Abstract
Adolescence is characterized by an increase in risk-taking and reward-seeking behaviors. In other populations, increased risk taking has been associated with tighter coupling between cortisol production and ventral striatum (VS) activation during reward anticipation; this relation has not yet been examined, however, as a function of adolescent development. This study examined the influence of pubertal development on the association between diurnal cortisol production and VS activity during reward anticipation. Pre- and post-menarcheal girls collected diurnal cortisol and completed an functional magnetic resonance imaging-based monetary incentive delay task, from which we extracted estimates of VS activity during the anticipation of reward, anticipation of loss and anticipation of non-incentive neutral trials. Post-menarcheal girls showed greater coupling between the cortisol awakening response and VS activation during anticipation of reward and loss than did their pre-menarcheal counterparts. Post-menarcheal girls did not differ from pre-menarcheal girls in their cortisol-VS coupling during anticipation of neutral trials, suggesting that puberty-related changes in cortisol-VS coupling are specific to affective stimuli. Interestingly, behavioral responses during the task indicate that post-menarcheal girls are faster to engage with affective stimuli than are pre-menarcheal girls. Thus, post-menarcheal girls exhibit neurobiological and behavioral patterns that have been associated with risk taking and that may underlie the dramatic increase in risk-taking behavior documented during adolescence.
Keywords: ventral striatum, cortisol, risk taking, reward anticipation, adolescence
INTRODUCTION
For many individuals, adolescence is a time of peak physical health. Despite this fact, however, rates of morbidity and mortality increase by as much as 200% from early childhood to late adolescence (Dahl, 2004; Ladouceur, 2012). This dramatic contrast between physical health and elevated rates of morbidity and mortality has been termed the ‘adolescent health paradox’ (Forbes and Dahl, 2010). Underlying the high rates of morbidity is the well-documented increase in risk-taking behavior that occurs in adolescence (Steinberg, 2004, 2007). Although this developmental surge in risk-taking behaviors has been posited to serve the adaptive function of preparing individuals for independence and a life away from their primary caregivers, these behaviors are also posited to contribute to the increased rates of motor vehicle accidents, accidental deaths and suicides in adolescence (Spear, 2000). Researchers have suggested that the general increase in risk-taking behaviors during adolescence is due to developmental changes in the neurobiological response to anticipating reward that serves to heighten the salience of potentially rewarding stimuli (Galvan et al., 2007; Chien et al., 2010).
In adults, neurobiological responses to the anticipation of reward have been examined using both animal and human models. Evidence from animal models indicates that anticipation of reward increases activation in the mesocorticolimbic dopamine pathways and related ventral striatum (VS) regions (Panksepp, 1998; Schultz et al., 2000). Researchers have successfully extended these findings to humans and have reliably documented that anticipation of both primary and secondary rewards increases activation specifically in the VS (Knutson et al., 2001; O’Doherty et al., 2002). Interestingly, glucocorticoids also play a critical role in reward processing, potentially through their influence on dopamine release in the VS (Fahlke et al., 1994; Campbell and Carroll, 2001; Marinelli and Piazza, 2002). For example, increased glucocorticoid secretion facilitates dopamine release in regions of the VS, including the left VS and dorsal putamen (Piazza and Le Moal, 1996; Oswald et al., 2005 for a review), regions known to be rich in glucocorticoid receptor expression (De Kloet et al., 2005; Oitzl et al., 2010). Importantly, evidence suggests that tighter coupling between glucocorticoid secretion and activation of the reward pathway could sensitize the reward system, which investigators posit may increase individuals’ vulnerability to engage in risk-taking behaviors (Marinelli and Piazza, 2002). For example, Li et al. (2014) observed tighter coupling between the glucocorticoid cortisol and VS activation during anticipation of reward in pathological gamblers than in healthy controls.
Although researchers have not yet examined changes in cortisol-VS coupling as a function of development, such changes have been documented in each of these systems independently. Importantly, these changes seem to be driven, in large part, by the biological consequences of pubertal development (Martin et al., 2002; Steinberg, 2004; Gunnar et al., 2009). Animal models suggest that dopamine receptors in the nucleus accumbens and VS increase in number around the onset of puberty, providing preliminary evidence for increased sensitivity of the VS in pubertal adolescents (for a review, see Sisk and Foster, 2004; Sisk and Zehr, 2005). More concrete evidence comes from studies in humans in which, relative to both younger children and adults, adolescents show increased VS activation when anticipating a reward (Galvan et al., 2006; Bjork et al., 2010; Forbes et al., 2010). Moreover, changes in these neural patterns have been associated with pubertal development through their relation with the increase in gonadal hormones. For example, higher testosterone levels in adolescent males have been linked to greater VS activity during anticipation of reward (Forbes et al., 2010). Puberty-related changes have also been observed in diurnal patterns of cortisol secretion (Adam, 2006; Oskis et al., 2009). Both Adam (2006) and Oskis et al. (2009) found greater diurnal cortisol secretion with increasing pubertal maturation. Nevertheless, despite evidence of a critical link between glucocorticoids and the mesolimbic dopamine system (Marinelli and Piazza, 2002), no studies have yet examined how pubertal development influences the coupling of cortisol production and VS activity.
