Abstract
BACKGROUND
A significant share of the cost of cancer care is concentrated in the end‐of‐life period. Although quality measures of aggressive treatment may guide optimal care during this timeframe, little is known about whether these metrics affect costs of care.
METHODS
This study used population data to identify a cohort of patients who died of cancer in Ontario, Canada (2005‐2009). Individuals were categorized as having received or having not received aggressive end‐of‐life care according to quality measures related to acute institutional care or chemotherapy administration in the end‐of‐life period. Costs (2009 Canadian dollars) were collected over the last month of life through the linkage of health system administrative databases. Multivariate quantile regression was used to identify predictors of increased costs.
RESULTS
Among 107,253 patients, the mean per‐patient cost over the final month was $18,131 for patients receiving aggressive care and $12,678 for patients receiving nonaggressive care (P < .0001). Patients who received chemotherapy in the last 2 weeks of life also sustained higher costs than those who did not (P < .0001). For individuals receiving end‐of‐life care in the highest cost quintile, early and repeated palliative care consultation was associated with reduced mean per‐patient costs. In a multivariate analysis, chemotherapy in the 2 weeks of life remained predictive of increased costs (median increase, $536; P < .0001), whereas access to palliation remained predictive for lower costs (median decrease, $418; P < .0001).
CONCLUSIONS
Cancer patients who receive aggressive end‐of‐life care incur 43% higher costs than those managed nonaggressively. Palliative consultation may partially offset these costs and offer resultant savings. Cancer 2015;121:3307–3315. © 2015 American Cancer Society.
Keywords: chemotherapy, costs, end‐of‐life care, palliative care, quality measures
Short abstract
Cancer patients who receive aggressive end‐of‐life care incur 43% higher costs than those managed nonaggressively; these costs are driven by a heavy dependence on acute institutional care. Palliative consultation may partially offset these costs by tempering the tendency toward aggressive management and offer resultant savings.
INTRODUCTION
The economic burden of cancer is high for both individuals and society as a whole. In 2009, the National Institutes of Health estimated the annual costs of cancer to have reached 216.6 billion dollars, with 86.6 billion dollars representing direct medical costs of cancer care.1 Because of the changing incidence, prevalence, and outcomes of malignant diseases, the financial consequences are anticipated to increase further.2 In particular, a disproportionate share of health care costs is concentrated in the end‐of‐life period.2, 3, 4 Prior research in the United States and Canada has established quality measures of aggressive management for the last months of life.5, 6, 7, 8, 9 High rates of unplanned medical encounters such as emergency room visits, hospitalizations, and intensive care stays, for example, may indicate inattention to symptomatic issues, a lack of advance directives, or inadequate utilization of home and hospice services. Chemotherapy administered in the last days of life may offer little chance of clinical benefit but notable consequences, including toxicities that impair quality of life and delay access to hospice care.10, 11 Although quality measures of aggressive management may signal suboptimal oncology care, little is known about whether quality‐based end‐of‐life care can result in cost savings.
Implicit in the assumption that aggressive care is suboptimal at the end of life is the suggestion that a palliative focus could improve the quality of care. When physicians simply engage in end‐of‐life conversations, their patients experience less aggressive medical care near death (including fewer intensive care unit [ICU] admissions) with improved quality of life.12, 13 With additional consultation with palliative care teams, further benefits in patient satisfaction and health care utilization have been demonstrated.14, 15 As such, it would be anticipated that palliation could reduce the use of costly resources. Supporting this contention, preliminary research has suggested that end‐of‐life discussions16 and conversations about spirituality17 might reduce costs of care. Conversely, palliative consultation and placements have their own intrinsic costs. The relation between palliative care, aggressiveness of care, and costs warrants further evaluation.
We used population‐level administrative databases to identify the costs of end‐of‐life care in patients managed with aggressive and nonaggressive intent on the basis of previously established quality measures. Distinctly from the aggressiveness of care received, we further assessed the impact of preceding palliative care services on costs of care. We hypothesized that patients receiving aggressive care near the end‐of‐life would use more resources and correspondingly incur greater costs of care in comparison with individuals who did not receive aggressive care.
MATERIALS AND METHODS
Study Design
We used public administrative databases to identify resources and costs associated with end‐of‐life cancer care for adult patients in Ontario, Canada who died between January 1, 2005 and December 31, 2009. We excluded cases if they did not have a valid provincial health insurance number, died within 30 days of the initial cancer diagnosis, or were younger than 20 years. Individuals in the decedent cohort were categorized as having received or having not received aggressive end‐of‐life management according to the following quality measures5, 7, 18: 1) chemotherapy administered within 14 days of death, 2) more than 1 emergency department (ED) visit within 30 days of death, 3) more than 1 hospitalization within 30 days of death, or 4) at least 1 ICU admission within 30 days of death. This study was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre.
