can also influence mate preferences (Wedekind et al., 1995; Roberts et al., 2008) and initiation of sexual behavior (Adams et al., 1978). Interestingly, behaviors seemingly unrelated to sex may also be influenced. Verbal memory is significantly better in OC users (Mordecai et al., 2008), and, in direct accordance with findings that amygdala activity is altered by OC use, emotional memory is also different. Women using hormonal contraceptives show a different pattern of memory for an emotional story (Nielsen et al., 2011), and the relationship between stress hormones and emotional memory is altered by OCs (Merz et al., 2012; Nielsen et al., 2013a,b).
Thus, due to mounting evidence that ovarian hormones influence amygdala reactivity, we hypothesized that dramatically altering sex hormones with the use of OCs may influence amygdala reactivity to emotionally arousing stimuli. To limit unconstrained heterogeneity in the sample, we included only OC users; however, no evidence shows or fails to show that other methods of delivery (e.g. transdermal or vaginal) would lead to a different outcome. Injectable depot medroxyprogesterone acetate (brand name Depo-Provera), subdermal levonorgestrel implants such as Norplant or Implanon and the Mirena IUD contain only a synthetic progestin component and no synthetic estrogen, and as a result may have different effects on the brain from those reported here.
METHODS
Participants
Recruitment details and inclusion/exclusion criteria have been previously reported in a published manuscript describing resting state functional connectivity in these participants (Petersen et al., 2014). A brief description of the overlapping methods and detailed description of those that diverged will be included here. Healthy women with no psychiatric, neurological or endocrine disorders were recruited for participation. Naturally cycling women not using hormonal contraception were assigned to either the follicular group or luteal group. Follicular women were scanned during cycle days 2–6, and luteal women during cycle days 18–24. OC users were assigned to either the inactive pill group and scanned during days 2–6 of the inactive phase of the OC cycle, or assigned to the active pill group and scanned 18–24 days after the beginning of the inactive pills (11–17 days of continuous active pill use). The specific formulations of each OC type used by the participants in this study are included in supplementary Table S1.
One participant was consented but withdrew before scanning due to feelings of claustrophobia, five were consented but all scans could not be completed due to technical problems at the imaging center and three participants in the naturally cycling group were scanned but later excluded for menstrual irregularities leading to abnormal cycles within the study cycle; their data were not analyzed. After data preprocessing, an additional two subjects were excluded for excessive head motion (>4 mm or 4°). Thus, the final study group comprised 83 participants: 20 follicular, 23 luteal, 20 active pill users and 20 inactive pill users.
Saliva collection and assay
Saliva was collected, stored and analyzed by immunoassay as previously reported.
MRI data collection and preprocessing
Before data were collected for this experiment, participants underwent a 424 s resting state scan. Immediately following, participants began the amygdala reactivity task described next. Participants were instructed before entering the scanner that they would see a series of images ranging in degree of emotional intensity, and that they should rate each image on a scale from 1 to 4, with 1 as the least emotional rating and 4 as the most emotional rating. Participants were given a response box with buttons corresponding to each number to enter their ratings, and images were projected onto a screen in their field of vision via a mirror.
The slideshow participants viewed comprised images from the International Affective Picture System (Lang et al., 2008). It included 72 emotionally arousing (M = 6.51, s.d. = 0.65), negatively valenced (M = 2.05, s.d. = 0.78) images; 72 non-arousing (M = 3.59, s.d. = 0.92), neutrally valenced (M = 5.77, s.d. = 1.21) images and 72 fixation crosses randomized into 3 presentations corresponding to 3 functional runs; each included 24 slides from each category.
Data were collected on a Philips Achieva 3T MRI scanner (Eindhoven, The Netherlands) equipped with an 8-channel SENSE head coil. Functional echoplanar imaging data were collected in 30 slices with an 80 × 79 acquisition matrix size, a 70° flip angle, 2 s repetition time, 30 ms echo time and 3.0 × 1.5 × 3 mm3 voxel size. Two hundred fourteen volumes were collected per run, and three functional runs were completed. Immediately following the functional runs, structural T1-weighted data were collected in 160 slices with 1.0 × 1.0 × 0.67 mm3 voxel size.
Imaging data were preprocessed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK) modules via the toolbox Data Processing Assistant for Resting-State fMRI (http://www.restfmri.net; Yan and Zang, 2010) implemented in MATLAB R2012b. The first volume of each run was dropped to allow for magnetic field stabilization. Subsequent volumes were slice timing corrected to the middle slice, realigned to the first slice, normalized to MNI space using a standard echoplanar imaging template and smoothed with a 4 × 4 × 4 mm full-width half-maximum Gaussian kernel.