The present study was designed to examine the effects of pubertal development on the association between diurnal cortisol production and VS activity during the anticipation of reward. Identifying changes across pubertal development in cortisol-VS coupling is necessary for understanding the increase in risk taking and sensation seeking that characterizes the mid- to late-adolescent period (Steinberg, 2008). We used a child version of the monetary incentive delay task (KIDMID; Knutson et al., 2008; Gotlib et al., 2010) to assess VS activity in anticipation of reward, and we assessed the presence or absence of menarche as an index of pubertal development (Oskis et al., 2009). In light of the particularly strong relation between cortisol production and VS activity during reward in other samples characterized by high levels of risk-taking behavior (e.g. Li et al., 2014), we expected greater coupling between diurnal cortisol production and VS activity during anticipation of reward in post-menarcheal than in pre-menarcheal participants. To date, no study has documented cortisol-VS coupling during anticipation of loss or non-incentive trials. Thus, we expected that the coupling between diurnal cortisol production and VS activity would be specific to anticipation of reward and would not be evident as participants anticipated loss or in non-incentive trials.
METHODS
Participants
Young girls without a history of any Axis I disorder were recruited as part of a larger longitudinal study of girls at risk for depression. Interested participants were first screened over the telephone with respect to inclusion and exclusion criteria. We then invited potentially eligible girls between the ages of 9 and 14 years to come to the laboratory with their mothers for a more extensive screening process and completion of the Kiddie Schedule for Affective Disorders and Schizophrenia interview (K-SADS; Kaufman et al., 2000). Girls were excluded if they met criteria for any current or past Axis I disorder (according to either mothers’ or adolescents’ report on the K-SADS), had a history of head trauma, had any major medical illnesses, or were taking psychotropic or other medication that could interfere with neuroendocrine activity. This study was approved by the Institutional Review Board at Stanford University, and all experiments were performed in accordance with ascribed guidelines and regulations. Of the 50 girls who were initially eligible for participation in the study, 12 were excluded due to poor quality imaging data, primarily due to the presence of motion artifacts or experimenter error while running the scan. In sum, 38 girls (21 pre-menarcheal and 17 post-menarcheal) participated in this part of the study. Approximately half of these girls (n = 20) had mothers with a history of depression during their daughter’s lifetime, and the other girls (n = 18) had mothers without any Axis I disorder. We included maternal history of depression as a covariate in all analyses.
Self-report measures
All participants completed the short form of the Childhood Depression Inventory (CDI-S; Kovacs, 1992) to assess symptoms of depression and also provided basic demographic information, including age and ethnicity. We assessed pubertal status via self-reported experience of menarche and self-reported Tanner Staging: each girl reported her developmental stage using schematic drawings of two secondary sex characteristics (breast and pubic hair; Tanner and Whitehouse, 1976). Ratings of Tanner Stage were made on a five-point scale, with Tanner Stage I representing an absence of secondary sexual characteristics and Tanner Stage 5 representing physiological sexual maturity.
Diurnal cortisol
Diurnal cortisol production was measured within 2 weeks of the diagnostic assessment via a 2 day, eight-sample collection procedure. Instructions on cortisol collection and storage were given to both children and their mothers, and the exact time of each cortisol collection was documented. On each day, cortisol samples were taken at the following times: immediately upon waking; 30 min after waking; 3:00pm and 30 min before bedtime. Samples were stored in participants’ freezers until they were returned to Stanford University, where they were then stored in a −20°F freezer until analysis. Cortisol levels were assayed by luminescence immunoassay reagents using a commercial kit from Immuno-Biological Laboratories Inc. (Hamburg, Germany). The assay sensitivity was set at 0.015 mg/dl. Samples were assayed together in large batches to control for interassay error, and control samples were included to evaluate variability. The intraassay variation on three saliva pools of the low, medium and high controls were averaged 2.78, 10.45 and 4.79%, respectively. The mean values of the low, medium and high controls were 0.054, 0.228 and 0.863 mg/dl, respectively. The interassay coefficients of the variations of the low, medium and high controls were 10.9, 10.5 and 5.5%, respectively.
Functional magnetic resonance imaging (fMRI) data acquisition
All functional magnetic resonance images were collected on a 1.5-T imaging system (Signa; GE Medical Systems, Milwaukee, WI). Functional images were acquired using a T2-weighted spiral-in/out pulse sequence (Preston et al., 2004) with the following parameters: 83-ms repetition time per second, 40-ms echo time, 90° flip angle, 24-cm field of view and 2 s acquisition time per frame, consisting of 24 sequential axial sections (3.75 mm2 × 3 mm × 1 mm). High-resolution structural images were obtained using a T1-weighted spoiled gradient-recalled acquisition in a steady state sequence (1 mm2 × 1.5 mm), 7 ms echo time and 15° flip angle.