Cohort Selection and Variable Definitions
Patients with cancer as the cause of death were identified in the Ontario Cancer Registry.19 International Classification of Diseases, Ninth Revision (ICD9) codes were used to identify the type of cancer death (breast [ICD9‐174], lung [ICD9‐162], colorectal [ICD9‐153 and ICD9‐154], prostate [ICD9‐185], hematologic [ICD9‐196 and ICD9‐200 to ICD9‐208], and other [all other ICD9 cancer codes]). As previously described, patient cases from the Ontario Cancer Registry were linked to administrative databases (Table 1) with encrypted insurance numbers to determine whether quality measures were performed.5
Table 1.
Administrative Databases
| Database | Description |
|---|---|
| Ontario Cancer Registry | Population‐based database with approximately 95% of all provincial cancer diagnoses captured |
| Ontario Health Insurance Plan | Contains all billing claims for physician services and certain other health professionals (including fee codes for chemotherapy administration) |
| Canadian Institute for Health Information Discharge Abstract Database | Contains demographic, administrative, and clinical data related to hospital admissions (including the provision of intensive care unit care) |
| National Ambulatory Care Reporting System | Contains information on ambulatory care visits (including emergency department care) |
| Home Care Database | Contains information on government‐funded care at home |
| Registered Persons Database | Contains basic demographics and dates of death for all Ontario residents eligible for Ontario Health Insurance coverage |
The neighborhood income quintile was assigned by the linkage of the patient's postal code (from the Registered Persons Database) to the median income by dissemination area identified from the Statistics Canada 2006 census. The rural status (communities with a population size < 10,000) and the regional designation in Ontario (based on the local health integration networks) were based on the city, postal code, and dissemination area of the patient's residence. Patient comorbidity was calculated with the Deyo adaptation20 of the Charlson comorbidity index measure21 according to inpatient diagnoses in the last 2 years of life.
Palliative care was defined by hospitalization, home care, or physician billing codes specific for palliative consultation (see online supporting information). To identify patients who had received repeated and earlier palliative care services before the final days of life, we defined patients as palliative if at least 2 palliative care codes were identified at least 30 days apart and in the last 365 days before death. The palliative care designation was a unique predictor variable mutually distinct from the aggressive quality measures.
Cost Calculation
We used database linkages to identify and determine the costs of all health‐related resources used by patients over the last month of life and paid for by the Ontario Ministry of Health and Long‐Term Care (see online supporting information). Costs were calculated for acute hospitalizations, ED visits, ambulatory oncology visits, same‐day surgery, complex continuing care (inclusive of palliative care placement), long‐term care, home care, physician assessments, certain intravenous chemotherapies, oral medications, and other resources (mental health admissions, dialysis, rehabilitation, and devices).
Statistical Analysis
We examined baseline descriptive characteristics for patients who experienced at least 1 indicator of aggressive management versus those who did not experience any aggressive intervention. Crude rates for each indicator of aggressive end‐of‐life care were computed per year from 2005 to 2009, and they were stratified according to the palliative designation.
Total and disaggregated mean costs per patient were presented descriptively for patients who experienced at least 1 indicator of aggressive care and for those who did not. Because of the higher intrinsic costs associated with the hospitalization, ED, and ICU quality measures, we separately analyzed the costs for those who received chemotherapy in the last 14 days of life and those who did not. Analyses were further stratified according to whether patients had a palliative designation.
Because of the skewness in the cost data and exponential characteristics of the distribution, univariate quantile regression was used to test the significance of the cost differential between aggressive and nonaggressive end‐of‐life care. Analyses were further stratified according to the palliative designation. Multivariate quantile regression analysis was used to estimate effects of the palliative designation and the aggressive quality measures on median costs. In quantile regression, the quantiles or percentiles of the distribution of costs are modeled as a function of predictor characteristics.22 Because mean costs are potentially more susceptible to outliers than median costs,23 we felt that this modeling was a more appropriate method to avoid overestimating the impact of the aggressive indicators on overall end‐of‐life costs. Models were adjusted for the age at death (continuous variable centered on the age of 20 years), sex, cancer type (hematologic vs nonhematologic), income quintile, Charlson score, duration of disease, residence (urban vs rural), and year of death (continuous variable centered on the year 2005). All analyses were performed with SAS 9.1.3 (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics and Aggressive Care Indicators
There were 107,253 patients who died of cancer in Ontario, Canada and met our inclusion criteria to form the cohort for the study. Table 2 presents demographic characteristics of patients who did and did not receive aggressive care. In the cohort, 24,095 patients (22.5%) experienced at least 1 quality measure of aggressive end‐of‐life care.
