Abstract
Predicting which individuals may engage in aggressive behavior is of interest in today’s society; however, there is little data on the neural basis of aggression in healthy individuals. Here, we tested whether regional differences in white matter (WM) microstructure were associated with later reports of aggressive tendencies. We recontacted healthy young adults an average of 3 years after they underwent research MRI scans. Via electronic survey, we administered the Buss Perry Aggression Questionnaire. We divided aggression into Aggressive Thoughts (Anger and Hostility subscales) and Aggressive Acts (Verbal and Physical subscales) and used Tract-Based Spatial Statistics to test the relationship of those measures to WM microstructure. In 45 individuals age 15–30 at baseline, we observed significant relationships between Aggressive Acts and fractional anisotropy (FA) in a parietal region consistent with the superior longitudinal fasciculus (SLF). As the SLF has an established relationship to executive function, we performed an exploratory analysis in a subset of individuals with working memory data. Decreased FA in executive network regions, as well as working memory performance, were associated with later self-reported aggressive tendencies. This has implications for our healthy behavior understanding of as well as that of patient populations known to have executive dysfunction.
Keywords: diffusion tensor imaging, aggression, imaging, young adults, working memory
INTRODUCTION
The increasing attention in our society on violent and aggressive acts by young adults has made understanding the basis of aggressive behavior, and predicting which individuals are most likely to engage in aggressive behavior, a high priority. In addition to some very salient but relatively rare instances of extreme aggression, ongoing day-to-day aggression can also have a profound impact on family, colleagues and the community at large (Macmillan and Hagan, 2004; Holt et al., 2007). For instance, estimates of the number of children affected by some type of bullying range from 20 to 30%, with up to 50% being affected by verbal bullying (Glew et al., 2008; Wang et al., 2009). In adulthood, 7.6% of men and up to 25% of women experience intimate partner violence (NIJCDC, 2000) and 7.8% of all US workers report experiencing a hostile work environment in the last year (Alterman et al., 2013). Thus, we believe that investigating neural differences associated with aggression in healthy individuals is important. Furthermore, findings from such work in healthy participants also have the potential to suggest new mechanisms and new treatment targets for future work on aggression in patient populations.
Aggression is a complex cognitive and emotional construct that includes aspects of anger, impulsivity and self-control (Schutter and Harmon-Jones, 2013). Given the complicated nature of aggressive behavior, it almost certainly is associated with multiple wide spread brain networks. Despite this, there are very little data on how individual differences in white matter (WM) microstructure, the anatomical basis of connections in such networks, relate to aggressive tendencies. There are currently very few neuroimaging studies of aggression in healthy individuals, with conflicting results. One study, using the Buss Perry Aggression Questionnaire (BPAQ) in young adult males in conjunction with a region of interest-based analysis, has shown a lack of association between WM microstructure in the uncinate fasciculus, a major fronto-limbic connection, and aggression (Beyer et al., 2014b). Another such study investigated a large sample ranging from childhood to young adulthood, also employed the BPAQ, and tested regional tract-based measures of WM microstructure in connections between frontal lobes, temporal lobes and subcortical regions as related to sex steroids (Peper et al., 2014). This study reported lower fractional anisotropy (FA) in individuals with higher levels of reported verbal aggression and hostility.
There is relatively more data on WM and aggression from patient populations, however, this literature is also limited. For example, WM in fronto-limbic networks have been implicated in individuals with schizophrenia (Hoptman et al., 2002, 2005) borderline personality disorder (Rusch et al., 2007), and conduct disorder (Zhang et al., 2014a). There have also been findings of callosal abnormalities in bipolar disorder (Saxena et al., 2012), antisocial psychopathy (Raine et al., 2003) and conduct disorder (Zhang et al., 2014b). In addition to work on WM differences, there have been studies of other modalities, with a meta-analysis of antisocial, violent, or psychopathic individuals, by Yang and Raine demonstrating grey matter differences in a number of frontal regions including orbitofrontal, dorsolateral frontal and anterior cingulate cortex (Yang et al., 2009).
Although violence and aggression in patient groups is a growing area of interest and a subgroup of individuals with mental illness may show increased violence, given the relatively small percentage of the population suffering from mental illness, the majority of violent acts on the whole are committed by individuals without psychiatric diagnoses (Walsh et al., 2002). Furthermore, while findings in clinical populations can provide valuable insight, they are complicated by the fact that changes associated with aggression occur against the background of disease-related change, and may thus be difficult to isolate. It is not known whether enhanced aggression in healthy individuals is associated with the same or different neurostructural alterations as those seen in patient groups.
