Abstract
Early timing of adrenarche, associated with relatively high levels of Dehydroepiandrosterone (DHEA) in children, has been associated with mental health and behavioral problems. However, little is known about effects of adreneracheal timing on brain function. The aim of this study was to investigate the effects of early adrenarche (defined by high DHEA levels independent of age) on affective brain function and symptoms of psychopathology in late childhood (N = 83, 43 females, M age 9.53 years, s.d. 0.34 years). Results showed that higher DHEA levels were associated with decreased affect-related brain activity (i) in the mid-cingulate cortex in the whole sample, and (ii) in a number of cortical and subcortical regions in female but not male children. Higher DHEA levels were also associated with increased externalizing symptoms in females, an association that was partly mediated by posterior insula activation to happy facial expressions. These results suggest that timing of adrenarche is an important moderator of affect-related brain function, and that this may be one mechanism linking early adrenarche to psychopathology.
Keywords: adrenarche, dehydroepiandrosterone, emotion, sex differences, puberty, externalizing symptoms
INTRODUCTION
Individual differences in the timing of puberty (i.e. the stage of puberty one is at relative to their same-aged peers—early or late) are a strong predictor of mental health problems, including depression, anxiety and behavioral disorders, each of which show a dramatic rise in incidence during the adolescent period (Patton et al., 2008; Paus et al., 2008). For example, advanced maturation of the hypothalamic-pituitary-gonadal axis (i.e. early timing of gonadarche) has been consistently associated with emotional and behavioral problems in girls (Stattin and Magnusson, 2003). The evidence for boys is more mixed, with reports of associations between both delayed (Graber et al., 1997) and advanced (Kaltiala-Heino et al., 2003) gonadal maturation and psychopathology.
Less work has investigated whether timing of adrenarche, a maturational process occurring prior to gonadarche, and characterized by a marked increase in the adrenal androgen dehydroepiandrosterone (DHEA) and its sulfate DHEA-S, also predicts mental health problems. This is despite evidence that adrenarcheal processes may be especially relevant for understanding risk for mental health problems. For example, cross-sectional studies have shown that high DHEA levels are associated with a number of mental health problems such as conduct disorder, oppositional defiant disorder and anxiety disorders in boys (Susman et al., 1996; Van Goozen et al., 1998; van Goozen et al., 2000; Dmitrieva et al., 2001). Only one group to date has assessed whether adrenarcheal timing is associated with current mental health. Dorn et al. (1999a, 2008) found that girls and boys with premature adrenarche (a clinical term characterizing high levels of DHEA and Tanner stage 2 pubic hair in 6–8 year olds) demonstrate increased levels of depression, anxiety and behavioral problems compared with their normatively developing peers.
It has been proposed that hormonal changes in puberty may create a general vulnerability for dysregulated affect and psychopathology via their effect on the development of cognitive and affective systems in the brain during sensitive periods (Blakemore et al., 2010; Ladouceur, 2012). Thus, the effect of early exposure to pubertal hormones on developmentally immature brain systems may partly explain the link between early pubertal timing and vulnerability to mental health problems. This has previously been supported by findings of early pubertal timing influencing internalizing symptoms via its effect on the structure of brain regions involved in emotional processing (Whittle et al., 2012). To date, there is extremely limited understanding of the relationship between adrenarcheal processes and affective brain function. A few studies have investigated the associations between DHEA and affective brain function in human adolescents or adults. For example, Goddings et al. (2012) found high DHEA levels to predict increased anterior temporal lobe activity for social > basic emotion processing in adolescent females. Sripada et al. (2013) found that in male adults, acute administration of DHEA affected activity in the amygdala, hippocampus and anterior cingulate cortex (ACC), during implicit emotion processing. Activation in the left hippocampus was in turn associated with negative emotional response to stimuli. Although no similar studies have been done in children during the adrenarcheal phase, the existing studies support a link between DHEA and affect-related brain function.
Thus, no studies to date have explored the associations between adrenarcheal processes, emotional brain function and mental health, specifically during the adrenarchal transition. Furthermore, no studies have investigated whether the timing of DHEA exposure during adrenarche affects emotional brain function and mental health problems. These are critical questions given that the effects of this exposure may be particularly important during this developmentally sensitive period.
Healthy individuals typically activate regions such as the superior temporal gyrus, anterior cingulate and basal ganglia in response to happy stimuli, the inferior frontal gyrus and parahippocampal gyrus in response to angry stimuli and the amygdala insula in response to fear stimuli (see Vytal and Hamann, 2010, for a meta-analysis of brain regions activated in response to emotional stimuli). Given preliminary evidence that early adrenarche may be associated with increased risk for psychopathology (both internalizing and externalizing), we propose that relatively high levels of DHEA during late childhood (i.e. early timing of adrenarche) will be associated with a pattern of brain activation that is typical of children with internalizing and/or externalizing symptoms. Specifically, we hypothesize that children with early timing of adrenarche will show brain activation consistent with childhood internalizing problems [i.e. increased limbic (e.g. amygdala) and decreased prefrontal activation to negative emotional stimuli, and decreased striatal and prefrontal activity to positive emotional stimuli (Hulvershorn et al., 2011)], and/or childhood externalizing problems [i.e. decreased prefrontal activation to negative emotional stimuli, reduced limbic (e.g. amygdala) activity to negative stimuli (Bellani et al., 2012) and increased striatal activity to rewarding positive stimuli (Bjork et al., 2010)]. We also hypothesize that these patterns of brain function will be associated with mental health problems. While there has been no brain imaging research that has investigated sex differences in the association between DHEA and affective brain function, and the literature regarding sex differences in the association between DHEA levels and psychopathology in childhood is mixed, given consistent support for sex differences in the effects of pubertal timing on psychopathology, we hypothesize that relatively early exposure to DHEA will have a differential effect on brain function and psychopathology in females and males.
METHODS
Participants
Ninety-six participants completed an affective face task during the neuroimaging component of the Imaging Brain Development in the Childhood to Adolescence Transition Study (iCATS). Data from 83 participants (43 females, M age 9.53 years, s.d. 0.34 years) were included in analyses after exclusions based on image acquisition problems (n = 8) and excessive motion (n = 5).