Table 2.
Baseline Demographic and Clinical Characteristics
| Characteristic | Patients Not Experiencing Any Aggressive Care (n = 83,158) | Patients Experiencing Any Aggressive Care (n = 24,095) | Total (n = 107,253) |
|---|---|---|---|
| Age, mean ± SD, y | 71.92 ± 12.84 | 68.14 ± 12.85 | 71.07 ± 12.94 |
| Female, No. (%) | 40,832 (49.1) | 10,329 (42.9) | 51,161 (47.7) |
| Charlson score per point, mean ± SD | 5.11 ± 2.65 | 5.63 ± 2.41 | 5.23 ± 2.61 |
| Duration of disease, mean ± SD, y | 3.43 ± 5.28 | 3.17 ± 5.05 | 3.37 ± 5.23 |
| Cancer type, No. (%) | |||
| Breast | 6976 (8.4) | 1950 (8.1) | 8926 (8.3) |
| Colorectal | 9715 (11.7) | 2464 (10.2) | 12,179 (11.4) |
| Hematologic | 6763 (8.1) | 3399 (14.1) | 10,162 (9.5) |
| Lung | 19,678 (23.7) | 5937 (24.6) | 25,615 (23.9) |
| Prostate | 5196 (6.2) | 1147 (4.8) | 6343 (5.9) |
| Other | 34,830 (41.9) | 9198 (38.2) | 44,028 (41.1) |
| Income quintile, No. (%) | |||
| Highest | 15,634 (18.8) | 4392 (18.2) | 20,026 (18.7) |
| Second highest | 15,627 (18.8) | 4530 (18.8) | 20,157 (18.8) |
| Middle | 15,945 (19.2) | 4782 (19.8) | 20,727 (19.3) |
| Second lowest | 17,719 (21.3) | 5291 (22.0) | 23,010 (21.5) |
| Lowest | 17,763 (21.4) | 5004 (20.8) | 22,767 (21.2) |
| Rural residence, No. (%) | 11,503 (13.8) | 4484 (18.6) | 15,987 (14.9) |
| Region of residence, No. (% by LHIN) | |||
| 01. Erie–St. Clair | 4879 (5.9) | 1437 (6.0) | 6316 (5.9) |
| 02. South West | 6835 (8.2) | 2169 (9.0) | 9004 (8.4) |
| 03. Waterloo | 4328 (5.2) | 1150 (4.8) | 5478 (5.1) |
| 04. Hamilton | 11,530 (13.9) | 2893 (12.0) | 14,423 (13.4) |
| 05. Central West | 3024 (3.6) | 1075 (4.5) | 4099 (3.8) |
| 06. Mississauga | 5404 (6.5) | 1439 (6.0) | 6843 (6.4) |
| 07. Toronto | 7228 (8.7) | 1707 (7.1) | 8935 (8.3) |
| 08. Central | 8075 (9.7) | 2532 (10.5) | 10,607 (9.9) |
| 09. Central East | 9290 (11.2) | 2973 (12.3) | 12,263 (11.4) |
| 10. South East | 4174 (5.0) | 1268 (5.3) | 5442 (5.1) |
| 11. Champlain | 8298 (10.0) | 2017 (8.4) | 10,315 (9.6) |
| 12. North Simcoe | 3181 (3.8) | 1210 (5.0) | 4391 (4.1) |
| 13. North East | 4937 (5.9) | 1682 (7.0) | 6619 (6.2) |
| 14. North West | 1804 (2.2) | 533 (2.2) | 2337 (2.2) |
| Designated palliative, No. (%) | 56,674 (68.2) | 13,041 (54.1) | 69,715 (65.0) |
| Quality measures, No. (%) | |||
| >1 ED visit in last 30 d of life | 0 (0.0) | 15,812 (65.6) | 15,812 (14.7) |
| Chemotherapy in last 14 d of life | 0 (0.0) | 3893 (16.2) | 3893 (3.6) |
| ICU admission in last 30 d of life | 0 (0.0) | 5923 (24.6) | 5923 (5.5) |
| >1 hospitalization in last 30 d of life | 0 (0.0) | 6765 (28.1) | 6765 (6.3) |
Abbreviations: ED, emergency department; ICU, intensive care unit; LHIN, local health integration network; SD, standard deviation.