Functional magnetic resonance imaging (fMRI) task protocol. Participants completed the KIDMID task in the scanner (for a full description of this task, see Gotlib et al., 2010). The KIDMID task was based on Knutson et al.’s (2008) adult monetary incentive delay task and was designed to probe children’s neural activation to the anticipation of reward and loss. Participants responded to a target as fast as possible in order to gain points (gain trials) or to avoid losing points (loss trials). Gain and loss trials were compared with non-incentive neutral trials, in which participants withheld a response and no points could be gained or lost.
The task consisted of a single run of 100 trials, each lasting 6 s. Each trial began with an anticipation phase, where a cue was presented to signify the trial type (circle = gain trial; square = loss trial; triangle = neutral trial). Following the anticipation phase, participants were presented with the target (a star) of variable duration, and they pressed a button as quickly as possible (or they did not press the button on neutral trials). Participants then received feedback about whether they responded quickly enough to gain points or to avoid losing points on that trial. For incentive trials (gain or loss trials), either 1 or 5 points were at stake. For neutral trials, a ‘0’ was presented because participants could neither win nor lose points on these non-incentive trials.
Each cue type (circle, square, triangle) appeared 20 times and each trial type was pseudorandomized across the run. The cue during anticipation was displayed for 250 ms and was followed by a variable interstimulus interval (ISI) to last for a total of 2000-2500 ms. The target was presented from 250-350 ms, determined through pilot testing to ensure 75% accuracy. A second variable ISI separated the offset of the target stimulus from the onset of the feedback phase that informed participants whether they had lost or won points. This second ISI was calibrated so that the length of the entire trial was consistently 6 s. The feedback phase followed immediately and lasted 1650 ms. For the purpose of these analyses, we focused on neural activation during the anticipation phase (anticipation of gain, anticipation of loss and non-incentive neutral trials). Reaction time and hit rates were recorded for each trial of the KIDMID task.
fMRI data analysis. Analyses were conducted in FSL Version 4.1.6 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl), using FEAT (FMRI Expert Analysis Tool). The first four volumes of each participant’s functional scan were discarded to allow for stabilization of longitudinal magnetization. The remaining images were preprocessed using a series of steps. Preprocessing included motion correction to the mean image (Jenkinson et al., 2002), spatial smoothing (Gaussian kernel FWHM = 5 mm) and high-pass temporal filtering (t > 0.01 Hz; Woolrich et al., 2001). Functional data were linearly registered to a common stereotaxic space by first registering to the in-plane T2 image (6 degrees of freedom) then to the MNI152 T1 2 mm brain (12 degrees of freedom; Jenkinson and Smith, 2001).
Statistical analysis was conducted using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL. A general linear model (GLM) analysis was conducted for each participant, including regressors for each anticipation condition (anticipation of gain, anticipation of loss and non-incentive neutral trials), as well as the target and all outcome conditions (not the focus of this manuscript). Motion-correction parameters were included as covariates of non-interest. Participants’ individual runs were then combined in a higher-level mixed effects model to investigate the average group response per condition of interest. Higher-level group analyses were conducted using FSL’s FLAME (FMRIB’s Local Analysis of Mixed Effects State) stage 1 and 2 (Beckmann et al., 2003; Woolrich et al., 2004; Woolrich, 2008). Within-group Z statistical images for each condition were thresholded at Z > 1.7, corrected for multiple comparisons at the cluster level using Gaussian random field theory as implemented in FSL (P < 0.05, whole brain correction).
Data analysis
A multistep analytic procedure was used to identify the association between diurnal cortisol patterns and VS activation in anticipation of gain and loss. First, we examined adolescents’ diurnal cortisol production. Because the salivary cortisol data were positively skewed, we winsorized values 2 s.d. above the mean to the 2-s.d. value. We then calculated adolescents’ cortisol awakening response (CAR), which measures the rise in cortisol levels from awaking to 30 min after awakening as a function of time (Pruessner et al., 1997). We also calculated adolescents’ total cortisol production throughout the day (area under the curve with respect to ground; AUCg) using methods recommended by Pruessner et al. (2003).
Next, we identified functional maps from the higher-level mixed effects model for all three anticipation conditions (gain, loss, neutral), and we isolated clusters within the VS to create functional region of interest (ROI) masks.1 Parameter estimates were then extracted from these functionally defined ROIs and used in the regression models to examine the association between diurnal cortisol patterns and VS activation to gain, loss and neutral.
