Abstract
Huntington’s disease (HD) is an inherited neurodegenerative condition. Patients with this movement disorder can exhibit deficits on tasks involving Theory of Mind (ToM): the ability to understand mental states such as beliefs and emotions. We investigated mental state inference in HD in response to ambiguous animations involving geometric shapes, while exploring the impact of symptoms within cognitive, emotional and motor domains. Forty patients with HD and twenty healthy controls described the events in videos showing random movements of two triangles (i.e. floating), simple interactions (e.g. following) and more complex interactions prompting the inference of mental states (e.g. one triangle encouraging the other). Relationships were explored between animation interpretation and measures of executive functioning, alexithymia and motor symptoms. Individuals with HD exhibited alexithymia and a reduced tendency to spontaneously attribute intentions to interacting triangles on the animations task. Attribution of intentions on the animations task correlated with motor symptoms and burden of pathology. Importantly, patients without motor symptoms showed similar ToM deficits despite intact executive functions. Subtle changes in ToM that are unrelated to executive dysfunction could therefore feature in basal ganglia disorders prior to motor onset.
Keywords: anthropomorphism, biological motion, frontostriatal dysfunction, movement disorders, theory of mind
INTRODUCTION
Huntington’s disease (HD) is an inherited neurodegenerative condition, characterized by choreiform movements and frontostriatal dysfunction. In addition to impairments in executive functions (Stout et al., 2012; Paulsen et al., 2013; Dumas et al., 2013; You et al., 2014), patients with manifest HD, as determined by onset of motor symptoms, can exhibit poor recognition of emotional facial expressions (Calder et al., 2010; Novak et al., 2012; Labuschagne et al., 2013; Trinkler et al., 2013) and altered emotional reactivity (e.g. Hayes et al. 2007; Eddy et al., 2011; Ille et al., 2011). Deficits in Theory of Mind (ToM): the ability to reason about mental states such as beliefs and emotions (Snowden et al., 2003; Allain et al., 2011; Brüne et al., 2011; Eddy et al., 2012, 2014) have also been reported. These deficits may in turn contribute to disturbances in social interaction in HD (Craufurd et al., 2001; Snowden et al., 2003; Duff et al., 2010) and reduced everyday perspective taking (Eddy et al., 2014).
This study investigated patients’ ability to infer mental states when viewing ambiguous stimuli in the form of video animations involving geometric shapes. Inspired by the early work of Heider and Simmel (1944), the animations task (AT) was developed to investigate ToM in autistic spectrum disorders (Abell et al., 2000; Castelli et al., 2000). The AT assesses the tendency to ascribe mental states when observing stimuli containing few cues about ToM as there are no facial or vocal expression cues (Abell et al., 2000). Each of this series of video clips features a small blue triangle and a large red triangle. In some video clips, the movements of the triangles appear random. In others, the triangles appear to interact. Individuals with intact ToM spontaneously attribute mental states (e.g. intentions) to the triangles when asked to describe what is happening in more complex animations depicting interaction. Mental state inferences are less likely in autism (Abell et al., 2000), dementia (Gregory et al., 2002) and schizophrenia (Horan et al., 2009).
Performance on the AT should rely on the ability to interpret mental states from analysis of visual-kinetic cues. Simply understanding that an interaction is depicted will require visual attention to perceive the contiguity of movement of the shapes and is likely to be based on the application of the Gestaltist principle of common fate. To draw higher-order inferences of intention, the visual-kinetic information must be additionally understood to portray movements that are goal-directed (GD) and imply intention. This may be possible because GD movements involving agents tend to have special kinematics such as asymmetrical velocity profiles, which are unlike the ballistic movements of non-biological projectiles (Jeannerod, 2006). Osaka et al. (2012) highlight the role of the posterior superior temporal sulcus in constructing an abstract visual description of another agent’s intentional actions. These authors suggest that incoming visual animation information is decoded perceptually and integrated with contextual interpretation, generating a product which can be understood in terms of perceptual- or intention-related behaviours. Castelli et al. (2002) further suggest that feedback from limbic and prefrontal regions may be important to interpret the social significance of the stimuli on the AT.
One advantage of using the AT in HD is that it relies on implicit non-verbal cues. This may help in reducing the impact of executive dysfunction. Executive functions are critically involved in ToM tasks (Isoda and Noritake, 2013; Fizke et al., 2014; Moriguchi, 2014) and deficits in ToM and executive functions can be correlated in HD (Brüne et al., 2011). Furthermore, assessing ToM in HD gene carriers who are yet to exhibit executive dysfunction could provide insight into the mechanisms underlying patients’ social reasoning impairments. Although emotional reactivity may be altered in pre-manifest HD (e.g. Hennenlotter et al., 2004; Sprengelmeyer et al., 2006; Johnson et al., 2007), a recent study suggested ToM was intact (Saft et al., 2013). The current study used a new task and included a subgroup of HD gene carriers who had yet to exhibit motor onset.
Additional strengths of using the AT is that the stimuli are non-facial as well as non-verbal, and the instructions given do not inform the participant (explicitly or implicitly) of the requirement to reason about thoughts and emotions. This may mean the task is a more sensitive and challenging measure for use with high-functioning pre-manifest HD patients. Previous studies have used tasks like the faux pas task (Stone et al., 1998; Gregory et al., 2002) and the reading the mind in the eyes task (Baron-Cohen et al., 2001), which make the ToM requirement explicit. The AT may provide a better indication of how patients may interpret social interaction in everyday life without prompts and cues.
Osaka et al. (2012) showed that some neural regions, including the premotor cortex, were activated when watching the intentional movements depicted on the AT but not the unintentional movements. As it is expected that the motor system is dysfunctional in HD, this could contribute to difficulties in interpreting the lower level non-verbal cues on this task. De Gelder et al. (2008) revealed difficulties in recognising instrumental body postures in manifest HD, and recognition was worse in patients with more severe motor dysfunction. We therefore hypothesised that patients with manifest HD would make fewer mental state attributions than healthy controls when describing AT video clips, leading to less accurate interpretations, but that any such deficits would be more subtle in pre-manifest HD.
Moriguchi et al. (2006) showed that interpretation of AT video clips can be related to alexithymia, i.e. difficulties in identifying and describing feelings and distinguishing them from physical states (Taylor et al., 1988). Although Trinkler et al. (2013) reported no evidence of alexithymia in 13 patients with HD, we assessed alexithymic traits in the current study to explore relationships between this factor and AT responses. As Ladegaard et al., (2014) found that patients with major depression and executive dysfunction showed less mentalising in their descriptions than controls on the AT, we also assessed psychiatric symptoms to explore relationships between these factors, alexithymia and mental state inference in patients with manifest and pre-manifest HD.