During the study period, 3.6% of the cohort received systemic chemotherapy in the last 2 weeks of life. The remaining indicators were noted more frequently: 14.7% experienced more than 1 ED visit in the last 30 days of life, 6.3% of patients experienced multiple hospitalizations in the final month of life, and 5.5% experienced ICU admissions in the final month of life. Figure 1 depicts the trends in aggressive management for each indicator over the study time horizon stratified by the palliative designation. At all time points, patients who were designated as palliative were less likely to receive aggressive care according to all 4 quality measures.
Figure 1.
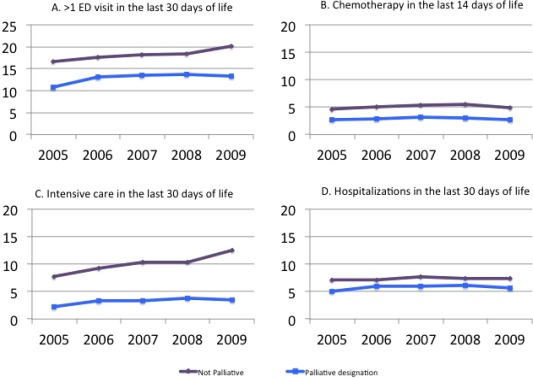
Trends in aggressive end‐of‐life care in Ontario according to the palliative designation (% of cohort on y‐axis): (A) >1 ED visit in the last 30 days of life, (B) chemotherapy in the last 14 days of life, (C) intensive care unit admissions in the last 30 days of life, and (D) hospitalizations in the last 30 days of life. ED indicates emergency department.
Costs of Care
The mean cost per patient in the last month of life was $13,903 Canadian dollars (median, $10,859; interquartile range [IQR], $5387‐$18,498). The predominant cost driver during this period was hospitalizations (contributing 64.7% of the total cost), with a mean cost of $8998.
Table 3 lists the overall mean costs and disaggregated costs for patients who received aggressive care and patients who did not. The mean per‐patient costs over the final month were $18,131 (median, $14,464; IQR, $9437‐$21,658) for patients receiving aggressive care and $12,678 (median, $9586; IQR, $4456‐$17,414) for patients receiving nonaggressive care; the difference was $5453 (P < .0001). For individuals with 1 (n = 16,867), 2 (n = 6220), 3 (n = 946), or all 4 indicators of care (n = 62), the mean per‐patient costs increased to $17,001, $20,304, $23,690, and $22,700, respectively.
Table 3.
Mean Costs for Aggressive and Nonaggressive Care (in Canadian dollars)
| Nonaggressive Care | Aggressive Care | |
|---|---|---|
| Hospitalizations | 7971 (12,483) | 12,541 (14,032) |
| Emergency department visits | 214 (325) | 826 (530) |
| Outpatient (same‐day) surgery | 17 (151) | 43 (251) |
| Outpatient cancer clinic | 237 (787) | 586 (1318) |
| Complex continuing care | 1046 (2951) | 273 (1259) |
| Long‐term care | 206 (718) | 75 (436) |
| Home care costs | 1370 (2229) | 988 (1306) |
| Chemotherapy (NDFP) | 28 (253) | 170 (699) |
| Oral/outpatient drugs | 401 (1089) | 454 (1044) |
| Physician billings | 1060 (1501) | 2041 (2201) |
| Other costsa | 127 (1912) | 133 (1841) |
| Total costs | 12,678 (12,754) | 18,131 (15,065) |
Abbreviation: NDFP, New Drug Funding Program.
Disaggregated costs may not add up to the total amount because of rounding. Data are presented as means and standard deviations.
Other costs include admissions for mental health, dialysis, rehabilitation, and devices.
Aggressive care patients incurred higher costs for hospitalization, ED visits, and physician services in comparison with nonaggressive care patients. In contrast, nonaggressive care was associated with higher costs for the provision of complex continuing care (including inpatient palliation) and home care. Hospitalizations remained the key cost drivers for patients managed both aggressively and nonaggressively ($12,541 and $7971, respectively).
We separately compared costs for individuals who received systemic chemotherapy in the last 14 days of life as the sole indicator of aggressive care. The mean per‐patient costs over the final month were $16,110 (median, $12,433; IQR, $8045‐$19,599) for patients receiving chemotherapy and $13,820 (median, $10,794; IQR, $5269‐$18,454) for patients who did not receive treatment (P < .0001). Patients treated with chemotherapy in the 2 weeks before death incurred higher hospitalization, ED, physician‐related, and chemotherapy drug costs, whereas those who did not receive this treatment incurred higher palliative care and home care costs (data not shown).