In this study, we assessed a sample of healthy individuals with no psychiatric history in order to understand whether individual differences in WM microstructure may be associated with aggression across the whole brain as assessed by the BPAQ. The BPAQ has been shown to correlate with real-life measures of violent and aggressive behavior (Archer and Webb, 2006). There is a sex difference on the BPAQ such that males score more highly on the verbal and physical subscales (Buss and Perry, 1992; Archer, 2004); therefore all analyses were controlled for sex. Verbal Aggression and Physical Aggression subscales are highly correlated with each other but are not as strongly related to Anger and Hostility (Buss and Perry, 1992), therefore, in the current analysis these two traits were combined into a single subscale representing the participants’ tendency to engage in Aggressive Acts. Likewise, Anger and Hostility subscales were combined into a single Aggressive Thoughts measure. Dividing the measures in this way allowed us to determine whether the WM differences associated with a tendency to experience emotions or have thoughts with an aggressive tone, were the same or different from WM differences associated with a tendency to engage in violent or aggressive actions. Given the established relationship between aggression and impulsivity (Buss and Perry, 1992; Krakowski, 2003), as well as growing evidence for a relationship between aggressive behavior and executive control in healthy individuals (Hoaken et al., 2003; Hancock et al., 2010; Sprague et al., 2011) it was of particular interest whether Aggressive Acts, as opposed to Aggressive Thoughts, might be related to individual differences in executive function and self-control.
MATERIALS AND METHODS
Participants were selected from a group of individuals who underwent diffusion tensor imaging (DTI) scanning with the same 31 direction research sequence at the Zucker Hillside Hospital Psychiatry Research Division. We identified a group of 109 healthy participants with no Axis I disorder, as determined by the Structured Clinical Interview for DSM-IV administered at initial assessment. Selected individuals were age 15–30 at time of initial scan, and had been scanned at least 18 months prior to contact. All had agreed to be re-contacted for future studies at the time of original consent. To be eligible to provide on-line consent, participants had to be at least 18 years of age at the time of recontact for this study. We contacted individuals via phone calls; in the absence of a working phone number or if the participant could not be reached by phone, letters were sent to the most recent address on file. Interested participants were provided with an individual PIN number which they used to log into the SurveyMonkey site where they completed assessments remotely. To comply with IRB regulations, no personal health information was collected as part of the survey, IP addresses were not logged by the program, and individuals were not allowed to navigate backwards through the survey, which prevented subsequent users of the same computer from accessing previously entered responses. We were able to reach 99 individuals (with contact defined as either reaching them by phone or mailing a letter that was not returned). In total, 48 individuals participated in the survey and 45 completed the entire survey. Participants were reimbursed either electronically with an Amazon gift card or via mail with a check, according to their stated preference.
The 45 participants were age 15.51–29.63 years at time of scan (mean age 22.71 ± 4.06) and participated an average of 3.09 ± 0.84 after their original scan session. At follow up, participants were 18.22–32.51 years old (mean age 25.80 ± 4.08 years); there were 23 male and 21 females in the final sample. One (female, 17 years) was eliminated due to insufficient DTI quality. Baseline characteristics of those individuals who did not elect to participate in the survey were similar to those who did participate (age range 15.71–29.95, 32 female/29 male), indicating that there was not an age or sex related response bias.
Aggression questionnaire
The BPAQ is a commonly used self-report measure used to assess aggressive traits based on 29 items in a Likert scale format from 0 (‘extremely uncharacteristic of me’) to 7 (‘extremely characteristic of me’) (Buss and Perry, 1992). The items are divided into four subscales, Physical Aggression, Verbal Aggression, Anger and Hostility. The Physical Aggression scale contains items such as ‘Once in the while I can’t control the urge to strike another person’ and ‘I have become so mad that I have broken things’. The Verbal Aggression subscale consists of items such as ‘I often find myself disagreeing with people’ and ‘I can’t help getting into arguments when people disagree with me’. The Anger subscale contains items such as ‘Sometimes I fly off the handle for no good reason’ and ‘Some of my friends think I’m a hothead’. Finally, Hostility contains items such as ‘I am suspicious of overly friendly strangers’ and ‘I am sometimes eaten up with jealousy’.
Scanning parameters
All individuals were scanned on a 3T GE HD × 3.0 T system (General Electric, Milwaukee, WI) at the NorthShore University Hospital (NorthShore-LIJ Healthy System, Manhasset, NY. The DTI sequence consisted of 31 directions, B0 = 1000, 5 B0 images, TR = 14s, matrix 128×128, 51 contiguous 2.5-mm axial slices. Other scans administered during the imaging session varied according to each research protocol.