Data analysis
Functional imaging data were analyzed in SPM8. First-level contrasts were generated by subtracting functional activity during neutral slide presentations from functional activity during emotional slide presentations (E–N contrast) for all three sessions for each subject. The E–N contrasts were then entered into a second-level (group-level) contrast as a one-way analysis of variance (ANOVA) including all subjects.
Because the amygdala is not a unitary, homogenously activated structure, a combined structural and functional amygdala region of interest (ROI) was generated to isolate the portion of the amygdala that was responsive to the stimuli used in this task across the group of all participants. The amygdala was first structurally defined in each hemisphere by the automated anatomical labeling (Tzourio-Mazoyer et al., 2002) template library in the MARSeille Boîte À Région d’Intérêt (MarsBaR; Brett et al., 2002) toolbox. Next, the functional definition was generated by subtracting the activity associated with presentation of fixation crosses from the activity associated with presentation of all slides (Slides–Fixation contrast). This Slides–Fixation contrast was explicitly masked with the structural ROI to generate the combined structural/functional amygdala ROIs. This ROI was extracted and transformed into a NifTI image in MarsBaR. Using small-volume correction thresholded at P = 0.001, this yielded a cluster in the left amygdala of 45 voxels centered at −27, −6, −18; cluster-level PFWE = 0.001, t = 6.39. The right amygdala cluster was 46 voxels centered at 27, 0, −18; cluster-level PFWE < 0.001, t = 4.61 (Figure 1).
Fig. 1.
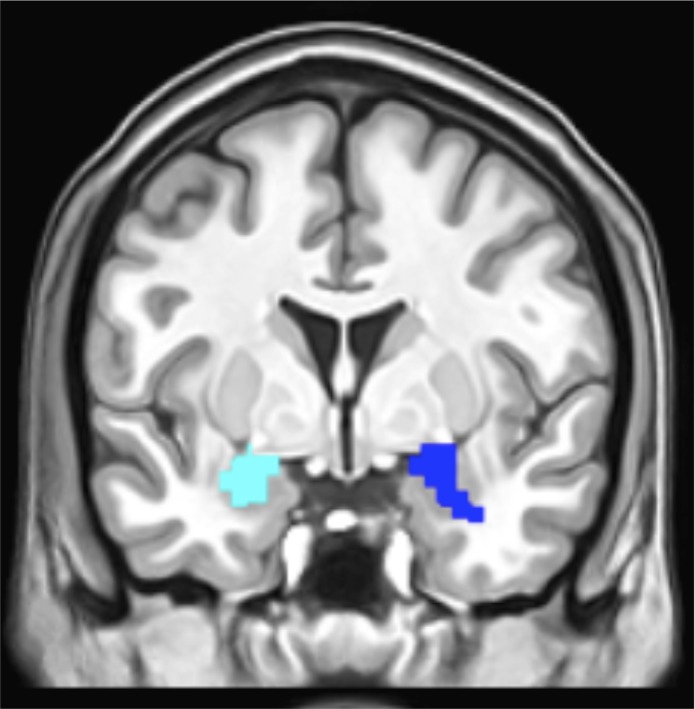
The final ROIs comprised areas of functional activity within anatomically defined amygdala masks. This figure shows the left amygdala ROI in pale blue and the right amygdala ROI in dark blue.
Activity in this amygdala ROI was compared between groups to measure any differences in amygdala reactivity with the emotional stimuli. First, cluster-level and peak-level differences were detected by one-way ANOVA in SPM8. Second, in a separate analysis, the average ROI signal was extracted and compared between groups in MarsBaR.
All ANOVAs and t-tests were performed in JMP10.0 (SAS Inc., Cary, NC).
RESULTS
Salivary assay results
Differences in hormone levels between the four groups (follicular, luteal, inactive pill and active pill) were analyzed by a one-way ANOVA. The groups did not differ significantly in mean salivary estradiol levels, F(3,83) = 1.46, P = 0.23 (Figure 2); however, the group of all OC users did have significantly lower estradiol levels compared with all NC (naturally-cycling) users, t(1,83) = 2.23, P = 0.028 (OC: M = 2.79, s.d. = 1.27; NC: M = 3.43, s.d. = 1.37).
Fig. 2.