In the overall cohort, 69,715 patients (65.0%) were designated as palliative on the basis of system billing codes associated with the provision of palliative services. For patients ultimately managed aggressively and patients managed nonaggressively, the proportions of patients initially designated as palliative were 54.1% and 68.2%, respectively. Palliative patients incurred lower mean costs (mean, $13,503 vs $14,644) but higher median costs (median, $10,988 vs $10,573) in comparison with patients who were not palliative. We further analyzed the relation of the palliative designation with quintiles of costs. For individuals receiving end‐of‐life care in the highest cost quintiles (the top 2 quintiles), palliative care consultation was associated with reduced mean per‐patient costs. However, for individuals receiving end‐of‐life care in the lowest cost quintiles (the bottom 2 quintiles), palliation was associated with increased per‐patient costs. This relation between palliative care and costs was seen in patients who were managed either aggressively or nonaggressively. The most significant reduction in mean per‐patient costs associated with the palliative designation occurred in aggressively managed patients in the highest cost quintile (Fig. 2).
Figure 2.
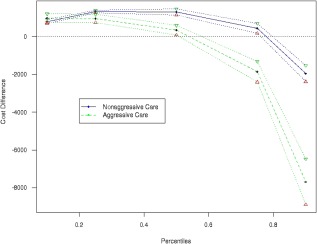
Effect of the palliative designation according to quintiles of end‐of‐life costs (in Canadian dollars) stratified by patients receiving aggressive care and patients receiving nonaggressive care.
Multivariate quantile regression models were constructed to study the effects of the palliative designation, aggressive care indicators, and patient variables on median end‐of‐life costs (Table 4). In the initial model including the palliative designation, individuals were more likely to have high end‐of‐life expenditures if they were younger, were female, were in the lowest income quintile, had been diagnosed with a hematologic malignancy, were affected by multiple comorbidities, had experienced a shorter duration of disease, or had died in the later years of the study period (for a model example, see online supporting information). The palliative designation independently reduced median costs by $423 (P < .0001). In subsequent models, the impact of the individual quality measures was studied (models 2‐5). In the model that incorporated chemotherapy in the last 14 days of life, this aggressive indicator was independently associated with increased costs (median increase, $536; P < .0001), whereas the palliative designation remained predictive of decreased costs (median decrease, $418; P < .0001).
Table 4.
Quantile Multivariate Regression to Estimate the Effects of Predictor Variables on Median End‐of‐Life Costs (in Canadian dollars)
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Level | Estimate (SE) | P | Estimate (SE) | P | Estimate (SE) | P | Estimate (SE) | P | Estimate (SE) | P |
| Intercept | 6562.99 (187.96) | <.0001 | 5991.87 (185.15) | <.0001 | 6509.6 (182.62) | <.0001 | 5902.1 (186.42) | <.0001 | 5651.37 (178.96) | <.0001 | |
| Agea | −64.22 (2.5) | <.0001 | −58.71 (2.44) | <.0001 | −63.41 (2.44) | <.0001 | −55.87 (2.51) | <.0001 | −52.93 (2.4) | <.0001 | |
| Sex | Maleb | — | .0447 | — | .0005 | — | .0287 | — | .0068 | — | .0006 |
| Female | 122.3 (60.93) | 192.53 (55.19) | 127.66 (58.36) | 165.58 (61.22) | 193.8 (56.57) | ||||||
| Year of deathc | 540.35 (21.86) | <.0001 | 523.55 (20.29) | <.0001 | 540.42 (20.81) | <.0001 | 515.36 (21.97) | <.0001 | 544.43 (20.33) | <.0001 | |
| Income quintile | Highestb | — | .1696 | — | .2253 | — | .1703 | — | — | .342 | |
| Second highest | 135.62 (98.73) | .0389 | 108.37 (89.37) | .0587 | 131.39 (95.82) | .0484 | 146.82 (99.31) | .1393 | 87.44 (92.02) | .1345 | |
| Middle | 204.26 (98.88) | .0864 | 153.43 (81.17) | .4671 | 188.09 (95.28) | .0863 | 201.37 (94.83) | .0337 | 130.92 (87.48) | .3506 | |
| Second lowest | 159.61 (93.09) | .0002 | 61.44 (84.48) | .0006 | 149.36 (87.07) | .0002 | 168.57 (91.51) | .0655 | 79.54 (85.22) | .0004 | |
| Lowest | 368.81 (98.97) | 297.01 (86.49) | 365.75 (96.94) | 404.14 (97.7) | <.0001 | 311.04 (87.31) | |||||
| Rurality | Ruralb | — | .5717 | — | .0093 | — | .6169 | — | .5622 | — | .0027 |
| Urban | 47.67 (84.28) | 214.26 (82.34) | 41.65 (83.26) | 48.47 (83.63) | 236.68 (78.96) | ||||||
| Cancer type | Nonhematologicb | — | <.0001 | — | <.0001 | — | <.0001 | — | <.0001 | — | <.0001 |
| Hematologic | 3749.67 (152.93) | 3554.97 (161.81) | 3728.2 (151.04) | 3054.94 (156.95) | 3333.51 (159.66) | ||||||
| Duration of cancer | — | <.0001 | — | <.0001 | — | <.0001 | — | <.0001 | — | <.0001 | |
| −23.94 (4.83) | −25.74 (2.7) | −23.59 (4.05) | −26.31 (4.97) | −24.82 (3.6) | |||||||