Imaging analyses
The acquired images were corrected using eddy correct (FMRIB Software Library (FSL)), and images were skull stripped using FSL’s Brain Extraction Tool. FA images were calculated using DTIFit (FMRIB’s Diffusion Toolbox), which fits a diffusion tensor model at each voxel, and then were registered to MNI-152 space using a 12-parameter affine registration with a mutual information cost function implemented in Flirt (FSL). A group map was created using Tract-Based Spatial Statistics (TBSS, Smith et al., 2006). An average FA image was created and the tracts were narrowed to generate an FA ‘skeleton’ representing the center of all tracts common to the entire group. The area around the skeleton in each subjects’ aligned FA map was searched and the highest local FA value was assigned to the skeleton. This ensured that each subject’s skeleton was in the group space, yet represented the center of that subject’s own unique fiber tracts.
Statistics were performed using the FSL randomize tool, which performed 2500 permutations at each voxel represented in the TBSS skeleton using the Threshold Free Cluster Enhancement as optimized for TBSS data, with multiple comparisons corrected for using family-wise error (P < 0.05). The BPAQ subscales were combined into Aggressive Thoughts or feelings (Anger and Hostility subscales) or Aggressive Actions (the Verbal and Physical subscales). BPAQ scores were mean centered and entered as primary variables as interest, with age and sex (both were mean centered) entered as covariates. In order to break down the overall results and determine whether the significant regions from the voxel-wise analysis were associated with any particular DTI metric, the radial and axial diffusion measures were extracted within the significant region, and entered into individual robust regressions in Stata (v.13) with FA predicting the score for each subscale (converted to a z-score), controlled for age and sex.
RESULTS
To test the relationship between FA and the Aggressive Thoughts and Aggressive Acts subscales, a whole brain voxel-wise analysis on the TBSS skeleton was performed. In the analysis of Aggressive Acts there was a cluster of significant voxels in the WM of the superior right parietal lobe consistent with the location of the superior longitudinal fasciculus (SLF) (Figure 1). However, in the analysis of Aggressive Thoughts, there were no voxels in which FA significantly related to the BPAQ subscale.
Fig. 1.
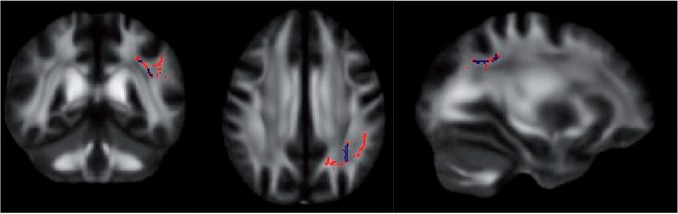
Voxel-wise analysis of Aggressive Acts (slice locations: x = −30, y = −45, z = 34). Red regions significant with one-tailed test, blue regions significant with two-tailed test.
To further probe which aspect of WM integrity might be influencing the significant relationship between Aggressive Acts and FA, we tested the relative differences in radial diffusivity (RD) and axial diffusivity (AD) in the region of interest. Axial and radial diffusion measures of the length of the longest and shortest axes of the elliptical area of diffusion, and have been previously described as putative indices of either tract organization or axonal integrity and myelination, axonal diameter or axonal packing density, respectively (Song et al., 2003, 2005; Wozniak and Lim, 2006). RD (controlled for age and sex) showed a significant relationship with Aggressive Acts [F(3,40) = 27.21, P < 0.0001] such that higher RD was associated with higher aggression, but AD (also controlled for age and sex) did not show a significant relationship [F(3,40) = 1.90, P = 0.1451] (Figure 2). This pattern of results is consistent with the idea that the observed FA effect may be due to lower myelination, narrower axons, or less axonal density in the fronto-parietal executive network in individuals with higher aggressive behavior.
Fig. 2.