Recruitment
Full details of the recruitment strategy and data acquisition can been found in the iCATS study protocol (Simmons et al., 2014). To summarize, equal numbers of male and female children were invited to participate in iCATS based on their age and adrenal androgen levels measured during participation in the broader Childhood to Adolescence Transition Study (CATS) (Mundy et al., 2013). In total, 1239 8- to 9-year-old students were recruited from 43 primary schools across Melbourne, Australia to participate in CATS. Male and female children were selected whose morning DHEA and testosterone levels (which were highly correlated, and obtained from passive drool saliva collection) fell in the lower and upper tertiles for each sex. The purpose of this selection was to obtain a sample of male and female children who varied widely in their exposure to adrenal androgens, capturing those who were relatively advanced or mature in their adrenal maturation to those where the hormone changes associated with adrenarche were not conspicuously underway. Groups were intentionally matched for age so as to maximize our ability to comment on the effects of relative timing of exposure to adrenal hormones (i.e. timing of adrenarche). Note that we did not examine testosterone in this study because DHEA is the key hormone primarily associated with the initiation of adrenarche, and acts as a precursor for production of more potent androgens including testosterone. Further, the strong correlation between these two hormones means that attempting to dissociate the influence of each would be difficult and interpretation of results would not be straight forward.
Ethics approval was granted by the Royal Children’s Hospital Human Research Ethics Committee (32171), and ratified by the University of Melbourne Human Research Ethics Office (1238745).
Procedure and measures
Magnetic resonance imaging assessment: image aqcuisition
Approximately 6 months after CATS selection (M 0.55 years, s.d. 0.15 years), participants took part in a magnetic resonance imaging (MRI) assessment. Neuroimaging data were acquired on the 3T Siemens TIM Trio scanner (Siemens, Erlangen, Germany) at the Murdoch Childrens Research Institute, Royal Children's Hospital, Melbourne. Participants lay supine with their head supported in a 32-channel head coil. For each of the affective face tasks, 70 whole-brain T2*-weighted echo-planar images (TR = 3000 ms, TE = 40 ms, pulse angle = 85°, FOV = 216 mm) were acquired, corresponding to 40 interleaved slices with a voxel size of 3 × 3 × 3 mm. T1-weighted images were acquired (TR = 1900 ms, TE = 2.24 ms, flip angle = 9°, FOV = 230 mm), producing 208 0.9 mm contiguous sagittal slices (voxel dimensions = 0.9 mm3).
MRI assessment: fMRI task
Participants were administered a modified version of a common face emotion-viewing task used in children (Gaffrey et al., 2011). As in these prior studies, children were presented with a series of faces varying in affective content and asked to complete a simple button press each time a face appeared. In addition to being more ‘child friendly’, a less constrained response was used due to evidence that neural responses associated with psychopathology may be more apparent during such tasks (Monk et al., 2008). Calm, happy, angry and fearful facial expressions from the Nimstim Set of Facial Expressions (Tottenham et al., 2009) were presented in two 3 min 40 s runs using a block design. Calm as opposed to neutral faces were used as a control expression due to evidence that neutral expressions can be perceived as mildly threatening (Tottenham et al., 2009). Each run contained one block of each face type, with each containing eight faces (four males and four female) presented in a random order for 3 s each, separated by a 1 s fixation. Blocks were separated by 15 s fixation rests. Block order was counterbalanced across participants.
Mental health symptoms
Externalizing, depressive and anxiety symptoms were assessed using The Child Behavior Checklist—Parent Report (CBCL-PR; Achenbach, 1999), The Children’s Depression Inventory 2—self-report (CDI-2; Kovaks, 2004), and The Spence Children’s Anxiety Scale—self report (SCAS; Spence, 1997, 1998), respectively.
Saliva samples
Saliva samples were collected on two consecutive days (including the day of the MRI assessment) immediately after waking. Samples were initially frozen at −30°C, and prior to analysis, defrosted and centrifuged, with the supernatant assayed for levels of DHEA. Hormonal assays were conducted using Salimetrics ELISA kits. The range of sensitivity was from 10.2 to 1000 mg/ml. The inter-assay coefficient of variation (CV) was 5.45%, and intra-assay CV 8.56%. Given that the majority of participants (88%) had detectable DHEA levels, and thus could be considered adrenarcheal (i.e. as having entered adrenarche; Ellis and Essex, 2007), and that there is no precedent for grouping children into different phases of adrenarche, DHEA levels were investigated as a continuous measure. Thus, DHEA levels were averaged across the two days and this average measure was used in subsequent analysis. Note that the correlation between DHEA measured at recruitment (not used in analyses) and average DHEA measured at scanning was high (Rho 0.714, P < 0.011). All DHEA values were log transformed due to their non-Gaussian distribution.
Physical maturation
Tanner Stage was assessed via parent report of pubertal development using the Sexual Maturity Status line drawing instrument (Morris and Udry, 1980).
Body mass index
Two measures of height (to nearest 0.1 cm) and weight (to nearest 0.1 kg) were obtained and averaged, and used to calculate a body mass index (BMI) for each participant.
Statistical analysis
Image pre-processing
Images were processed on a Linux platform running MATLAB version 7 and using Statistical Parametric Mapping 8 (SPM8,www.fil.ion.ucl.ac.uk/spm/). Motion correction involved the alignment of each participant’s time series to their first image using least squares minimization with a six-parameter (rigid-body) spatial transformation. Translation and rotation estimates (x, y, z) were required to be <3 mm or 3°, respectively, for all subjects. The realigned functional sequences were then co-registered to the participants’ respective anatomical scans. As the brain structure of children differs from that of adults, spatial normalization was conducted using the ‘DARTEL’ (Diffeomorphic Anatomical Registration using Exponential Lie Algebra; Ashburner, 2007) technique, rather than transformation to a standard adult template. This involved first creating a study-specific T1 template, by segmenting the subjects’ T1 images into gray matter, white matter, and cerebrospinal fluid, and warping them together in a series of iterations. This study-specific template was then warped to the Montreal Neurological Institute (MNI) standard template. Next, at a single-subject level, the individuals’ echo planar images were warped into MNI space using the deformation information from the previous steps. Functional images were then smoothed with a 6 mm (full-width at half-maximum) Gaussian filter. All time-series were routinely inspected for potential normalization artifacts.