| Charlson score | 0b | — | — | — | — | — | |||||
| 1 | 1543.29 (192.49) | <.0001 | 1436.88 (166.97) | <.0001 | 1550.24 (193.89) | <.0001 | 1443.00 (169.11) | <.0001 | 1525.72 (175.7) | <.0001 | |
| ≥2 | 7045.36 (58.42) | <.0001 | 6609.97 (54.34) | <.0001 | 7039.43 (55.24) | <.0001 | 6697.68 (60.63) | <.0001 | 6517.95 (56.61) | <.0001 | |
| Palliative designation | Nob | — | <.0001 | — | <.0001 | — | <.0001 | — | .6155 | — | |
| Yes | −423.29 (65.45) | −236.50 (60.14) | −418.26 (62.21) | −33.21 (66.11) | −221.14 (61.37) | .0003 | |||||
| >1 ED visit | Nob | — | <.0001 | ||||||||
| Yes | 2636.41 (73.15) | ||||||||||
| Chemotherapy indicator | Nob | — | <.0001 | ||||||||
| Yes | 536.35 (89.26) | ||||||||||
| ICU indicator | Nob | — | <.0001 | ||||||||
| Yes | 9546.38 (258.61) | ||||||||||
| >1 hospitalization | Nob | — | <.0001 | ||||||||
| Yes | 6077.94 (102.11) | ||||||||||
Abbreviations: ED, emergency department; ICU, intensive care unit; SE, standard error.
Continuous variable centered on the age of 20 years.
Reference group.
Continuous variable centered on the year 2005.
DISCUSSION
Quality measures for the end‐of‐life period were initially derived to determine health care systems that might struggle in providing appropriate patient‐centered care.7 Although aggressive care may be warranted or requested for individual patients, on a system level, the associated indicators of such care might denote inadequate preparation or a lack of supportive services for those in the last days of life.24 These indicators have thus been positioned as system measures for poor‐quality care. Our results indicate that aggressive end‐of‐life care is more expensive than nonaggressive care. In the final month of life, the care for aggressively managed patients was $5453 more (or 43% costlier) than nonaggressive care. Aggressively managed patients were more likely to incur higher costs for acute care and physician services, whereas nonaggressively managed patients generated higher costs for home care and complex continuing care, which included the provision of inpatient palliation.
Because 3 of the 4 quality measures were intrinsically linked with more expensive resource utilization, namely, institutional care, we separately evaluated the costs with the chemotherapy indicator as the sole reflection of aggressive end‐of‐life care. Once again, patients treated aggressively according to this definition incurred higher costs than their nonaggressively treated counterparts.
Our study is the first to examine the unique relation of a palliative designation with aggressive management and end‐of‐life expenditures. We chose a palliative designation to reflect repeated and earlier access to palliation. For patients destined to receive more costly (and aggressive) care, we found that earlier access to palliative consultation and services could meaningfully reduce costs. For patients who would ultimately consume few health care resources (and potentially less aggressive care), palliative care was associated with higher costs, likely because of the intrinsic costs required for such care. The palliative designation remained predictive for decreased costs in multivariate models after adjustments for clinical variables. Notably, the sole model in which the cost savings with palliation did not achieve statistical significance (median decrease, $33; P = .6155) was the model in which the ICU indicator dramatically increased costs (median increase, $9546; P < .0001). In the remaining analyses, the palliative designation continued to independently predict lower costs. This is in keeping with prior research demonstrating less intensive care and resource consumption when earlier palliative care is instituted.14 In randomized trials of patients with advanced cancer, patients allocated to earlier palliative care reported improved quality of life and were less likely to receive aggressive end‐of‐life care.15, 25, 26 In an observational study, Zhang et al16 studied the impact of end‐of‐life conversations between cancer patients and physicians on costs in the last week of life. Patients who reported discussions about palliation incurred costs of $1876 (2008 US dollars), whereas the cost was $2917 for those who did not. We speculate that our palliative designation might serve as a surrogate for conversations about end‐of‐life preferences or the availability of palliative services and might potentially modulate medical expenditures even among those destined to receive aggressive care. Further research is needed to define the optimal timing and nature of palliative consultation to optimize resource utilization and, more importantly, patient outcomes.