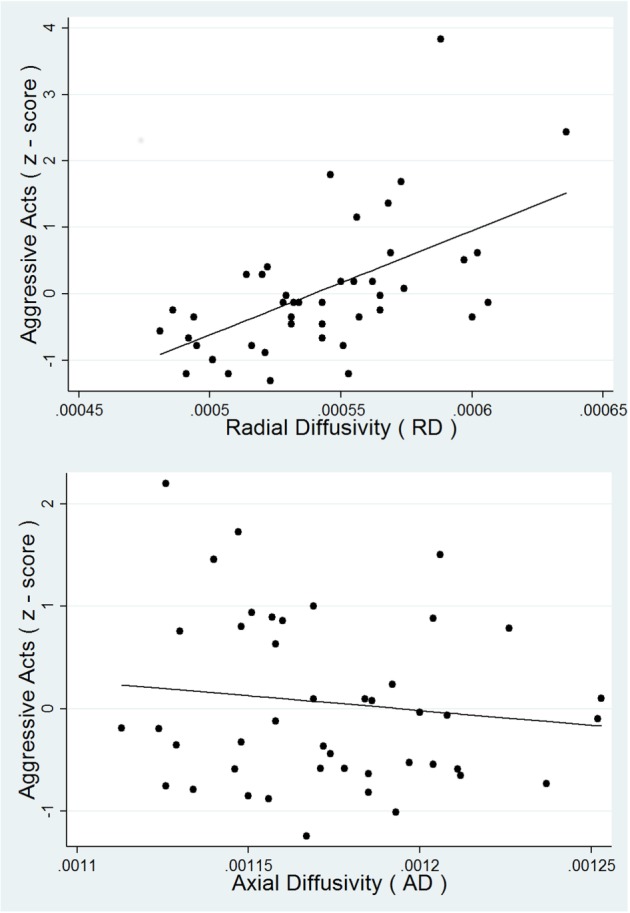
Regression of RD (top panel) and AD (bottom panel) predicting Aggressive Acts.
To test for any potential influence of sex-differences, in addition to controlling for sex in all analyses, we carried out a series of post-hoc tests. First, we tested whether the level of aggression differed between sexes. There was a moderately significant sex difference in aggressive acts [F(1,42) = 4.20, P = 0.0468] but not in aggressive thoughts F(1,42) = 1.30, P = 0.2605. Second, we compared FA in the region of interest between sexes. There was no significant sex difference in FA: [F(1,42) = 0.71, P = 0.403]. There was also no significant sex difference in RD [F(1,42) = 0.17, P = 0.684] or AD [F(1,42) = 0.28, P = 0.602]. Finally, we performed a post-hoc correlation analysis separately for each sex. For females, WM microstructure in the significant ROI was associated with both aggressive thoughts [F(2,18) = 3.73, P = 0.019] and acts [F(2,18) = 10.31, P < 0.001]. For males the region was associated with aggressive acts [F(2,20) = 12.96, P < 0.001] but not thoughts [F(2,20) = 0.36, P = 0.415]. It should be noted, however, that the relationship with thoughts would not survive multiple comparison correction, and that these analyses can only be considered exploratory as they use data extracted from the already significant region of interest.
Next, given the known relationship of the parietal lobe and the SLF to executive function and specifically to working memory (Karlsgodt et al., 2008, 2010), an analysis was performed to determine whether individual differences in working memory were associated with differences in ratings of aggression in a subset of 27 individuals who had digit span data acquired at the time of the initial scan. Due to the retrospective nature of the design, not all participants included in the data set had participated in neuropsychological testing at the time of their scan. Of those who had neuropsychological data, the demographics were similar to the overall sample: mean age at scan was 22.7 ± 4.36 (14 males, 13 females). After a normal transform, robust regression of digit span predicting Aggressive Thoughts (z-scored) and then Acts (also z-scored) was performed in Stata (v13), controlled for age and sex. Digit span score measured at baseline predicted Aggressive Acts [F(3,23) = 5.75, P = 0.0044] with significant effects of digit span (P = 0.033) and sex (P = 0.044) but not age (P = 0.757) (Figure 3). Specifically, a lower digit span score was associated with a higher rating on Aggressive Acts. No relationship between Aggressive Thoughts and digit span score was detected [F(3,23) = 0.71, P = 0.5534]. To further test the relationship of these results to age and sex, we tested the overall relationship between digit span and sex and age, finding that age was not correlated with digit span [F(1,25) = 0.02, P = 0.8984], and sex showed only a trend-level relationship to digit span [F(1,25) = 3.55, P = 0.0714], indicating that there is an effect of sex in the relationship of digit span to aggressive acts but not overall.
Fig. 3.
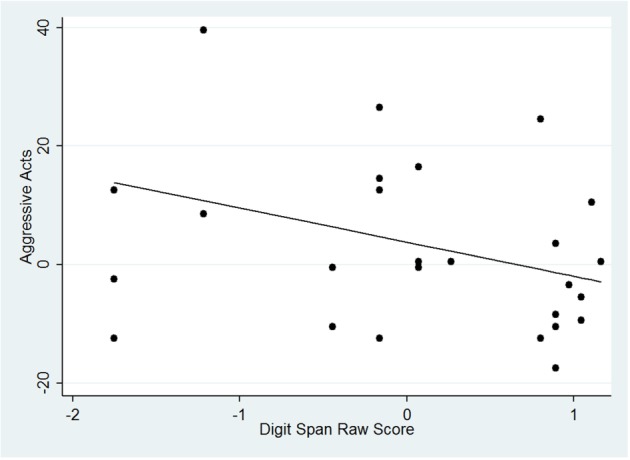
Regression of digit span predicting Aggressive Acts.