METHOD
Participants
Participants were 40 patients with a positive genetic test for HD [20 males; mean age: 50.08 years, standard deviation (s.d.) = 11.73, median = 53, range = 20–64; mean education 13.3 years, s.d. = 2.43, median = 12.5, range = 11–19] and 30 healthy controls (13 males; mean age 47.87 years, s.d. = 9.86, median = 51.5, range = 20–65; mean education 13.97 years, s.d. = 2.05, median = 13, range = 11–19). They were screened with Mini Mental State Examination (Folstein et al., 1975) and were included if they achieved a score of at least 27/30. Eight patients reported depression, one had anxiety problems and five reported both depression and anxiety. Twenty-three patients were taking medication to help with motor symptoms or psychiatric difficulties (carbamazepine = 2, venlafaxine = 1, tetrabenazine = 3, citalopram = 4, sertraline = 3, amitryptiline = 1, fluoxetine = 3, mirtazapine = 2, risperidone = 1, citalopram+lorazepam = 1, sertraline + tetrabenazine = 1, fluoxetine+risperidone = 1). Patient clinical characteristics are listed in Table 1.
Table 1.
Characteristics of participants with the HD gene
| Measure | Manifest HD (n=25) |
Pre-manifest HD (n=15) |
|||
|---|---|---|---|---|---|
| Mean (s.d.) | Median (range) | Mean (s.d.) | Median (range) | ||
| CAG | 43.35 (1.66) | 43 (41–47) | 41.92 (2.21) | 42 (38–45) | |
| Burden of pathology (CAG–35.5) x age | 430.26 (67.97) | 437 (297–529) | 261.19 (83.99) | 264 (110–357.5) | |
| UHDRS Motor Symptom Score | 33.28 (11.38) | 30 (18–67) | 3.33 (3.81) | 2 (0–10) | |
| PBA-S Depression | Frequency | 1.32 (1.25) | 2 (0–3) | 1.2 (1.42) | 0 (0–3) |
| Severity | 1.48 (1.53) | 2 (0–4) | 1.4 (1.76) | 0 (0–4) | |
| PBA-S Anxiety | Frequency | 1.40 (1.08) | 2 (0–3) | 0.8 (1.26) | 0 (0–3) |
| Severity | 1.56 (1.33) | 2 (0–4) | 1 (1.65) | 0 (0–4) | |
| PBA-S Irritability | Frequency | 1.76 (1.20) | 2 (0–4) | 0.93 (1.33) | 0 (0–4) |
| Severity | 1.68 (1.16) | 2 (0–4) | 0.93 (1.33) | 0 (0–4) | |
| PBA-S Aggression | Frequency | 1.16 (1.00) | 1 (0–4) | 0.73 (1.33) | 0 (0–4) |
| Severity | 1.00 (1.15) | 1 (0–3) | 0.40 (0.74) | 0 (0–2) | |
| PBA-S Apathy | Frequency | 1.08 (1.12) | 1 (0–3) | 0.33 (0.72) | 0 (0–2) |
| Severity | 1.24 (1.27) | 1 (0–4) | 0.53 (1.25) | 0 (0–4) | |
Notes. CAG: Cytosine adenine guanine repeat number; UHDRS, Unified HD Rating Scale, motor score max 100; PBA-S: Problem Behaviours Assessment Short form.
The study was approved by the local National Health Service Research Ethics Committee. All participants gave written informed consent and then completed the following tasks in pseudorandom order.
Measures
Unified HD rating scale: motor symptoms subscale (Huntington Study Group, 1996)
A neuropsychiatrist (H.E.R.) examined each patient with the Huntingtin gene for motor signs (e.g. finger tapping, dysarthia and chorea). Scores were summed to generate a total motor score (possible range = 0–124). Higher scores indicate more severe symptoms. To examine ToM in a subgroup of patients who could be objectively classified as pre-manifest, specific thresholds used in previous studies based on this motor scale and diagnostic confidence intervals were applied (e.g. Majid et al., 2011; Wolf et al., 2012; Seibert et al., 2012). Fifteen patients had no definite motor signs of HD on examination (Diagnostic Confidence Index rating below 2) with the remaining 25 showing motor signs yielding a diagnostic certainty of more than 50% confidence of motor onset. The mean motor score for the pre-manifest subgroup (Table 1) is similar to that reported in other studies (Jurgens et al., 2008, 2010; Majid et al., 2011; Enzi et al., 2012; Wolf et al., 2012). These criteria led to a modestly sized pre-manifest group but allowed greater confidence in categorisation.
Problem Behaviours Assessment short-form (PBA-S: Craufurd et al., 2001)
This interview scale was developed for HD. The frequency and severity of problem behavioural and psychiatric symptoms were rated for the previous 4 weeks. The subscales to assess mood (anxiety, depression and apathy) and problematic social behaviour (aggression, irritability) were the focus of this study. Each symptom was rated 0–4.
Verbal fluency tests
Phonological fluency was tested by asking participants to say as many words as they could think of beginning with a given letter. The letters F, A and S were used in turn. One minute was given for each and people’s names and repeats were not counted. To assess semantic fluency, three categories (fruit, animals and vegetables) were the cues. Scoring was based on the total number of different items generated over the three letters or categories (Lezak, 1995).
Digit ordering test-adapted (DOT-A)
For this working memory measure, participants listened to mixed strings of digits (e.g. 3, 8, 4, 7) and were asked to recall these digits in ascending order immediately after presentation (Cooper et al., 1991; Werheid et al., 2002). String length ranged from 3 to 8, with two strings presented of each length. If a participant responded to two strings of the same length incorrectly, testing was terminated. Half a point was deducted from the maximum span if only one string of that length was correctly answered.
Trail making test
This task (Reitan and Wolfson, 1985) involves sustained attention and switching. Participants were first presented with a page containing 25 small circles, each containing a number from 1 to 25. Each circle has to be joined with a line according their number (i.e. 1 to 2, 2 to 3, etc.). For the second condition, stimuli were 24 circles containing the numbers 1–12 and the letters A–L. Participants were required to join these circles with a line, alternating from number to letter, ascending in both categories (i.e. 1 - A, A - 2, 2 – B, etc.). They were given a short demonstration before testing. When an error was made, the participant was prompted to correct it, and the time taken to complete each condition was recorded, to generate a score of the difference between conditions (2 − 1).
Stroop task
The Stroop task (Stroop, 1935) assesses inhibition. The baseline condition required participants to speak the colour of the ink of each item on a page of 40 groups of XXXs, going across the rows from left to right. In the test condition, items were colour names written in coloured inks, which did not match the name (e.g. ‘blue’ printed in red ink). Participants were asked to name the colour of the ink of each word as before. Recorded measures were errors and time taken for each condition, yielding a measure of inhibitory interference (test minus baseline).
Toronto Alexithymia Scale
The Toronto Alexithymia Scale (TAS) (Taylor et al., 1988) contains 26 statements. Participants rate their agreement with each using a 5-point Likert scale (strongly disagree; disagree; neither agree nor disagree; agree; strongly agree). Higher scores indicate greater evidence of alexithymia. The test authors suggest that non-alexithymic individuals score up to 62, although a more conservative cut-off of 74 yields greater diagnostic confidence. The TAS demonstrates excellent psychometric properties including validity (Bagby et al., 1990; Taylor et al., 1990).