Our results are in keeping with prior costing efforts for cancer patients at the end of life in both public27 and private health care systems.4 In the United States, Chastek et al4 used claims data to identify cancer‐related costs for 28,530 patients with active cancer 6 months before death. They established costs related to inpatient stays, hospice care, and outpatient services of $74,212 (2009 US dollars) and identified inpatient care as the key cost driver. This analysis focused on a select population of commercially insured individuals and did not stratify outcomes according to aggressive indicators of care. In contrast, a study from Kyoto, Japan did analyze costs according to select aggressive quality measures, albeit in a smaller population (n = 3143) of predominantly self‐employed, unemployed, or elderly individuals (with the majority older than 75 years).28 The investigators studied the costs associated with the last 3 months of life but exclusively for those who received institutional care. Death in an acute‐care hospital (rate ratio [RR], 1.32; P < .001) and chemotherapy in the last month of life (RR, 1.25; P < .001) were associated with higher end‐of‐life costs. In contrast to our results, palliative consultation did not abrogate costs (RR, 0.98; P = .66). This inconsistency may be attributable to differences in our definition of palliation; earlier and repeated access to palliative care consultation might be required to have a downstream impact on resource use and costs. Our research further builds on these data by calculating a broad range of cancer and non–cancer‐related costs in a large, unrestricted, and diverse cohort that includes the entire population of Ontario.
It is important to note potential limitations of this study. The quality measures of aggressive management are unable to consider patient preferences or define optimal care on an individual basis. There may be occasions when aggressive care is most appropriate and patient‐centered. Our results are subject to the limitations of the individual data sets used and the biases inherent to a retrospective design. In particular, we appreciate that not all relevant societal costs (eg, lost productivity and out‐of‐pocket costs) were captured, and they can impart a substantial financial burden borne by patients and their caregivers.29, 30 Finally, our results are based on the public insurance system, and their generalizability to private or mixed systems, as found in the United States, should be considered. It is likely that the cost implications related to aggressive end‐of‐life care are considerable in the United States. A parallel increase in aggressive end‐of‐life care over time has been documented in both countries. Moreover, the absolute intensity of care, reflected by higher utilization of chemotherapy and ICU care near the end of life, is notably higher in the United States versus Canada.5 Differences in physician and hospital remuneration may further drive this variability; for example, financial incentives for physicians to prescribe chemotherapy do not exist in Canada, and the public‐payer system may limit certain aspects of aggressive care, including the provision of chemotherapy for refractory disease.24, 31, 32 Ultimately, the goal of improving the quality of end‐of‐life care, increasing the availability of palliative care, and optimizing the distribution of limited resources is a universal pursuit common to all modern health care systems.
This study established the costs associated with the end‐of‐life period according to aggressive measures of care. Costs are substantially higher for patients managed aggressively in the final weeks of life, and they are driven by a heavy dependence on acute institutional care. Health care systems that seek to optimize the costs and quality of end‐of‐life care should explore the possibility that earlier palliation might temper the tendency toward aggressive management and ultimately offset costs.
FUNDING SUPPORT
This work was funded in part by the National Cancer Institute (grant R01 CA91753‐02) and an Ontario Institute for Cancer Research Health Services Program Grant.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosure.
Supporting information
Supplementary Information
REFERENCES
- 1. National Heart, Lung, and Blood Institute. Fact Book Fiscal Year 2012. http://www.nhlbi.nih.gov/files/docs/factbook/FactBook2012.pdf. Accessed March 17, 2015.