Finally, we examined whether the individual differences in WM microstructure within the significant parietal region could explain the relationship between executive function and aggression using a hierarchical regression mediation model. This analysis revealed that WM FA in the parietal region mediated the relationship between digit span score and scores on the aggressive acts subscale. Specifically, we found that digit span was significantly related to Aggressive Acts (b = −0.41608, t = −2.27, P = 0.033) and to FA in the significant parietal region (b = 0.3814, t = 2.84, P = 0.009) but the relationship between digit span and Aggressive Acts was no longer significant when FA in the parietal region was added to the model (b = −0.11604, t = −1.00, P = 0.329).
DISCUSSION
To our knowledge, this is the first report of a relationship between the neural underpinnings of the executive network WM and self-report of aggressive traits in healthy young adults. This is also the first study to use baseline imaging data to predict later reports of aggression. Moreover, a behavioral index of working memory performance was also predictive of aggressive measures, further supporting the notion that executive control plays an important role in the exhibition of aggressive behavior and WM microstructure mediated the relationship between executive function and aggression. Taken together these findings can influence how we conceptualize the roots of aggression in healthy individuals as well as the difference between individuals likely to feel aggressive, and those likely to translate those feelings into actions.
The parietal lobe, including the region implicated in the current analysis, has long been associated with a working memory and cognitive control (Quintana et al., 1989; Jonides et al., 1998; Owen et al., 2005), as a part of the core fronto-parietal network that supports executive function. Coordinated fronto-parietal activity is likely supported by the SLF, a WM tract which in part connects Brodmann’s Areas 9/46 with the supramarginal gyrus (BA 40) (Petrides and Pandya, 2002). FA in the SLF has been associated with working memory performance across a wide range of samples (Karlsgodt et al., 2008, 2010; Burzynska et al., 2011). Moreover, training of working memory has been associated with increased FA in the parietal lobe (Takeuchi et al., 2010). Therefore, the WM region implicated in our current analysis is in an area that has been repeatedly shown to be associated with executive function. In the other existing studies on WM microstructure in healthy individuals with aggressive traits (Beyer et al. 2004a; Peper et al., 2014), differences in the executive network were not found, however unlike our whole-brain analysis, both of these studies employed region of interest-based analyses that did not include assessment of fronto-parietal connections.
The current finding is supported by previous behavioral and cognitive evidence for a relationship between executive function and aggressive behavior. There have been a number of behavioral studies to this end in healthy individuals. For example, some studies have investigated laboratory measures of induced aggression. Such manipulations have demonstrated that participants with lower executive function showed higher levels of lab induced aggressive behavior (Hoaken et al., 2003). In addition, it has been shown that the level of executive function moderated the relationship of functional activation and aggression during social rejection (Chester et al., 2014). There is also evidence that the role of executive function may interact with other factors, such as sex and acute intoxication (Giancola, 2004). Importantly, these results are not just relevant to lab-based measures, but also have real life implications. For instance, in an incarcerated sample, low scores on the D-KEFS executive function battery were associated with violent, but not non-violent, offenses (Hancock et al., 2010). Moreover, in people with low executive function, the level of executive function served as a moderator of the relationship between real life stressors and aggressive behaviors (Sprague et al., 2011). In addition to findings in healthy individuals, there is some existing data in patient populations. For instance, in schizophrenia patients, executive function predicted outcome after pharmacological intervention aimed at curbing aggressive behavior (Krakowski and Czobor, 2012). Taken together, these previous findings indicate that across testing environments and populations, lower executive function is consistently associated with higher evoked and real life aggression. The current data, including the mediation model, indicate that these observed effects in executive function performance may be explained by underlying WM changes.