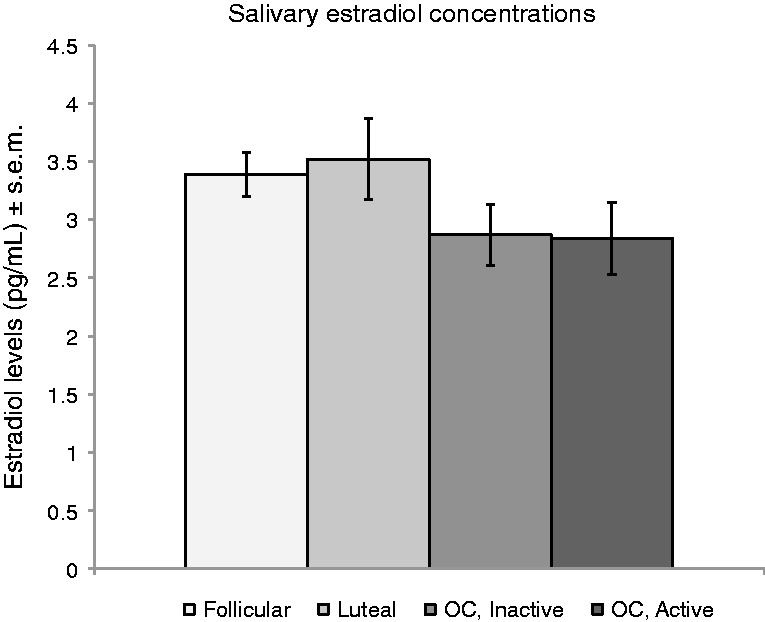
Mean estradiol levels did not differ between groups.
The groups did differ in baseline salivary progesterone levels (F(3,83) = 5.64, P < 0.0015). Post hoc t-tests found that the luteal group differed significantly from the inactive pill group (P = 0.0013) and from the active pill group (P = 0.0031). The follicular group differed significantly from the inactive pill group (P = 0.03) (Figure 3). The luteal and follicular groups differed significantly only when a one-tailed t-test was employed (P = 0.0456). Using this test, the luteal group had significantly higher progesterone levels compared with the follicular group.
Fig. 3.
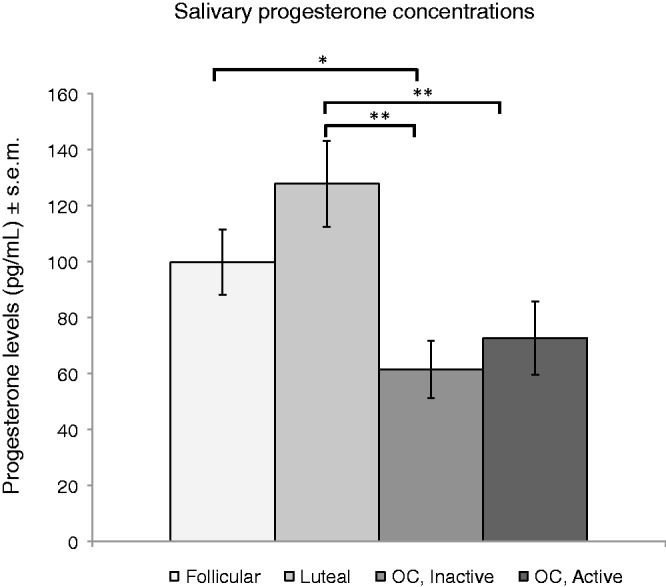
The luteal group had significantly higher baseline progesterone levels than either OC group (**P < 0.01). The follicular group had significantly higher baseline progesterone than the inactive OC group (*P < 0.05).
Functional imaging results
To investigate differences in brain activity associated with emotional reactivity, activity associated with emotional slide presentation was contrasted with that associated with neutral slide presentation. These emotional–neutral contrasts were compared between groups.
Effect of contraceptive status: OC vs NC comparisons
For a preliminary voxel-wise analysis, the two naturally cycling groups were combined to form an NC group, and the two contraceptive user groups were combined to form an OC group. No differences were observed in the left amygdala; however, in the right amygdala, the NC group showed significantly higher peak-level activation (t(251) = 3.21, PFWE = 0.029). At P < 0.05 (small-volume corrected), this forms the peak of an 8 voxel cluster.
Further analysis revealed different patterns of activation bilaterally that depended on subgroup. Follicular women showed more amygdala activation than active pill users, but only in the right amygdala. Two significantly higher peaks were identified: PFWE = 0.014 at 21, −3, −18 and PFWE = 0.020 at 30, −3, −12; however, the corresponding clusters were only 2 and 1 voxels, respectively. At a less conservative threshold (small-volume corrected at 0.01), these form a single, 13 voxel cluster, still significant at both the cluster level (PFWE = 0.039) and the peak level (PFWE = 0.021 and PFWE = 0.035, respectively).