Animations task
The AT (Abell et al., 2000; Castelli et al., 2000) comprises a set of 12 computer-presented animations, each lasting around 40 s, featuring a big red triangle and a small blue triangle. There are four each of three different types of animations: random movements, GD movements and movements which imply the triangles have mental states (ToM). Participants were asked to watch each animation and explain what was happening on screen. If a participant had failed to speak by the time the animation had almost finished, we asked ‘what’s happening?’ or ‘tell me what you are thinking’. We did not give specific feedback in relation to the content of the responses but offered positive encouragement for any remarks. Two raters scored responses for appropriateness, intentionality and word length using the developers’ coding system (Abell et al., 2000; Castelli et al., 2000). For appropriateness, scores ranged from 0 (a response with no description related to the depicted interaction) to 2 (demonstrating understanding of all key features of the interaction and some reference to mental states if appropriate). Intentionality was scored based on the presence of specific terms (e.g. 4 point answers involved mental state terms and 5 point answers involved descriptions of intent to influence the mental state of another). Response length was scored from 0 to 4 based on number of clauses. One rater was blinded to participant group to reduce the impact of rater bias. For scores where raters did not reach agreement, averages were used. Inter-rater reliability for final scores (length, intention and accuracy) on the AT was high (Cohen’s kappa = 0.74, 0.76 and 0.77, respectively).
Analysis
Analyses involved non-parametric Mann–Whitney U (MWU) tests and Spearman’s r correlations as the patient and control groups were of different sizes and examination of data plots and Shapiro–Wilk tests indicated that data were not normally distributed. The control group was split into two subgroups to match the two subgroups of patients: pre-manifest gene carriers and manifest HD. Within group correlational analysis for patients examined relationships between scores on the AT (length, accuracy, intentionality) and alexithymia, burden of pathology (calculated using age and genetic information: Penney et al., 1997), motor symptom severity, PBA scores (anxiety, depression, apathy, aggression, irritability) and executive functions (verbal fluency, working memory, sustained attention and shifting, inhibition).
RESULTS
Manifest patients and their control group were not significantly different in terms of age (MWU = 195, P = 0.847) or education (MWU = 201.5, P = 0.699). This was also the case for pre-manifest patients and controls for age (MWU = 105, P = 0.775) and education (MWU = 156, P = 0.074).
Manifest HD patients vs controls
As listed in Table 2, patients with manifest HD exhibited a range of significant deficits in executive functions such as verbal fluency (phonological: MWU = 359, P < .001; semantic: MWU = 374.5, P < .001), working memory (MWU = 350.5, P < .001) and sustained attention and shifting (MWU = 47, P < .001) but no deficits in response inhibition (Stroop errors: MWU = 140.5, P = 0.192; times: MWU = 137, P = 0.164).
Table 2.
Task performance for patients with the HD gene and healthy controls
| Variable | Manifest patients |
Manifest group healthy controls |
Pre-manifest patients |
Pre-manifest group healthy controls |
||||
|---|---|---|---|---|---|---|---|---|
| Mean (s.d.) | Median (range) | Mean (s.d.) | Median (range) | Mean (s.d.) | Median (range) | Mean (s.d.) | Median (range) | |
| Age (years) | 54.64 (6.79) | 54 (44–66) | 55.52 (6.67) | 56 (43–65) | 42.47 (14.32) | 42 (20–65) | 40.53, (13.05) | 40 (20–62) |
| Education (years) | 13.28 (2.44) | 12 (11–19) | 13.33 (2.29) | 13 (11–17) | 13.33 (2.50) | 13 (11–19) | 14.60 (1.80) | 15 (12–17) |
| Phonological fluency test | 22.5 (9.63) | 21 (8–39) | 50 (15.15) | 47 (30–80) | 44.33 (18.64) | 42 (23–100) | 48.40 (12.26) | 45 (35–79) |
| Semantic fluency test | 27.72 (8.44) | 26 (11–41) | 55.60 (8.55) | 54 (41–73) | 45.8 (11.50) | 46 (29–66) | 54.60 (11.34) | 55 (35–71) |
| Stroop task errors | 3.13 (3.35) | 2 (0–13) | 1.6 (2.13) | 1 (0–8) | 1.73 (2.12) | 1 (0–7) | 1.07 (1.10) | 1 (0–3) |
| Stroop task times (seconds) | 68.84 (46.86) | 50.08 (4.10–198.13) | 43.89 (20.30) | 37.86 (11.91–81.03) | 34.32 (14.63) | 30.08 (12.16–65.27) | 35.46 (8.70) | 35.18 (25.22–50.42) |
| Trail making test times (seconds) | 107.03 (72.58) | 97.75 (26.12–303.59) | 31.15 (18.58) | 26.01 (9.81–51.86) | 28.44 (21.10) | 24.19 (0.19–81.35) | 30.45 (18.35) | 22.94 (6.34–70.51) |
| Digit ordering test | 4.18 (0.97) | 4.5 (2.5–5.5) | 6 (0.76) | 6 (4.5–7.5) | 5.7 (0.75) | 6 (4–6.5) | 5.67 (0.75) | 5.5 (4.5–6.5) |
| TAS | 74.52 (10.46) | 74 (56–5–95) | 58.33 (12.91) | 55 (34–83) | 67.13 (12.64) | 66 (46–95) | 52.47 (9.54) | 55 (41–71) |
| AT RAN: length | 9.24 (2.50) | 9.5 (5.5–13.5) | 11.60 (2.47) | 10 (8–16) | 8.13 (2.07) | 8 (4–13.5) | 11.73 (2.28) | 12 (8–15) |
| AT RAN: intention | 1.38 (1.54) | 0.5 (0–4.5) | 1.47 (2.00) | 1 (0–6) | 1.8 (1.54) | 1.5 (0–5.5) | 0.93 (0.96) | 1 (0–3) |
| AT RAN: accuracy | 6.22 (1.4) | 6.5 (4–8) | 7.07 (1.10) | 7 (5–8) | 6.03 (1.59) | 6.5 (3–8) | 7.33 (0.61) | 7 (6–8) |
| AT GD: length | 10.38 (2.67) | 11 (5–14.5) | 13.60 (2.38) | 14 (9–16) | 7.9 (2.11) | 8 (4.5–12) | 12.60 (2.20) | 13 (9–15) |
| AT GD: intention | 7.16 (2.19) | 8 (3–10) | 9.80 (1.57) | 10 (7–12) | 7.03 (2.58) | 8 (0–10) | 9.60 (1.76) | 9 (6–12) |
| AT GD: accuracy | 4.94 (1.88) | 5 (2–8) | 7.53 (0.64) | 8 (6–8) | 5 (1.90) | 5 (1–8) | 7.53 (0.64) | 8 (6–8) |
| AT TOM: length | 12.38 (2.84) | 13.5 (5–14.5) | 15.20 (1.61) | 16 (13–18) | 9.63 (3.01) | 9 (6–16) | 14.40 (1.55) | 14 (41–71) |
| AT TOM: intention | 11.5 (3.61) | 11.5 (3.5–18.5) | 16 (1.96) | 16 (13–20) | 8.97 (2.75) | 9 (4–13.5) | 16.27 (1.94) | 17 (13–19) |
| AT TOM: accuracy | 4.28 (1.53) | 4 (0.5–8) | 5.8 (1.15) | 5 (4–8) | 3.77 (1.18) | 4 (1.5–6) | 6.40 (1.18) | 6 (5–8) |
Notes. AT: Animations Task; GD, goal directed action video clips; RAN, random movement video clips; TOM, theory of mind video clips; maximum scores: length = 16; intention = 20; accuracy = 8. TAS: Toronto Alexithymia Scale.