- 2. Tangka FK, Trogdon JG, Richardson LC, et al. Cancer treatment cost in the United States: has the burden shifted over time? Cancer. 2010;116:3477‐3484. [DOI] [PubMed] [Google Scholar]
- 3. Fireman BH, Quesenberry CP, Somkin CP, et al. Cost of care for cancer in a health maintenance organization. Health Care Financ Rev. 1997;18:51‐76. [PMC free article] [PubMed] [Google Scholar]
- 4. Chastek B, Harley C, Kallich J, et al. Health care costs for patients with cancer at the end of life. J Oncol Pract. 2012;8:75s‐80s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ho TH, Barbera L, Saskin R, et al. Trends in the aggressiveness of end‐of‐life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol. 2011;29:1587‐1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barbera L, Paszat L, Chartier C. Indicators of poor quality end‐of‐life cancer care in Ontario. J Palliat Care. 2006;22:12‐17. [PubMed] [Google Scholar]
- 7. Earle CC, Park ER, Lai B, et al. Identifying potential indicators of the quality of end‐of‐life cancer care from administrative data. J Clin Oncol. 2003;21:1133‐1138. [DOI] [PubMed] [Google Scholar]
- 8. Barnato AE, McClellan MB, Kagay CR, et al. Trends in inpatient treatment intensity among Medicare beneficiaries at the end of life. Health Serv Res. 2004;39:363‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nappa U, Lindqvist O, Rasmussen BH, et al. Palliative chemotherapy during the last month of life. Ann Oncol. 2011;22:2375‐2380. [DOI] [PubMed] [Google Scholar]
- 10. Harrington SE, Smith TJ. The role of chemotherapy at the end of life: "when is enough, enough?". JAMA. 2008;299:2667‐2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saito AM, Landrum MB, Neville BA, et al. The effect on survival of continuing chemotherapy to near death. BMC Palliat Care. 2011;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wright AA, Zhang B, Ray A, et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mack JW, Cronin A, Keating NL, et al. Associations between end‐of‐life discussion characteristics and care received near death: a prospective cohort study. J Clin Oncol. 2012;30:4387‐4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howie L, Peppercorn J. Early palliative care in cancer treatment: rationale, evidence and clinical implications. Ther Adv Med Oncol. 2013;5:318‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greer JA, Pirl WF, Jackson VA, et al. Effect of early palliative care on chemotherapy use and end‐of‐life care in patients with metastatic non–small‐cell lung cancer. J Clin Oncol. 2012;30:394‐400. [DOI] [PubMed] [Google Scholar]
- 16. Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end‐of‐life conversations. Arch Intern Med. 2009;169:480‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Balboni T, Balboni M, Paulk ME, et al. Support of cancer patients’ spiritual needs and associations with medical care costs at the end of life. Cancer. 2011;117:5383‐5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grunfeld E, Lethbridge L, Dewar R, et al. Towards using administrative databases to measure population‐based indicators of quality of end‐of‐life care: testing the methodology. Palliat Med. 2006;20:769‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clarke EA, Marrett LD, Kreiger N. Cancer registration in Ontario: a computer approach. IARC Sci Publ. 1991;95:246‐257. [PubMed] [Google Scholar]
- 20. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45:613‐619. [DOI] [PubMed] [Google Scholar]
- 21. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 22. Koenker R, Bassett G. Regression quantiles. Econometrica. 1978;46:33‐50. [Google Scholar]
- 23. Austin PC, Ghali WA, Tu JV. A comparison of several regression models for analysing cost of CABG surgery. Stat Med. 2003;22:2799‐2815. [DOI] [PubMed] [Google Scholar]
- 24. Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: is it a quality‐of‐care issue? J Clin Oncol. 2008;26:3860‐3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small‐cell lung cancer. N Engl J Med. 2010;363:733‐742. [DOI] [PubMed] [Google Scholar]
- 26. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster‐randomised controlled trial. Lancet. 2014;383:1721‐1730. [DOI] [PubMed] [Google Scholar]
- 27. Walker H, Anderson M, Farahati F, et al. Resource use and costs of end‐of‐life/palliative care: Ontario adult cancer patients dying during 2002 and 2003. J Palliat Care. 2011;27:79‐88. [PubMed] [Google Scholar]
- 28. Morishima T, Lee J, Otsubo T, et al. Association of healthcare expenditures with aggressive versus palliative care for cancer patients at the end of life: a cross‐sectional study using claims data in Japan. Int J Qual Health Care. 2014;26:79‐86. [DOI] [PubMed] [Google Scholar]
- 29. Longo CJ, Deber R, Fitch M, et al. An examination of cancer patients’ monthly ‘out‐of‐pocket’ costs in Ontario, Canada. Eur J Cancer Care (Engl). 2007;16:500‐507. [DOI] [PubMed] [Google Scholar]
- 30. Dumont S, Jacobs P, Turcotte V, et al. The trajectory of palliative care costs over the last 5 months of life: a Canadian longitudinal study. Palliat Med. 2010;24:630‐640. [DOI] [PubMed] [Google Scholar]
- 31. Jacobson M, O'Malley AJ, Earle CC, et al. Does reimbursement influence chemotherapy treatment for cancer patients? Health Aff (Millwood). 2006;25:437‐443. [DOI] [PubMed] [Google Scholar]
- 32. Matsuyama R, Reddy S, Smith TJ. Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J Clin Oncol. 2006;24:3490‐3496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