The luteal vs active pill contrast revealed significantly more amygdala activation in the left amygdala of the luteal women relative to active pill OC users, peak-level PFWE = 0.007. This activation was also significant at the cluster level, PFWE = 0.012, but again with a very small voxel extent of only 2. Reducing the significance threshold to small-volume corrected at 0.01 does not substantially increase the cluster size; this more liberal threshold yields a cluster of 3 voxels that are no longer significant after familywise error (FWE) correction (PFWE = 0.094). However, this does not alter the peak-level significance.
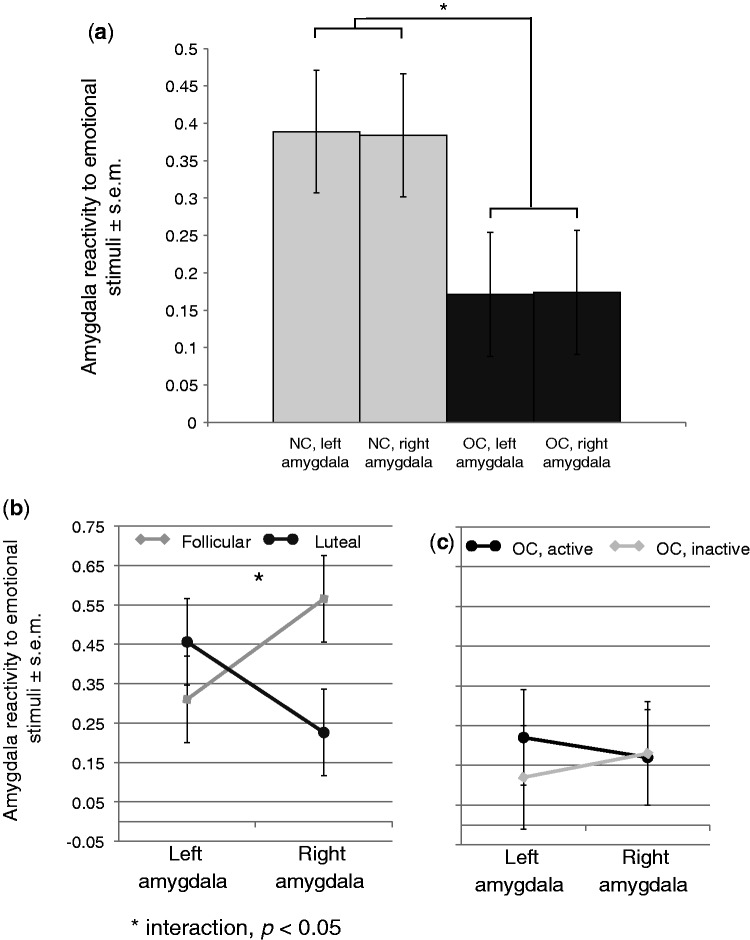
In addition to the voxel-wise analysis previously described, an ROI-wise analysis was also performed to compare the ROI-wise amygdala activity in each hemisphere in NC and OC women. A two-way ANOVA including contraceptive use and hemisphere as factors found a main effect of contraceptive use (F(1,508) = 6.67, P = 0.010), but not hemisphere (F(1,508) = 0.00, P = 0.99), indicating that NC women had significantly more amygdala reactivity to emotional stimuli compared with OC women. No interaction was found between the two factors (P = 0.96) (Figure 4a).
Fig. 4.
(a) The contrast between activity associated with emotional stimuli vs that associated with neutral activity in the amygdala was significantly greater in NC women compared with OC users (P < 0.05). No effect of hemisphere was found, nor was an interaction between contraceptive use and hemisphere present. (b) Within the naturally cycling women, a significant interaction was found between hemisphere and menstrual cycle phase. (c) No interaction between pill phase and hemisphere was observed in the OC users.
Within the group of OC users, ROI-wise amygdala activity was compared between the active and inactive pill users. No main effect of group or hemisphere was found, nor was the interaction term significant (all Ps > 0.6) (Figure 4c).
Menstrual phase effects: follicular vs luteal comparisons
Within the group of NC women, ROI-wise amygdala activity was compared between the luteal and follicular women. Here, no main effect of group or hemisphere was found (P > 0.3); however, a significant interaction emerged (F(3,254) = 4.85, P = 0.03) (Figure 4b). Post hoc testing revealed a significant difference in the right, but not left amygdala (F(1,127) = 5.82, P = 0.02), indicating significantly more activity in the right amygdala of the follicular group compared with that of the luteal group.