Estimating face viewing activation
Estimates of functional activation during each condition were obtained using block-design analyses. First-level (i.e. single-subject) whole-brain, linear regression analyses were conducted in SPM8, including motion parameters as covariates. A high-pass filter set at 128 s was used to remove low-frequency drifts of less than approximately 0.008 Hz. Contrast images for each participant were generated, comparing each of the emotional face conditions (happy, angry and fear) to the calm face condition.
Emotional face processing and DHEA levels
In order to assess the potential associations between DHEA levels and activation to emotional faces at the group level, participants’ blood oxygenation level-dependent (BOLD) activation maps were included in second-level (i.e. group-based) random-effects analyses. These involved a design with run (first and second) as a within-subjects factor, including DHEA as the primary regressor of interest. Analyses were run separately for each of the emotion vs calm face comparisons, and for the whole sample as well as separately for males and females, given our a priori hypotheses for differences in associations for each sex. Age and BMI were treated as nuisance factors in all analyses. Based on our a priori hypotheses that DHEA would be associated with activation in specific regions whose activity has previously been shown to be (i) modulated by DHEA (Sripada et al., 2013), and (ii) associated with emotional and behavioral dysregulation in children (Hulvershorn et al., 2011), potential associations between DHEA levels and activation to emotional faces were explored using a region-of-interest (ROI) analysis. Our ROI mask included the following regions [defined by WFU Pick Atlas Toolbox (http://www.fmri.wfubmc.edu/; Maldjianet al., 2003)]: bilateral amygdala, hippocampus, cingulate cortex, insula, dorsolateral prefrontal cortex (DLPFC, i.e. Brodmann’s Area 46) and striatum. Correction for multiple comparisons was performed using Monte Carlo simulation. A corrected threshold of P < 0.05 (two-tailed) was derived from a combined threshold of P < 0.01 for each voxel and a cluster size of >146 voxels was determined using AlphaSim (Ward, 2000), implemented in the SPM Rest toolbox (Song et al., 2011). Input parameters to AlphaSim included 1000 iterations, a full-width, half maximum (FWHM) of 10 mm, and a single voxel threshold of P < 0.01.
Relationship with psychiatric symptoms
Relationships between DHEA-related brain activation and psychiatric symptoms were explored for each of the significant clusters identified in analyses. Measures of activation for each significant cluster were obtained by extracting the first eigenvariate from ROIs formed by 3-mm-radius spheres around the peak voxels. Partial correlations (covarying for age and BMI) were conducted to assess associations between DHEA-related brain activation and CDI, SCAS and CBCL-externalizing scores. The B-Y method (Benjamini and Yekutieli, 2001) was used to correct for multiple comparisons. Unlike Bonferroni corrections, which are considered to be too conservative in dependent data sets (Armitage et al., 2002), or earlier false discovery rate (FDR) approaches (Benjamini and Hochberg, 1995), which can lead to large Type 1 error rates (Narum, 2006), the method described by Benjamini and Yeketuli was designed to accommodate dependence among hypothesis tests while providing a more conservative Type 1 error rate than earlier FDR approaches (Narum, 2006).
For any significant cluster identified in analyses where activation was found to be associated with symptoms, mediation analyses were performed to investigate the possibility of an indirect association whereby brain activity mediates the association between DHEA levels and symptoms. Mediation was tested regression analyses and a bootstrapping method to test the significance of the indirect (mediation) effect (Hayes, 2009). Bootstrapping generates an empirical approximation of the sampling distribution of the indirect effect statistic by repeated random resampling from the available data, and uses this distribution to construct bias corrected and accelerated confidence intervals. Five thousand resamples were taken and 95% confidence intervals were used. Significant mediation is indicated if the confidence intervals do not contain 0.
In order to increase confidence that any associations found were due to the timing of exposure to DHEA, rather than overt physical signs or early gonadarche, all analyses were re-run using Tanner stage as a nuisance factor.
RESULTS
Descriptive data for the sample can be found in Tables 1 and 2. Of note, DHEA levels were positively associated with parent reported externalizing symptoms in female but not male children.
Table 1.
Sample demographics
| Females (n = 54) |
Males (n = 46) |
|||
|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | |
| DHEA | 60.20 | 54.01 | 42.34 | 31.51 |
| TST | 31.43 | 14.88 | 29.48 | 9.637 |
| Age* | 9.44 | 0.31 | 9.56 | 0.38 |
| Tanner stage | 1.36 | .59 | 1.29 | .51 |
| BMI | 18.07 | 2.52 | 17.06 | 1.89 |
| CBCL | 6.52 | 6.16 | 5.52 | 4.42 |
| SCAS | 20.56 | 12.58 | 18.22 | 10.18 |
| CDI | 3.48 | 2.90 | 4.15 | 3.43 |
*Significant sex difference P < 0.05. TST, testosterone; CBCL, child behavior checklist—externalizing; SCAS, Spence Children’s Anxiety Scale; CDI, child depression inventory 2.
Table 2.
Pearson’s bivariate correlations between demographic and symptom data (females above diagonal and males below diagonal)
| DHEA | TST | Age | Tanner stage | BMI | CBCL | SCAS | CDI | |
|---|---|---|---|---|---|---|---|---|
| DHEA | 1 | 0.833** | 0.108 | 0.391** | 0.526** | 0.364* | 0.057 | 0.123 |
| TST | 0.679** | 1 | 0.036 | 0.345* | 0.411** | 0.354* | −0.027 | 0.156 |
| Age | 0.488** | 0.292 | 1 | 0.281 | 0.285 | 0.156 | 0.226 | 0.354* |
| Tanner stage | 0.021 | −0.180 | −0.011 | 1 | 0.452** | 0.359* | −0.008 | 0.153 |
| BMI | 0.307 | 0.332* | 0.132 | −0.133 | 1 | 0.226 | 0.210 | 0.149 |
| CBCL | −0.271 | −0.143 | −0.193 | −0.109 | −0.171 | 1 | 0.342* | 0.466** |
| SCAS | −0.007 | −0.128 | 0.140 | −0.361* | 0.071 | −0.056 | 1 | 0.633** |
| CDI | −0.251 | −0.321* | −0.124 | −0.339* | −0.056 | 0.227 | 0.732** | 1 |
*P < 0.05, **P < 0.01. TST, testosterone, CBCL, child behavior checklist—externalizing, SCAS, Spence Children’s Anxiety Scale, CDI, Child Depression Inventory 2.