TAS mean scores indicated alexithymia in these patients (MWU = 57.5, P < .001), regardless of mood symptoms. The pattern of responses in HD suggested that high scores were spread across most items. Responses ranged across at least 4 of the 5 points on the Likert scale in almost all patients, showing normal use of the scale. Three of the 20 controls (15%) exhibited a score above the non-alexithymic cut-off (62) but just one (5%) reached the more conservative diagnostic threshold level indicative of alexithymia (74: Taylor et al., 1988). Of the patients with manifest HD, 23/25 (92%) scored above the non-alexithymic cut-off of 62 points, with 14 scoring above the higher threshold (52%).
Patients with manifest HD also exhibited some differences when compared to healthy controls on the AT. Scores for appropriateness (MWU = 255.5, P = 0.057) and intention ratings (MWU = 179.5, P = 0.825) were not significantly different for random video clips. Response length was shorter for patients on this condition (MWU = 283, P = 0.007), though this difference would not survive correction for multiple comparisons. For GD action video clips, patients showed reduced attribution of intentions (MWU = 316.5, P < .001) and poorer accuracy of interpretation (MWU = 341, P < .001), with shorter answers (MWU = 305.5, P = 0.001) than controls. For the ToM video clips, patients with HD again exhibited reduced attribution of intentions to the triangles (MWU = 326.5, P < .001), reduced appropriateness (MWU = 305.5, P = 0.001) and shorter answers (MWU = 303.5, P = 0.001).
Pre-manifest HD group vs controls
For the subgroup of patients below threshold of motor onset, no significant executive deficits were apparent (phonological fluency: MWU = 139, P = 0.285; semantic fluency: MWU = 157.5, P = 0.061; Stroop errors: MWU = 100, P = 0.624; Stroop times: MWU = 132, P = 0.436; DOT-A: MWU = 108.5, P = 0.870; Trail making test: MWU = 122, P = 0.713). However, there was evidence of alexithymia in pre-manifest patients (MWU = 36.5, P = 0.001), with 66% of patients scoring above the cut-off for non-alexithymic.
For the AT, pre-manifest patients seemed to respond more similarly to manifest patients than healthy controls. For the random videos, pre-manifest patients offered shorter responses than controls (MWU = 197.5, P < .001) but achieved similar intention (MWU = 72.5, P = 0.098) ratings. Appropriateness ratings were lower for pre-manifest HD (MWU = 173.5, P = 0.010), but this difference would not survive correction for multiple comparisons. For both GD action and ToM video clips, patients’ responses indicated reduced attribution of intention (GD: MWU = 181, P = 0.004; ToM: MWU = 222, P < .001) to the triangles and less accurate interpretations (GD: MWU = 205.5, P < .001; ToM: MWU = 212.5, P < .001). Their answers were also shorter than controls’ (GD: MWU = 211.5, P < .001; ToM: MWU = 203, P < .001).
Correlational analyses
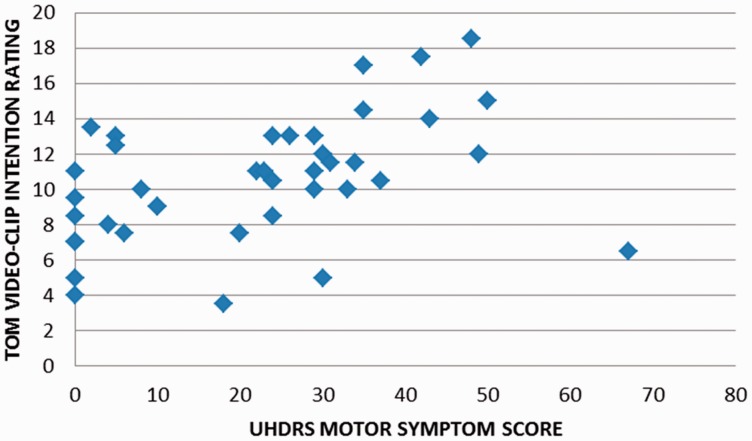
Correlations for all patients with HD are listed in Table 3. Relationships were explored between the AT, TAS, executive tasks, motor symptoms and burden of pathology. A number of correlations were significant P < 0.05, including relationships between AT scores, semantic fluency, motor symptoms and burden of pathology. However, only four correlations would survive correction for multiple comparisons (P = 0.001). These were positive relationships between burden of pathology and the length of answers for both GD movement and ToM video clips, as well as the intentions rating for ToM video clips. Patients’ intention ratings for ToM video clips were also higher in association with increased motor symptom severity (Figure 1) and burden of pathology. Positive relationships between ToM accuracy scores, burden of pathology and motor symptoms would not survive correction.
Table 3.
Correlations between ToM tasks, executive tasks and clinical variables for patients with the HD gene
| Measure | Phonological fluency | Semantic fluency | Stroop errors | Stroop times | TMT | DOT-A | UHDRS motor | BOP | TAS |
|---|---|---|---|---|---|---|---|---|---|
| AT RAN length | −0.037, 0.818 | 0.048, 0.771 | −0.003, 0.983 | −0.031, 0.850 | 0.113, 0.487 | −0.111, 0.497 | 0.282, 0.078 | 0.478, 0.009* | −0.125, 0.444 |
| AT RAN intention | 0.098, 0.546 | 0.240, 0.136 | −0.195, 0.227 | −0.187, 0.249 | −0.136, 0.403 | 0.244, 0.130 | −0.164, 0.311 | 0.077, 0.690 | −0.081, 0.619 |
| AT RAN appropriateness | −0.022, 0.893 | −0.077, 0.635 | 0.266, 0.097 | 0.249, 0.122, | 0.044, 0.787 | −0.123, 0.449 | −0.003, 0.986 | −0.041, 0.832 | 0.168, 0.301 |
| AT GD length | −0.206, 0.203 | −0.242, 0.133, | 0.045, 0.781 | 0.218, 0.176 | 0.258, 0.109 | −0.310, 0.052 | 0.454, 0.003** | 0.581, 0.001** | −0.205, 0.204 |
| AT GD intention | 0.118, 0.469 | −0.011, 0.947 | 0.038, 0.817 | 0.037, 0.822 | 0.040, 0.805 | −0.009, 0.954 | 0.122, 0.453 | 0.234, 0.222 | −0.060, 0.711 |
| AT GD appropriateness | 0.089, 0.587 | 0.029, 0.860 | 0.133, 0.413 | 0.091, 0.578 | 0.087, 0.595 | −0.101, 0.534 | 0.079, 0.629 | 0.176, 0.362 | 0.010, 0.951 |
| AT TOM length | −0.167, 0.304 | −0.199, 0.218 | 0.161, 0.322 | 0.247, 0.124 | 0.243, 0.131 | −0.257, 0.109 | 0.454, 0.003** | 0.637, <0.001*** | −0.204, 0.206 |
| AT TOM intention | −0.205, 0.204 | −0.326, 0.040* | 0.134, 0.409 | 0.249, 0.121 | 0.192, 0.235 | −0.220, 0.173 | 0.515, 0.001** | 0.592, 0.001** | 0.024, 0.882 |
| AT TOM appropriateness | −0.055, 0.734 | −0.194, 0.231 | 0.051, 0.754 | 0.198, 0.222 | 0.078, 0.631 | −0.112, 0.490 | 0.342, 0.031* | 0.417, 0.024* | 0.002, 0.992 |
| TAS | −0.296, 0.064 | −0.237, 0.140 | 0.067, 0.679 | −0.008, 0.963 | 0.395, 0.014* | −0.387, 0.014* | 0.342, 0.031* | 0.051, 0.793 | X |
Notes. AT: Animations Task; BOP: Burden of pathology (CAG-35.5) x age; CAG: Cytosine adenine guanine repeat number; DOT-A: Digit Ordering Test-Adapted; GD: goal directed action video clips; RAN: random movement video clips; TOM: theory of mind video clips; TAS: Toronto Alexithymia Scale; TMT: Trail Making Test; UHDRS motor: Unified HD Rating Scale motor symptom severity score. Values in bold remain significant after correction for multiple comparisons. *significant at p<.05, **significant at p<.01; ***significant at p<.001.