Because the follicular and luteal groups’ progesterone levels differed only when a one-tailed test of significance was used, it is possible that group assignment was incorrect despite the narrow windows used to assign participants to the follicular and luteal groups. To strengthen our conclusions regarding menstrual phase, follicular and luteal women were further divided according to progesterone levels. A median split was performed, dividing participants into a low progesterone group (progesterone levels were below the median) and a high progesterone group (progesterone levels were above the median). Thus, luteal women with relatively low progesterone were excluded, and follicular women with relatively high progesterone were excluded. This group assignment was determined a priori once the hormone levels were observed due to the authors’ concerns that hormone levels were only marginally different, and no other manipulations of group assignment were attempted.
ROI-wise amygdala activity was then compared between only low progesterone follicular (N = 9) women and high progesterone luteal (N = 11) women. Despite the reduction in statistical power due to the reduced sample size, a significant phase by hemisphere interaction persisted (F(3,116) = 2.52, P = 0.03).
Because progesterone is a continuous variable, a regression analysis was performed in JMP10.0 to fit progesterone levels in NC women against the amygdala reactivity compared in the ROI analyses. No significant correlation emerged in either hemisphere (all Ps > 0.10).
Negative control to test for the specificity of the emotional reactivity effect
Because this difference in amygdala reactivity was measured by subtracting activity associated with neutral slides from activity associated with emotional slides, we considered the possibility that it could be explained by an increased response to neutral material in the OC women (decreasing the emotional vs neutral contrast) or by an increased response to emotional material. To test this, the fixation cross presentation trials were selected as a baseline against which to contrast neutral slides, and the neutral–fixation cross trials were analyzed in a 2 × 2 ANOVA including group (NC, OC) and hemisphere (left, right) as factors. Neither group nor hemisphere emerged as a significant main effect, and no interaction was detected (all Ps > 0.09). The same analysis was performed comparing activity associated with emotional slides with that associated with fixation crosses; again no significant main effects or interactions were detected (all Ps > 0.10).
DISCUSSION
In this experiment, we found that OC pill use is associated with decreased amygdala reactivity to emotional stimuli, and that the menstrual cycle may be associated with a shift in amygdala hemispheric laterality in response to emotional stimuli. The comparison of NC with OC women showed that in a voxel-wise analysis, OCs are associated with a decrease in activity in a small region of the left amygdala relative to luteal women, and in a small region of the right amygdala relative to follicular women. When the signal is averaged across the entire amygdala ROI in each hemisphere, OC users show significantly less amygdala reactivity to emotional stimuli than do NC women. Within the group of NC women alone, a significant interaction was found between hemisphere and group, indicating that the amygdala in each hemisphere responds to emotional stimuli differently depending on menstrual cycle phase (follicular or luteal). This was true using our full dataset of naturally cycling women or a subset of naturally cycling women divided both by self-reported phase and by progesterone levels. However, given the marginal difference in progesterone levels observed in the two naturally cycling groups, this finding should be considered to be provisional.
Previous literature has demonstrated menstrual cycle effects on amygdala reactivity; however, the direction of the effect previously detected is not entirely consistent with that observed in this investigation. In a very similar experimental design, Andreano and Cahill (2010) showed a bilateral increase in amygdala reactivity in the luteal phase relative to the follicular phase, in apparent contrast to our observation here that follicular women showed increased right amygdala activity relative to luteal women. A 5 voxel cluster in the left amygdala of the luteal group in this experiment did show significantly more activity than the follicular group, but this difference did not survive multiple comparison correction.
Additionally, the Andreano and Cahill (2010) study did not look at average signal across the ROI, and the amygdala ROI definitions differed considerably between studies. Andreano and Cahill used anatomically defined ROIs >200 voxels, whereas those in this study were built out of regions of increased functional activity within a structurally defined amygdala ROI. This reduced the ROI sizes to 45 and 46 voxels. The amygdala is not a homogenous structure, and it may be the case that these different ROIs involved different subnuclei within the amygdala, which may in turn have different sensitivities to menstrual cycle effects.
Another potential contributing factor to inconsistencies seen in amygdala response concerns the use of between- vs within-subjects designs. A within-subjects design necessarily means that participants undergo two scanning sessions, which can potentially lead to some unquantified degree of habituation, whereas a between-subjects design requires only one session, eliminating the possibility of habituation to stimuli and increasing the net novelty of the experience. Previous literature has shown that the amygdala is particularly sensitive to novelty and habituation effects (Baas et al., 2004) and furthermore, that a laterality effect also exists, such that the right amygdala habituates more quickly to emotional stimuli than does the left (Wright et al., 2001; Fischer et al., 2003; Andreano et al., 2014).