Behavioral
Across face types, 78–88% of participants successfully made a button press to all faces. After correcting for multiple comparisons (four face types: alpha = 0.024 as per the B-Y method), DHEA was not associated with omissions or reaction time in either boys or girls. This suggests that any associations between DHEA and brain activation were unlikely to be attributed specifically to attention.
Associations with DHEA
Fear vs calm
In the right insula and mid-cingulate cortex, higher DHEA levels were associated with less activation to fear vs calm faces (see Table 3 and Figure 1). Analyses conducted separately for each sex showed that for females, there were negative associations between DHEA and activation in the insula, cingulate and DLPFC bilaterally, and in the left caudate (see Figure 2a). No associations were found for males.
Table 3.
DHEA-related brain activation associated with viewing fear vs calm faces
| Region | Cluster size | Peak Z | Peak MNI coordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| Negative association with DHEA (whole sample) | |||||
| Right posterior insula | 179 | 3.3 | 42 | −12 | −3 |
| Right anterior mid-cingulate | 248 | 3.19 | 4 | 0 | 33 |
| Negative association with DHEA (females) | |||||
| Right posterior insula | 2598 | 4.32 | 38 | −4 | 3 |
| Right posterior mid-cingulate | 760 | 4.11 | 6 | −10 | 30 |
| Left posterior insula | 3062 | 3.87 | −30 | −18 | 7 |
| Left dorsolateral prefrontal | 538 | 3.32 | −45 | 33 | 21 |
| Right dorsolateral prefrontal | 202 | 3.27 | 45 | 45 | 9 |
| Left caudate | 158 | 3.24 | −18 | 18 | 7 |
| Right dorsal anterior cingulate | 182 | 2.92 | 10 | 20 | 34 |
Note: Clusters of significant activation that survived statistical threshold for multiple comparisons (montecarlo simulation determined cluster size >146 voxels).
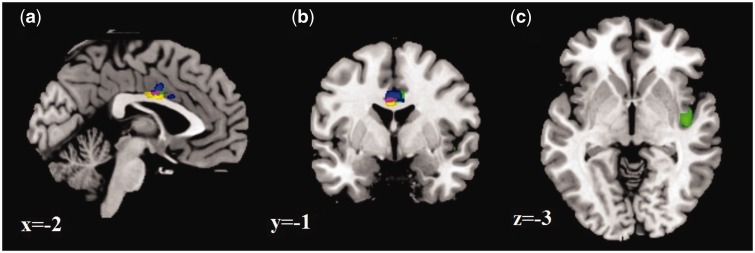
Fig. 1.
(a) Sagittal, (b) coronal and (c) axial slices showing negative BOLD signal associations with DHEA in the whole sample for fear > calm (green), angry > calm (blue), and happy > calm (yellow). Purple, overlap.
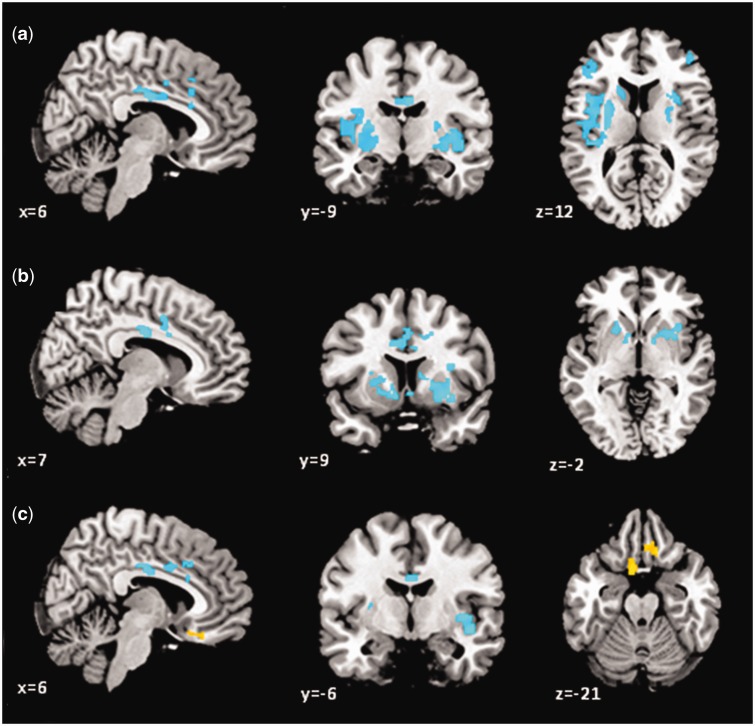
Fig. 2.
BOLD signal associations with DHEA in females for (a) fear > calm, (b) angry > calm and (c) happy > calm. Cool colors, negative associations; warm colors, positive associations.
Angry vs calm
In the right mid-cingulate cortex, higher DHEA levels were associated with less activation to angry vs calm faces (see Table 4 and Figure 1). In females, DHEA was negatively associated with activation in the right mid-cingulate and putamen, and left caudate (see Figure 2b). No associations were found for males.
Table 4.
DHEA-related brain activation associated with viewing angry vs calm faces
| Region | Cluster size | Peak Z | Peak MNI coordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| Negative association with DHEA (whole sample) | |||||
| Right mid-cingulate | 409 | 3.1 | 3 | 0 | 34 |
| Negative association with DHEA (females) | |||||
| Right mid-cingulate | 161 | 3.48 | 8 | −10 | 30 |
| Right putamen | 1277 | 3.4 | 27 | 9 | −6 |
| Right mid-cingulate | 636 | 3.29 | 12 | 0 | 45 |
| Left caudate | 789 | 3.23 | −20 | 18 | 3 |
Note: Clusters of significant activation that survived statistical threshold for multiple comparisons (montecarlo simulation determined cluster size >146 voxels).