Fig. 1.
Correlation between HD patients’ motor symptom severity and mean intention ratings for AT ToM video clips.
DISCUSSION
Reduced attribution of mental states in HD
When compared with healthy controls, individuals with HD exhibit a reduced tendency to infer mental states, including intentions, during observation of animated non-human stimuli whose movements imply social interaction. For example, when asked to describe the ‘seducing’ video clip, healthy control answers included: ‘the big [triangle] won't allow the small [triangle] to come out … the small is trying to manipulate the big one, he tricked him and went out’ and ‘it’s goading the big one, it looks like it is teasing it, it's stroking it, enticing it into the square … and now gone’. Patient responses were often more concrete with fewer references to mental states, e.g. ‘the large one [triangle] is stopping the small one [triangle] from exiting the building … the smaller one escaped’ and ‘they’re both inside, he's blocking the door and stopping blue [triangle] going out, he’s trying to find a way out, he turned, he's gone now’. These deficits extended to gene carriers with no motor symptoms.
Although HD was associated with less accurate interpretation of the social interactions depicted in the AT for both GD action and ToM video clips, responses to random movement video clips were similar to controls’. The GD movement video clips on the AT feature situations such as chasing and dancing. Although these interactions are less complex than those in the ToM scenarios (e.g. deception and persuasion), the GD actions are likely to implicitly suggest a desire or intention to interact (e.g. to follow another) and therefore a mental state. Indeed, intention attribution ratings are higher for the GD action video clips than the random movement video clips (e.g. Castelli et al., 2000, 2002). Differences in mentalising ability could therefore explain why patients with HD differed to controls in their responses to GD movement as well as ToM video clips.
Cognitive factors which may contribute to patients’ impairments in ToM
Previous studies have debated whether patients with manifest HD have a deficit in ToM per se or whether impairments are a result of executive dysfunction (e.g. Brüne et al., 2011). In this study, executive dysfunction was apparent in manifest HD. However, there was only one correlation between executive function and AT scores, and this was not significant after correction. Moreover, the pre-manifest group exhibited deficits on the AT in the absence of executive dysfunction, indicating that ToM impairment can be independent of executive dysfunction in HD.
An important consideration for the AT is the length of the descriptions. Shorter answers could reflect either the natural form of less complex concrete interpretations or deficits in verbal communicative ability. Although no participants exhibited significant speech impairments according to Unified HD rating scale motor assessment, executive deficits could be accompanied by impaired verbal communication. However, manifest patients with more executive deficits frequently provided longer answers than pre-manifest individuals, as indicated by positive correlations between both motor symptoms and burden of pathology with length scores. For the pre-manifest group, there was little evidence of executive dysfunction and no speech impairment. It is therefore unlikely that this subgroup performed poorly on the AT simply because they were unable to communicate their thoughts.
HD gene carriers are typically very high functioning prior to motor onset. Perhaps, the ambiguity of the AT, which offers fewer clues about the purpose of assessment, makes this a sensitive measure to alterations in cognitive reasoning and reduced mentalising in pre-manifest HD. Aviezer et al. (2009) found that despite impaired explicit recognition of facial expressions in pre-manifest HD, preserved processing of the same facial configurations was evident when these stimuli were embedded in context. Intact understanding of accompanying contextual cues may therefore help maintain performance, potentially obscuring subtle differences in social cognitive ability. Our findings support this possibility by illustrating that measures containing fewer cues to meaning can be sensitive to impairment in pre-manifest HD.
Mentalising and motor function
Correlational analyses revealed an interesting relationship, such that higher intentionality ratings for ToM video clips were linked to more severe motor signs and greater burden of pathology. That is, although patients with HD were less accurate than controls when using ToM to describe AT scenarios, the amount of intention attributed increased with disease progression. This finding may seem counterintuitive, as greater motor symptoms and high burden of pathology scores would suggest more extensive neuropathology. One explanation for this relationship could lie in mediating factors such as mood disorder or medication. For example, ToM may have been impacted by undiagnosed depression or apathy in pre-manifest individuals (e.g. apathy has been shown to affect ToM in Parkinson’s disease: Santangelo et al., 2012), and patients with more severe motor symptoms may have been taking pharmacological agents, which helped with some aspects of ToM performance. The possibility of neural compensation may also be considered.
Perhaps, a more compelling explanation for increased attribution of intentions in association with advancing HD may lie in the relationship between dopamine and disease progression. It is thought that during the early hyperkinetic stage of manifest HD, dopamine levels are actually increased, and it is much later in the disease course that the level of this neurotransmitter is low (Chen et al., 2013). Dopamine levels may influence ToM. For example, increased attribution of intentions in particular may occur in paranoid schizophrenia in association with increased tonic dopamine (Abu-Akel and Shamay-Tsoory, 2011). It is therefore possible that the more symptomatic patients recruited into this study exhibited elevated levels of dopamine, which was in turn linked to increased attribution of intentions in response to ToM video clips.
Linking motion to emotion
As humans have an innate tendency to draw meaning from experience, basic factors relating to motion of objects can convey the sense of intention or emotion (Michotte, 1950). For example, the trajectory of a door (opening as the person walks towards it but then abruptly closing) can lead to attributions of intent that the door intends to keep the person out (Ju and Takayama, 2009). Studies have even shown an effect of the speed of the movement of inanimate objects on emotional interpretations (Ju and Takayama, 2009; Hiraga and Takahashi, 2011). Emotional changes in HD could therefore affect patients’ responses on the AT.
In this study, both manifest and pre-manifest patients exhibited alexithymia. Traits associated with alexithymia may have the potential to affect performance on a range of ToM tasks, from recognition of emotional facial expressions (Grynberg et al., 2012) to performance on the AT (Moriguchi et al., 2006). However, there was no evidence for a relationship between alexithymia ratings and deficits in AT in this study, suggesting at least some of patients’ ToM deficits may be independent of alexithymia. Tavares et al. (2008) reported that when viewing a similar task showing animated shapes engaging in social interaction, high trait emotional awareness was linked to temporal activation, whereas low emotional awareness (more akin to alexithymia) was associated with greater activity within hypothalamus and premotor cortex, indicating more action-oriented and visceral processing. Neuroimaging may therefore help clarify the contribution of emotional dysfunction to patients’ deficits on the AT.