As a consequence of this habituation effect, an experimental design including multiple scanning sessions introduces the possibility of habituation within the amygdala, which may differ both between hemispheres and between phases. In a within-subjects study, Gingnell et al. (2013) observed more amygdala activity in the left amygdala during the luteal phase, but importantly, also observed more activity in the right amygdala in the first scanning session irrespective of phase. Ossewaarde et al. (2010) also observed more amygdala activity in the luteal phase relative to follicular phase in a within-subjects design. It is possible, then, that differences in habituation between the amygdala in the left and right hemispheres are contributing to the somewhat confusing pattern of menstrual cycle differences documented in the literature. If an interaction exists between phase, laterality and habituation, even appropriate counterbalancing measures could lead to inaccurate conclusions about phase and laterality when multiple scanning sessions are employed. Thus, the differences in outcomes may be due to subtle but important differences in the studies’ designs.
Other literature has supported an increase in amygdala reactivity in the follicular phase relative to the luteal phase. In a between-subjects investigation of amygdala reactivity to facial expressions, follicular women showed significantly more amygdala reactivity than did luteal women. This effect was seen bilaterally in response to images of disgusted faces, and in the right amygdala in response to happy faces (Derntl et al., 2008). An investigation of reward circuitry using a monetary reward paradigm also found increased amygdala reactivity in the right amygdala of follicular women relative to luteal (Dreher et al., 2007) women. In the context of this literature showing a contradictory pattern of menstrual phase effects, as well as literature showing a contradictory pattern of laterality effects (for review, see Baas et al., 2004), the data presented here suggest that menstrual phase, hemisphere and novelty may all influence the pattern of amygdala reactivity to emotional stimuli. Indeed, our data are the first to formally document an interaction between two of these variables (hemisphere, menstrual phase) on amygdala reactivity to emotional stimuli.
In comparison with investigations of menstrual cycle effects, potential effects of OCs on amygdala activity have received scarce attention in previous literature. Merz et al. (2012) found no difference in amygdala reactivity in OC users compared with follicular women, luteal women, or men, in a fear conditioning task with cortisol administration, although attenuated activity in the hippocampus and left parahippocampal gyrus was observed in OC users relative to both menstrual cycle groups. In an emotion processing task involving emotional faces and neutral shapes, the right amygdala showed an attenuation of activity in response to emotional stimuli between two scanning sessions only in naturally cycling women; in contrast, no attenuation of amygdala activity to emotional stimuli occurred in women using OCs (Gingnell et al., 2013). However, these results may not generalize to a healthy population as all participants had previously reported adverse mood effects of OCs. To our knowledge, these two studies constitute the entire existing literature exploring OC effects on the amygdala in humans. Thus, our finding that OC users show less amygdala activity in response to emotional stimuli has not been previously demonstrated. This decrease in amygdala responsiveness to emotional stimuli cannot be explained by an increase in responsiveness to neutral slides, as no significant differences in reactivity to neutral slides (relative to fixation crosses) were found.
In a voxel-wise comparison, differences between the OC group and NC women were found to be hemisphere dependent. It has been hypothesized that the amygdala in the left hemisphere and in the right hemisphere may play different roles, as the left hemisphere of the brain has been shown to be associated with local or detail processing, while the right hemisphere is associated with global or holistic processing (Hugdahl and Davidson, 2004). Furthermore, a sex difference exists such that in men, the right amygdala reliably predicts subsequent memory, and in women, the left amygdala does (Cahill et al. 2001, 2004; Canli et al., 2002). Men and women show sex differences in the kind of emotional information retained: In experiments using an emotional story as the encoding stimulus, men tend to show an emotional memory enhancement for the central information essential to the meaning of the story, while women tend to show an emotional memory enhancement for peripheral details of the story (Nielsen et al., 2013a), and pharmacological manipulations of the stress system suggest this effect may be related to the previously described amygdala laterality effect (Cahill and van Stegeren, 2003).
Nielsen et al. (2013a) showed that the emotional memory enhancement for details observed in women is in fact a menstrual cycle phase-dependent effect found only in luteal women, and is not observed in follicular women or hormonal contraceptive users. In fact, hormonal contraceptive users show a central information enhancement similar to that seen in men (Nielsen et al., 2011). This suggests that OC users may have increased right amygdala reactivity relative to naturally cycling women that corresponds with increased memory for central, rather than peripheral, information. However, that is not the pattern of amygdala activity observed in this investigation.