RESULTS
Participant characteristics
Demographic and clinical characteristics of the pre-menarcheal and post-menarcheal groups are presented in Table 1. As expected, girls in the pre-menarcheal group were significantly younger than girls in the post-menarcheal group, t(36) = 4.806, P < 0.001. In contrast, there were no significant group differences in CDI-S scores, t(35) = 0.657, P = 0.515, and CDI-S scores for both groups were well below the recommended clinical cutoff of 8 for the likely presence of depression (Kovacs, 1992). There were also no significant group differences in the proportion of girls whose mothers had a history of depression, χ2(1, N = 38) = 0.001, P = 0.973, or ethnicity, χ2(4, N = 38) = 3.484, P = 0.480. Within the pre-menarcheal group, 71% of participants self-identified as Caucasian, 10% as biracial, 10% as Black, 5% as Hispanic and 5% as Asian; within the post-menarcheal group, 71% self-identified as Caucasian, 18% as biracial and 12% as Hispanic.
Table 1.
Participant characteristics
| Variable | Pre-menarcheal (n = 21) | Post-menarcheal (n = 17) |
|---|---|---|
| Age (in months), M (SD) | 11.286 (1.178)a | 13.284 (1.386)a |
| CDI-S, M (SD) | 1.476 (1.721) | 1.875 (1.962) |
| Maternal depression, % | 52.381 | 52.941 |
| Caucasian, % | 71.429 | 70.588 |
| Reaction time, M (SD) | ||
| Gain trials | 245.672 (35.642)b | 221.179 (26.610)b |
| Loss trials | 248.214 (39.876)c | 223.629 (33.896)c |
| Hit rates, % | ||
| Gain trials | 88.452 | 86.471 |
| Loss trials | 88.810 | 85.441 |
| Salivary cortisol, M (SD) (ug/dl) | ||
| Wake | 0.419 (0.167) | 0.555 (0.293) |
| Wake + 30 min | 0.618 (0.275) | 0.779 (0.347) |
| 3:00pm | 0.216 (0.178) | 0.228 (0.139) |
| Bedtime | 0.078 (0.111) | 0.076 (0.077) |
Notes: CDI-S (Childhood Depression Inventory) (Kovacs, 1992). Superscripts indicate differences between pre-menarcheal and post-menarcheal groups. aP < 0.001, bP = 0.024, cP = 0.051.
Behavioral results
We present behavioral data from the KIDMID task in Table 1. To examine group differences in behavioral responses during the KIDMID task, we conducted mixed-model analyses of variance (ANOVA) on mean reaction times and mean hit rates with menarcheal group (pre-menarcheal, post-menarcheal) as the between-subjects factor and trial type (gain, loss) as the within-subject factor. The two-way ANOVA conducted on reaction time yielded a significant main effect of menarcheal group, F(1,36) = 4.828, P = 0.035, η2 = 0.118: post-menarcheal girls were significantly faster than were pre-menarcheal girls to respond to the target in anticipation of both gain and loss [t(36) = 2.350, P = 0.024 and t(36) = 2.018, P = 0.051, respectively].2 Neither the main effect of trial type, F(1,36) = 1.653, P = 0.207, η2 = 0.044, nor the interaction of trial type and menarcheal group, F(1,36) = 0.001, P = 0.981, η2 = 0.000, was significant. There were no significant main or interaction effects predicting hit rates for gain or loss trials.
We also examined the relation between response time and VS response to the anticipation of gain and loss to ensure that our neural differences were not due simply to differences in reaction time. There were no significant correlations between reaction time and VS activity on either gain or loss trials (all rs > −0.253, all ps > 0.174).
Diurnal cortisol activity
Diurnal cortisol data are presented in Table 1. To examine patterns of diurnal cortisol production, we conducted a repeated-measures ANOVA on the four cortisol samples with menarcheal group (pre-menarcheal, post-menarcheal) as the between-subject factor and time as the within-subject factor. This analysis yielded a significant main effect of time, F(3,108) = 91.286, P < 0.001, η2 = 0.717. Cortisol level increased significantly within the first 30 min of awakening, tpaired(37) = 4.945, P < 0.001, and then declined significantly throughout the day, tspaired(37) > 5.244, ps < 0.001. Neither the main effect of menarcheal group, F(1,36) = 2.454, P = 0.126, η2 = 0.064, nor the interaction of time and menarcheal group, F(3,108) = 2.077, P = 0.108, η2 = 0.055, was significant. This pattern of results was confirmed when using estimates of the CAR and total cortisol production (AUCg): there were no significant group differences in either CAR, t(36) = 0.038, P = 0.970, or AUCg, t(36) = 0.922, P = 0.363.
Menarcheal status, neural activation and diurnal cortisol
Overview
We conducted a series of regression analyses to examine the unique associations of diurnal cortisol production and menarcheal group, and the interaction of diurnal cortisol production and menarcheal group on each estimate of VS activity during the KIDMID task. In all analyses, we controlled for maternal history of depression, given prior findings in an overlapping sample showing decreased recruitment of reward-related neural circuitry in girls with a maternal history of depression (Gotlib et al., 2010). Given their non-independence, estimates of diurnal cortisol production (CAR and AUCg) were modeled separately. The significant interactions of puberty and cortisol that are reported below remained significant when Tanner Staging was used to define puberty status. Because Tanner Staging data were missing for a subset of participants, we used menarcheal status in all analyses.