Trinkler et al. (2013) reported no evidence of alexithymia in a sample of 13 patients with manifest HD. This difference may be due to the heterogeneity of patient samples or because Trinkler et al. used a different version of the TAS to this study. In any case, the current findings provide compelling evidence that regardless of disease stage, HD is likely to be associated with changes in the interpretation and experience of emotions. No correlations were found between mood and alexithymia ratings and psychiatric symptoms were mild across the patient group. However, many patients were taking medications, and difficulties with reflecting on one’s own emotional state could explain both alexithymia and a tendency to under-report psychiatric symptoms. Further work is needed to determine whether alterations in emotional reactivity in HD reflect a psychological reaction to diagnostic awareness or early signs of neural dysfunction.
Neuropathological implications
Studies have indicated that determining intentional causality as opposed to physical causality activates a network of ToM regions including medial prefrontal cortex, temporal poles, precuneus and posterior cingulate in addition to superior temporal sulcus and parietal regions (den Ouden et al., 2005). In HD, striatal degeneration may impair ToM via dysfunction within wider frontostriatal networks, with the potential to affect many of the aforementioned structures. However, it has been suggested that the basal ganglia may play a more specific role in social cognition by allowing motor simulation of emotion as opposed to determination of mental states via more abstract reasoning processes (e.g. Van Overwalle and Baetens, 2009), therefore making an important contribution to affective ToM (Bodden et al., 2013).
More basic requirements of the AT include visual attention and perceptual processing of the visual-kinetic cues depicted, to determine whether the movements are random or show contiguity and therefore imply movements made by animate objects. A recent fMRI study in healthy participants (Straube and Chatterjee, 2010) showed that individual differences in sensitivity to time or space in relation to perceiving movement causality amongst objects were related to activation of the left basal ganglia or right parietal lobe, respectively. In the current study, individuals with HD were often able to draw these lower-level inferences, as they commonly recognised that interaction was occurring between the triangles. The deficit on the AT appeared to arise more from the finding that patients explained these interactions on a more concrete basis using fewer mental state terms.
Impaired attribution of mental states in HD could reflect poor integration of perceptual information with cognitive or emotional processes required to draw higher-order (i.e. ToM based) inferences from observed actions. Castelli et al. (2002) suggest a more specific explanation for the AT deficits in people with autistic spectrum disorder is a problem with feedback from higher level processing areas (i.e. limbic or prefrontal regions), which is needed to interpret the social significance of the viewed stimuli. Kana et al. (2014) reported weaker functional connectivity between temporoparietal regions and motor areas in autistic spectrum disorder patients who were processing intentional causality. Fractional anisotropy further showed alterations in temporal white matter in these patients, leading the authors to conclude that ToM deficits in autism are linked to a disconnection between ToM and mirror neuron systems. Other studies in clinical groups have revealed a relationship between ToM impairment and reduced integrity of the inferior longitudinal fasciculus, which connects the temporal and occipital lobes (e.g. Mike et al., 2013; Crespi et al., 2014). Decreased fractional anisotropy in tracts including the inferior frontooccipital fasciculus and inferior longitudinal fasciculus in HD (Della Nave et al., 2010) could contribute to the proposed integration difficulties.
Potential experimental confounds
One limitation of this study is that as we used an early version of the TAS, so it would be useful to assess another group of HD patients with the more recent and widely applied version. It is also important to consider the possibility of insight problems in HD (e.g. Ho et al., 2006) with the use of self-report measures. As patients with HD who have insight problems are generally thought to under-report problems, this does not appear to have been a difficulty with this study as many significant differences were reported by the patient group. However, further work in HD applying more objective measures of emotional response (e.g. galvanic skin response) may be of value.
No correlations were found between psychiatric diagnoses and either alexithymia scores or responses on the AT, implying that ToM was probably not significantly influenced by mood disorders in this sample. Having said this, further work is needed to determine the potential links between alexithymia, psychiatric diagnoses and medications in manifest and pre-manifest HD. Catergorisation of patients as pre-manifest HD has intrinsic limitations as our understanding of the disorder progresses. Although the majority of studies have applied similar criteria to this study in terms of the DCI cut-off (Seibert et al., 2012; Wolf et al., 2012), it is possible that subtle differences in eye movements may have affected performance on the AT. Finally, longitudinal research will be needed to evaluate the influence of factors such as functional neural compensation on ToM task performance, in addition to determining whether our findings can be replicated in larger samples of gene carriers without any motor abnormalities.
CONCLUSIONS
In conclusion, patients with the HD gene can show a reduced tendency to spontaneously attribute mental states. Alexithymia can be prominent in HD, although these emotional changes were not correlated with ToM deficits in this study. The possibility that a link between burden of pathology and attribution of intentions in HD may be mediated by dopamine dysfunction is intriguing, although neuroimaging research will be required to establish the exact neural basis of patients’ deficits. Importantly, the findings of this study imply that alterations in ToM can be detected prior to motor symptom onset in HD, and these impairments may be independent of executive dysfunction.
Acknowledgments
We are grateful to all participants and to Dr Sridevi Sira Mahalingappa who assisted with data collection. The reviewers of this paper also made many helpful comments. This work was supported by the European Huntington’s Disease Network.