The BPAQ is often used as a trait-based measure of aggression (Buss and Perry, 1992; Kramer et al., 2011), and as such, future research on the role of the executive network in aggression based on behavioral reports of specific aggressive or violent acts will be an important addition to the literature. However, the BPAQ, in particular the Verbal and Physical subscales that were significant here, have been shown to correlate with measures of violent behavior against others and against the self (Bushman and Wells, 1998; Archer and Webb, 2006; Zhang et al., 2012), thus we believe that BPAQ ‘Acts’ scores can serve as a good index of the likelihood of an individual engaging in real-life aggressive behaviors. Findings of a relationship between executive function and aggression also are consistent with findings that impulsivity is an important factor in aggressive behavior (Buss and Perry, 1992; Krakowski, 2003). For instance, a recent study using the Urgency, Premeditation, Perseverance and Sensation Seeking scale found that in a sample of patients with schizophrenia, this measure of impulsivity correlated with aggression (Hoptman et al., 2014). Although we did not obtain a measure of impulsivity here, future studies addressing this relationship in healthy subjects may help us understand the more subtle role of executive functions in control of aggressive behavior. Moreover, in addition to impulsivity, future studies would be well-served by assessing the relationship with other potentially related constructs such as anxiety and depression.
A number of previous studies have reported sex differences in aggression (Archer, 2004; Potegal and Archer, 2004; Hess and Hagen, 2006; Kalmoe, 2014; Peper et al., 2014), and in particular in direct verbal and physical aggression. Our results support this, with a difference in the level of aggressive acts between males and females such that males showed more aggression. However, we did not find overall sex differences in either the working memory measure or in WM microstructure in the region of interest. The relationship between aggressive acts and WM FA in the region of interest was significant in both sexes, although females also showed a moderate relationship with thoughts. Interestingly, the relationship between digit span predicting aggressive acts also showed an effect of sex. Although our work supports a relationship between executive network connectivity and aggressive acts across both sexes, future work specifically designed to compare the neural basis of aggression between males and females will be of great interest.
As we did not collect aggression measures at baseline, we cannot know whether FA predicted newly aggressive behavior that arose across follow up or if the level of aggressive behavior is a stable trait and the relationship would also have existed at baseline. However, whether baseline FA predicts new aggression, or predicts the continued presence of previously existing aggression, it remains that the baseline imaging data were still relevant to behavior almost 3 years later. The retrospective nature of our study had limitations, in that we were not able to obtain neurocognitive measures uniformly on every individual who contributed imaging data, with the further limitation that the data were self-report. We also cannot know the degree to which the aggressive behavior may or may not have caused functional impairment in the subjects. However the design was also strength, in that it provided the unique opportunity to test whether baseline imaging data were associated with later reports of aggressive attitudes, which is a first step towards prediction. Furthermore, while all participants were free from Axis-1 disorders at baseline, we did not repeat a clinical assessment due to the on-line nature of the study. However, based on our demographic questions, we did determine that none of the participants reported use of any medications associated with psychiatric conditions at time of filling out the BPAQ. Reported medication use included oral contraceptives, anti-inflammatories, blood pressure and thyroid medication, but no psychoactive medication. In addition, no subjects reported being on long term disability (for psychiatric or other disorders) or being a day patient in a treatment program. Future longitudinal studies with larger samples and a broader set of measures on other aspects of aggression as well as on Axis-II disorders will be informative, as will studies in patient populations.
Overall, this study has demonstrated that one of the consequences of impairment in the executive function network may be increased verbal and physical aggression. Furthermore, the finding that the implicated SLF region was associated with Aggressive Acts, but not Thoughts, may indicate that a lack of executive control is specifically a risk factor for exhibiting aggressive behavior, while aggressive emotional states may be associated with different neural circuitry. This finding has implications for typically developing individuals who are at stages in the lifespan associated with decreased frontal lobe microstructure such as adolescence and old age. It also has implications for populations known to have frontal lobe impairment such as fronto-temporal dementia and schizophrenia.
Conflict of Interest
None declared.
Acknowledgments
This work was supported by the National Institute of Mental Health (NIMH): P30MH090590 (to A.K.M.), P50MH080173 (to A.K.M.) and MH086756 (to P.D.). We would like to acknowledge the assistance of Jamie Wagner, Ashley Moyett, and Melina Manolas with this project.