A number of important limitations constrain the interpretation of these findings. Importantly, participants were not randomized to the four groups, leaving open the possibility that a third variable explains both the choice to use OCs and changes in amygdala reactivity. On the basis of this and other evidence showing effects of OCs on brain function, we urge future investigators to use a randomized controlled trial when feasible to better demonstrate a causal relationship between OCs and cognitive changes. Additionally, we did not control for the type of OC, including the type of progestin used. A complete list of the OCs included in this investigation can be found in supplementary Table S1, including details about the dosage and types of progestins in each formulation. The progestin type is of particular importance due to previous findings that different progestins may have different cognitive effects. Women using OCs with androgenic progestins performed significantly worse on a test of verbal fluency compared with women using OCs with anti-androgenic progestins, and also had longer reaction times on a test of mental rotation compared with naturally cycling women (Griksiene and Ruksenas, 2011). A similar investigation found that users of an anti-androgenic OC performed more poorly on a mental rotation task compared with either naturally cycling women or users of an androgenic OC (Wharton et al., 2008). To our knowledge, the effects of different synthetic progestins (differing in androgenicity, in dose, by generation or any other feature) on emotion have not been previously demonstrated, and we consider this to be an important variable for future experiments to explicitly investigate, and a limitation of our own study.
In conclusion, we present here the first evidence of which we are aware that OCs reduce amygdala reactivity to emotional stimuli, and that women in the follicular phase of the menstrual cycle have increased right amygdala reactivity to emotional stimuli compared with women in the luteal phase. These findings are consistent with previous evidence that OCs (Nielsen et al., 2011) and menstrual phase (Ertman et al., 2011) influence emotional memory. We suggest that experimenters performing functional imaging studies of the amygdala and emotional memory studies may wish to control for menstrual phase and hormonal contraceptive use in female participants. Furthermore, this finding adds to the growing evidence that the amygdala is influenced by sex hormones, thus future investigations may find that both healthy and pathological behaviors that are amygdala dependent are also influenced by sex hormones. We believe this finding provides a compelling basis for future investigators to consider the role of OCs and menstrual phase in behavioral and psychiatric disorders that include the amygdala, such as anxiety disorders and substance-related and addictive disorders.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We thank NIH grant R01MH057508 to L.C. for financial support. We also thank Azaadeh Goharzad, Annie Hu, and the staff at the Research Imaging Center for providing technical support.
REFERENCES
- Adams DB, Gold AR, Burt AD. Rise in female-initiated sexual activity at ovulation and its suppression by oral contraceptives. The New England Journal of Medicine. 1978;299:1145–50. doi: 10.1056/NEJM197811232992101. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. NeuroImage. 2010;53:1286–93. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Arjomandi H, Cahill L. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology. 2014;33:874–82. doi: 10.1016/j.psyneuen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn R. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Research Reviews. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. 2002 Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan. Available at NeuroImage 16(2) [Google Scholar]
- Cahill L, van Stegeren A. Sex-related impairment of memory for emotional events with β-adrenergic blockade. Neurobiology of Learning and Memory. 2003;79:81–8. doi: 10.1016/s1074-7427(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377:295–6. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, et al. Sex-related differences in amygdala activity during emotionally influenced memory storage. Neurobiology of Learning and Memory. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an fMRI investigation. Learning & Memory. 2004;11:261–6. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci U S A. 2002;99:10789–94. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli J, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bondt T, Van Hecke W, Veraart J, et al. Does the use of hormonal contraceptives cause microstructural changes in cerebral white matter? Preliminary results of a DTI and tractography study. European Radiology. 2013;23:57–64. doi: 10.1007/s00330-012-2572-5. [DOI] [PubMed] [Google Scholar]
- Derntl B, Windischberger C, Robinson S, et al. Facial emotion recognition and amygdala activation are associated with menstrual cycle phase. Psychoneuroendocrinology. 2008;33:1031–40. doi: 10.1016/j.psyneuen.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher J, Schmidt P, Kohn P, Furman D, Rubinow D, Berman K. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007;104:2465–70. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertman N, Andreano JM, Cahill L. Progesterone at encoding predicts subsequent emotional memory. Learning & Memory. 2011;18:759–63. doi: 10.1101/lm.023267.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Research Bulletin. 2003;59:387–92. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Gingnell M, Engman J, Frick A, et al. Oral contraceptive use changes brain activity and mood in women with previous negative affect on the pill—a double-blinded, placebo-controlled randomized trial of a levonorgestrel-containing combined oral contraceptive. Psychoneuroendocrinology. 2013;38:1133–44. doi: 10.1016/j.psyneuen.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Gogos A, Wu CW, Williams AS, Byrne LK. The effects of ethinylestradiol and progestins (“the pill”) on cognitive function in pre-menopausal women. Neurochemical Research. 2014;39(12):2288–300. doi: 10.1007/s11064-014-1444-6. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. Journal of Neuroscience. 2005;25:9309–16. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griksiene R, Ruksenas O. Effects of hormonal contraceptives on mental rotation and verbal fluency. Psychoneuroendocrinology. 2011;36(8):1239–48. doi: 10.1016/j.psyneuen.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Hamann S. Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences. 2001;5:394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Davidson RJ, editors. The Asymmetrical Brain. Cambridge: MIT Press; 2004. [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lang, P.J., Bradley, M.M., Cuthbert, B.N. (2008). International Affective Picture System (IAPS): affective ratings of pictures and instruction manual. Technical Report A-8. Gainesville, FL: University of Florida.