Happy vs calm
In the left dorsal cingulate cortex, higher DHEA levels were associated with less activation to happy vs calm faces (see Table 5 and Figure 1). In females, DHEA was negatively associated with activation in the insula bilaterally and in the right cingulate, and positively associated with activation in the left subgenual and right ventromedial prefrontal cortex (see Figure 2c).
Table 5.
DHEA-related brain activation associated with viewing happy vs calm faces
| Region | Cluster size | Peak Z | Peak MNI coordinates |
||
|---|---|---|---|---|---|
| x | y | Z | |||
| Negative association with DHEA (whole sample) | |||||
| Left dorsal cingulate | 224 | 3.39 | −3 | −1 | 30 |
| Negative association with DHEA (females) | |||||
| Right posterior insula | 388 | 3.72 | 44 | −6 | −8 |
| Right mid insula | 217 | 3.63 | 32 | 4 | 19 |
| Right posterior cingulate | 1017 | 3.57 | 8 | −13 | 33 |
| Left anterior insula | 679 | 3.31 | −28 | 21 | 1 |
| Positive association with DHEA (females) | |||||
| Left subgenual cingulate | 214 | 4.34 | −8 | 12 | −21 |
| Right ventromedial prefrontal | 239 | 3.94 | 14 | 27 | −20 |
Note: Clusters of significant activation that survived statistical threshold for multiple comparisons (montecarlo simulation determined cluster size >146 voxels).
See Supplementary Figure S1 for scatterplots of associations for males and females separately for all regions where there were sex-specific findings. Note that including Tanner Stage as a nuisance factor did not change the pattern of significant results for any contrast.
Attenuation to affective faces vs potentiation to calm faces
Follow-up analyses were run to assess the association between DHEA and activation for calm faces (relative to baseline). These analyses produced null results, indicating that the associations found in females reflected a diminished response to affective faces rather than a potentiated response to calm faces.
Associations with symptoms
Critical alpha values were set based on the number of correlations performed for each contrast and each group (i.e. based on the number of significant clusters found for each contrast for the whole sample and for females) using the B-Y method. Thus, for the whole sample, the critical value was set at 0.050 for fear vs calm, and 0.033 for angry vs calm and happy vs calm. Symptoms were not associated with ROI activation for any contrast.
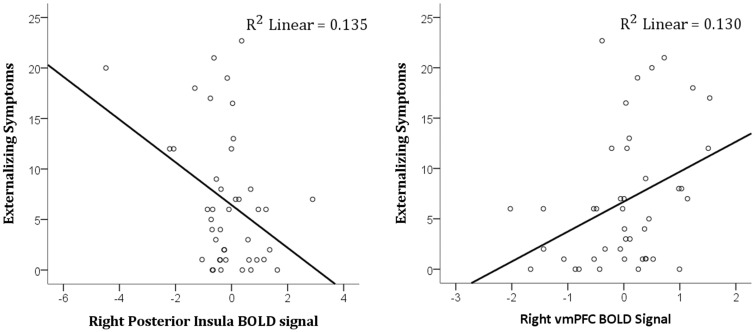
For females, the critical value was set at 0.020 for happy vs calm ROIs, 0.019 for fear vs calm ROIs and 0.024 for angry vs calm ROIs. Higher externalizing symptoms were associated with decreased activation in the anterior insula for happy faces (r = −0.42, P = 0.007), and (at a trend level) increased activation in the ventromedial prefrontal cortex for happy faces (r = 0.33, P = 0.035) (see Figure 3). Winsorizing the apparent anterior insula outlier did not alter the association substantially (r = −0.354, P = 0.023). There were no other significant associations between symptoms and ROI activation for females. Mediation analyses revealed that in females, high DHEA levels indirectly predicted higher externalizing symptoms via decreased insula activation to happy faces (indirect effect = 5.89, SE = 3.59, 95% confidence interval = 0.42–5.71). Controlling for Tanner Stage did not change the significant indirect effect (indirect effect = 5.23, SE = 3.14, 95% confidence interval = 0.51–13.55).
Fig. 3.
Association between externalizing symptoms and (left) right posterior insula and (right) right ventromedial prefrontal activation to happy > calm faces in females.
DISCUSSION
In this unique study of adrenarcheal timing, we found that male and female children with high DHEA levels for their age showed reduced cingulate cortex activation to emotional (angry, fear and happy) face stimuli. Furthermore, there were a number of female-specific findings: in females higher DHEA levels were\associated with reduced activation in the cingulate cortex (in response to all emotional faces), insula cortex (happy and fearful faces), striatum (angry and fearful faces) and DLPFC (fearful faces), and increased activation in the subgenual cingulate/ventromedial prefrontal cortex (happy faces). Higher DHEA levels were also associated with parent-reported externalizing symptoms in females, and this association was partly mediated by decreased posterior insula activity to happy faces.
Our findings are inconsistent with the only study to date to investigate associations between DHEA levels and neural response to ‘basic’ affective stimuli. Sripada et al. (2013) found that acute administration of DHEA in adult males was associated with increased rostral ACC and deceased amygdala and hippocampus activation to emotional faces. However, a number of methodological factors are likely to explain the discrepant results, including the age and gender distribution of participants, measure of DHEA (i.e. acute administration vs baseline levels) and type of fMRI paradigm. Our findings are largely consistent, however, with our hypothesis that early exposure to DHEA during childhood would be associated with brain function reflecting emotion dysregulation. Interestingly, early exposure to DHEA (particularly in females) was associated with a pattern of brain function more typically associated with externalizing as opposed to internalizing symptoms (Bellani et al., 2012). Higher DHEA levels in females were also directly associated with higher parent-reported externalizing symptoms. These findings might suggest an association between early adrenarche in girls and externalizing problems via a dampening of brain responses to emotionally laden stimuli. This idea is discussed further below in relation to the specific indirect effects of DHEA on externalizing symptoms found.