References
- Alterman T, Luckhaupt SE, Dahlhamer JM, Ward BW, Calvert GM. Job insecurity, work-family imbalance, and hostile work environment: prevalence data from the 2010 National Health Interview Survey. American Journal of Industrial Medicine. 2013;56(6):660–9. doi: 10.1002/ajim.22123. [DOI] [PubMed] [Google Scholar]
- Archer J. Sex differences in aggression in real-world settings: a meta-analytic review. Review of General Psychology. 2004;8:291–322. [Google Scholar]
- Archer J, Webb IA. The relation between scores on the Buss-Perry Aggression Questionnaire and Aggressive Acts, Impulsiveness, Competitiveness, Dominance, and Sexual Jealousy. Aggressive Behavior. 2006;32:464–73. [Google Scholar]
- Beyer F, Munte TF, Wiechert J, Heldmann M, Kramer UM. Trait aggressiveness is not related to structural connectivity between orbitofrontal cortex and amygdala. PLoS One. 2014a;9(6):e101105. doi: 10.1371/journal.pone.0101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer F, Munte TF, Wiechert J, Heldmann M, Kramer UM. Trait aggressiveness is not related to structural connectivity between orbitofrontal cortex and amygdala. PLoS One. 2014b;9(6):e101105. doi: 10.1371/journal.pone.0101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Nagel IE, Preuschhof C, et al. Microstructure of frontoparietal connections predicts cortical responsivity and working memory performance. Cerebral Cortex. 2011;21(10):2261–71. doi: 10.1093/cercor/bhq293. [DOI] [PubMed] [Google Scholar]
- Bushman BJ, Wells GL. Trait aggressiveness and hockey penalties: predicting hot tempers on the ice. Journal of Applied Psychology. 1998;83(6):969–74. doi: 10.1037/0021-9010.83.6.969. [DOI] [PubMed] [Google Scholar]
- Buss AH, Perry M. The aggression questionnaire. Journal of Personality and Social Psychology. 1992;63(3):452–9. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Chester DS, Eisenberger NI, Pond RS, Jr, Richman SB, Bushman BJ, Dewall CN. The interactive effect of social pain and executive functioning on aggression: an fMRI experiment. Social Cognitive and Affective Neuroscience. 2014;9(5):699–704. doi: 10.1093/scan/nst038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR. Executive functioning and alcohol-related aggression. Journal of Abnormal Psychology. 2004;113(4):541–55. doi: 10.1037/0021-843X.113.4.541. [DOI] [PubMed] [Google Scholar]
- Glew GM, Fan MY, Katon W, Rivara FP. Bullying and school safety. Journal of Pediatrics. 2008;152(1):123–8, 128 e121. doi: 10.1016/j.jpeds.2007.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock M, Tapscott JL, Hoaken PN. Role of executive dysfunction in predicting frequency and severity of violence. Aggressive Behavior. 2010;36(5):338–49. doi: 10.1002/ab.20353. [DOI] [PubMed] [Google Scholar]
- Hess NH, Hagen EH. Sex differences in indirect aggression: psychological evidence from young adults. Evolution and Human Behavior. 2006;27:231–45. [Google Scholar]
- Hoaken PNS, Shaughnessy VK, Pihl RO. Executive cognitive functioning and aggression: is it an issue of impulsivity? Aggressive Behavior. 2003;29(1):15–30. [Google Scholar]
- Holt MK, Finkelhor D, Kantor GK. Multiple victimization experiences of urban elementary school students: associations with psychosocial functioning and academic performance. Child Abuse and Neglect. 2007;31(5):503–15. doi: 10.1016/j.chiabu.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Antonius D, Mauro CJ, Parker EM, Javitt DC. Cortical thinning, functional connectivity, and mood-related impulsivity in schizophrenia: relationship to aggressive attitudes and behavior. American Journal of Psychiatry. 2014;171(9):939–48. doi: 10.1176/appi.ajp.2014.13111553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Volavka J, Johnson G, Weiss E, Bilder RM, Lim KO. Frontal white matter microstructure, aggression, and impulsivity in men with schizophrenia: a preliminary study. Biological Psychiatry. 2002;52(1):9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Volavka J, Weiss EM, et al. Quantitative MRI measures of orbitofrontal cortex in patients with chronic schizophrenia or schizoaffective disorder. Psychiatry Research. 2005;140(2):133–45. doi: 10.1016/j.pscychresns.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, et al. The role of parietal cortex in verbal working memory. Journal of Neuroscience. 1998;18(13):5026–34. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmoe NP. Trait aggression in two representative U.S. surveys: testing the generalizability of college samples. Aggressive Behavior. 2014 doi: 10.1002/ab.21547. doi: 10.1002/ab/21547. [DOI] [PubMed] [Google Scholar]
- Karlsgodt K, van Erp TG, Poldrack R, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biological Psychiatry. 2008;63(5):512–8. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Kochunov P, Winkler AM, et al. A multimodal assessment of the genetic control over working memory. Journal of Neuroscience. 2010;30(24):8197–202. doi: 10.1523/JNEUROSCI.0359-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowski M. Violence and serotonin: influence of impulse control, affect regulation, and social functioning. The Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15(3):294–305. doi: 10.1176/jnp.15.3.294. [DOI] [PubMed] [Google Scholar]
- Krakowski MI, Czobor P. Executive function predicts response to antiaggression treatment in schizophrenia: a randomized controlled trial. Journal of Clinical Psychiatry. 2012;73(1):74–80. doi: 10.4088/JCP.11m07238. [DOI] [PubMed] [Google Scholar]
- Kramer UM, Kopyciok RP, Richter S, Rodriguez-Fornells A, Munte TF. The role of executive functions in the control of aggressive behavior. Frontiers in Psychology. 2011;2:152. doi: 10.3389/fpsyg.2011.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan R, Hagan J. Violence in the transition to adulthood: adolescent victimization, education, and socioeconomic attainment in later life. Journal of Research on Adolescence. 2004;14(2):127–58. [Google Scholar]
- National Institute of Justice/Centers for Disease Control and Prevention (NIJCDC). Extent, nature, and consequences of intimate partner violence. In: Justice, D.O., editor. Findings from the National Violence Against Women Survey.