CAR predicting VS activation in anticipation of gain
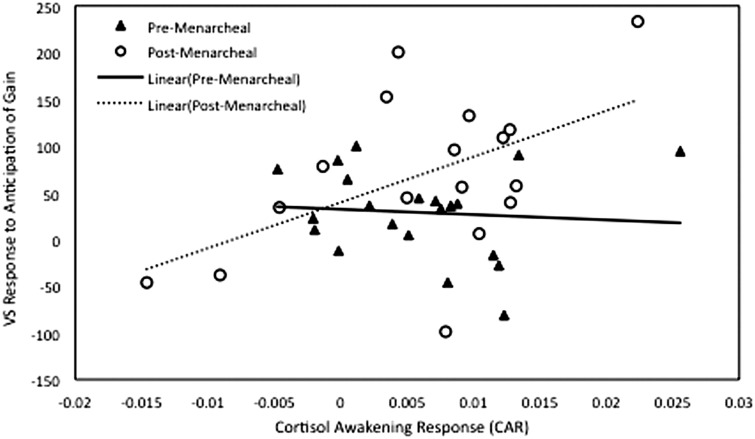
VS activation in anticipation of gain was marginally greater in girls whose mothers had no history of depression than girls whose mothers had a history of depression, β = −0.263, t(33) = 1.750, P = 0.089, η2 = 0.085, marginally greater in post-menarcheal than pre-menarcheal girls, β = 0.288, t(33) = 2.015, P = 0.052, η2 = 0.110, and was marginally associated with a steeper CAR, β = 0.295, t(33) = 1.992, P = 0.055, η2 = 107. As expected, these main effects were qualified by a significant interaction of menarcheal group and CAR predicting VS activation in anticipation of gain, β = 0.361, t(33) = 2.435, P = 0.020, η2 = 0.152. The interaction term remained significant when age was included as a covariate, β = 0.331, t(32) = 2.088, P = 0.045, η2 = 0.120, and age did not significantly predict neural activation in anticipation of gain, β = 0.116, t(32) = 0.579, P = 0.566, η2 = 0.010. In order to decompose the significant interaction of menarcheal group and CAR, we examined the correlation between CAR and VS activation in anticipation of gain separately for each menarcheal group. Whereas the correlation between CAR and VS activation was not significant for girls who had not experienced menarche, r(21) = −0.082, P = 0.722, r2 = 0.007, post-menarcheal girls showed a significant positive correlation between CAR and VS activation in anticipation of gain, r(17) = 0.517, P = 0.034, r2 = 0.267 (Figure 1).
Fig. 1.
Scatter plot depicting the relation of CAR and VS response to anticipation of gain for the pre-menarcheal and post-menarcheal groups.
CAR predicting VS activation to anticipation of loss
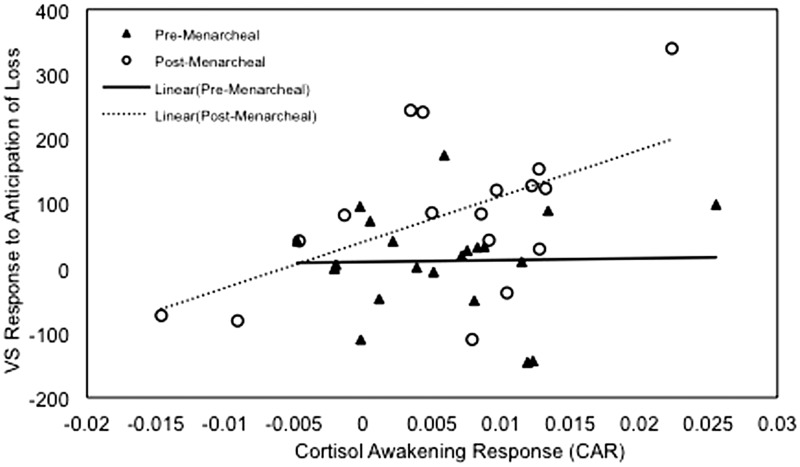
VS activation in anticipation of loss was greater in girls whose mothers had no history of depression than girls whose mothers had a history of depression β = −0.305, t(33) = 2.101, P = 0.043, η2 = 0.118, greater in post-menarcheal than in pre-menarcheal girls, β = 0.343, t(33) = 2.476, P = 0.019, η2 = 0.157, and was associated with a steeper CAR, β = 0.335, t(33) = 2.340, P = 0.025, η2 = 0.142. There was also a significant interaction of menarcheal group and CAR predicting VS activation in anticipation of loss, β = 0.316, t(33) = 2.205, P = 0.035, η2 = 0.128. This interaction term remained significant when age was included in the model, β = 0.308, t(32) = 1.996, P = 0.055, η2 = 0.111, and the main effect of age was not significant, β = 0.034, t(32) = 0.175, P = 0.863, η2 = 0.001. In order to decompose the significant interaction of menarcheal group and CAR, we examined the correlation between CAR and VS activation in anticipation of loss for each group separately. Although there was no significant correlation between CAR and anticipation of loss for pre-menarcheal girls, r(21) = 0.025, P = 0.916, r2 = 0.001, for post-menarcheal girls greater CAR was associated with greater VS activation in anticipation of loss, r(17) = 0.533, P = 0.028, r2 = 0.284 (Figure 2).