REFERENCES
- Abell F, Happé F, Frith U. Do triangles play tricks? Attribution of mental states to animated shapes in normal and abnormal development. Journal of Cognitive Development. 2000;15:1–20. [Google Scholar]
- Abu-Akel A, Shamay-Tsoory S. Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia. 2011;49:2971–84. doi: 10.1016/j.neuropsychologia.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Allain P, Havet-Thomassin V, Verny C, et al. Evidence for deficits on different components of theory of mind in Huntington's disease. Neuropsychology. 2011;25(6):741–51. doi: 10.1037/a0024408. [DOI] [PubMed] [Google Scholar]
- Aviezer H, Bentin S, Hassin RR, et al. Not on the face alone: perception of contextualized face expressions in Huntington's disease. Brain. 2009;132:1633–44. doi: 10.1093/brain/awp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby RM, Taylor GJ, Parker JD, Loiselle C. Cross-validation of the factor structure of the Toronto Alexithymia Scale. Journal of Psychosomatic Research. 1990;34(1):47–51. doi: 10.1016/0022-3999(90)90007-q. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The ‘‘reading the mind in the eyes’’ test revised version: a study with normal adults, adults with Asperger’s syndrome or high functioning autism. Journal of Child Psychology and Psychiatry. 2001;42:241–51. [PubMed] [Google Scholar]
- Bodden ME, Kübler D, Knake S, et al. Comparing the neural correlates of affective and cognitive theory of mind using fMRI: involvement of the basal ganglia in affective theory of mind. Advances in Cognitive Psychology. 2013;9(1):32–43. doi: 10.2478/v10053-008-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne M, Blank K, Witthaus H, Saft C. “Theory of Mind” is impaired in Huntington’s disease. Movement Disorders. 2011;26:671–8. doi: 10.1002/mds.23494. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Young AW, Lawrence AD, Mason S, Barker RA. The relation between anger and different forms of disgust: implications for emotion recognition impairments in Huntington's disease. Neuropsychologia. 2010;48(9):2719–29. doi: 10.1016/j.neuropsychologia.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–49. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. NeuroImage. 2000;12:314–25. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Chen JY, Wang EA, Cepeda C, Levine MS. Dopamine imbalance in Huntington's disease: a mechanism for the lack of behavioral flexibility. Frontiers in Neuroscience. 2013;7:114. doi: 10.3389/fnins.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early untreated Parkinson’s disease and its relationship to motor disability. Brain. 1991;114:2095–122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- Craufurd D, Thompson JC, Snowden JS. Behavioural changes in Huntington’s disease. Neuropsychiatry Neuropsychology and Behavioral Neurology. 2001;14:219–26. [PubMed] [Google Scholar]
- Crespi C, Cerami C, Dodich A, et al. Microstructural white matter correlates of emotion recognition impairment in Amyotrophic Lateral Sclerosis. Cortex. 2014;53:1–8. doi: 10.1016/j.cortex.2014.01.002. [DOI] [PubMed] [Google Scholar]
- De Gelder B, Van den Stock J, de Diego Balaguer R, Bachoud-Lévi AC. Huntington's disease impairs recognition of angry and instrumental body language. Neuropsychologia. 2008;46(1):369–73. doi: 10.1016/j.neuropsychologia.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Della Nave R, Ginestroni A, Tessa C, et al. Regional distribution and clinical correlates of white matter structural damage in Huntington disease: a tract-based spatial statistics study. AJNR American Journal of Neuroradiology. 2010;31(9):1675–81. doi: 10.3174/ajnr.A2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Ouden HE, Frith U, Frith C, Blakemore SJ. Thinking about intentions. NeuroImage. 2005;28(4):787–96. doi: 10.1016/j.neuroimage.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Duff K, Paulsen JS, Beglinger LJ, et al. Frontal behaviours before the diagnosis of Huntington’s disease and their relationship to markers of disease progression: evidence of early lack of awareness. Journal of Neuropsychiatry and Clinical Neurosciences. 2010;22:196–207. doi: 10.1176/appi.neuropsych.22.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas EM, van den Bogaard SJ, Middelkoop HA, Roos RA. A review of cognition in Huntington's disease. Frontiers in Bioscience. 2013;5:1–18. doi: 10.2741/s355. [DOI] [PubMed] [Google Scholar]
- Eddy CM, Mitchell IJ, Beck SR, Cavanna AE, Rickards HE. Altered subjective fear responses in Huntington's disease. Parkinsonism and Related Disorders. 2011;17:386–9. doi: 10.1016/j.parkreldis.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Eddy CM, Sira Mahalingappa S, Rickards HE. In Huntington’s disease associated with deficits in theory of mind? Acta Neurologica Scandinavia. 2012;126:376–83. doi: 10.1111/j.1600-0404.2012.01659.x. [DOI] [PubMed] [Google Scholar]
- Eddy CM, Sira Mahalingappa S, Rickards HE. Putting things into perspective: the nature and impact of theory of mind impairment in Huntington's disease. European Archives of Psychiatry and Clinical Neurosciences. 2014;264:697–705. doi: 10.1007/s00406-014-0498-4. [DOI] [PubMed] [Google Scholar]
- Enzi B, Edel MA, Lissek S, et al. Altered ventral striatal activation during reward and punishment processing in premanifest Huntington's disease: a functional magnetic resonance study. Experimental Neurology. 2012;235(1):256–64. doi: 10.1016/j.expneurol.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Fizke E, Barthel D, Peters T, Rakoczy H. Executive function plays a role in coordinating different perspectives, particularly when one's own perspective is involved. Cognition. 2014;130(3):315–34. doi: 10.1016/j.cognition.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gregory C, Lough S, Stone V, et al. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer's disease: theoretical and practical implications. Brain. 2002;125:752–64. doi: 10.1093/brain/awf079. [DOI] [PubMed] [Google Scholar]
- Grynberg D, Chang B, Corneille O, et al. Alexithymia and the processing of emotional facial expressions (EFEs): systematic review, unanswered questions and further perspectives. PLoS One. 2012;7(8):e42429. doi: 10.1371/journal.pone.0042429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes CJ, Stevenson RJ, Coltheart M. Disgust and Huntington's disease. Neuropsychologia. 2007;45(6):1135–51. doi: 10.1016/j.neuropsychologia.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Heider F, Simmel M. An experimental study of apparent behavior. American Journal of Psychology. 1944;57:243–9. [Google Scholar]
- Hennenlotter A, Schroeder U, Erhard P, et al. Neural correlates associated with impaired disgust processing in pre-symptomatic Huntington's disease. Brain. 2004;127:1446–53. doi: 10.1093/brain/awh165. [DOI] [PubMed] [Google Scholar]
- Hiraga R, Takahashi K. Conveying emotion with moving images: relationship between movement and emotion. Affective Computing and Intelligent Interaction. Lecture Notes in Computer Science. 2011;6974:558–67. [Google Scholar]
- Ho AK, Robbins AO, Barker RA. Huntington's disease patients have selective problems with insight. Movement Disorders. 2006;21(3):385–9. doi: 10.1002/mds.20739. [DOI] [PubMed] [Google Scholar]
- Horan WP, Nuechterlein KH, Lee J, Castelli F, Green MF. Disturbances in the spontaneous attribution of social meaning in schizophrenia. Psychological Medicine. 2009;39(4):635–43. doi: 10.1017/S0033291708003838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington Study Group (1996) Unified Huntington’s Disease Rating Scale: reliability and consistency. Movement Disorders. 1996;11:136–42. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- Ille R, Holl AK, Kapfhammer HP, Reisinger K, Schäfer A, Schienle A. Emotion recognition and experience in Huntington's disease: is there a differential impairment? Psychiatry Research. 2011;188(3):377–82. doi: 10.1016/j.psychres.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda M, Noritake A. What makes the dorsomedial frontal cortex active during reading the mental states of others? Frontiers in Neuroscience. 2013;7:232. doi: 10.3389/fnins.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M. Motor Cognition: What Actions Tell the Self. New York: Oxford University Press Inc; 2006. [Google Scholar]