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, de Reus MA, van den Heuvel MP, Schutter DJ. Short fused? associations between white matter connections, sex steroids, and aggression across adolescence. Human Brain Mapping. 2014;36(3):1043–52. doi: 10.1002/hbm.22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association pathways of the prefrontal cortex and functional observations. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2002. pp. 31–50. [Google Scholar]
- Potegal M, Archer J. Sex differences in childhood anger and aggression. Child and Adolescent Psychiatric Clinics of North America. 2004;13(3):513–28. doi: 10.1016/j.chc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Quintana J, Fuster JM, Yajeya J. Effects of cooling parietal cortex on prefrontal units in delay tasks. Brain Research. 1989;503(1):100–10. doi: 10.1016/0006-8993(89)91709-5. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Taylor K, et al. Corpus callosum abnormalities in psychopathic antisocial individuals. Archives of General Psychiatry. 2003;60(11):1134–42. doi: 10.1001/archpsyc.60.11.1134. [DOI] [PubMed] [Google Scholar]
- Rusch N, Weber M, Il’yasov KA, et al. Inferior frontal white matter microstructure and patterns of psychopathology in women with borderline personality disorder and comorbid attention-deficit hyperactivity disorder. Neuroimage. 2007;35(2):738–47. doi: 10.1016/j.neuroimage.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Saxena K, Tamm L, Walley A, et al. A preliminary investigation of corpus callosum and anterior commissure aberrations in aggressive youth with bipolar disorders. Journal of Child and Adolescent Psychopharmacology. 2012;22(2):112–9. doi: 10.1089/cap.2011.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter DJ, Harmon-Jones E. The corpus callosum: a commissural road to anger and aggression. Neuroscience and Biobehavioral Reviews. 2013;37(10 Pt 2):2481–8. doi: 10.1016/j.neubiorev.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–22. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–40. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sprague J, Verona E, Kalkhoff W, Kilmer A. Moderators and mediators of the stress-aggression relationship: executive function and state anger. Emotion. 2011;11(1):61–73. doi: 10.1037/a0021788. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, et al. Training of working memory impacts structural connectivity. Journal of Neurosciences. 2010;30(9):3297–303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E, Buchanan A, Fahy T. Violence and schizophrenia: examining the evidence. British Journal of Psychiatry. 2002;180:490–5. doi: 10.1192/bjp.180.6.490. [DOI] [PubMed] [Google Scholar]
- Wang J, Iannotti RJ, Nansel TR. School bullying among adolescents in the United States: physical, verbal, relational, and cyber. Journal of Adolescent Health. 2009;45(4):368–75. doi: 10.1016/j.jadohealth.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Lim KO. Advances in white matter imaging: a review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neuroscience and Biobehavioral Reviews. 2006;30(6):762–74. doi: 10.1016/j.neubiorev.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Abnormal temporal and prefrontal cortical gray matter thinning in psychopaths. Molecular Psychiatry. 2009;14(6):561–62, 555. doi: 10.1038/mp.2009.12. [DOI] [PubMed] [Google Scholar]
- Zhang J, Gao J, Shi H, et al. Sex differences of uncinate fasciculus structural connectivity in individuals with conduct disorder. Biomed Research International. 2014a;2014:673165. doi: 10.1155/2014/673165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhu X, Wang X, et al. Increased structural connectivity in corpus callosum in adolescent males with conduct disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2014b;53(4):466–75 e461. doi: 10.1016/j.jaac.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Zhang P, Roberts RE, Liu Z, et al. Hostility, physical aggression and trait anger as predictors for suicidal behavior in Chinese adolescents: a school-based study. PLoS One. 2012;7(2):e31044. doi: 10.1371/journal.pone.0031044. [DOI] [PMC free article] [PubMed] [Google Scholar]