Fig. 2.
Scatter plot depicting the relation of CAR and VS response to anticipation of loss for the pre-menarcheal and post-menarcheal groups.
CAR predicting VS activation to anticipation of neutral
To ensure that these results specifically reflected neural activation in anticipation of gain and loss rather than in anticipation of non-incentive neutral trials, we also conducted a linear regression analysis predicting VS activation during anticipation of non-incentive neutral trials (in which participants withheld a response and could not gain or lose any points). This analysis yielded no main effects of menarcheal group or CAR, nor was the interaction of menarcheal group and CAR significant. When age was included in this model, the interaction of menarcheal group and CAR remained non-significant, and the main effect of age was not significant.
AUCg predicting VS activation to anticipation of gain
Across the two menarcheal groups, greater AUCg was associated with greater VS activation to the anticipation of gain, β = 0.382, t(33) = 2.418, P = 0.021, η2 = 0.151. No other main or interaction effects were significant.
AUCg predicting VS activation to anticipation of loss
There were no significant main or interaction effects predicting neural activation in anticipation of loss.
AUCg predicting VS activation to anticipation of neural
There were no significant main or interaction effects predicting neural activation in anticipation of neutral.
Neural activation related to age and diurnal cortisol patterns
To examine whether these results could be attributed to age rather than to menarcheal status, we conducted a parallel series of regression analyses to test the unique associations of diurnal cortisol production and age, and the interaction of diurnal cortisol production and age on each estimate of neural activation during the KIDMID task. As before, in all analyses we controlled for maternal history of depression, and estimates of diurnal cortisol production (CAR and AUCg) were modeled separately. There were no significant main effects of age, and age did not interact with either CAR or AUCg to predict any estimate of neural activation.
DISCUSSION
The present study is the first to examine the association between diurnal cortisol production and VS activity during anticipation of reward in pre- and post-menarcheal girls. We found that menarcheal status moderated the association between the CAR and VS activity in anticipation of both reward and loss: post-menarcheal girls exhibited tighter coupling between the CAR and VS activation than did pre-menarcheal girls. Menarcheal status did not moderate the association between any other measure of diurnal cortisol production and VS activation. This foundational study provides information about biological changes that occur across pubertal development and that may underlie the increases in risk-taking behavior that characterize mid- to late-adolescents (Steinberg, 2008).
As hypothesized, post-menarcheal girls exhibited greater coupling between the CAR and VS activation in anticipation of reward than did pre-menarcheal girls. Marinelli and Piazza (2002) posited that tighter coupling between glucocorticoid production and neural activation of the reward pathway activation sensitizes the reward system and increases individuals’ vulnerability to engage in risk-taking behaviors. Tighter coupling between cortisol production and VS activity in anticipation of reward has been found in pathological gamblers (Li et al., 2014) and is posited to increase vulnerability to drug abuse (Marinelli and Piazza, 2002, for a review). Therefore, tighter cortisol-VS coupling may also contribute to the increased risk taking that is characteristic of later adolescence. Indeed, behavioral responses during the KIDMID task support this interpretation: post-menarcheal girls responded faster than did pre-menarcheal girls on trials in which there was the possibility that they would receive a reward. This measure of behavioral activation and reward reactivity suggests that post-menarcheal girls are faster to engage with rewarding stimuli, which may leave them more susceptible to rush into potentially rewarding but risky situations. It will be important in future research to examine whether cortisol-VS coupling is associated with risk-taking behavior outside the laboratory or with self-report measures of risk taking.
In addition, post-menarcheal girls showed greater CAR-VS coupling in anticipation of a potential loss. We hypothesized that CAR would be uniquely related to VS activation during anticipation of reward given the consistency of previous findings that document VS recruitment in the anticipation and receipt of reward (Ikemoto and Panksepp, 1999). Interestingly, however, researchers have also reported VS activation in anticipation of loss (Bjork et al., 2010; Lamm et al., 2014). Inherent to each loss trial is the motivation to not lose money, and participants could be responding to the goal of avoiding loss, rather than to the expectation of losing. It is also possible that this CAR-VS coupling may indicate a more general anticipatory arousal or emotional reactivity. Adolescents have been found to exhibit increased reactivity to both rewarding and aversive cues (Somerville et al., 2010, for a review); thus, the relation between CAR and VS activation in anticipation of both gain and loss may be the mechanism that underlies this increased salience of affective stimuli. Greater cortisol-VS coupling in anticipation of loss might also be relevant to understanding risk for depression or anxiety in post-pubertal girls. Greater coupling may sensitize individuals to loss and, in the context of environmental stressors, may potentiate susceptibility to depression or increased anxiety.