- LeDoux JE. Emotional memory systems in the brain. Behavioural Brain Research. 1993;58:69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, et al. Oral contraceptive usage alters the effects of cortisol on implicit fear learning. Hormones and Behavior. 2012;62:531–8. doi: 10.1016/j.yhbeh.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Mordecai KL, Rubin LH, Maki PM. Effects of menstrual cycle phase and oral contraceptive use on verbal memory. Hormones and Behavior. 2008;54:286–93. doi: 10.1016/j.yhbeh.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Nielsen SE, Ahmed I, Cahill L. Sex and menstrual cycle phase at encoding influence emotional memory for gist and detail. Neurobiology of Learning and Memory. 2013a;106:56–65. doi: 10.1016/j.nlm.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Ertman N, Lakhani YS, Cahill L. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiology of Learning and Memory. 2011;96:378–84. doi: 10.1016/j.nlm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Segal SK, Worden IV, Yim IS, Cahill L. Hormonal contraception use alters stress responses and emotional memory. Biological Psychology. 2013b;92:257–66. doi: 10.1016/j.biopsycho.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde L, Hermans EJ, van Wingen GA, et al. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology. 2010;35:47–55. doi: 10.1016/j.psyneuen.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Petersen N, Kilpatrick LA, Goharzad A, Cahill L. Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. NeuroImage. 2014;90:24–32. doi: 10.1016/j.neuroimage.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer B, Kerschbaum HH. 50 years of hormonal contraception—time to find out, what it does to our brain. Frontiers in Neuroscience. 2014;8:256. doi: 10.3389/fnins.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer B, Kronbichler M, Aichhorn M, Bergmann J, Ladumer G, Kerschbaum HH. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Research. 2010;1348:55–62. doi: 10.1016/j.brainres.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Roberts SC, Gosling LM, Carter V, Petrie M. MHC-correlated odour preferences in humans and the use of oral contraceptives. Proceedings of the Royal Society B. 2008;275:2715–22. doi: 10.1098/rspb.2008.0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell, J. (2007). Contraceptive Efficacy. In: Hatcher, R.A., Trussell, J., Nelson, A.L., Cates, W., Stewart, F.H., Kowal, D., editors. Contraceptive Technology, New York: Ardent Media.
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, Ossewaarde L, Bäckström T, Hermans EJ, Fernández G. Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience. 2011;191:38–45. doi: 10.1016/j.neuroscience.2011.04.042. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, van Broekhoven F, Verkes RJ, et al. Progesterone selectively increases amygdala reactivity in women. Molecular Psychiatry. 2008;13:325–33. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, Zylicz SA, Pieters S, et al. Testosterone increases amygdala reactivity in middle-aged women to a young adulthood level. Neuropsychopharmacology. 2009;34:539–47. doi: 10.1038/npp.2008.2. [DOI] [PubMed] [Google Scholar]
- Wedekind C, Seebeck T, Bettens F, Paepke AJ. MHC-dependent mate preferences in humans. Proceedings of the Royal Society B. 1995;260:245–9. doi: 10.1098/rspb.1995.0087. [DOI] [PubMed] [Google Scholar]
- Wharton W, Hirshman E, Merritt P, Doyle L, Paris S, Gleason C. Oral contraceptives and androgenicity: influences on visuospatial task performance in younger individuals. Experimental and Clinical Psychopharmacology. 2008;16(2):156–64. doi: 10.1037/1064-1297.16.2.156. [DOI] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–83. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- Yan C, Zang Y. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Frontiers in Systems Neuroscience. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological Psychiatry. 2011;70:920–7. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.