- Johnson SA, Stout JC, Solomon AC, et al. Beyond disgust: impaired recognition of negative emotions prior to diagnosis in Huntington's disease. Brain. 2007;130:1732–44. doi: 10.1093/brain/awm107. [DOI] [PubMed] [Google Scholar]
- Ju W, Takayama L. Approachability: how people interpret automatic door movement as gesture. International Journal of Design. 2009;3(2):77–86. [Google Scholar]
- Jurgens CK, Bos R, Luyendijk J, et al. Magnetization transfer imaging in ‘premanifest' Huntington's disease. Journal of Neurology. 2010;257(3):426–32. doi: 10.1007/s00415-009-5339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens CK, van de Wiel L, van Es AC, et al. Basal ganglia volume and clinical correlates in ‘preclinical' Huntington's disease. Journal of Neurology. 2008;255(11):1785–91. doi: 10.1007/s00415-008-0050-4. [DOI] [PubMed] [Google Scholar]
- Kana RK, Libero LE, Hu CP, Deshpande HD, Colburn JS. Functional brain networks and white matter underlying theory-of-mind in autism. Social Cognitive and Affective Neuroscience. 2014;9(1):98–105. doi: 10.1093/scan/nss106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I, Jones R, Callaghan J, et al. Emotional face recognition deficits and medication effects in pre-manifest through stage-II Huntington's disease. Psychiatry Research. 2013;207(1–2):118–26. doi: 10.1016/j.psychres.2012.09.022. [DOI] [PubMed] [Google Scholar]
- Ladegaard N, Larsen ER, Videbech P, Lysaker PH. Higher-order social cognition in first-episode major depression. Psychiatry Research. 2014;216(1):37–43. doi: 10.1016/j.psychres.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological assessment. New York: Oxford University Press; 1995. [Google Scholar]
- Majid DS, Stoffers D, Sheldon S, et al. Automated structural imaging analysis detects premanifest Huntington's disease neurodegeneration within 1 year. Movement Disorders. 2011;26(8):1481–8. doi: 10.1002/mds.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michotte A. The emotions regarded as functional connections. In: Reymert ML, editor. Feelings and Emotions: The Mooseheart Symposium. New York: McGraw-Hill; 1950. pp. 114–25. [Google Scholar]
- Mike A, Strammer E, Aradi, et al. Disconnection mechanism and regional cortical atrophy contribute to impaired processing of facial expressions and theory of mind in multiple sclerosis: a structural MRI study. PLoS One. 2013;8(12):e82422. doi: 10.1371/journal.pone.0082422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y. The early development of executive function and its relation to social interaction: a brief review. Frontiers in Psychology. 2014;5:388. doi: 10.3389/fpsyg.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Lane RD, et al. Impaired self-awareness and theory of mind: an fMRI study of mentalizing in alexithymia. NeuroImage. 2006;32:1472–82. doi: 10.1016/j.neuroimage.2006.04.186. [DOI] [PubMed] [Google Scholar]
- Novak MJ, Warren JD, Henley SM, Draganski B, Frackowiak RS, Tabrizi SJ. Altered brain mechanisms of emotion processing in pre-manifest Huntington's disease. Brain. 2012;135:1165–79. doi: 10.1093/brain/aws024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka N, Ikeda T, Osaka M. Effect of intentional bias on agency attribution of animated motion: an event-related fMRI study. PLoS One. 2012;7(11):e49053. doi: 10.1371/journal.pone.0049053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Smith MM, Long JD, PREDICT HD investigators and Coordinators of the Huntington Study Group Cognitive decline in prodromal Huntington Disease: implications for clinical trials. Journal of Neurology, Neurosurgery and Psychiatry. 2013;84(11):1233–9. doi: 10.1136/jnnp-2013-305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney JB, Jr., Vonsattel JP, MacDonald ME, Gusella JF, Myers RH. CAG repeat number governs the development rate of pathology in Huntington's disease. Annals of Neurology. 1997;41:189–92. doi: 10.1002/ana.410410521. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. 1985 The Halstead–Reitan neuropsychological test battery: therapy and clinical interpretation. Tucson: Neuropsychological Press. [Google Scholar]
- Saft C, Lissek S, Hoffmann R, et al. Mentalizing in preclinical Huntington's disease: an fMRI study using cartoon picture stories. Brain Imaging and Behavior. 2013;7(2):154–62. doi: 10.1007/s11682-012-9209-9. [DOI] [PubMed] [Google Scholar]
- Santangelo G, Vitale C, Trojano L, et al. Neuropsychological correlates of theory of mind in patients with early Parkinson's disease. Movement Disorders. 2012;27(1):98–105. doi: 10.1002/mds.23949. [DOI] [PubMed] [Google Scholar]
- Seibert TM, Majid DS, Aron AR, Corey-Bloom J, Brewer JB. Stability of resting fMRI interregional correlations analyzed in subject-native space: a one-year longitudinal study in healthy adults and premanifest Huntington's disease. NeuroImage. 2012;59(3):2452–63. doi: 10.1016/j.neuroimage.2011.08.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Gibbons ZC, Blackshaw A, et al. Social cognition in frontotemporal dementia and Huntington's disease. Neuropsychologia. 2003;41(6):688–701. doi: 10.1016/s0028-3932(02)00221-x. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Schroeder U, Young AW, Epplen JT. Disgust in pre-clinical Huntington's disease: a longitudinal study. Neuropsychologia. 2006;44(4):518–33. doi: 10.1016/j.neuropsychologia.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. Journal of Cognitive Neuroscience. 1998;10:640–56. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Stout JC, Jones R, Labuschagne I, et al. Evaluation of longitudinal 12 and 24 month cognitive outcomes in premanifest and early Huntington's disease. Journal of Neurology, Neurosurgery and Psychiatry. 2012;83(7):687–94. doi: 10.1136/jnnp-2011-301940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube B, Chatterjee A. Space and time in perceptual causality. Frontiers in Human Neuroscience. 2010;4:28. doi: 10.3389/fnhum.2010.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–62. [Google Scholar]
- Tavares P, Lawrence AD, Barnard PJ. Paying attention to social meaning: an FMRI study. Cerebral Cortex. 2008;18(8):1876–85. doi: 10.1093/cercor/bhm212. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM, Ryan DP, Parker JD. Validation of the alexithymia construct: a measurement-based approach. Canadian Journal of Psychiatry. 1990;35(4):290–7. doi: 10.1177/070674379003500402. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM, Ryan DP, Parker JD, Doody KF, Keefe P. Criterion validity of the Toronto Alexithymia Scale. Psychosomatic Medicine. 1988;50(5):500–9. doi: 10.1097/00006842-198809000-00006. [DOI] [PubMed] [Google Scholar]
- Trinkler I, Cleret de Langavant L, Bachoud-Lévi AC. Joint recognition-expression impairment of facial emotions in Huntington's disease despite intact understanding of feelings. Cortex. 2013;49(2):549–58. doi: 10.1016/j.cortex.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. NeuroImage. 2009;48:564–84. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Werheid K, Hoppe C, Thone A, Muller U, Mungersdorf M, Yves von Cramon D. The adaptive digit ordering test: clinical application, reliability, and validity of a verbal working memory test. Archives of Clinical Neuropsychology. 2002;17:547–65. [PubMed] [Google Scholar]
- Wolf RC, Sambataro F, Vasic N, et al. Default-mode network changes in preclinical Huntington's disease. Experimental Neurology. 2012;237(1):191–8. doi: 10.1016/j.expneurol.2012.06.014. [DOI] [PubMed] [Google Scholar]
- You SC, Geschwind MD, Sha SJ, et al. Executive functions in premanifest Huntington's disease. Movement Disorders. 2014;29(3):405–9. doi: 10.1002/mds.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]