Across the whole sample (i.e. males and females), and consistent across all emotions, high DHEA levels were associated with lower activation in the mid-cingulate region. This finding is consistent with our hypothesis that relatively early adrenarche would be associated with a reduction of activation in prefrontal areas involved in emotional and behavioral regulation. The mid-cingulate has been ascribed a general role in evaluative functions (Etkin et al., 2011), and more specific roles in emotion regulation, interoceptive processes and emotional appraisal, conflict, pain and empathy (Etkin et al., 2011). As such, it is difficult to speculate about the specific meaning of the observed association. However, given that greater maturity (i.e. adulthood vs young adolescence) is associated with increased activation in this region during emotional face processing (Passarotti et al., 2009), decreased activity associated with higher DHEA levels may reflect an immaturity of prefrontal control during affective challenge.
The most striking findings relate to female-specific associations between DHEA and brain activation to emotional faces (no associations between DHEA levels and brain activation were observed for males). Although the regions implicated and the direction of associations were not as expected (e.g. a positive relation between DHEA and limbic activation to negative faces was not observed), the general finding of sex-specific results is consistent with hypotheses. This finding is also consistent with other research supporting a complex interaction between sex and pubertal hormones with respect to brain organizational processes, whereby male and female brains respond differentially to the impact of exposure to pubertal hormones (Peper et al., 2011). However, it is of note that a study of brain structure found no sex differences in the association between DHEA levels and cortical thickness in a group of pre-pubertal children (Nguyen et al., 2013). Further research is required to understand why during childhood, individual differences in DHEA levels may have an effect on female, but not male, emotional brain function.
In females, but not males, DHEA levels were negatively associated with insula activity for both fearful and happy faces. While the posterior insula was implicated in fearful faces, both anterior and posterior insula cortex were implicated in happy faces. The insula cortex is an important area for integrating emotional and homeostatic information to and from limbic and cortical areas. The insula can trigger and map bodily states, and represent the relationship between changes in the bodily states and the objects that elicited them (Bechara and Damasio, 2005). It has been suggested that interoceptive information regarding the physiological condition of the entire body is received by the posterior insula which is then projected to the anterior insula for subjective evaluation of internal conditions (Craig, 2002; Critchley et al., 2004). Further, connections between the insula and the mid-cingulate have been suggested to form a general salience and action system (Taylor et al., 2009). That higher levels of DHEA were associated with reduced activity in both of these regions in females for happy and fearful faces might suggest a decreased ability to process bodily states associated with experiencing affect.
Higher DHEA levels in females were also associated with reduced striatal activity for fearful and angry faces, and reduced DLPFC activity for fearful faces. While caudate nucleus activation has been shown with negative affective stimuli in some research (Goldin et al., 2008), increased striatal activation to happy faces is a more consistent finding, and it has been suggested that this activation might represent reward processing (Mende-Siedlecki et al., 2013). Further, disorders marked by deficits in reward processing show decreased striatal activation to negative affective stimuli (Townsend et al., 2010). As such, it is possible that reduced striatal activation to negative faces in females with high DHEA represents difficulties in reward-related processing. DLPFC activation has been consistently implicated in the regulation of negative emotion (e.g. Goldin et al., 2008). Decreased activity in this region has also been found during exposure to negative affective stimuli in disorders marked by deficits in emotion regulation (Lee et al., 2008; Townsend et al., 2010). As such, it is possible that reduced DLPFC activation to fearful faces in females with high DHEA represents difficulties in the regulation of responses to fearful affective stimuli.
In addition to the negative associations described above, DHEA levels were positively associated with activation to happy faces in the subgenual and ventromedial prefrontal cortices in females. While activation in the subgenual prefrontal area has been predominantly seen in relation to negative affective stimuli, activation by happy faces and in reward paradigms has also been reported in healthy populations (Elliott et al., 2000a, b), suggesting that this region may play a specific role in positive emotional states. Activation in the ventromedial prefrontal cortex has been associated with reward processing and emotional decision making (Rushworth et al., 2011). The significance of the current finding of an association between DHEA and activation in these regions to happy faces in females is unclear; however, the trend level association between ventromedial prefrontal cortex activation to happy faces and externalizing symptoms in females may provide some clues.
As mentioned above, in females, but not males, high DHEA levels were associated with high parent-reported externalizing symptoms. One study in animals suggests that DHEA may be important in influencing aggression, independent of testosterone levels (Soma et al., 2015). While a link between DHEA levels and aggression in boys has been more consistently documented, there is some evidence for a link in girls (Berenbaum and Resnick, 1997). Further, premature adrenarche has been associated with increased behavioral problems in both boys and girls (Dorn et al., 1999b, 2008), although it is of note that our participants experiencing relatively early exposure to DHEA cannot be classified as undergoing premature adrenarche as it is usually defined. We found some evidence, albeit cross-sectional, that relatively early exposure to DHEA in girls may [independently of physical development (i.e. Tanner stage)] influence externalizing symptoms via its effects on posterior insula activation to happy affective stimuli. As noted above, the posterior insula is an important region for interoceptive processing, and therefore its decreased activation to happy faces may reflect a deficit in the processing of bodily states associated with experiencing positive affect, which may in turn have an effect on behavior. There is some evidence that externalizing problems may be associated with insula dysfunction in the processing of positive affective stimuli. For example, youth with a family history of substance use disorders have been shown to have reduced insula activity to happy faces (Cservenka et al., 2014). Also, young females with conduct disorder have been shown to have reduced insula volumes in comparison to healthy females, and males with conduct disorder (Fairchild et al., 2013). Given the cross-sectional nature of the finding, further research is required to establish the causal nature of the identified associations.