It is noteworthy that the association between glucocorticoid production and VS activation was largely specific to the CAR. In fact, although there was a significant association between cortisol AUCg and VS activation in anticipation of reward, this relation was not moderated by menarcheal status, and there were no significant associations between cortisol AUCg and VS activation in anticipation of loss or in anticipation of non-incentive neutral trials. The fact that VS activation was associated predominantly with CAR is consistent with research suggesting that CAR is distinct from diurnal cortisol variations as measured by AUCg (Fries et al., 2009; Golden et al., 2013). The CAR, in particular, has been linked to anticipation of the upcoming day (Rohleder et al., 2007), prospective memory representations about the self or the day (Wilhelm et al., 2007; Fries et al., 2009), and daily stressors (Buchanan et al., 2004). Thus, our finding that VS activation is associated specifically with the CAR could indicate that the connectivity between cortisol and VS is unique to the anticipatory response system.
It is important to note that the association between cortisol production and VS activation was moderated by puberty, and not by age. Although it is difficult to fully disentangle the effects of pubertal development from the effects of chronological age, the interaction between menarcheal group and CAR remained after controlling for chronological age; moreover, age did not predict VS activation either as a main effect or in interaction with diurnal cortisol production. Thus, our findings appear to be driven by the biological consequences of pubertal development. It is possible that greater density of dopamine receptors in the VS (Sisk and Foster, 2004) or greater diurnal cortisol secretion (Adam, 2006) contributed to the observed group differences in cortisol-VS coupling. Future research should explore these and other mechanisms through which puberty might contribute to CAR-VS coupling. In this study, we used menarcheal status as a proxy for pubertal development, and then confirmed these results using self-report Tanner staging. Future studies should replicate these findings using more precise measures of pubertal status, such as physical exams of secondary sex characteristics or salivary levels of gonadal hormones (Shirtcliff et al., 2009).
We should note several limitations of this study. First, we included a wide age range in our sample. In order to fully eliminate the effects of chronological age on our results, it is necessary to examine these constructs in an age-restricted sample of girls at different stages of pubertal development. Similarly, the use of self-report menarche as a proxy for pubertal development is a limitation of this research, given that some pre-menarche girls may have hormone levels similar to girls who just experienced menarche. Thus, future research should examine the association between CAR and VS response to anticipation as a function of gonadal hormones or physician-reported pubertal development. Second, including only girls in this study limits the generalizability of our findings. Future research should examine whether the relation between CAR and VS response to affective cues is also moderated by pubertal development in adolescent males. Third this project was part of a larger study examining the effects of maternal depression on risk for depression in adolescents. Although we found no effect of maternal depression history on our results, it is nonetheless important to replicate these findings in an unselected sample. Finally, we did not include a self-report or parent-report measure of risk taking. Behavioral performance on the KIDMID task supports our interpretation that tighter cortisol-VS coupling may contribute to the increased risk taking that is characteristic of later adolescence. Nevertheless, it will be important to substantiate this interpretation with self-report or observational measures of risk taking.
In this study, we demonstrated that, compared with pre-menarcheal girls, post-menarcheal girls exhibited tighter coupling between the morning rise in cortisol production (CAR) and VS activation in anticipation of both reward and loss. We also documented behaviorally that post-menarcheal girls exhibit greater reactivity to affective material than do pre-menarcheal girls. Together, these findings provide a potential mechanism for the increase in risk-taking behavior observed in mid- to late-adolescence. This increase in risk-taking behavior occurs in parallel with rising levels of gonadal hormones (Romeo, 2010), suggesting that gonadal hormones contribute to increases in cortisol-VS coupling observed in post-menarcheal girls. It will be important in future work to examine explicitly the role played by pubertal hormones in the developmental changes in cortisol-VS coupling. Furthermore, it is imperative that future work investigate the association between tighter cortisol-VS coupling and increased risk-taking outside the laboratory. Although this coupling may be a potential mechanism for increased risk-taking behavior in adolescence, it is necessary to link these psychophysiological measures to real-world risk-taking.
Acknowledgments
We thank Melissa Henry, Hannah Burley and Maria Lemus for their help in recruiting and running the participants. This work was supported by the National Institute of Mental Health (R01-MH59259 to IHG and F32-MH102013 to JL), the National Science Foundation (awarded to NLC), and the Brain & Behavior Research Foundation (formerly NARSAD; Distinguished Investigator Award to IHG).
Footnotes
1 When we conducted a whole-brain analysis examining the interaction between menarche status and CAR, we observed a significant cluster in the VS that is similar to that used in our ROI-based analyses (gain: x = 16, y = 12, z = −6 and loss: x = −6, y = 14, z = −10).