As alluded to, one limitation of our study is its cross-sectional design, which limits our ability to make assumptions about the temporal ordering of effects. Second, we attempted to measure adrenarchal timing by implementing a design that specifically selected individuals who were matched for age but differed in their exposure to DHEA. Although DHEA levels were correlated with age at the time of scanning (although not in the female sample), we controlled for age in all analyses, allowing stronger conclusions to be drawn about relative timing of exposure to DHEA (i.e. adrenarcheal timing). However, further research is needed that measures changes in measures over time, in order to more comprehensively investigate adrenarcheal timing and its relation to brain function. Third, although DHEA levels were not statistically significantly different between boys and girls in the current sample, other research suggests that the rise in DHEA levels characteristic of adrenarche may occur 1–2 years earlier in girls (Ducharme et al., 1976). This difference supports our decision to examine associations separately for males and females. However, it is possible that fMRI effects observed for females and not males may be partly attributable to females being more advanced in their adrenarcheal development. On a related note, it is important to emphasize that we did not formally test for sex differences in associations, and as such, while we can say that there are no statistically significant associations between DHEA and brain activation specifically for males, we cannot say that our female-specific associations are statistically significantly different from those in males. Fourth, it is possible that the effects of DHEA on brain function may reflect the influence of some unmeasured variable. Predictors of adrenarche are far less well understood as compared with gonadarche, although there is some evidence for environmental (Ellis and Essex, 2007) and other influencing factors such as birth weight (Auchus and Rainey, 2004). Thus, future work is needed to investigate the influence of potential confounding factors on brain function. Fifth, only parent report of physical maturation was obtained. Future studies should also include self-report and physical exam to gain a potentially more accurate measure of physical maturation. Finally, due to the design of this study, we are not able to comment on the mechanisms of action of DHEA on brain function. Future research should investigate the meaning of the found associations at a more mechanistic level.
In conclusion, we found that relatively earlier exposure to DHEA was associated with decreased affect-related brain activity (i) in the mid-cingulate cortex in the whole sample, and (ii) in a number of cortical and subcortical regions in female children. Relatively early exposure to DHEA in late childhood was also associated with increased externalizing symptoms in females, and this association was partly mediated by reduced posterior insula activation to happy facial expressions. One interpretation of these findings is that early adrenarche might put children (particularly females) at risk for the development of psychopathology as a result of the effects of early DHEA exposure on affective brain function. However, it is also possible that existing psychopathology might affect adrenarcheal timing. Further longitudinal research is now needed to better understand the temporal ordering of these effects.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The authors like to thank all of the families who have participated in this study, and all investigators and staff working on the CATS at the Murdoch Childrens Research Institute (MCRI) for making this investigation possible. MCRI research is supported by the Victorian Government’s Operational Infrastructure Program. The authors also thank the research staff who contributed to the collection of research data (Dr Megan Dennison, Dr Rebecca Davenport-Thomas and Rachel Ellis). This work was supported by the Australian Research Council (Discovery Project grant: DP120101402), the National Health and Medial Council (NHMRC; Career Development Fellowship ID 1007716 to S.W.).
REFERENCES
- Achenbach TM. Child Behavior Checklist. In: Kazdin AE, editor. Encyclopedia of Psychology. New York: Oxford University Press; 1999. [Google Scholar]
- Armitage P, Berry G, Matthews J. Statistical Methods in Medical Research. Oxford: Blackwell; 2002. [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Rainey WE. Adrenarche–physiology, biochemistry and human disease. Clinical Endocrinology. 2004;60(3):288–96. doi: 10.1046/j.1365-2265.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: a neural theory of economic decision. Games and Economic Behavior. 2005;52(2):336–72. [Google Scholar]
- Bellani M, Garzitto M, Brambilla P. Functional MRI studies in disruptive behaviour disorders. Epidemiology And Psychiatric Sciences. 2012;21(01):31–3. doi: 10.1017/s2045796011000692. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Benjamini Y, Yekutieli D. The control of false discovery rate under dependency. Annals of Statistics. 2001;29:1165–88. [Google Scholar]
- Berenbaum SA, Resnick SM. Early androgen effects on aggression in children and adults with congenital adrenal hyperplasia. Psychoneuroendocrinology. 1997;22(7):505–15. doi: 10.1016/s0306-4530(97)00049-8. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Smith AR, Hommer DW. Incentive—elicited mesolimbic activation and externalizing symptomatology in adolescents. Journal of Child Psychology and Psychiatry. 2010;51(7):827–37. doi: 10.1111/j.1469-7610.2009.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Human Brain Mapping. 2010;31(6):926–33. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Fair DA, Nagel BJ. Emotional processing and brain activity in youth at high risk for alcoholism. Alcoholism: Clinical and Experimental Research. 2014 doi: 10.1111/acer.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva T, Oades RD, Hauffa B, Eggers C. Dehydroepiandrosterone sulphate and corticotropin levels are high in young male patients with conduct disorder: comparisons for growth factors, thyroid and gonadal hormones. Neuropsychobiology. 2001;43(3):134–40. doi: 10.1159/000054881. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Hitt SF, Rotenstein D. Biopsychological and cognitive differences in children with premature vs on-time adrenarche. Archives of Pediatrics and Adolescent Medicine. 1999a;153(2):137–46. doi: 10.1001/archpedi.153.2.137. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Hitt SF, Rotenstein D. Biopsychological and cognitive differences in children with premature vs on-time adrenarche. Archives of Pediatrics and Adolescent Medicine. 1999b;153(2):137–46. doi: 10.1001/archpedi.153.2.137. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Rose SR, Rotenstein D, et al. Differences in endocrine parameters and psychopathology in girls with premature adrenarche versus on-time adrenarche. Journal of Pediatric Endocrinology and Metabolism. 2008;21(5):439–48. doi: 10.1515/jpem.2008.21.5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme J-R, Forest MG, Peretti ED, Sempe M, Collu R, Bertrand J. Plasma adrenal and gonadal sex steroids in human pubertal development. The Journal of Clinical Endocrinology and Metabolism. 1976;42(3):468–76. doi: 10.1210/jcem-42-3-468. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. The Journal of Neuroscience. 2000a;20(16):6159–65. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport. 2000b;11(8):1739–44. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ. Family environments, adrenarche, and sexual maturation: a longitudinal test of a life history model. Child Development. 2007;78(6):1799–817. doi: 10.1111/j.1467-8624.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ, Goodyer IM. Brain structure abnormalities in adolescent girls with conduct disorder. Journal of Child Psychology and Psychiatry. 2013;54(1):86–95. doi: 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Luby JL, Belden AC, Hirshberg JS, Volsch J, Barch DM. Association between depression severity and amygdala reactivity during sad face viewing in depressed preschoolers: an fMRI study. Journal of Affective Disorders. 2011;129(1):364–70. doi: 10.1016/j.jad.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings AL, Burnett Heyes S, Bird G, Viner RM, Blakemore SJ. The relationship between puberty and social emotion processing. Developmental Science. 2012;15(6):801–11. doi: 10.1111/j.1467-7687.2012.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(12):1768–76. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Communication Monographs. 2009;76(4):408–20. [Google Scholar]
- Hulvershorn LA, Cullen K, Anand A. Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging and Behavior. 2011;5(4):307–28. doi: 10.1007/s11682-011-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Marttunen M, Rantanen P, Rimpela M. Early puberty is associated with mental health problems in middle adolescence. Social Science and Medicine. 2003;57(6):1055–64. doi: 10.1016/s0277-9536(02)00480-x. [DOI] [PubMed] [Google Scholar]
- Kovaks M. Children's Depression Inventory. Toronto, Canada: Multi-Health Systems; 2004. [Google Scholar]
- Ladouceur CD. Neural systems supporting cognitive-affective interactions in adolescence: the role of puberty and implications for affective disorders. Frontiers in Integrative Neuroscience. 2012;6:65. doi: 10.3389/fnint.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B-T, Seok J-H, Lee B-C, et al. Neural correlates of affective processing in response to sad and angry facial stimuli in patients with major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32(3):778–85. doi: 10.1016/j.pnpbp.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mende-Siedlecki P, Said CP, Todorov A. The social evaluation of faces: a meta-analysis of functional neuroimaging studies. Social Cognitive and Affective Neuroscience. 2013;8(3):285–99. doi: 10.1093/scan/nsr090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Klein R, Telzer E, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. American Journal of Psychiatry. 2008;165(1):90–8. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. Journal of Youth and Adolescence. 1980;9(3):271–80. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Mundy LK, Julian GS, Nicholas BA, et al. Study protocol: the Childhood to Adolescence Transition Study (CATS) BMC pediatrics. 2013;13(1):160. doi: 10.1186/1471-2431-13-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narum SR. Beyond Bonferroni: less conservative analyses for conservation genetics. Conservation Genetics. 2006;7(5):783–7. [Google Scholar]
- Nguyen T-V, McCracken JT, Ducharme S, et al. Interactive effects of dehydroepiandrosterone and testosterone on cortical thickness during early brain development. The Journal of Neuroscience. 2013;33(26):10840–8. doi: 10.1523/JNEUROSCI.5747-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of incidental and directed facial emotion processing in adolescents and adults. Social Cognitive and Affective Neuroscience. 2009;4(4):387–98. doi: 10.1093/scan/nsp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Olsson C, Bond L, et al. Predicting female depression across puberty: a two-nation longitudinal study. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(12):1424–32. doi: 10.1097/CHI.0b013e3181886ebe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9(12):947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Hulshoff Pol HE, Crone EA, van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;191(0):28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70(6):1054–69. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Simmons JG, Sarah LW, George CP, et al. Study protocol: Imaging brain development in the Childhood to Adolescence Transition Study (iCATS) BMC pediatrics. 2014;14(1):115. doi: 10.1186/1471-2431-14-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Rendon NM, Boonstra R, Albers HE, Demas GE. DHEA effects on brain and behavior: insights from comparative studies of aggression. The Journal of Steroid Biochemistry and Molecular Biology. 2015;145:261–72. doi: 10.1016/j.jsbmb.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Song X, Dong Z, Long X, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6(9):e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SH. Structure of anxiety symptoms among children: a confirmatory factor-analytic study. Journal of Abnormal Psychology. 1997;106(2):280–97. doi: 10.1037//0021-843x.106.2.280. [DOI] [PubMed] [Google Scholar]
- Spence SH. A measure of anxiety symptoms among children. Behaviour Research and Therapy. 1998;36(5):545–66. doi: 10.1016/s0005-7967(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, et al. DHEA enhances emotion regulation neurocircuits and modulates memory for emotional stimuli. Neuropsychopharmacology. 2013;38(9):1798–807. doi: 10.1038/npp.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stattin H, Magnusson D. Pubertal Maturation in Female Development. Hillsdale: Erlbaum; 2003. [Google Scholar]
- Susman EJ, Granger DA, Murowchick E, Ponirakis A, Worrall BK. Gonadal and adrenal hormones developmental transitions and aggressive behaviora. Annals of the New York Academy of Sciences. 1996;794(1):18–30. doi: 10.1111/j.1749-6632.1996.tb32506.x. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping. 2009;30(9):2731–45. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JD, Eberhart NK, Bookheimer SY, et al. fMRI activation in the amygdala and the orbitofrontal cortex in unmedicated subjects with major depressive disorder. Psychiatry Research: Neuroimaging. 2010;183(3):209–17. doi: 10.1016/j.pscychresns.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goozen SH, Matthys W, Cohen-Kettenis PT, Thijssen JH, van Engeland H. Adrenal androgens and aggression in conduct disorder prepubertal boys and normal controls. Biological Psychiatry. 1998;43(2):156–8. doi: 10.1016/S0006-3223(98)00360-6. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, van den Ban E, Matthys W, Cohen-Kettenis PT, Thijssen JH, van Engeland H. Increased adrenal androgen functioning in children with oppositional defiant disorder: a comparison with psychiatric and normal controls. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(11):1446–51. doi: 10.1097/00004583-200011000-00020. [DOI] [PubMed] [Google Scholar]
- Vytal K, Hamann S. Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. Journal of Cognitive Neuroscience. 2010;22(12):2864–85. doi: 10.1162/jocn.2009.21366. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous Interference for fMRI Data. AFNI AlphaSim Documentation. Milwaukee, WI: Biophysics Research Institute, Medical College of Wisconsin; 2000. [Google Scholar]
- Whittle S, Yücel M, Lorenzetti V, et al. Pituitary volume mediates the relationship between pubertal timing and depressive symptoms during adolescence. Psychoneuroendocrinology. 2012;37(7):881–91. doi: 10.1016/j.psyneuen.2011.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.