2 Age was significantly correlated with reaction time to anticipation of gain, P = 0.023, and loss, P = 0.011; however, when age was included as a covariate in the two-way (menarcheal group repeated over trial type) ANOVA conducted on reaction time, it did not significantly predict reaction time either as a main effect or in interaction with trial type, ps > 0.05.
REFERENCES
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–79. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20(2):1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5:1–14. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Kern S, Allen JS, Tranel D, Kirschbaum C. Circadian regulation of cortisol after hippocampal damage in humans. Biological Psychiatry. 2004;56(9):651–6. doi: 10.1016/j.biopsych.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Effects of ketoconazole on the acquisition of intravenous cocaine self-administration under different feeding conditions in rats. Psychopharmacology. 2001;154(3):311–8. doi: 10.1007/s002130000627. [DOI] [PubMed] [Google Scholar]
- Chien J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2010;14(2):F1–10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R. Adolescent brain development: a period of vulnerabilities and opportunities. Annals of the New York Academy of Science. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience. 2005;6(6):463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Engel JA, Peter Eriksson CJ, Hård E, Söderpalm B. Involvement of corticosterone in the modulation of ethanol consumption in the rat. Alcohol. 1994;11(3):195–202. doi: 10.1016/0741-8329(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain and Cognition. 2010;72:66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, et al. Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(2):162––172e5. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. International Journal of Psychophysiology. 2009;72(1):67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26(25):6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Voss H, Glover G, Casey BJ. Risk taking and the adolescent brain: who is at risk? Developmental Science. 2007;10(2):F8–14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Golden SH, Sánchez BN, Wu M, et al. Relationship between the cortisol awakening response and other features of the diurnal cortisol rhythm: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2013;38(11):2720–8. doi: 10.1016/j.psyneuen.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Archives of General Psychiatry. 2010;67(4):380–7. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Development and Psychopathology. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Research Reviews. 1999;31(1):6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-Sads-Pl. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1208–9. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biological Psychiatry. 2008;63(7):686–92. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–7. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children’s Depression Inventory: Manual. New York: Multi-Health Systems; 1992. [Google Scholar]
- Ladouceur C. Neural systems supporting cognitive-affective interactions in adolescence: the role of puberty and implications for affective disorders. Frontiers in Integrative Neuroscience. 2012;6(65):1–11. doi: 10.3389/fnint.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Benson BE, Guyer AE, et al. Longitudinal study of striatal activation to reward and loss anticipation from mid-adolescence into late adolescence/early adulthood. Brain and Cognition. 2014;89:51–60. doi: 10.1016/j.bandc.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sescousse G, Dreher JC. Endogenous cortisol levels are associated with an imbalanced striatal sensitivity to monetary versus non-monetary cues in pathological gamblers. Frontiers in Behavioral Neuroscience. 2014;8:1–8. doi: 10.3389/fnbeh.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs*. European Journal of Neuroscience. 2002;16(3):387–94. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, et al. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(12):1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33(5):815–26. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Champagne DL, van der Veen R, De Kloet ER. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neuroscience and Biobehavioral Reviews. 2010;34(6):853–66. doi: 10.1016/j.neubiorev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Oskis A, Loveday C, Hucklebridge F, Thorn L, Clow A. Diurnal patterns of salivary cortisol across the adolescent period in healthy females. Psychoneuroendocrinology. 2009;34:307–16. doi: 10.1016/j.psyneuen.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, McCaul M, et al. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30(4):821–32. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective Neuroscience: The Foundations of Human and Animal Emotions. New York: Oxford University Press; 1998. [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends in Pharmacological Sciences. 1998;19(2):67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Preston AR, Thomason ME, Oschner KN, Cooper JC, Glover GH. Comparison of spiral-in/out and spiral-out BOLD fMRI at 1.5 and 3 T. NeuroImage. 2004;21(1):291–301. doi: 10.1016/j.neuroimage.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–31. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sciences. 1997;61(26):2539–49. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Beulen SE, Chen E, Wolf JM, Kirschbaum C. Stress on the dance floor: the cortisol stress response to social-evaluative threat in competitive ballroom dancers. Personality and Social Psychology Bulletin. 2007;33(1):69–84. doi: 10.1177/0146167206293986. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Pubertal maturation and programming of hypothalamic–pituitary–adrenal reactivity. Frontiers in Neuroendocrinology. 2010;31(2):232–40. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10(3):272–83. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child development. 2009;80(2):327–37. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7(10):1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology. 2005;26(3):163–74. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–33. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk-taing in adolescence: what changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–8. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence new perspectives from brain and behavioral science. Current Directions in Psychological Science. 2007;16(2):55–9. [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–196. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Archives of Disease in Childhood. 1976;51(3):170–9. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wüst S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32(4):358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Woolrich MW. Robust group analysis using outlier inference. Neuroimage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilinear linear modelling for FMRI group analysis using bayesian inference. Neuroimage. 2004;21(4):1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]